Abstract
The following work was initiated to determine the scope of application methodology and fumigant combinations for increasing efficacy of 1,3-dichloropropene (1,3-D) and metam sodium for management of root-knot nematodes (Meloidogyne spp.) in Florida. A series of five experiments were set up during spring and fall seasons to evaluate the potential of different fumigants, alone or in combination, in polyethylene film tomato production. The most promising chemical alternatives to methyl bromide, in terms of root-knot nematode management, were the combinations 1,3-D-chloropicrin, chloropicrin-proprietary solvent ,and 1,3-D-metam sodium. Sprayed or injected metam sodium generally provided only short-term nematode management and by harvest nematode infection was not different from the nontreated control. Drip-applied metam sodium gave good nematode management under high nematode pressure, but needs further verification to establish (i) the importance of soil moisture and temperature on treatment efficacy and (ii) whether similar management can be obtained with fewer than three drip tubes. Broadcast applications of 1,3-D showed better efficacy as compared to applications on a preformed raised bed. Fumigation did not increase tomato yields in spring when root-knot nematode pressure was low, but during fall all chemical treatments increased yields three to five-fold, as root-knot nematode was a major yield-limiting factor.
Keywords: 1,3-dichloropropene; metam sodium; fumigation; plasticulture
Fumigation with methyl bromide has been used for more than 50 years to manage soilborne pests and diseases, including plant-parasitic nematodes, in a wide range of horticultural crops. Because methyl bromide was categorized as a Class I ozone-depleting substance by the Montreal Protocol, production and use of methyl bromide was phased out internationally in developed countries in 2005 with scheduled reductions planned during the interim (Anonymous, 1992, 2000). Preplant fumigation with methyl bromide in the United States is no longer used, except for a limited amount on California strawberries (Arling, 2015).
Since the phase out, a significant amount of research has been dedicated to finding alternative fumigants to methyl bromide. Much of this research was focused on evaluating existing fumigants that had limited usage as long as methyl bromide was available, such as 1,3-dichloropropene (1,3-D), metam sodium, and chloropicrin, as well as some new fumigants, such as dimethyl disulfide (DMDS) and allyl isothiocyanate (AITC). Currently, the most commonly used soil fumigants for nematode management in vegetables are 1,3-D (Locascio et al., 1997; Lamberti, 2000; Gilreath and Santos, 2004; Gilreath et al., 2005), DMDS (Rosskopf et al., 2006; Cabrera et al., 2014; Leocata et al., 2014), and a 3-way combination of 1,3-D + metam sodium or metam potassium + chloropicrin (Gilreath and Santos, 2008; Schneider et al., 2008; Noling et al., 2010). When 1,3-D is formulated with chloropicrin broad-spectrum management of plant-parasitic nematodes and most soilborne fungi is attained; however, it provides little if any weed management. In Florida, nematode management with 1,3-D + chloropicrin (C-35) has given mixed results but when applied by appropriate methods it mostly has been similar to methyl bromide with the exception of weed management (Dickson et al., 1999; Gilreath et al., 2004a, 2004b; Santos et al., 2006a, 2006b). There are, however, limitations and restrictions that pose problems for its use, e.g., restricted use pattern in the southern most region of Florida to protect groundwater. Most of Florida has karst topography, to which application of 1,3-D is not allowed, and worker protection issues and restrictions on distances from dwelling pose hardships for growers (Overman and Jones, 1984, 1986; Anonymous 2002; Telone C-35 label, Dow AgroSciences LLC, Indianapolis, IN).
The main disadvantage that all alternative fumigants have compared to methyl bromide is their lower vapor pressure (volatility), which means that uniform soil distribution is more difficult to attain. This is especially true for metam-type products (a.i. = methyl isothiocyanate [MIT]), which are about 100 times less volatile than methyl bromide (Ajwa et al., 2002; Ruzo, 2006). Fumigating vegetable crops with metam sodium or potassium, which have a more broad-spectrum pesticidal activity, has given variable results, particularly against root-knot nematodes (Locascio and Dickson, 1998; Desaeger and Csinos, 2006; Zasada et al., 2010). The high variation in product efficacy indicates the need for a better understanding of the specific requirements with regard to application methods and soil and climatic conditions.
Soil cultivation, rototillage, soil sealing by power rolling, and deep placement with chisels are some of the practices that may improve the effectiveness of traditional metam sodium/potassium applications. An alternative application method is to deliver the chemical through the drip irrigation system. This could not only improve its distribution in the soil (Gerstl et al., 1977; McGovern et al., 1998), but would also reduce worker exposure. One must first ensure the product will work reliably when delivered through drip irrigation, and then determine how to match satisfactory delivery with minimum cost. For the moment only combinations of different chemicals can offer the broad-spectrum pest–pathogen management provided by methyl bromide. Chemical combinations, such as chloropicrin + 1,3-D (C-35) mixtures, plus a suitable herbicide increase nematicidal efficacy and broaden the range of toxicological activity (Munnecke and Van Gundy, 1979; Rich et al., 2003; Schneider et al., 2008).
The following work was initiated to determine appropriate application methodology and fumigant combinations for increasing efficacy of 1,3-D and metam sodium for management of root-knot nematodes (Meloidogyne spp.) in Florida. A total of five experiments were set up during spring and fall seasons to evaluate the effectiveness of different fumigants, alone or in combination, in raised bed polyethylene film tomato production.
Materials and Methods
Experiments were conducted at the Plant Science Research and Education Unit at Citra, Marion County, Florida, during spring and fall seasons of 2001. The experimental area was previously covered with grasses and trees, and used as grazing land. Soils are fine sandy Arredondo and Sparr type (sand 95%, silt 3%, clay 2%; organic matter 1.5%; pH 6.5), 1 to 2 m in thickness, with some underlying loamy materials. Moisture content of the soil at field capacity was 13.5% to 30-cm deep and bulk density of the planting beds was 1.4 g/cm3.
Spring and fall tests were conducted on separate, adjacent blocks of 12-m wide × 183-m long. Root-knot nematodes were inoculated into each of the blocks by spreading heavily galled chopped tomato roots in to 15-cm deep furrows spaced 45-cm apart, followed by rototillage, and cropping okra at least one season before the area was used for testing chemical efficacy.
The two spring test sites were inoculated with Meloidogyne arenaria race 1 and planted with okra in the previous spring. A rye crop was planted during the following winter. The sites were disked and rototilled during late January (1,3-D test site) or mid-February (metam sodium test site). Final land preparations and fumigants were applied mid-February (1,3-D test site) and 23 to 29 March, 2001 (metam sodium test site). In the metam sodium site, about 2.5 cm of water was applied by overhead irrigation in the late afternoon before fumigation was to occur. The following morning broadcast applications of metam sodium were applied to a flat soil surface. A preplant application of fertilizer was made, and the beds were fumigated and immediately covered with black polyethylene mulch. Soil moisture content at fumigation was 75% of field capacity and soil temperature at 10 cm deep was 19 °C.
A double-wall single drip tube (Chapin Watermatics, Watertown, NY) with emitters spaced 30-cm apart and a flow rate of 62 liters/minute per 30.5 m of row was placed under the polyethylene mulch before transplanting for application of water, fertilizer, calcium, and metam sodium. Drip irrigation was applied once or twice daily (depending on the need) throughout the season. Before fumigation about 2.5 cm of water was applied by overhead irrigation to ensure sufficient moisture for building firm raised beds and 76 liters of water/bed was applied before and after applying metam sodium through the drip system.
Methyl bromide, 1,3-D, and 1,3-D + chloropicrin were applied with a closed-system mulch-laying fumigant injection rig (Kennco, Ruskin, FL), through three backswept chisels spaced 30-cm apart and adjusted to deliver the fumigants ca. 30-cm deep beneath the final bed surface. Chloropicrin was chisel injected with two sweeps (each with three outlets spaced 15-cm apart) at 23-cm deep. Methyl bromide was applied on preformed beds in all tests. For 1,3-D and 1,3-D + chloropicrin, both in-bed and broadcast (on the flat) applications were tested. The broadcast fumigants were custom applied by Dow AgroSciences experimental fumigation rig equipped with 65-cm diam. cutting coulters, skid shoes, and V-closing press wheels (Yetter Farm Equipment, Colchester, IL). The fumigants were placed ca. 30-cm deep.
Metam sodium was applied (i) through chisel injection, (a) using the same rig as for chloropicrin and (b) using a rig with four sweeps, each with three outlets spaced 15-cm apart (spring test 2); (ii) by broadcast spraying on the flat using a rig that sprays metam sodium in front of a powered rototiller, followed by a powered roller (spring and fall tests); and (iii) through drip application, using three drip tubes per bed, spaced 25- to 30-cm apart.
Metam sodium was applied both on preformed beds and on the flat soil surface in spring test 1, and only on the flat (except for the drip application) in spring test 2 and the fall test. Nitrogen gas was used to pressurize the fumigant cylinders and a power take-off pump was used in the delivery of metam sodium via the powered rototiller application.
Fumigants were applied midmorning to midafternoon and beds covered with black polyethylene mulch (1.25-mm thick × 1.5-m wide) immediately after fumigation, using a mini-combo unit attached to a separate tractor. A more impermeable mulch type (virtually impermeable film [VIF]) was included as an extra treatment in the metam sodium fall test.
After a 20-day waiting period, tomato cv. Florida 47 bare-root seedlings were transplanted by hand on 19 April in holes made by a hole puncher (45-cm apart). Beds were wet by the drip line prior to transplant, and 150 ml of transplant water added per seedling. Any dead or dying seedlings were reset over the next week. The plants were staked on 7 May and tied on 15 May and again 3 weeks later. Harvest dates were 27 June and 10 July.
The fall test site was infested with Meloidogyne javanica and planted with okra in the spring. The land was disked and rototilled on 20 July, 2001, and final land preparations were done on 25 July. Irrigation was done as in spring and fumigants were applied on 26, 27, and 31 July in the metam sodium and 1,3-D tests. Soil moisture content at fumigation was determined at 70% of field capacity and soil temperature at 10 cm deep was 31 °C.
Before transplanting tomato, the black plastic mulch in fall was painted white to avoid heat damage to the crop. Tomato (cv. Florida 47) seedlings were transplanted on 23 August as described earlier for spring. Any dead or dying seedlings were reset over the next 14 days. The plants were staked on 27 and 28 September (metam sodium and 1,3-D tests).
Temperature and rainfall were recorded within 500 m of the experimental site. Total rainfall was 421 mm for the spring season and 411 mm for the fall season. Minimum and maximum temperatures at 60 cm above ground at planting, flowering, and harvest of the crop were respectively 5 to 31 °C, 12 to 31 °C, and 19 to 35 °C during spring, and 20 to 35 °C, 15 to 32 °C, and 10 to 29 °C during fall.
Experimental set-up and treatments:
Plots were separate single row raised beds 22-cm tall, covered with polyethylene mulch, 12.2-m long on 1.8-m centers with a 90-cm-bed width, arranged in a randomized complete block, and replicated four times. Alleyways between tests were 9-m wide. All five tests were conducted on adjacent blocks. Metam sodium was evaluated in three tests, two in spring and one in fall; 1,3-D was evaluated in two tests each, one in spring and one in fall.
Metam sodium, spring test 1:
All dosages are for broadcast application unless otherwise noted. Treatments were (i) methyl bromide (67%) + chloropicrin (33%) injected at 393 kg/ha on preformed raised beds (Mbr); (ii) 1,3-D (65%) + chloropicrin (35%) injected on preformed raised beds at 327 liters/ha (C-35); (iii) a combination of 1,3-D injected at 168 liters/ha and metam sodium sprayed and rototilled at 351 liters/ha (on preformed raised beds) (MST); (iv) a combination of metam sodium sprayed and rototilled at 702 liters/ha on 1.8 m wide flat soil surface and chloropicrin injected at 280 kg/ha (on preformed bed) metam sodium-chloropicrin (MSC); (v) metam sodium sprayed and rototilled at 702 liters/ha on flat soil surface (broadcast) (MS-s); (vi) metam sodium injected at 702 liters/ha with two sweeps each rigged with three outlet ports spaced 15-cm apart 30-cm deep (on preformed raised beds) (MS-i); (vii) metam sodium sprayed and rototilled at 421 liters/ha on flat soil surface (broadcast) + injected with the two sweeps at 281 liters/ha (preformed raised beds) (MS-si); (viii) metam sodium applied via drip irrigation at 468 liters/ha (preformed raised beds) dripped MS (MS-d); and (ix) nontreated control.
Metam sodium, spring test 2:
Treatments were (i) methyl bromide (67%) + chloropicrin (33%) injected at 393 kg/ha on preformed raised beds (Mbr); (ii) C-35 at 243 liters/ha; (iii) MST, with the same rate of 1,3-D and 468 liters/ha of MS sprayed and rototilled, both on flat soil surface (broadcast) applied and with a 5-day interval; (iv) MSC, with 168 kg/ha of chloropicrin broadcast injected and the same rate of MS sprayed and rototilled; (v) MS-s at the same rate; (vi) MS-si, with 351 liters/ha MS injected with four sweeps and 351 liters/ha MS sprayed and rototilled, both applied on flat soil surface (broadcast) applied; (vii) MS-d at the same rate; and (viii) nontreated control.
Metam sodium, fall test:
Treatments were (i) at the same rate; (ii) C-35 at 337 liters/ha; (iii) MST with the same rate of 1,3-D and metam sodium sprayed at 468 liters/ha, both broadcast applied; (iv) MSC with the same rate of metam sodium broadcast applied and 140 kg/ha of chloropicrin, injected in the bed; (v) MSs at the same rate; (vi) same as (v) with VIF mulch; (vii) MS-d at the same rate; (viii) methyl bromide (70%) + 1,3-D (30%) injected at 393 kg/ha (on preformed raised beds) (MbrT); (ix) DeSolve 80 (50% chloropicrin + 50% proprietary solvent [Hendrix and Dail, Cairo, GA]) injected at 224 kg/ha (on preformed raised beds) (CDeS); and (x) nontreated control. Pebulate at 4.5 kg/ha was sprayed for nutsedge management in both spring tests as part of MST treatments.
1,3-D, spring test:
Treatments were (i) at the same rate; (ii) 1,3-D at 168 liters/ha applied on a flat soil surface (broadcast); (iii) same as (ii), but on preformed bed; (iv) C-35 at 243 liters/ha on flat soil surface (broadcast); (v) same as (iv), but on preformed bed; (vi) chloropicrin at 264 kg/ha (on preformed bed); (vii) a combination of 1,3-D at 168 liters/ha (broadcast) and chloropicrin at 132 kg/ha (in bed); (viii) 1,3-D (65%) + chloropicrin (35%) at 243 liters/ha + oxamyl dripped at 4.7 liters/ha at 4 and 6 weeks after transplanting (all on preformed raised beds); and (ix) nontreated control.
1,3-D, fall test:
Treatments (i) to (vi) and treatment (ix) were identical to the 1,3-D spring test. Treatment (vii) had the same rate of 1,3-D, but with chloropicrin at 157 kg/ha (broadcast) and metam sodium dripped in the bed at 468 liters/ha; treatment (viii) had C-35 injected at 337 liters/ha + oxamyl dripped at 4.7 liters/ha at 4 and 6 weeks. Three additional treatments were included: (ix) 1,3-D (65%) + chloropicrin (35%) at 243 liters/ha + chloropicrin at 157 kg/ha applied on the flat soil surface (broadcast); (x) oxamyl dripped at 4.7 liters/ha at planting, 2, 4, 6 and 8 weeks after planting (on preformed raised beds); (xi) oxamyl dripped at 4.7 liters/ha at 4 weeks after planting (on preformed raised beds). Pebulate at 4.5 kg/ha was sprayed in spring as part of all treatments, except for Mbr and nontreated beds.
Tomato yellow leaf curl virus (TYLCV) was a major problem in the fall tests. In the metam sodium fall test, two entire plots, where the infestation started, were rogued at 1 month, and several individual plants were removed during the next 2 weeks.
Data collection:
Tomato fruits were harvested at color break from the 12 central plants in each plot. Picking was done twice with 1 to 2 week intervals. The fruits were graded according to size (extra large, large, and medium size) and weighed. Small-sized fruit (culls) were discarded.
Root-knot nematode galling was assessed immediately after the final picking for all 12 plants on an index scale of 0 to 10 (0 = no galls, 1 = very few small galls, 2 = numerous small galls, 3 = numerous small galls of which some are grown together, 4 = numerous small and some big galls, 5 = 25% of roots severely galled, 6 = 50% of roots severely galled, 7 = 75% of roots severely galled, 8 = no healthy roots but plant is still green, 9 = roots rotting and plant dying, and 10 = plant and roots dead) (Zeck, 1971).
Nematode soil populations at harvest of tomato were determined in the metam sodium tests by taking 12-soil cores (2.5-cm-diam., 20-cm-deep, using a cone-shaped sampling tube) from each plot (11 July for spring, and 14 December for fall tests). Soil cores from each plot were combined and nematodes extracted from a 100 cm3 (170 g wet weight) subsample using a centrifugal-flotation method (Jenkins, 1964). Soil samples also were collected at pretreatment (20 March) in the spring tests.
In the metam sodium fall test, additional samples were collected 1 month after transplanting (24 September). Three arbitrarily selected plants outside the center of the bed were rated for root galling (0–10 scale) and a 0.1 to 0.5 g subsample (depending on the amount of roots) stained for Meloidogyne egg masses using Phloxine B (Holbrook et al., 1983). Nematode soil populations were measured by taking soil cores from the same three planting holes. Plant growth at this stage was determined by taking fresh and dry weights of roots and shoots (after drying in an oven for constant weight). As significant seedling mortality occurred in the fall test, survival of tomato transplants was counted throughout the first month after 5, 7, 9, 15, and 30 days. TYLCV affected tomato plants in fall, and virus-infested plants were counted 1, 2, and 3 months after transplanting.
All data were subjected to analysis of variance using a SAS statistical package, and mean differences were separated by Duncan’s multiple-range test.
Results
Metam sodium tests, spring season:
Initial nematode populations were low, with an average 5 M. arenaria juveniles and <1 Paratrichodorus spp./100 cm3 of soil in test 1, and practically undetectable levels in test 2 (data not given).
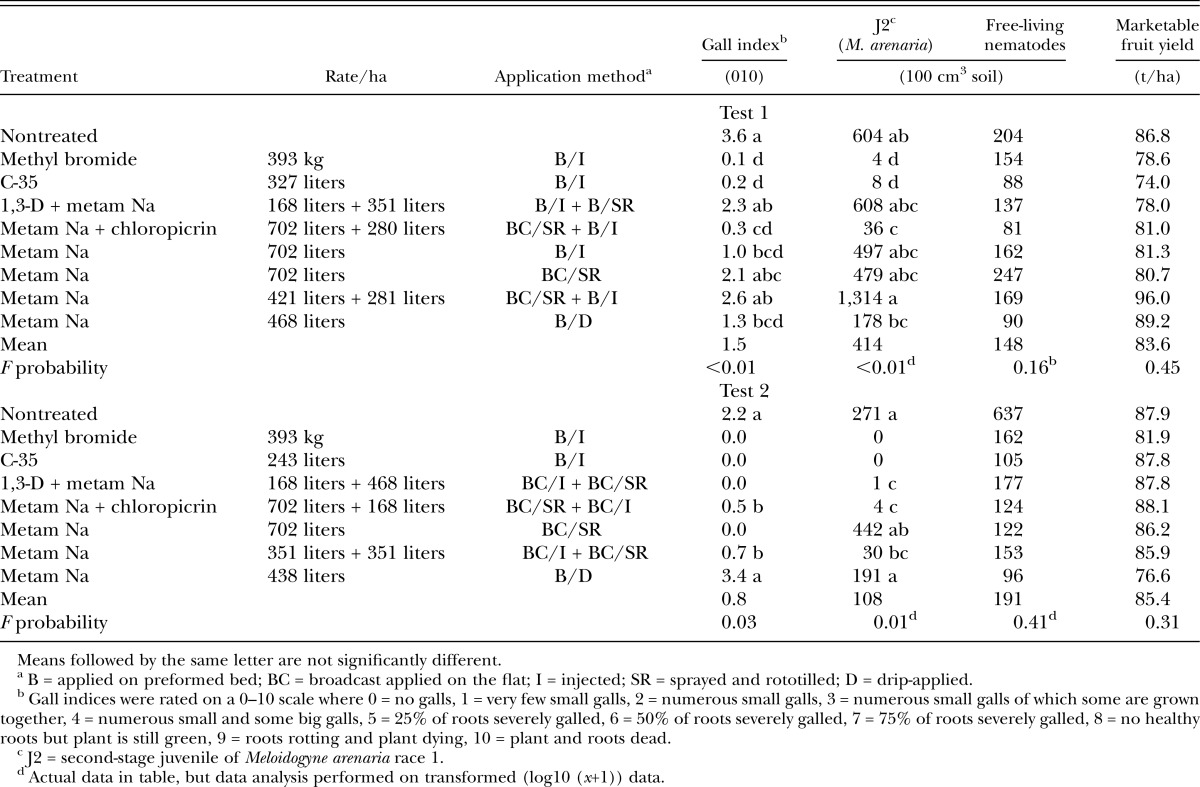
By harvest, tomato showed moderate root-knot nematode galling in test 1 and (very) low galling in test 2 (Table 1). Gall indices (GI) were significantly reduced with methyl bromide (Mbr)-chloropicrin, C-35, and MSC in both tests. Metam sodium (MS) by itself—be it injected, sprayed, or dripped—generally provided poor management of root-knot nematodes. In test 2, root galling was only noted in the nontreated control and the MS-d application.
Table 1.
Effect of multipurpose soil fumigants and different application methods of metam sodium on root-knot nematode gall index, nematode soil populations and yield of spring-planted tomato cv. Florida 47 at harvest in Plant Science Unit, Florida (metam sodium spring tests).
Root-knot nematode soil population densities in the nontreated plots at harvest also were greater in test 1 (ca. 600 J2/100 cm3 soil) than in test 2 (ca. 270/100 cm3 of soil) (Table 1). Much lower populations were detected for the C-35 and MSC applications. High root-knot nematode soil population densities, similar to the nontreated control, were noted in the other MS treatments, including the metam sodium-1,3-D (MST) combination in test 1.
Very few other parasitic nematodes were noted. Both Criconemella spp. (ring nematodes) and Paratrichodorus spp. (stubby root nematodes) were found at about 10 nematodes/100 cm3 of soil, most of them in the nontreated plots. Populations of free-living nematodes at tomato harvest were largely made up of bacterivorous rhabditidae (>90% of the total free-living population), and were not affected by any of the chemical treatments (Table 1). Tomato yields in both tests averaged 42 t/ha and there were no differences among treatments (Table 1).
MS tests, fall season:
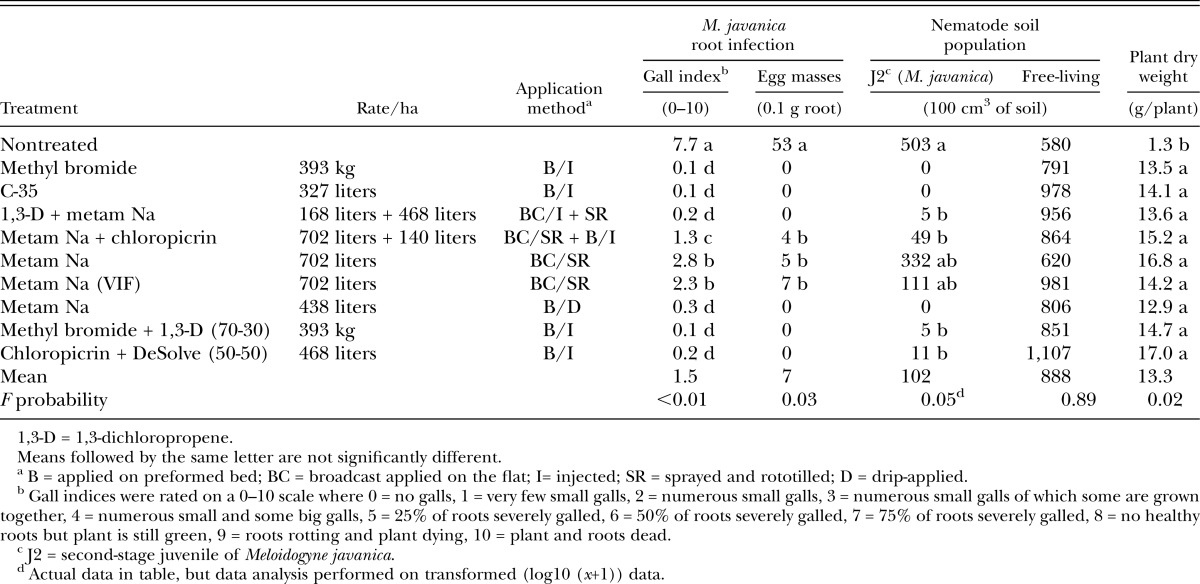
High seedling mortality was observed in the nontreated beds during the first month after transplanting. Almost half of the seedlings (45%) in nontreated plots died, even after replacing them three times. Mortality in any of the fumigated beds varied from 1% to 7% during the first 10 days, and from 0% to 1% additional later on. Seedlings were checked for fungal diseases, but only traces of root rot causing fungi (Pythium spp. and Fusarium oxysporum) were found. Root-knot nematode (M. javanica) infection was severe in nontreated beds, with an average GA of 7.7 and 53 egg masses/0.1 g root (Table 2). Nematode galls, although significantly less than in nontreated beds, also were observed in MS- and MSC-treated beds. VIF-mulch did not affect root galling. In any of the other applications, including the drip-applied MS, tomato roots were largely free of root-knot nematodes. Similarly, Meloidogyne J2 soil population densities at 1 month after planting were significantly higher in nontreated beds as compared to all other treatments, except for the MS sprayed beds, which had intermediate population densities (Table 2).
Table 2.
Effect of multipurpose soil fumigants on root-knot nematode infection, nematode soil populations and plant biomass of fall-planted tomato cv. Florida 47 at 1 month after planting in Plant Science Unit, Florida (metam sodium fall test).
Other plant-parasitic nematodes at this stage were few, only four Paratrichodorus spp./100 cm3 of soil were noted. Free-living nematodes, mainly bacterivorous rhabditidae, were common, and did not show any difference among treatments (Table 2). Omnivorous dorylaimid nematodes were mainly found in the nontreated beds (55 nematodes/100 cm3 of soil versus <8 in any of the treated plots, data not given). Initial plant growth (dry weights) was very poor in the nontreated beds and was significantly better in any of the fumigated beds (P = 0.05) (Table 2).
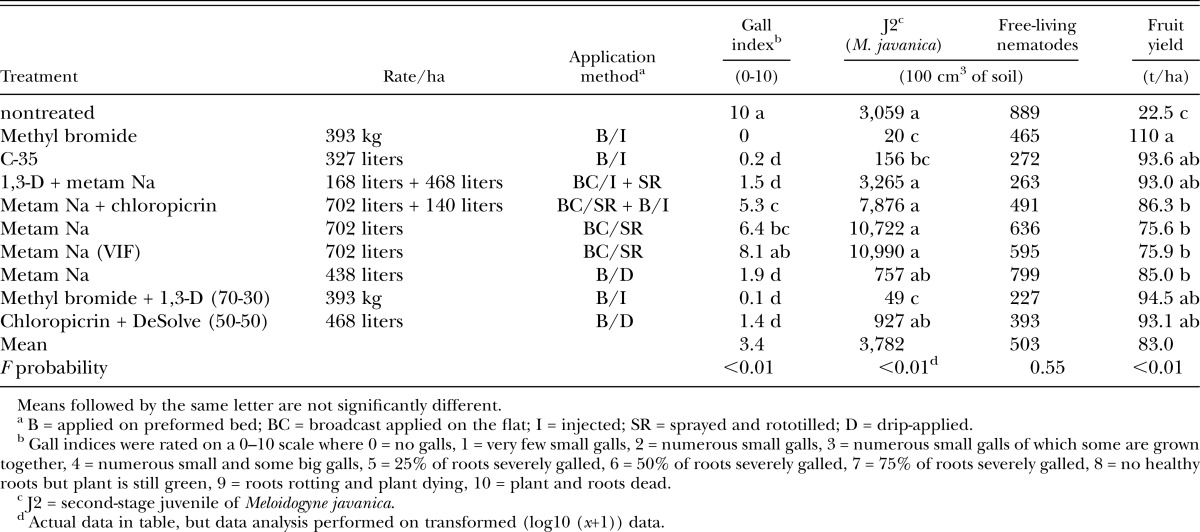
By harvest, roots were 100% galled (GI = 10) throughout all nontreated beds (Table 3). High GI also were observed in the MS-fumigated plots, with or without VIF. No or very few galls were noted for methyl bromide and C-35-treated beds. Also the MST and the chloropicrin-DeSolve (CDeS) combinations, as well as the MS-d application showed only minor root galling (GI = 1–2). The MSC mixture showed intermediate root galling (GI = 5).
Table 3.
Effect of multipurpose soil fumigants on root-knot nematode infection, nematode soil populations and marketable fruit yield at harvest of fall-planted tomato cv. Florida 47 at Plant Science Unit, Florida (metam sodium fall test).
Root-knot nematode soil population densities at harvest varied greatly, from only 20 J2/100 cm3 of soil in the treated beds, to more than 3,000 J2 in the nontreated beds, up to almost 11,000 J2/100 cm3 of soil in the MS-treated beds with VIF mulch (Table 3). Root-knot nematode males also were most common with the latter treatments (30 males/100 cm3 of soil, as compared to less than five for the other treatments, data not given). In general, methyl bromide-1,3-D (MbrT) and C-35 fumigation gave lowest root-knot nematode population densities, whereas MS, alone or in combination with chloropicrin (MSC) or 1,3-D (MST), gave highest populations. The MS drip application and the CDeS fumigation had populations in between.
Paratrichodorus spp. were more numerous at harvest than at 1 month (28 nematodes/100 cm3 of soil, no difference between treatments). Few Criconemella spp. (ring nematodes) also were present, though exclusively in the nontreated beds (45 nematodes/100 cm3 of soil). Free-living nematode populations were slightly lower than at 1 month and again did not show any difference among treatments (Table 3). Dorylaimid population, however, was still smaller in the fumigated beds (81 nematodes/100 cm3 of soil in the nontreated control versus <35 in any of the treated plots, data not given).
Poor yields, only 22.5 t/ha, were recorded in the nontreated beds (Table 3). Any of the fumigated beds gave three to five times greater yields. The standard fumigation had highest yields at 110 t/ha, which was largely due to more extra large tomato fruit. If tomato yields are expressed relative to the yield obtained with maximum nematode management (GI <1, as found with MbrT and C-35), yields were 5% less for MST and CDeS treatments (93 t/ha), 15% less for the MSC and dripped MS treatments (86 t/ha), and 25% less for the sprayed MS treatments (irrespective of mulch type) (76 t/ha).
1,3-D tests:
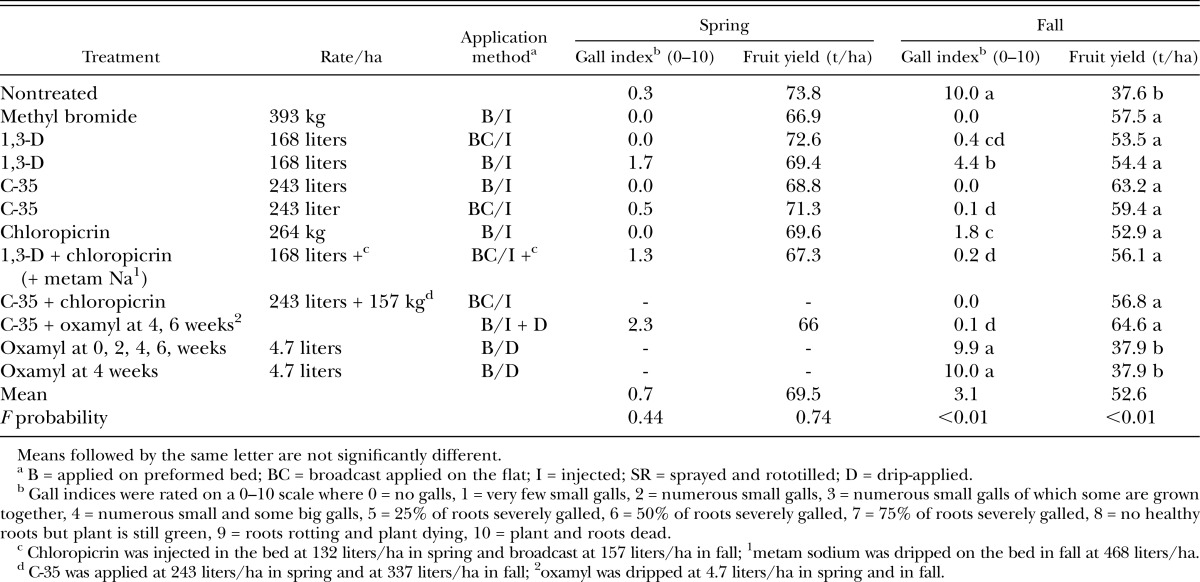
Similarly to the MS spring test, root galling in the 1,3-D spring test was low, with an average GI of 0.3 in the nontreated control, and no difference among treatments (Table 4). Also, tomato yield was an average of almost 70 t/ha, and were not different among treatments.
Table 4.
Effect of multipurpose soil fumigants and broadcast versus in-bed applications of 1,3-dichloropropene (1,3-D) on root-knot nematode gall index and marketable yield of tomato cv. Florida 47 at Plant Science Unit, Florida during spring and fall (1,3-D tests).
Severe root-knot nematode infection occurred in the fall test. At harvest, tomato roots were 100% galled in the nontreated control and in the two sole oxamyl treatments (Table 4). No galling was noted in the Mbr and C-35-treated beds. Low GI also were observed with chloropicrin and with 1,3-D, although the latter had significantly more root galls when applied on preformed beds. Tomato yields averaged just more than 52 t/ha and were about 35% less in the nontreated control and sole oxamyl treatments as compared to the average of all other treatments.
Other pest-disease problems:
No major problems occurred during spring, with few scattered plants showing symptoms of tomato spotted wilt virus. During fall, tomato suffered serious infestation by the white fly-transmitted TYLCV. Despite transplants being treated with the systemic insecticide imidacloprid before planting, the virus was observed on tomato as soon as 4 weeks after planting. The virus causes stunting of tomato and flower abortion, and the earlier the plants are infected, the greater the impact. Once the virus has set in, little fruit is produced. The MS test was infected first and suffered greatest infection by the virus. Despite removing the earliest infected plants, repeated applications (at 1, 2, and 3 months) of the insecticide imidacloprid through the drip system, as well as biweekly spraying against white flies, the virus rapidly spread through the entire trial. Out of four blocks, two were 100% infected, one about 50% and one about 10%. Tomato yields were different according to blocks, with the block having the least TYLCV giving 60% higher yields than the heavily virus-infected blocks (53 t/ha versus 30 t/ha). The virus was much less of a problem in the other two fall tests, and affected on average 10% of plants in the 1,3-D-test (mostly in one block).
As the virus affected all treatments similarly, data analysis was done including all virus-infested beds, except for two missing plots in the MS test that had been entirely rogued at 1 month.
Nutsedge (Cyperus spp.) was relatively common during fall, especially on the bed-ends and in the MS test. High nutsedge cover (>50%) in bed-centers was observed in one block where tomato plants suffered first and most severe TYLCV infection.
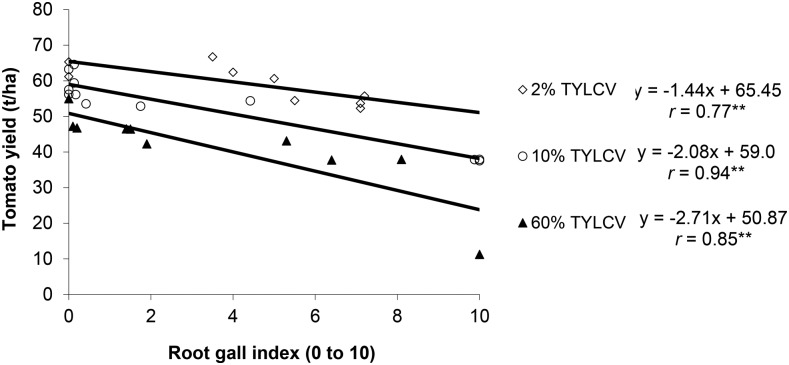
Tomato yields in fall were similar to spring for the MS tests, and 25% less than in spring for the 1,3-D tests. Nematode damage was apparent in all of the fall tests, and yield loss was most severe in the MS test, which was worst hit by TYLCV. Significant negative relationships between nematode galling at harvest and tomato yield were found in all three tests, but baseline yields decreased and regression slopes became steeper with higher TYLCV-infestation (Fig. 1).
Fig. 1.
Effect of nematode root gall index at harvest on marketable yield of fall-planted tomato in three separate tests, having different incidence of tomato yellow leaf curl virus (TYLCV), Plant Science Unit, Florida.
Early-growth nematode assessments in the MS test showed even better relations between M. javanica infection and yield (y) of tomato (y = 48.6 − 4.73x, r = −0.97**, where x is root GI (0–10); y = 47.6 − 0.06x, r = −0.90**, where x is J2/100 cm3 of soil).
Discussion
Nematode pressure:
Very low nematode infection in spring as opposed to very high infection in fall was probably due to a combination of factors. (i) The spring site was infested with M. arenaria, while the more aggressive root-knot nematode species, M. javanica, infested the fall test site. (ii) Nematode survival ahead of the tests was probably greater for the fall test, as nematode inoculum had not been exposed to a cold season. (iii) Higher soil temperatures in fall allowed more rapid reproduction of the nematode.
During the fall, tomato roots in nontreated beds were heavily galled and yellowing and wilting were observed as soon as 1 to 2 weeks after transplanting. Even with ample supply of water (twice daily) and fertilizer (weekly), tomato root growth in these plots was not able to overcome the damage inflicted by root-knot nematodes.
Nematode pressure in the spring tests was too low to have any negative effect on tomato growth, and all treatments within the same test had similar yields. During fall significant yield differences were seen in all tests, 35% (1,3-D test) and 80% (MS test) less yield than the methyl bromide-chloropicrin standard. Yield losses were well correlated with root-knot nematode incidence. Early growth nematode assessments (root gall ratings and nematode soil populations) proved good predictors of future yield loss. At-harvest nematode soil populations rarely correlate with yield loss, as by that time nematodes on the most severely affected plants usually have depleted their food source.
Root GI among all tests seem to indicate a yield loss threshold level in tomato when root galling reaches about 50% (GI = 5), and no yield reductions were observed when root galling was below that level. However, although low nematode levels do not seem to pose a threat during the same season, the higher build up could cause yield loss in the second crop.
Tomato plants were more severely affected by root-knot nematode infection when incidence of TYLCV was high, as the latter decreased the plants’ vigor and photosynthetic capacity. Limited yield differences between both seasons, despite higher pest pressure of root-knot nematodes, TYLCV and nutsedge in fall, was probably related to seasonal effects, the use of different tomato cultivars, and the different preplant fertilizer rate that was applied in fall (40% less N, 70% more P, and 60% more K than in spring).
Populations of other plant-parasitic nematodes than Meloidogyne were low. Criconemella spp. (ring nematodes) were exclusively noted in the nontreated plots, indicating that they were very susceptible to any of the chemical treatments. Ring nematodes are not known as being virulent pathogens of tomato. Paratrichodorus spp. (stubby root nematodes) may be pathogenic to tomato, but only at much greater levels than observed in this trial. Some species of Paratrichodorus are known to migrate vertically in the soil to escape unfavorable conditions, which may explain why they appeared to be somewhat less susceptible to fumigation (Ingham et al., 2000).
Populations of free-living nematodes, mostly bacterivorous Rhabditidae (>90% of the total free-living population), recovered rapidly following fumigation. Bacterial feeding nematodes are typical colonizers, and rapidly dominate empty niches (Neher, 2001), as can be expected after fumigation. Populations of free-living Dorylaimidae, which have much longer life cycles, were very sensitive to fumigation, irrespective of the chemical that was used.
Fumigant efficacy:
Methyl bromide, mixed with chloropicrin or with 1,3-D, and 1,3-D + chloropicrin were similarly effective and superior to other treatments in managing root-knot nematodes. Chiseled or sprayed MS as a single treatment gave poor nematode management, confirming several previous reports from Florida (Dickson and Hewlett, 1988; Locascio et al., 1997; Nelson et al., 2004). Drip-applied MS, however, provided good nematode management in fall when nematode pressure was high. The continuous delivery of MS in irrigation water possibly ensured a more uniform soil distribution of MIT, the active ingredient of MS (Gerstl et al., 1977). MS has low vapor pressure (and fumigant activity) compared to other fumigants and distribution of the chemical in the soil is largely dependent on soil water movement (Goring, 1967; Desaeger et al., 2004; Candole et al., 2007). Good nematode management with MS in irrigation water has been reported before and is usually explained by the high affinity of MS for water (Roberts et al., 1988; Noling and Becker, 1994; McGovern et al., 1998).
There remain many questions to be answered before MS can be effectively applied through the irrigation system in Florida. Certainly, farmers will not be interested in a system with three drip tubes, which was used in our tests, because of the cost and labor involved. Achieving adequate soil movement from one or two drip tubes in the highly sandy Florida soils (>90 % sand) is difficult. Drip studies using blue dye in Georgia and Florida for instance indicated that probably a minimum of 6 to 8 hours of watering is required for adequate wetting of standard-size (90-cm-wide) beds (Eger et al., 2001; Csinos et al., 2002; Desaeger et al., 2004). Practices such as burying drip tubes, using narrower planting beds, increasing the application rate, and creating a uniformly wetted bed can be expected to improve efficacy, but may also have practical and/or cost limitations.
Tomato yields with sprayed MS were only 70% of the methyl bromide-chloropicrin standard, with significant nematode infection noted as soon as 1 month after transplanting and very high nematode populations at harvest. Although more impermeable plastic mulch (VIF) has shown to improve retention of MITC in soil (Ou et al., 2006), VIF did not improve nematode management with MS in our trials. Instead there were greater numbers of Meloidogyne males in the VIF plastic treated plots, indicating overpopulation or stress conditions, possibly because soil temperatures under the more impermeable VIF plastic were higher.
Overall, reports on the efficacy of different application methods of MS have been contradictory, and seem to indicate that the product is very sensitive to variations in soil and environmental conditions (Braun and Supkoff, 1994). The deep sandy soils in Florida do not provide optimum conditions for MS applications. On one hand, sufficient water is needed to increase lateral dispersion of MS in these soils, on the other hand, excess water will increase the risk of MIT being drained from the root zone into the deeper sandy subsoil. In addition, the hot climate and high soil temperatures will increase the decomposition rate of MIT (Smelt and Leistra, 1974; Boesten et al., 1991).
The combined applications, MS + chloropicrin and especially MS + 1,3-D, both had high nematode soil population densities at harvest, but provided good nematode management at early growth, when nematodes are most damaging. Yields were, respectively, 12% and 20% greater than with the sole sprayed MS applications.
Broadcast injection of 1,3-D followed by rototillage was more effective in managing root-knot nematode than applications on preformed beds, possibly because of improved soil distribution of the chemical. No difference was noted for MS, though broadcast and in-bed applications of MS were only compared during spring when nematode pressure was low.
In summary, the most promising chemical alternatives to methyl bromide, in terms of root-knot nematode management, were the combinations 1,3-D-chloropicrin, CDeS, and 1,3-D-MS. Sprayed or injected MS generally provided only short-term nematode management and by harvest nematode infection was not different from the nontreated control. Drip-applied metam gave good nematode management under high nematode pressure, but needs further verification to establish (i) the importance of soil moisture—temperature on treatment efficacy and (ii) whether similar management can be obtained with less than three drip tubes. Broadcast applications of 1,3-D showed better efficacy as compared to applications on a preformed bed.
Fumigation did not increase tomato yields in spring when root-knot nematode pressure was low, but during fall all chemical treatments increased yields three- to five-fold, as root-knot nematode was a major yield-limiting factor.
Literature Cited
- Ajwa HA, Trout T, Mueller J, Wilhelm S, Nelson SD, Soppe R, Shatley D. Application of alternative fumigants through drip irrigation systems. Phytopathology. 2002;92:1349–1355. doi: 10.1094/PHYTO.2002.92.12.1349. [DOI] [PubMed] [Google Scholar]
- Anonymous 1992. Methyl bromide: Its atmospheric science, technology and economics. Synthesis report. Methyl bromide interim scientific and technology and economic assessment. Nairobi, Kenya: United Nations Environment Programme.
- Anonymous 2000. Economic implications of the methyl bromide phaseout. An economic research service report. Agriculture Information Bulletin Number 756. United States Department of Agriculture (USDA). http://www.ers.usda.gov/publications/aib756/aib756.pdf.
- Anonymous 2002. Chemical methods of nematode management. http://plpnemweb.ucdavis.edu/nemaplex/Mangmnt/Chemical.htm.
- Arling J. 2015. Methyl bromide and the Montreal Protocol. Proceedings of 2015 Annual International Research Conference on Methyl bromide Alternatives and Emissions Reductions. https://mbao.org/static/docs/confs/2015-sandiego/papers/arling.pdf.
- Boesten JJ, Van der Pas LJ, Smelt JH, Leistra M. Transformation rate of methylisothiocyanate and 1,3-dichloro-propene in water-saturated sandy subsoils. Netherlands Journal of Agricultural Science. 1991;39:179–190. [Google Scholar]
- Braun AL, Supkoff DM. 1994. Options to methyl bromide for the control of soil-borne diseases and pests in California with reference to the Netherlands. Pest management analysis and planning program, 24 pp. http://www.cdpr.ca.gov/docs/dprdocs/methbrom/alt-anal/soilsol.htm.
- Cabrera JA, Wang D, Gerik JS, Gan J. Spot drip application of dimethyl disulfide as a post-plant treatment for the control of plant parasitic nematodes and soilborne pathogens in grape production. Pest Management Science. 2014;70:1151–1157. doi: 10.1002/ps.3666. [DOI] [PubMed] [Google Scholar]
- Candole BL, Csinos AS, Wang D. Concentrations in soil and efficacy of drip-applied 1,3-D+chloropicrin and metam sodium in plastic-mulched sandy soil beds. Crop Protection. 2007;26:1801–1809. doi: 10.1002/ps.1363. [DOI] [PubMed] [Google Scholar]
- Csinos AS, Laska JE, Childers S. Dye injection for predicting pesticide movement in micro-irrigated polyethylene film mulch beds. Pest Management Science. 2002;58:381–384. doi: 10.1002/ps.465. [DOI] [PubMed] [Google Scholar]
- Desaeger JAJ, Eger JE, Csinos AS, Gilreath JP, Olson SM, Webster TM. Movement and biological activity of drip-applied 1,3-dichloropropene and chloropicrin in raised mulched beds in the southeastern USA. Pest Management Science. 2004;60:1220–1230. doi: 10.1002/ps.950. [DOI] [PubMed] [Google Scholar]
- Desaeger J, Csinos A. Root-knot nematode management in double crop plasticulture vegetables. Journal of Nematology. 2006;38:59–67. [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Hewlett TE. Efficacy of fumigant and non-fumigant nematicides for control of Meloidogyne arenaria on peanut. Annals of Applied Nematology. (Journal of Nematology vol. 20, Supplement) 1988;2:95–101. [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Locascio SJ, Mitchell DJ. 1999. Evaluating methyl bromide alternative fumigants on tomato under polyethylene mulch in Florida. http://www.ars.usda.gov/is/np/mba/jan99/altfum.htm.
- Eger JE, Gilreath JP, Noling JP. 2001. Effect of irrigation times on wetting patterns in Florida vegetable soils. Annual International Research. Conference on Methyl Bromide Alternatives and Emissions Reductions, San Diego, CA, Abstr. 48. http://mbao.org.
- Gerstl Z, Milgelgrin U, Krikun J, Yaron B. 1977. Behavior and effectiveness of vapam applied to soil in irrigation water. Pp. 42–50 in Behavior of pesticides in soil. M. Horowitz, ed. Proceedings of the Israel-France Symposium, 1975. Agricultural Research Organization. Bet Dagam, Israel: Special Publication 82.
- Gilreath JP, Santos BM. Methyl bromide alternatives for weed and soilborne disease management in tomato (Lycopersicon esculentum) Crop Protection. 2004;23:1193–1198. [Google Scholar]
- Gilreath JP, Santos BM, Gilreath PR, Jones JP, Noling JW. Efficacy of 1,3-dichloropropene + chloropicrin application methods in combination with pebulate and napropamide in tomato. Crop Protection. 2004a;23:1187–1191. [Google Scholar]
- Gilreath JP, Noling JW, Santos BM. Methyl bromide alternatives for pepper (Capsicum annuum) and cucumber (Cucumis sativus) rotations. Crop Protection. 2004b;23:347–351. [Google Scholar]
- Gilreath JP, Santos BM, Gilreath PR, Noling JW. Validation of 1,3-dichloropropene plus chloropicrin broadcast application in tomato grower fields. Journal of Vegetable Science. 2005;11:133–139. [Google Scholar]
- Gilreath JP, Santos BM. Managing weeds and nematodes with combinations of methyl bromide alternatives in tomato. Crop Protection. 2008;27:648–652. [Google Scholar]
- Goring CAI. Physical aspects of soil in relation to the action of fungicides. Annual Review of Phytopathology. 1967;5:285–318. [Google Scholar]
- Holbrook CC, Knauft DA, Dickson DW. A technique for screening peanut for resistance to Meloidogyne arenaria. Plant Disease. 1983;57:957–958. [Google Scholar]
- Ingham RE, Hamm PB, Williams RE, Swanson WH. Control of Paratrichodorus allius and corky ringspot disease of potato in the Columbia Basin of Oregon. Supplement to the Journal of Nematology. 2000;32:566–575. [PMC free article] [PubMed] [Google Scholar]
- Lamberti F. 2000. 1,3-D, a valid alternative to methyl bromide for the control of plant-parasitic nematodes. http://www.epa.gov/ozone/mbr/airc/2000/45lamberti.pdf.
- Leocata S, Pirruccio G, Medico E, Myrta A, Greco N. Dimethyl disulfide (DMDS): A new soil fumigant to control root-knot nematodes, Meloidogyne spp., in Protected crops in Sicily, Italy. Acta Horticulturae (ISHS) 2014;1044:415–420. [Google Scholar]
- Locascio SJ, Gilreath JP, Dickson DW, Kucharek TA, Jones JP, Noling JW. Fumigant alternatives to methyl bromide for polyethylene-mulched tomato. HortScience. 1997;32:1208–1211. [Google Scholar]
- Locascio SJ, Dickson DW. 1998. Metam sodium combined with chloropicrin as an alternative to methyl bromide. http://www.epa.gov/ozone/mbr/airc/1998/028locascio.pdf.
- McGovern RJ, Vavrina CS, Noling J, Datnoff LA, Yonce HD. Evaluation of application methods of metham sodium for management of fusarium crown and root rot in tomato in southwest Florida. Plant Disease. 1998;82:919–923. doi: 10.1094/PDIS.1998.82.8.919. [DOI] [PubMed] [Google Scholar]
- Munnecke DE, Van Gundy SD. Movement of fumigants in soil, dosage responses, and differential effects. Annual Review of Phytopathology. 1979;17:405–429. doi: 10.1146/annurev.py.17.090179.002201. [DOI] [PubMed] [Google Scholar]
- Neher D. Role of nematodes in soil health and their use as indicators. Journal of Nematology. 2001;33:161–168. [PMC free article] [PubMed] [Google Scholar]
- Nelson SD, Dickson DW, Ajwa HA, Sullivan DA. Efficacy of metam sodium under drip and surface spray application in Florida tomato production. Subtropical Plant Science. 2004;56:16–20. [Google Scholar]
- Noling JW, Becker JO. The challenge of research and extension to define and implement alternatives to methyl bromide. Supplement to the Journal of Nematology. 1994;26:573–586. [PMC free article] [PubMed] [Google Scholar]
- Noling JW, Botts DA, MacRae AW. 2010. Chapter 43: Alternatives to methyl bromide soil fumigation for florida fruit and vegetable production. Pp. 301–316 in S. M. Olson and E. H. Simonne, eds. Vegetable Production Handbook for Florida. University of Florida, SP170.
- Ou L, Thomas JE, Allen LH, Jr, Vu JC, Dickson DW. Effects of application methods of metam sodium and plastic covers on horizontal and vertical distributions of methyl isothiocyanate in bedded field plots. Archives of Environmental Contamination and Toxicology. 2006;51:164–173. doi: 10.1007/s00244-005-0185-6. [DOI] [PubMed] [Google Scholar]
- Overman AJ, Jones JP. Soil fumigants for control of nematodes, fusarium wilt and fusarium crown rot on tomato. Proceeding of the Florida State Horticultural Society. 1984;97:194–197. [Google Scholar]
- Overman AJ, Jones JP. Soil solarization, reaction and fumigation effects on double-cropped tomato under full-bed mulch. Proceeding of the Florida State Horticultural Society. 1986;99:315–318. [Google Scholar]
- Rich JR, Olson SM, Noling JW. Management of root-knot nematodes and nutsedge with fumigant alternatives to methyl bromide in north Florida U.S.A. tomato production. Nematologia Mediterranea. 2003;31:163–168. [Google Scholar]
- Roberts PA, Magyarosy AC, Matthews WC, May DM. Effects of metam-sodium applied by drip irrigation on root-knot nematodes, Pythium ultimum and Fusarium sp. in soil and on carrot and tomato roots. Plant Disease. 1988;72:213–217. [Google Scholar]
- Rosskopf EN, Church G, Holzinger J, Yandoc-Ables C, Noling J. Efficacy of dimethyl disulfide (DMDS) for management of nematodes and fungal plant pathogens. Phytopathology. 2006;96:S100. [Google Scholar]
- Ruzo LO. Physical, chemical and environmental properties of selected chemical alternatives for the pre-plant use of methyl bromide as soil fumigant. Pest Management Science. 2006;62:99–113. doi: 10.1002/ps.1135. [DOI] [PubMed] [Google Scholar]
- Santos BM, Gilreath JP, Motis TN, Noling JW, Jones JP, Norton JA. Comparing methyl bromide alternatives for soilborne disease, nematode and weed management in fresh market tomato. Crop Protection. 2006a;25:690–695. [Google Scholar]
- Santos BM, Gilreath JP, Motis TN, von Hulten M, Siham MN. Effects of mulch types and concentrations of 1,3-dichloropropene plus chloropicrin on fumigant retention and nutsedge control. HortTechnology. 2006b;16:637–640. [Google Scholar]
- Schneider SM, Ajwa HA, Trout TJ, Gao S. Nematode control from shank-and-drip-applied fumigant alternatives to methyl bromide. HortScience. 2008;43(6):1826–1832. [Google Scholar]
- Smelt JH, Leistra M. Conversion of metham-sodium to methyl isothiocyanate and basic data on the behaviour of methyl isothiocyanate. Pesticide Science. 1974;5:401–407. [Google Scholar]
- Zasada IA, Halbrendt JM, Kokalis-Burelle N, LaMondia J, McKenry MV, Noling JW. Managing nematodes without methyl bromide. Annual Review of Phytopathology. 2010;48:311–328. doi: 10.1146/annurev-phyto-073009-114425. [DOI] [PubMed] [Google Scholar]
- Zeck WM. A rating scheme for field evaluation of root-knot nematode infestation. Pflanzenschutz Nachrichten Bayer. 1971;24:141–144. [Google Scholar]