Summary
The aim of this study was to investigate whether transcranial static magnetic field stimulation (tSMS) delivered using a compact cylindrical NdFeB magnet over the cerebellum modulates the excitability of the cerebellum and contralateral primary motor cortex, as measured using cerebellar brain inhibition (CBI), motor evoked potentials (MEPs), and resting motor threshold (rMT). These parameters were measured before tSMS or sham stimulation and immediately, 5 minutes and 10 minutes after stimulation. There were no significant changes in CBI, MEPs or rMT over time in the sham stimulation condition, and no changes in MEPs or rMT in the tSMS condition. However, CBI was significantly decreased immediately after tSMS as compared to that before and 5 minutes after tSMS. Our results suggest that tSMS delivered to the cerebellar hemisphere transiently reduces cerebellar inhibitory output but does not affect the excitability of the contralateral motor cortex.
Keywords: static magnetic field, transcranial magnetic stimulation, cerebellar brain inhibition, motor evoked potential
Introduction
Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) over the motor cortex can modulate the excitability of the primary motor cortex (M1) (Priori et al., 2009; Grimaldi et al., 2014), because rTMS changes the efficiency of excitatory synaptic transmission (Funke and Benali, 2011), while tDCS modulates the resting membrane potential of neurons (Filmer et al., 2014). Non-invasive brain stimulation techniques are frequently used and studied for their potential applications in physical medicine and rehabilitation (Hoyer and Celnik, 2011; Adeyemo et al., 2012; Wessel et al., 2015). However, rTMS and tDCS are not comfortable for subjects submitted to these techniques (Hardwick et al., 2014).
Recently, transcranial static magnetic field stimulation (tSMS), a technique that is comfortable for the subject (Oliviero et al., 2011) and safe for healthy humans (Oliviero et al., 2015), was developed. With this method, stimulation is produced by placing a small neodymium magnet over the human motor cortex, which can modulate the excitability of M1 (Oliviero et al., 2011). tSMS over the motor cortex transiently inhibits motor evoked potentials (MEPs), but does not modulate the resting motor threshold (rMT) (Rosen, 2003), indicating that tSMS may not affect the membrane excitability of pyramidal neurons, but may instead induce changes at synaptic level. Furthermore, a reduction in MEPs was caused by short-latency intracortical inhibition (Nojima et al., 2015), indicating that the modulation induced by tSMS was mediated by plastic changes in the intracortical inhibitory circuits that are associated with gamma-aminobutyric acid receptors (Ziemann, 2004). However, it remains unclear whether tSMS over the cerebellum induces plastic changes in the neural system of the cerebellum.
Conditioning cerebellar TMS inhibits contralateral corticospinal excitability (Ugawa et al., 1995; Pinto and Chen, 2001; Daskalakis et al., 2004; Jayaram et al., 2011; Schlerf et al., 2012) via the dentatothalamocortical pathway (Ugawa et al., 1997; Daskalakis et al., 2004; Iwata and Ugawa, 2005; Kikuchi et al., 2011; Groiss and Ugawa, 2012; Grimaldi et al., 2014). This cerebellar brain inhibition (CBI) was found to be reduced after motor learning, which induced long-term depression-like changes in the synapses associated with Purkinje cells (Celnik, 2015), indicating that changes in CBI reflect plastic changes in the neural system of the cerebellum. On the basis of these findings we hypothesized that tSMS over the cerebellum would modulate cerebellar excitability by changing the efficiency of excitatory synaptic transmission in the cerebellum. Thus, we tested this hypothesis by examining the CBI before and after applying tSMS over the cerebellum.
Previous studies have shown that rTMS over the cerebellum modulates CBI (Celnik, 2015) and the excitability of the contralateral M1 (Oliveri et al., 2005; Fierro et al., 2007; Popa et al., 2010). Thus, if the excitability of the cerebellum is changed by tSMS, the excitability of the contralateral M1 may be modulated by applying tSMS over the cerebellum. To explore these hypotheses, we examined whether application of tSMS over the cerebellum would modulate the rMT and MEPs, as evaluated by applying single-pulse TMS over the contralateral motor cortex.
Materials and methods
Participants
Fourteen healthy volunteers (mean age: 21.3 ± 1.9 years) with no history of epilepsy or other neurological diseases were recruited for these experiments. All the participants were right handed.
Informed consent and approval
After the experimental protocol had been explained to them, the subjects gave their written informed consent to participate in these experiments. The ethics committee of Shijonawate Gakuen University approved the experimental procedures. This study was conducted according to the principles and guidelines of the Declaration of Helsinki.
tSMS
To deliver tSMS to the cerebellum, a cylindrical nickel-plated NdFeB magnet was used (Kirimoto et al., 2014; Nojima et al., 2015). The magnet was 50 mm in diameter and 30 mm thick, and its surface magnetic flux density was approximately 5340 G (Model N-50, NeoMag, Chiba, Japan). A non-magnetic steel cylinder of the same size and appearance was used for the sham stimulations in all experiments.
TMS
Motor cortex TMS was delivered by a figure-of-eight coil (YM-132B, Nihon Kohden), and cerebellar TMS was delivered by a double-cone coil (YM-133B, Nihon Kohden, Tokyo, Japan) connected to a magnetic stimulator (SMN-1200, Nihon Kohden, Tokyo, Japan).
The figure-of-eight coil was positioned at the hotspot for MEPs on the right first dorsal interosseous muscle (FDI), and the current in the coil was directed anterior to posterior. The center of the junction region of the double-cone coil was placed 1 cm below and 3 cm to the right of the inion to stimulate the right cerebellum (Théoret et al., 2001; Hiraoka et al., 2009; Hiraoka et al., 2010; Matsugi et al., 2012; Matsugi et al., 2013; Hardwick et al., 2014; Matsugi et al., 2014; Matsugi et al., 2015), and the current in the coil was directed downward, thus inducing an upwardly traveling current in the brain. To record electromyography (EMG) signals, two Ag/Ag-Cl surface-recording electrodes were placed 2 cm apart on the right FDI. The EMG signals were amplified via an amplifier (MEG-1200, Nihon Kohden, Tokyo, Japan) with a band-pass filter of 15 Hz to 3 kHz. The EMG signals were converted to digital signals at a sampling rate of 10 kHz using an A/D converter (PowerLab 800S, ADInstruments, Colorado Springs, CO), and the digital signals were stored on a personal computer.
Experiment 1: Effect of tSMS on the rMT of MEPs
The effective time of tSMS over the M1 was around 6 min (Oliviero et al., 2011) or a few minutes (Nojima et al., 2015) after the magnet was removed from the scalp. Therefore, the effective time of tSMS over the cerebellum may be very short, and similar to times in previous reports. However, because of the short effective time, we could not measure CBI and the rMT simultaneously, thus the rMT measurements were performed separately from the CBI measurements.
For experiment 1, seven subjects (mean age: 19.7 ± 0.5 years) were recruited. During the experiment, the subject sat in a chair with a backrest with the right forearm fixed to a metal frame to prevent unwanted movements from occurring following TMS. To deliver the tSMS to the cerebellar hemisphere, the center of the cylindrical magnet was placed 1 cm below and 3 cm to the right of the inion using a stand with a clip for 15 min, because it was previously reported that the effect obtained with tSMS lasted for 10 min or more (Oliviero et al., 2011; Kirimoto et al., 2014; Nojima et al., 2015). The south pole was used in the same way as in previous research (Oliviero et al., 2011) because the effect does not depend on the polarity of the magnet (Oliviero et al., 2011; Kirimoto et al., 2014).
To estimate the effect, on the excitability of the left motor cortex, of delivering the tSMS and sham stimulation over the right cerebellum, the rMT of the right FDI muscle was evaluated at the following times: pre stimulation (pre), immediately after stimulation (post), 5 min after stimulation (post5), and 10 min after stimulation (post10). The rMT was defined as the lowest stimulation intensity that produced a 100-μV response of the right FDI on the EMG recording immediately after the left M1-TMS in five out of 10 consecutive stimuli.
The interval between the tSMS condition and the sham condition was 1 day or more. This experiment was conducted using a single-blind design, in which information regarding whether magnetic or non-magnetic stimulation was being applied was not provided to the subjects before the experiment.
Experiment 2: Effect of tSMS over the cerebellum on CBI
Seven subjects (mean age: 22.9 ± 1.2 years) participated in experiment 2. The subjects were seated in a chair and the tSMS and sham stimulation conditions were implemented as described for experiment 1.
To calculate CBI, the conditioned MEPs induced by cerebellar TMS and the unconditioned MEPs were measured at the following time points: pre, post, post5, post10. To deliver sham stimulation, a non-magnetic cylinder was placed at the same position for 15 min and unconditioned and conditioned MEPs were measured at the pre, post, post5 and post10 time points. The interval between the tSMS condition and the sham condition was 1 day or more, and the subjects were not informed as to which type of stimulation was being applied.
To measure CBI, the inter-stimulus intervals (ISIs) were set at 5, 6 or 7 ms (Ugawa et al., 1995; Pinto and Chen, 2001; Iwata and Ugawa, 2005). For each subject, the ISI corresponding to the maximal inhibition was used in the experiment. The intensity of the conditioning TMS was set at 90% of the rMT of the cervicomedullary MEP (CMEP) on the right FDI when the junction center of the double-cone coil was placed 1 cm below and 3 cm to the right of the inion (Théoret et al., 2001; Hiraoka et al., 2009; Hiraoka et al., 2010; Matsugi et al. 2012; Matsugi et al., 2013; Hardwick et al., 2014; Matsugi et al., 2014; Matsugi et al., 2015). When the CMEP could not be induced with the maximum intensity of the magnetic stimulator, the intensity of the conditioning cerebellar TMS was set at 90% of the maximum output of the magnetic stimulator (Hiraoka et al., 2010; Matsugi et al., 2013).To be certain that we obtained CBI, the test MEP size was set at 0.5 mV to 1 mV (Pinto and Chen, 2001). The magnetic stimulator was adjusted so that the intensity of the unconditioned MEP amplitude was approximately 1 mV before the examination, and this intensity was used at the pre, post, post5 and post10 time points. Ten unconditioned MEPs and 10 conditioned MEPs were measured at the pre, post, post5 and post10 time points. The inter-test interval was approximately 3 s.
The interval between the tSMS condition and the sham condition was 1 day or more. This experiment was conducted using a single-blind design, thus the subjects were not told which type of stimulation was being applied.
Statistical analysis
In experiment 1, the rMT was measured at the pre, post, post5 and post10 time points. In experiment 2, the unconditioned and conditioned MEP amplitudes were measured at the pre, post, post5 and post10 time points. CBI was calculated as the averaged conditioned MEP amplitude/averaged unconditioned MEP amplitude. A repeated measures one-way analysis of variance (ANOVA) was used to test differences in the means of the rMT in experiment 1 and the unconditioned MEP amplitude and CBI in experiment 2 between the pre, post, post5 and post10 time points in the tSMS and sham stimulation conditions. A post-hoc multiple comparison Bonferroni’s test was conducted when the means differed between the conditions. Furthermore, paired t-tests were conducted to compare the conditions, tSMS and sham, at each time point. The alpha level was set at 0.05.
Results
None of the subjects showed any side effects or complained of discomfort during tSMS or sham stimulation in any of the experiments.
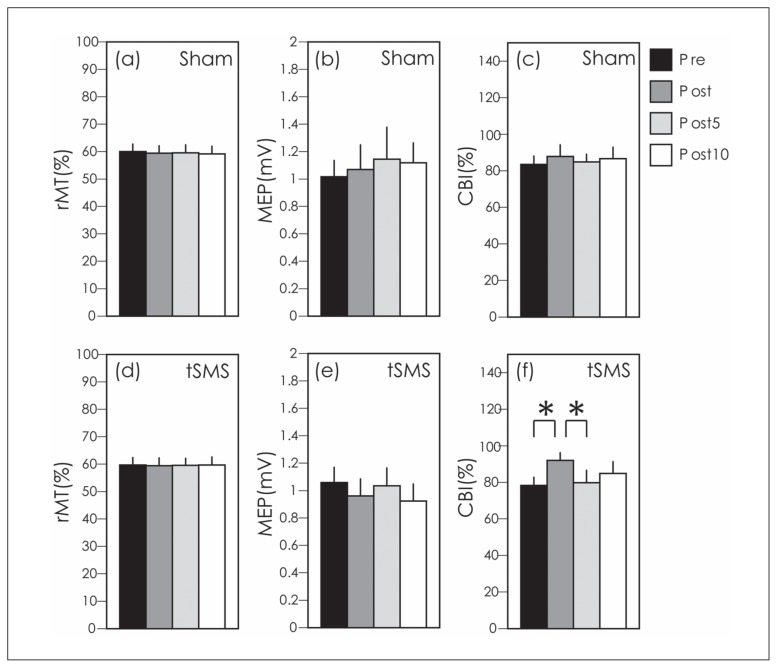
For experiment 1, a one-way ANOVA revealed no significant differences in the rMT for sham stimulation (F[3, 27] = 1.33, p = 0.3) or tSMS (F[3, 27] = 0.04, p = 0.99) between the pre, post, post5 and post10 time points (Figure 1a, d). A paired t-test revealed that there was no significant difference between tSMS and sham at the pre (t=0.3, p=0.78), post (t=0, p=1), post5 (t=0, p=1) or post10 (t=-1, p=0.36) time points.
Figure 1.
Resting motor thresholds (rMTs) are shown for the sham condition in (a) and the tSMS condition in (d). Unconditioned motor evoked potentials (MEPs) are shown for the sham condition in (b) and the tSMS condition in (e). Percent cerebellar brain inhibition (CBI) is shown for the sham condition in (c) and the tSMS condition in (f). Bars indicate the mean and error bars indicate the standard error of the mean. * indicates significance at p < 0.05.
The unconditioned MEP amplitudes in the sham stimulation condition for the pre, post, post5 and post10 time points were 1 ± 0.1 mV, 1 ± 0.2 mV, 1.1 ± 0.2 mV and 1.1 ± 0.1 mV, while the amplitudes in the tSMS condition were 1 ± 0.1 mV, 1 ± 0.1 mV, 1 ± 0.1 mV and 0.9 ± 0.1 mV. A one-way ANOVA revealed no differences in the unconditioned MEP amplitude of the sham condition between the pre, post, post5 and post10 time points (F[3, 27] = 0.7, p = 0.56), or of the tSMS condition (F[3, 27] = 1.06, p = 0.39) (Fig. 1b, e). In other words, the test MEP was set at approximately 1 mV in all test conditions. A paired t-test revealed that there was no significant difference between tSMS and sham at any time point: pre (t=−0.64, p=0.55), post (t=0.67, p=0.53), post5 (t=0.66, p=0.53) or post10 (t=1.9, p=0.1).
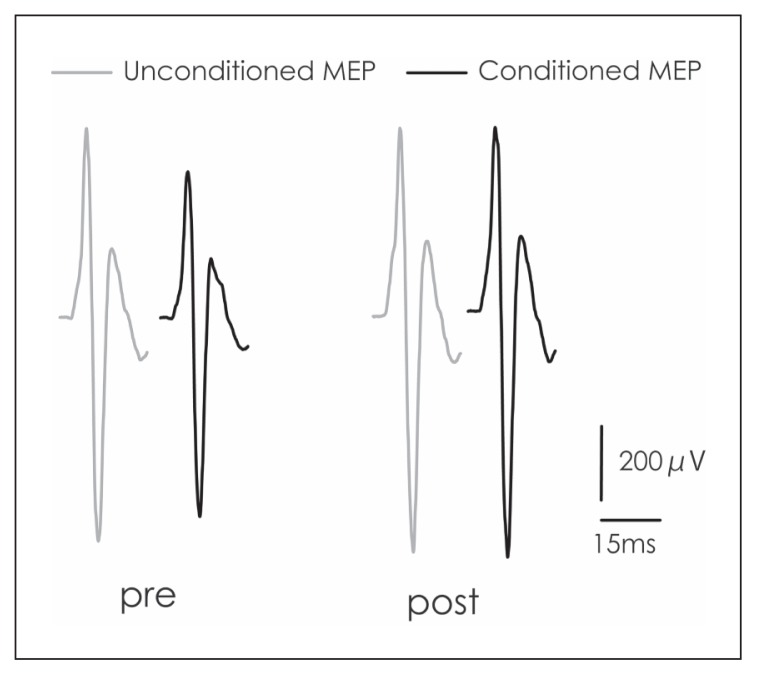
Figure 2, a trace of the averaged conditioned and unconditioned MEPs at the pre and post time points in one subject, indicates that the MEPs at post were suppressed. A one-way ANOVA revealed no differences in the CBI in the sham condition between the pre, post, post5 and post10 time points (F[3, 27] = 0.29, p = 0.83]; however, a difference in the CBI in the tSMS condition was noted (F[3, 27] = 4.9, p = 0.012). A post-hoc test revealed that the CBI at the post time point was significantly smaller than that at the pre (p = 0.016) and post5 (p = 0.038) time points (Fig. 1c, f). A paired t-test revealed that there was no significant difference between tSMS and sham at any time point: pre (t=0.85, p=0.43), post (t=−0.74, p=0.49), post5 (t=0.96, p=0.37) and post10 (t=0.2, p=0.85).
Figure 2.
Traces of the unconditioned and conditioned MEPs.
Discussion
In the present study, we found that CBI was significantly decreased post tSMS as compared to the pre and post5 time points, while no significant differences were observed in the sham stimulation condition. Moreover, no significant differences in MEPs or rMTs were observed between the pre, post, post5 and post10 time points in the tSMS or sham stimulation conditions. These results indicate that tSMS transiently inhibited the cerebellar excitability, but did not alter the excitability of the contralateral motor cortex.
A previous study reported that the distance from the scalp to M1 was approximately 10 mm, while the distance from the scalp to the cerebellar gray matter, from the point on the scalp that is 3 cm lateral and 1 cm below the inion, was 15 mm (Hardwick et al., 2014). Another study reported that the magnetic field strength ranges from 120 to 200 mT at 20–30 mm from the magnet (Rivadulla et al., 2014), which is approximately the same as the strength of the magnetic fields in this study. Static magnetic field stimulation with 125 mT modulates the electrophysiological properties of voltage-gated sodium and calcium channels in the neurons (Lu et al., 2015). Therefore, the static magnetic field generated with the NdFeB magnet may have been strong enough to stimulate at least the cerebellar gray matter to produce the biological effects observed in this study.
tSMS over the motor cortex modulates MEPs but does not modulate the rMT (Rosen, 2003), indicating that tSMS may not affect the membrane excitability of pyramidal neurons but may instead induce alterations at synaptic level. Therefore, in this study, tSMS modulated the synaptic excitability of a neural system consisting of parallel fibers, climbing fibers, mossy fibers, basket cells, Purkinje cells and other fibers in the cerebellar gray matter. Conditioning cerebellar TMS affected the Purkinje fibers and depressed the dentatothalamocortical pathway, resulting in depression of the excitability of the contralateral motor cortex (Pinto and Chen, 2001; Daskalakis et al., 2004; Iwata and Ugawa, 2005). These findings indicate that tSMS over the cerebellum affected the synaptic excitability associated with Purkinje fibers, such as that of the synapses between Purkinje fibers and dentate nuclei. Furthermore, it has been suggested that the magnetic fields in tSMS can alter the function of membrane ion channels (Coots et al., 2004) and that this stimulation reduces the synaptic excitability in the motor cortex (Rosen, 2003). A possible explanation for these findings is that tSMS reduced the synaptic excitability associated with Purkinje cells or fibers, resulting in reduced inhibitory output from the cerebellum, as measured by CBI.
tSMS over the cerebellum, during which the stimulus intensity may have been 0.1–0.2 Tesla, did not affect the excitability of the contralateral motor cortex, and the duration of the aftereffect was very short in this study. On the other hand, delivering 1-Hz rTMS with an intensity of approximately 1 Tesla to the contralateral cerebellum can inhibit the excitability of the contralateral motor cortex and the effect can reportedly continue for over 20 min (Fierro et al., 2007). On the basis of these findings, we speculate that the ability of the stimulation to affect the excitability of the contralateral motor cortex, as well as the duration of the effect, may be related to the type of magnetic field, for instance, a static or varying magnetic field, or the strength of the magnetic field. Therefore, if we want to modulate the cerebellar excitability but not the excitability of the contralateral motor cortex, then we should most likely choose tSMS.
tSMS may be a substitute for cerebellar tDCS (Ferrucci et al., 2016) or rTMS (Koch et al., 2009), which are used in therapy for Parkinson’s disease, and may be applied in cerebellar stroke patients for intermittent theta burst stimulation (Bonni et al., 2014). However, from our results, the effect of cerebellar tSMS is mild and transient compared to other non-invasive brain stimulation techniques, which could be a cause for concern in the clinical use of this technique. Thus, further investigations are needed to determine whether the effects of tSMS in Parkinson’s disease or cerebellar stroke are mild and transient.
In conclusion, tSMS transiently reduced the ipsilateral CBI, but did not modulate the excitability of the contralateral motor cortex. This reduction of CBI can be induced by static magnetic field stimulation, which has an effect on the synapses associated with Purkinje cells in the cerebellum. Our results suggest that tSMS modulates the cerebellar neural system through a mechanism different from those underlying rTMS and tDCS. This cerebellar neuromodulation technique using tSMS may be usable for rehabilitation in the same way as rTMS and tDCS.
Acknowledgments
The Authors are particularly grateful to Shijonawate Gakuen University and all the co-workers contributing to this study.
Footnotes
Funding
This work was supported by JSPS KAKENHI (Grant Number 15K16422).
Compliance with ethical standards
Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of interest
The Authors declare that they have no conflicts of interest.
References
- Adeyemo BO, Simis M, Macea DD, et al. Systematic review of parameters of stimulation, clinical trial design characteristics, and motor outcomes in non-invasive brain stimulation in stroke. Front Psychiatry. 2012;3:88. doi: 10.3389/fpsyt.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnì S, Ponzo V, Caltagirone C, et al. Cerebellar theta burst stimulation in stroke patients with ataxia. Funct Neurol. 2014;29:41–45. [PMC free article] [PubMed] [Google Scholar]
- Celnik P. Understanding and modulating motor learning with cerebellar stimulation. Cerebellum. 2015;14:171–174. doi: 10.1007/s12311-014-0607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coots A, Shi R, Rosen AD. Effect of a 0.5-T static magnetic field on conduction in guinea pig spinal cord. J Neurol Sci. 2004;222:55–57. doi: 10.1016/j.jns.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK, et al. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci R, Cortese F, Bianchi M, et al. Cerebellar and motor cortical transcranial stimulation decrease levodopa-induced dyskinesias in Parkinson’s disease. Cerebellum. 2016;15:43–47. doi: 10.1007/s12311-015-0737-x. [DOI] [PubMed] [Google Scholar]
- Fierro B, Giglia G, Palermo A, et al. Modulatory effects of 1 Hz rTMS over the cerebellum on motor cortex excitability. Exp Brain Res. 2007;176:440–447. doi: 10.1007/s00221-006-0628-y. [DOI] [PubMed] [Google Scholar]
- Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014;37:742–753. doi: 10.1016/j.tins.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Funke K, Benali A. Modulation of cortical inhibition by rTMS - findings obtained from animal models. J Physiol. 2011;589:4423–4435. doi: 10.1113/jphysiol.2011.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G, Argyropoulos GP, Boehringer A, et al. Non-invasive cerebellar stimulation: a consensus paper. Cerebellum. 2014;13:121–138. doi: 10.1007/s12311-013-0514-7. [DOI] [PubMed] [Google Scholar]
- Groiss SJ, Ugawa Y. Cerebellar stimulation in ataxia. Cerebellum. 2012;11:440–442. doi: 10.1007/s12311-011-0329-3. [DOI] [PubMed] [Google Scholar]
- Hardwick RM, Lesage E, Miall RC. Cerebellar transcranial magnetic stimulation: the role of coil geometry and tissue depth. Brain Stimul. 2014;7:643–649. doi: 10.1016/j.brs.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka K, Sugiyama K, Abe K. Effects of transcranial magnetic stimulation over the cerebellum on triphasic electromyographic pattern. Int J Neurosci. 2009;119:1523–1537. doi: 10.1080/00207450902938248. [DOI] [PubMed] [Google Scholar]
- Hiraoka K, Horino K, Yagura A, et al. Cerebellar TMS evokes a long latency motor response in the hand during a visually guided manual tracking task. Cerebellum. 2010;9:454–460. doi: 10.1007/s12311-010-0187-4. [DOI] [PubMed] [Google Scholar]
- Hoyer EH, Celnik PA. Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Restor Neurol Neurosci. 2011;29:395–409. doi: 10.3233/RNN-2011-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata NK, Ugawa Y. The effects of cerebellar stimulation on the motor cortical excitability in neurological disorders: a review. Cerebellum. 2005;4:218–223. doi: 10.1080/14734220500277007. [DOI] [PubMed] [Google Scholar]
- Jayaram G, Galea JM, Bastian AJ, et al. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex. 2011;21:1901–1909. doi: 10.1093/cercor/bhq263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S, Mochizuki H, Moriya A, et al. Ataxic hemiparesis: neurophysiological analysis by cerebellar transcranial magnetic stimulation. Cerebellum. 2011;11:259–263. doi: 10.1007/s12311-011-0303-0. [DOI] [PubMed] [Google Scholar]
- Kirimoto H, Tamaki H, Matsumoto T, et al. Effect of transcranial static magnetic field stimulation over the sensorimotor cortex on somatosensory evoked potentials in humans. Brain Stimul. 2014;7:836–840. doi: 10.1016/j.brs.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Koch G, Brusa L, Carrillo F, et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73:113–119. doi: 10.1212/WNL.0b013e3181ad5387. [DOI] [PubMed] [Google Scholar]
- Lu XW, Du L, Kou L, et al. Effects of moderate static magnetic fields on the voltage-gated sodium and calcium channel currents in trigeminal ganglion neurons. Electromagn Biol Med. 2015:1–8. doi: 10.3109/15368378.2014.906448. [DOI] [PubMed] [Google Scholar]
- Matsugi A, Kamata N, Tanaka T, et al. Long latency fluctuation of the finger movement evoked by cerebellar TMS during visually guided manual tracking task. Indian J Physiol Pharmacol. 2012;56:193–200. [PubMed] [Google Scholar]
- Matsugi A, Iwata Y, Mori N, et al. Long latency electromyographic response induced by transcranial magnetic stimulation over the cerebellum preferentially appears during continuous visually guided manual tracking task. Cerebellum. 2013;12:147–154. doi: 10.1007/s12311-012-0402-6. [DOI] [PubMed] [Google Scholar]
- Matsugi A, Mori N, Uehara S, et al. Task dependency of the long-latency facilitatory effect on the soleus H-reflex by cerebellar transcranial magnetic stimulation. Neuroreport. 2014;25:1375–1380. doi: 10.1097/WNR.0000000000000275. [DOI] [PubMed] [Google Scholar]
- Matsugi A, Mori N, Uehara S, et al. Effect of cerebellar transcranial magnetic stimulation on soleus Ia presynaptic and reciprocal inhibition. Neuroreport. 2015;26:139–143. doi: 10.1097/WNR.0000000000000315. [DOI] [PubMed] [Google Scholar]
- Nojima I, Koganemaru S, Fukuyama H, et al. Static magnetic field can transiently alter the human intracortical inhibitory system. Clin Neurophysiol. 2015;126:2314–2319. doi: 10.1016/j.clinph.2015.01.030. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Koch G, Torriero S, et al. Increased facilitation of the primary motor cortex following 1 Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci Lett. 2005;376:188–193. doi: 10.1016/j.neulet.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Carrasco-López MC, Campolo M, et al. Safety study of transcranial static magnetic field stimulation (tSMS) of the human cortex. Brain Stimul. 2015;8:481–485. doi: 10.1016/j.brs.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Mordillo-Mateos L, Arias P, et al. Transcranial static magnetic field stimulation of the human motor cortex. J Physiol. 2011;589(20):4949–4958. doi: 10.1113/jphysiol.2011.211953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res. 2001;140:505–510. doi: 10.1007/s002210100862. [DOI] [PubMed] [Google Scholar]
- Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3:161–169. doi: 10.1016/j.brs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2:241–245. doi: 10.1016/j.brs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Rivadulla C, Foffani G, Oliviero A. Magnetic field strength and reproducibility of neodymium magnets useful for transcranial static magnetic field stimulation of the human cortex. Neuromodulation. 2014;17:438–441. doi: 10.1111/ner.12125. [DOI] [PubMed] [Google Scholar]
- Rosen AD. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem Biophys. 2003;39:163–173. doi: 10.1385/CBB:39:2:163. [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Galea JM, Bastian AJ, et al. Dynamic modulation of cerebellar excitability for abrupt, but not gradual, visuomotor adaptation. J Neurosci. 2012;32:11610–11617. doi: 10.1523/JNEUROSCI.1609-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théoret H, Haque J, Pascual-Leone A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett. 2001;306:29–32. doi: 10.1016/s0304-3940(01)01860-2. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Terao Y, Hanajima R, et al. Magnetic stimulation over the cerebellum in patients with ataxia. Electroencephalogr Clin Neurophysiol. 1997;104:453–458. doi: 10.1016/s0168-5597(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, et al. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- Wessel MJ, Zimerman M, Hummel FC. Non-invasive brain stimulation: an interventional tool for enhancing behavioral training after stroke. Front Hum Neurosci. 2015;9:265. doi: 10.3389/fnhum.2015.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]