Summary
The present national survey seeking to identify unmet needs in the management of spasticity with botulinum toxin type A focused on the use of OnabotulinumoxinA, since this is the brand with the widest range of licensed indications in Italy. Physicians from twenty-four Italian neurorehabilitation units compiled a questionnaire about “real-life” post-stroke spasticity management. OnabotulinumtoxinA was reported to be used in the following average doses: upper limb 316.7 ± 79.1 units; lower limb 327.8 ± 152.3; upper and lower limb 543.7 ± 123.7 units. Of the physicians surveyed, 37.5% felt that increasing the frequency of OnabotulinumtoxinA injection would improve its efficacy; 70.8% use electrical stimulation/electromyography guidance (one fourth of injections with no instrumental guidance). Instrumental evaluation was used by 41.7% of the physicians.
The participants expressed the view that early identification of post-stroke spasticity would be facilitated by the availability of a post-stroke checklist, and that this should be used by physiotherapists (91.7%), physiatrists (58.3%), family doctors (50%), stroke unit physicians (25%), patients and caregivers (79.2%).
According to our findings, the management of post-stroke spasticity has several unmet needs that, were they addressed, might improve these patients’ clinical outcomes and quality of life. These needs concern patient follow-up, where a clearly defined pathway is lacking; furthermore, there is a need to use maximum doses per treatment and to ensure early intervention on post-stroke spasticity.
Keywords: botulinum toxins, disease management, muscle spasticity, rehabilitation
Introduction
Stroke is the fourth cause of death worldwide (in-hospital mortality rates for ischemic stroke have been estimated to stand at between 11% and 15%) and the second leading cause of disability in Europe (about half of stroke survivors are left with some degree of physical or cognitive impairment) (Bustamante et al., 2016). Spasticity, defined as a disorder of the sensorimotor system characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks (Lance, 1980), is a common complication of stroke. It is considered a “positive” sign of upper motor neuron syndrome, since it represents excessive muscle tone and stretch reflexes (other so-called positive consequences include clonus and spasms) (Li and Francisco, 2015). The prevalence of post-stroke spasticity ranges widely (from 19% to 92%), as does the timing of its onset after stroke (Ward, 2012; Wissel et al., 2013; Li and Francisco, 2015); in most cases, however, it emerges between 1 and 6 weeks after the initial damage (Balakrishnan and Ward, 2013). It has been suggested that early recognition of post-stroke spasticity could result in earlier treatment and possibly better outcomes (Wissel et al., 2015). Botulinum toxin type A (BoNT-A) has been proven to be effective and safe in the treatment of focal post-stroke spasticity (Simpson et al., 2008). It acts in the cytosol of nerve endings and inhibits acetylcholine release by cleaving the synaptosomal-associated 25 kDa protein, which is required for vesicle docking; consequently, it also inhibits neurotransmitter release (Aoki, 2003). Currently, three brands of BoNT-A are marketed in Italy: OnabotulinumoxinA (Allergan, Botox®, Irvine, CA, USA), AbobotulinumtoxinA (Ipsen, Dysport®, Boulogne-Billancourt, France) and IncobotulinumtoxinA (Merz, Xeomin®, Frankfurt am Main, Germany) (Albanese, 2011). These products are not considered interchangeable, as they differ significantly in terms of their biological manufacturing processes (i.e. isolation and purification techniques), molecular structures and formulations, which may affect local migration from the injection site, and also in terms of their potency characteristics, which may influence their efficacy, safety profile and antigenic potential (Albanese, 2011; US Food and Drug Administration, 2010; Caputi and Rossi, 2013).
In daily clinical practice, a number of organizational and methodological aspects have to be taken into account when planning a treatment strategy that includes the administration of BoNT-A. These include the treatment goals and methods, the method of assessment of clinical improvement, the injection schedule (e.g. the muscles to be injected, injection technique, number of injection sites per muscle, dose and dilution) and other treatment modalities (e.g. rehabilitation procedures) to be integrated into the treatment plan (Franceschini et al., 2014; Smania et al., 2013; Carda et al., 2011; Smania et al., 2010). Taking these aspects into account, a post-stroke checklist has been proposed in order to identify persistent long-term problems and improve long-term care for stroke survivors (Paolucci and Smania, 2015; Philp et al., 2013).
Although a growing number of physicians use BoNT-A to treat a range of clinical conditions, there are still some open practical problems and conjectures concerning BoNT-A injection-based therapeutic strategies (Smania et al., 2013). For this reason, we decided to conduct a national survey aimed at identifying the unmet needs in the management, with BoNT-A, of patients suffering from post-stroke spasticity. Specifically, the survey focused on the use of OnabotulinumoxinA, since this is the brand with the widest range of licensed indications in Italy (upper and lower limb spasticity associated with stroke in adults, focal spasticity associated with pediatric cerebral palsy, cervical dystonia, blepharospasm and hemifacial spasm, primary axillary hyperhidrosis, chronic migraine, overactive bladder and neurogenic detrusor overactivity).
The present paper reports the main findings of the Italian Real-Life Post-Stroke Spasticity Survey.
Methods
Thirty-eight Italian neurorehabilitation units selected from the Italian Society of Neurological Rehabilitation (SIRN) database qualified for inclusion in this survey. Each unit involved was required to compile a self-administered two-part questionnaire. Tables I and II show the full questionnaire.
Table I.
Survey questionnaire – Part A.
| Rehabilitation Center and OnabotulinumtoxinA Treatment – Organizational Structure | |
|---|---|
|
| |
| Question | Answer |
|
| |
| 1 Does your center have a dedicated OnabotulinumtoxinA outpatient service? | Yes/No |
| 2 How many physicians use OnabotulinumtoxinA in your center? | Number |
| 3 What are the main licensed indications that OnabotulinumtoxinA is used for in your center? | spasticity/dystonia/blepharospasm/chronic migraine/bladder/other |
| 4 How many patients are treated with OnabotulinumtoxinA in your center per year? | Number |
| 5 How many OnabotulinumtoxinA treatments are performed in your center per year? | Number |
| 6 Are the patients you inject under your clinical care? | Yes, always/Yes, at least in part/No, I only inject patients on the indication of other physicians |
| 7 What criteria do you follow to select the injection sites? | Independent clinical evaluation of the injector/Indication of referring physician |
| 8 What criteria do you follow to decide the injection dose? | |
| 9 Who decides the timing of OnabotulinumtoxinA treatment? | The injector/The referring physician/On patient’s request |
| 10 What instrumental support do you use for the injection of OnabotulinumtoxinA? | EMG/Ultrasonography/None |
| 11 Do you use kinematic of EMG analysis of gait to select the targeted sites of injection? | Yes/No |
| 12 What outcome indicators do you use to evaluate treatment efficacy? | Clinical evaluation/Scales/History |
| 13 Is the caregiver important to facilitate the identification of spasticity at an early stage? | Yes/No |
| 14 Could being aware of the PSC help to improve the identification of disability due to spasticity? | Yes/No |
| 15 Who is the most appropriate target for the PSC? | PT/GP/Stroke unit physician/Neurorehabilitation specialist/Other |
| 16 Do you believe it would be helpful to have a PSC version aimed at the patient? | Yes/No |
Abbreviations: EMG=electromyography; PSC=Post-Stroke Checklist; PT=physical therapist; GP=general medical practitioner.
Table II.
Survey questionnaire – Part B.
| Clinical Approach and Pathways in Real-Life Practice of Spasticity Management with OnabotulinumtoxinA for Upper and Lower Limb Spasticity | |
|---|---|
|
| |
| Question | Answer |
|
| |
| 1 What are the clinical conditions related to the development of spasticity that are usually treated in your center? | Description (%) |
| 2 How long have the patients currently treated with OnabotulinumtoxinA suffered from spasticity? | <3 m (%)/3–6 m (%)/6–12 m (%)/1–3 yrs (%)/> 3 yrs (%) |
| 3 How long is the follow-up of patients OnabotulinumtoxinA-treated patients in your center? | Mean and longest duration (years) |
| 4 What muscles do you usually treat with OnabotulinumtoxinA in patients with upper limb spasticity? | Description |
| 5 What muscles do you usually treat with OnabotulinumtoxinA in patients with lower limb spasticity? | Description |
| 6 What dose of OnabotulinumtoxinA do you usually inject for the treatment of spasticity? | Upper limb (U)/Lower limb (U)/Upper and lower limbs (U) |
| 7 What is the incidence of the different patterns of spasticity in your center? | One upper limb (%)/One lower limb (%)/One upper and one lower limb (%)/Both lower limbs (%)/All four limbs (%) ≤10 wk/11–12 wk/13–14 wk/14–15 wk/ |
| 8 What is the interval between two consecutive OnabotulinumtoxinA injections in your series? | 15–16 wk/≥17 wk |
| 9 Could increasing the frequency of OnabotulinumtoxinA injection help to improve its efficacy? (If yes) What, in your opinion, is the ideal interval between two consecutive OnabotulinumtoxinA injections? | Yes/No (Description) |
| 10 What proportion of patients do not come back for the planned treatment? | % |
| 11 What are the main reasons for this? | Unsatisfactory results/Difficulty reaching the hospital/Lack of a standardized pathway |
| 12 What, in your opinion, is the level of satisfaction with OnabotulinumtoxinA treatment among patients? | 0/1/2/3/4 |
| 13 What, in your opinion, is the level of satisfaction with OnabotulinumtoxinA treatment among physicians? | |
| 14 What, in your opinion, are the main reasons for patients’ satisfaction after treatment with OnabotulinumtoxinA? | Description |
| 15 What, in your opinion, are the main reasons for dissatisfaction after treatment with OnabotulinumtoxinA? | Description |
| 16 What, in your opinion, are the main reasons for delayed treatment of spasticity with OnabotulinumtoxinA? | Description |
Abbreviations: U=Units; mo=months; ys=years; wk=weeks.
Statistical analysis
Descriptive statistics were used for all the items investigated. Statistical analysis was carried out using the Statistical Package for Social Science for Macintosh, version 20.0 (SPSS Inc, Chicago, IL).
Results
Twenty-four of the selected units compiled the questionnaire during the period from July to October 2015 and were included in this survey.
Part A. Rehabilitation center and BoNT-A treatment – organizational structure
Eighteen (75%) units had a dedicated outpatient service for the treatment of patients with BoNT-A (on average, each center had 3.4 ± 2.4 clinicians specialized in and responsible for performing BoNT-A injections). The physicians included in this survey reported that they used OnabotulinumtoxinA for the following licensed indications: spasticity 56.6% ± 30.9%; dystonia 1.4% ± 11.1%; blepharospasm 13.5% ± 13.8%; chronic migraine 6.4% ± 7.8%; overactive bladder 0%; others 7.3% ± 12.3%. On average, each center treated 334.6 ± 327.2 patients per year, corresponding to a mean of 461.7 ± 322.8 BoNT-A treatments performed in each center per year. In 66.7% of cases, the clinicians reported that, at least in part, the patients they treated were under their own clinical care; in 91.7% of cases, the choice both of target muscles (injection sites) and of the injection doses were reported to be based on the independent clinical evaluation of the injector. With regard to the timing of BoNT-A treatment, the decision was made by the injector in 87.5% of cases, and by the referring physician in the remaining 12.5% of cases (in 20.8% of cases, the BoNT-A treatment has been requested beforehand by the patient). With regard to the assessment procedures used to select the BoNT-A injection sites, instrumental evaluation (e.g. EMG analysis of gait) was used by 41.7% of the physicians included in this survey. As for the use of instrumental support in performing BoNT-A injections, 70.8% of clinicians reported using electrical stimulation/electromyography guidance, and 50% ultrasonography guidance; the clinicians used no instrumental guidance for 25% of the treatments administered. For evaluation of the treatment, the surveyed clinicians mainly evaluated BoNT-A treatment efficacy by means of clinical evaluation (87.5%) and scales (70.5%).
With regard to post-stroke spasticity, according to 83.3% of the clinicians, caregivers play a key role in the early identification of muscle hypertonia after stroke onset, while 91.7% considered the post-stroke spasticity checklist a useful tool, indicating that it should be used by physical therapists (91.7%), physiatrists (58.3%), family doctors (50%), stroke unit physicians (25%), and others
Part B. Clinical approach and pathways in real-life practice of spasticity management: the use of BoNT-A for upper and lower limb spasticity
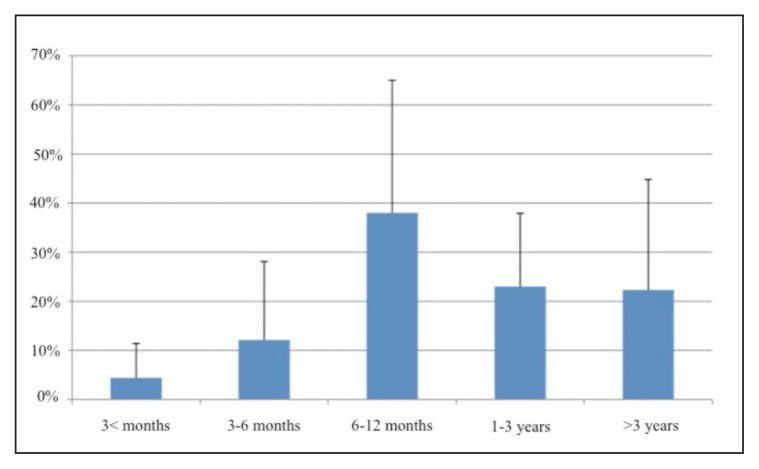
The physicians included in this survey used OnabotulinumtoxinA to treat spasticity resulting from the following clinical conditions: stroke 55.4% ± 23.0%; acquired brain injury 10.5% ± 16.7%; cerebral palsy 10.1% ± 12.7%; multiple sclerosis 9.9% ± 11.9%; spinal cord injury 4.8% ± 7.6%; myelopathy 2.3% ± 0.9%; others 5.5% ± 11.9%. Figure 1 details the duration of spasticity prior to the first treatment.
Figure 1.
Spasticity duration before the first BoNT-A treatment.
On average, the duration of spasticity follow-up was 4.9 ± 1.9 years (with a mean maximum of 10.9 ± 4.0 years). The muscles usually treated with OnabotulinumtoxinA in patients with upper limb spasticity were the following: pectoralis major, biceps brachii, brachioradialis, pronator teres, flexor carpi radialis, flexor carpi ulnaris, flexor digitorum superficialis, flexor digitorum profundus, flexor pollicis longus. The muscles usually treated with OnabotulinumtoxinA in patients with lower limb spasticity were as follows: rectus femoris, medial and lateral hamstrings, adductor longus, medial and lateral gastrocnemius, soleus, tibialis posterior, flexor digitorum longus. Table 3 provides data on the OnabotulinumtoxinA dose per muscle.
Table III.
Treatment parameters (dose per muscle).
| Pectoralis | Latissimus dorsi | Subscapularis | Teres major | Biceps brachii | Brachioradialis | Brachialis | Triceps brachii | Ext. carpi radialis | Ext. carpi ulnaris | Flex carpi radialis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average dose (mean) | 76 U | 73 U | 51 U | 35 U | 73 U | 55 U | 58 U | 66 U | 36 U | 36 U | 53 U |
| Minimum dose (mean) | 37 U | 36 U | 32 U | 21 U | 41 U | 32 U | 36 U | 40 U | 21 U | 20 U | 34 U |
| Maximum dose (mean) | 123 U | 103 U | 81 U | 57 U | 121 U | 82 U | 92 U | 120 U | 62 U | 62 U | 95 U |
|
| |||||||||||
| Flex. carpi ulnaris | Pronator teres | Pronator quadratus | Ext. dig. communis | Flex. dig. superficialis | Flex. dig. profundus | Flex. pollicis longus | Adductor pollicis | Thenar | Lumbrical | ||
|
| |||||||||||
| Average dose (mean) | 48 U | 43 U | 31 U | 27 U | 57 U | 56 U | 30 U | 21 U | 21 U | 33 U | |
| Minimum dose (mean) | 28 U | 23 U | 19 U | 19 U | 28 U | 28 U | 16 U | 13 U | 14 U | 19 U | |
| Maximum dose (mean) | 87 U | 74 U | 51 U | 42 U | 102 U | 95 U | 53 U | 39 U | 36 U | 47 U | |
|
| |||||||||||
| Adductor longus | Adductor magnus | Gracilis | Iliopsoas | Rectus femoris | Medial hamstrings | Biceps femoris | Quadriceps femoris | Gastrocnemius medialis | Gastrocnemius lateralis | Soleus | |
|
| |||||||||||
| Average dose (mean) | 75 U | 89 U | 69 U | 73 U | 72 U | 78 U | 82 U | 101 U | 72 U | 66 U | 73 U |
| Minimum dose (mean) | 44 U | 49 U | 49 U | 46 U | 41 U | 42 U | 48 U | 62 U | 37 U | 34 U | 44 U |
| Maximum dose (mean) | 105 U | 142 U | 92 U | 109 U | 110 U | 105 U | 125 U | 185 U | 108 U | 105 U | 121 U |
|
| |||||||||||
| Tibialis posterior | Tibialis anterior | Ext. hallucis longus | Flex. hallucis longus | Flex. dig. longus | Flex. dig. brevis | ||||||
|
| |||||||||||
| Average dose (mean) | 71 U | 63 U | 41 U | 44 U | 55 U | 39 U | |||||
| Minimum dose (mean) | 39 U | 36 U | 25 U | 28 U | 33 U | 30 U | |||||
| Maximum dose (mean) | 112 U | 95 U | 62 U | 69 U | 85 U | 55 U | |||||
Abbreviations: U=units; Ext=extensor; Flex=flexor; dig=digitorum.
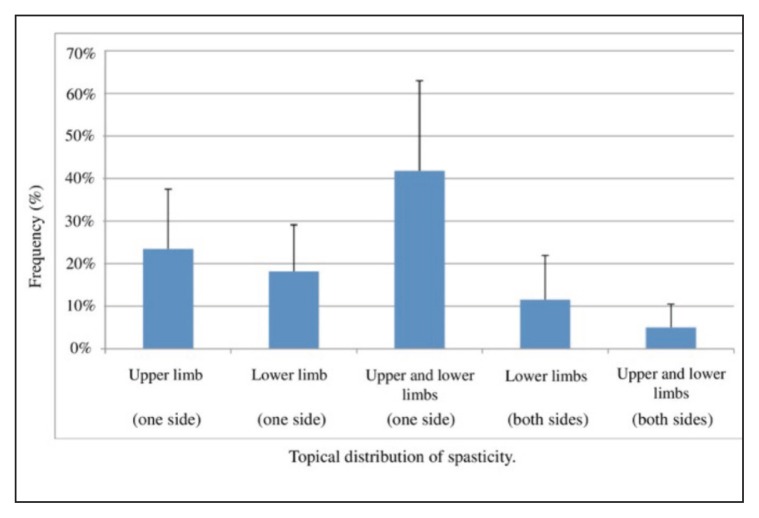
The mean OnabotulinumtoxinA doses used to treat spasticity were as follows: upper limb spasticity 316.7 ± 79.1 units; lower limb spasticity 327.8 ± 152.3 units; upper and lower limb spasticity 543.7 ± 123.7 units. Figure 2 shows the different distribution patterns of the spasticity.
Figure 2.
Distribution of spasticity.
The surveyed physicians reported that they allowed the following intervals between two consecutive OnabotulinumtoxinA injections: ≤ 10 weeks (2.2% ± 3.9%); 11–12 weeks (28.9% ± 34.7%); 13–14 weeks (14.4% ± 13.8%); 14–15 weeks (10.8% ± 11.8%); 15–16 weeks (16.0% ± 16.1%); ≥ 17 weeks (28.0% ± 34.7%). Meanwhile, 37.5% believed that increasing the frequency of OnabotulinumtoxinA injection would help to improve its efficacy (ideal interval between two consecutive injections: ≤ 7 weeks 4.2%; 7–8 weeks 12.5%; 11–12 weeks 12.5%; 13–14 weeks 8.3%). The proportion of patients who fail to return for the second treatment was reported to be 8.9% ± 9.2%, and the survey participants attributed this phenomenon mainly to: unsatisfactory results 20.8%; difficulty reaching the hospital 29.2%; the lack of a standardized pathway 29.2%; other reasons 12.5%.
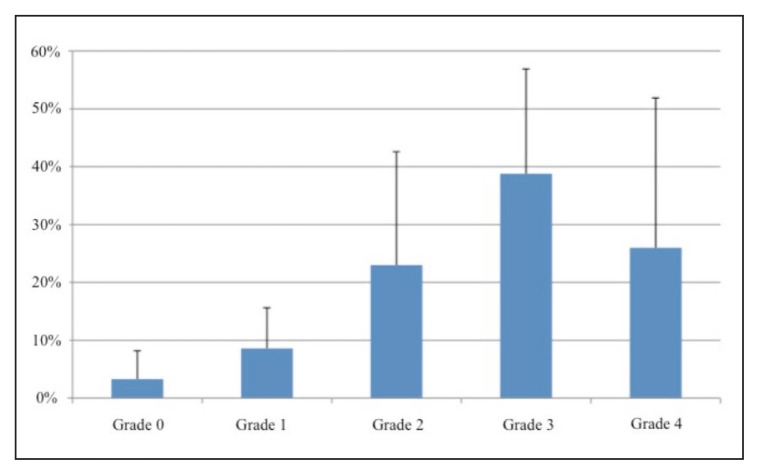
Figure 3 shows the participants’ opinions on the levels of patient satisfaction with the treatment.
Figure 3.
Level of satisfaction regarding treatment with OnabotulinumtoxinA: patients’ opinion.
In their view, patient satisfaction after treatment with OnabotulinumtoxinA could be attributed mainly to the following factors: for treatment of the upper limb, improvements in activities of daily living (29.2%), improvements in personal hygiene management (45.8%), pain reduction (62.5%), other reasons, such as improvements in esthetics, posture, body image and muscle stiffness (20.8%); for treatment of the lower limb: improved gait (79.2%), improved posture (29.8%), reduced clonus (50.0%), reduced pain (45.8%) and other reasons, such as improvements in social life (8.3%). The main reasons for dissatisfaction after treatment with BoNT-A were considered to be: low doses, physical weakness, a short or weak antispastic effect, unavailability of specific (integrated and innovative) post-injection management protocols, insufficient awareness of the benefits of the treatments, muscle tone improvements that are not reflected in an improved functional profile or better quality of life, goals that are not made clear or agreed with the physician, and delayed post-injection rehabilitation treatment. The participants gave the following main reasons for delayed treatment of spasticity with OnabotulinumtoxinA: delayed diagnosis of spasticity; difficulty in reaching the treatment centers; lack of a hub and spoke organizational model; lack of information about BoNT-A among family doctors and physical therapists; shortage of dedicated hospital staff.
Discussion
The main aim of this survey was to provide an overview of some important issues concerning the use of BoNT-A to treat patients with post-stroke spasticity, and to highlight related unmet needs. We surveyed a representative sample of Italian physicians from 24 neurorehabilitation units that use BoNT-A (corresponding to more than 80 clinicians). They provided feedback based on their current practice, and reported spasticity as the main licensed indication treated with OnabotulinumtoxinA (56.6%) in their centers.
The first unmet need in the management of post-stroke spasticity with BoNT-A, identified from present survey findings, is the need to define a consistent pathway able to ensure that patients will be diagnosed and assisted promptly after the onset of stroke and then regularly followed up. In particular, it was striking, in a negative sense, that 25% of the clinical units surveyed do not have a dedicated outpatient service delivering BoNT-A treatments. Along the same lines, we observed that 25% of BoNT-A treatments were delivered with no instrumental guidance, despite the growing evidence of the usefulness of electrical stimulation/electromyography and ultrasonography guidance (Picelli et al., 2012a, b; Picelli et al., 2014 a, b). Furthermore, a considerable proportion of patients with post-stroke spasticity are treated late (over 2 or 3 years after the onset of stroke).
The second unmet need concerns the OnabotulinumtoxinA doses used to treat post-stroke spasticity and the limbs treated. Our personal observations of real-life practice suggest that there is a need to re-consider the maximum dose administered per single treatment of both the upper and the lower limb (also when the two are treated together), since the use of high doses of OnabotulinumtoxinA is an established practice that, moreover, addresses the very real need to improve the quality of life of patients with post-stroke spasticity (Baricich et al., 2015). In line with these findings, our results showed a high percentage of patients (up to about 62%) who need a combined BoNT-A treatment of upper and lower limb, while only a very low proportion (< 25% on average) require treatment in the upper or lower limb alone in a single session. In addition, there is a lack of long-term followup studies on the use of BoNT-A in post-stroke spasticity, with scant data supporting the sustained efficacy of the BoNT-A treatment. Our anecdotal, unpublished, 10-year follow-up observations showed that a tendency to increase BoNT-A doses over time was paralleled by a tendency of patients to be more satisfied.
The third unmet need regards early intervention on post-stroke spasticity, which should be understood as early detection as well as early treatment. With regard to the early development of spasticity after stroke, several possible predictors have been identified, including development of increased muscle tone, initial paresis, hemihypesthesia, a low Barthel Index score, a low Fugl-Meyer Assessment score, and lesion location (Wissel et al., 2015; Opheim et al., 2015; Picelli et al., 2014c, d; Urban et al., 2010). Predictors of spasticity development have proved useful; indeed, accurate prediction of outcome after stroke not only leads to early treatment, but also assists in rehabilitation planning and supports realistic goal setting by clinicians and patients (Fietzek et al., 2014; Hesse et al., 2012; Rosales et al., 2012; Stinear, 2010). Along these lines, our findings support the need for a post-stroke checklist designed to identify treatable post-stroke problems, facilitate referral for treatment, and improve the standard of long-term management of stroke survivors (Paolucci and Smania, 2015; Philip et al., 2013). According to our survey observations, this checklist should be used not only by hospital medical doctors (e.g. physiatrists and neurologists), but also by physical therapists and family doctors. Furthermore, the involvement of patients and caregivers could be crucial for promptly identifying the development of muscle hypertonia after stroke.
This survey has several limitations. First, even though our sample is representative of the most important neurorehabilitation units in Italy (corresponding to about 80 Italian specialists with expertise in the field of botulinum toxin), it is possible that the small population size may have limited our evaluation of some aspects of the management of post-stroke spasticity with BoNT-A. Second, we focused only on the use of OnabotulinumtoxinA, because it is the product with the widest range of licensed indications in Italy. Thus, we cannot draw any conclusions about the use of AbobotulinumtoxinA and IncobotulinumtoxinA in patients with post-stroke spasticity. Future studies should take the above issues into account, also comparing the observations of this survey with those available across Europe.
In conclusion, our findings show that Italy lacks a consistent clinical care model for the treatment of post-stroke spasticity with BoNT-A. This is mainly due to the lack of an established clinical pathway, but also to the existence of different regional laws. Furthermore, our results highlighted the need for combined treatment of the upper and lower limbs, and also the need for doses higher than the licensed ones in order to improve our patients’ clinical outcomes and quality of life. Thus, as shown by our observations of current daily practice, there is a practical need to optimize our treatment paradigms in terms of muscles/limbs/doses, taking into account published clinical evidence and consensus, clinical experience showing a good safety profile both with short- and long-term use, and that the fact that the optimal OnabotulinumtoxinA dose is determined by the patient’s characteristics and specific treatment goals (Ghasemi et al., 2013; Naumann et al., 2006; Naumann and Jankovic, 2004). The present survey set out to highlight unmet needs of patients suffering from post-stroke spasticity, and it is hoped that its findings may help to improve the use of BoNT-A in their clinical and rehabilitation management.
Acknowledgment
The “Italian Real-Life Post-Stroke Spasticity Survey” was supported by an unrestricted grant from Allergan.
References
- Albanese A. Terminology for preparations of botulinum neurotoxins: what a difference a name makes. JAMA. 2011;305:89–90. doi: 10.1001/jama.2010.1937. [DOI] [PubMed] [Google Scholar]
- Aoki KR. Pharmacology and immunology of botulinum toxin type A. Clin Dermatol. 2003;21:476–480. doi: 10.1016/j.clindermatol.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Balakrishnan S, Ward AB. The diagnosis and management of adults with spasticity. Handb Clin Neurol. 2013;110:145–160. doi: 10.1016/B978-0-444-52901-5.00013-7. [DOI] [PubMed] [Google Scholar]
- Baricich A, Grana E, Carda S, et al. High doses of OnabotulinumtoxinA in post-stroke spasticity: a retrospective analysis. J Neural Transm. 2015;122:1283–1287. doi: 10.1007/s00702-015-1384-6. [DOI] [PubMed] [Google Scholar]
- Bustamante A, García-Berrocoso T, Rodriguez N, et al. Ischemic stroke outcome: a review of the influence of post-stroke complications within the different scenarios of stroke care. Eur J Intern Med. 2016;29:9–21. doi: 10.1016/j.ejim.2015.11.030. [DOI] [PubMed] [Google Scholar]
- Caputi AP, Rossi F Tossina botulinica. Italian Society of Pharmacology. 2013. [Accessed 05 May 2016]. http://www.sifweb.org/docs/sif_position_paper_tox_botulinica_mar13.pdf.
- Carda S, Invernizzi M, Baricich A, et al. Casting, taping or stretching after botulinum toxin type A for spastic equinus foot: a single-blind randomized trial on adult stroke patients. Clin Rehabil. 2011;25:1119–1127. doi: 10.1177/0269215511405080. [DOI] [PubMed] [Google Scholar]
- Fietzek UM, Kossmehl P, Schelosky L, et al. Early botulinum toxin treatment for spastic pes equinovarus--a randomized double-blind placebo-controlled study. Eur J Neurol. 2014;21:1089–1095. doi: 10.1111/ene.12381. [DOI] [PubMed] [Google Scholar]
- Franceschini M, Iocco M, Molteni F, et al. Management of stroke patients submitted to botulinum toxin type A therapy: a Delphi survey of an Italian expert panel of specialist injectors. Eur J Phys Rehabil Med. 2014;50:525–533. [PubMed] [Google Scholar]
- Ghasemi M, Salari M, Khorvash F. A literature review on the efficacy and safety of botulinum toxin: an injection in poststroke spasticity. Int J Prev Med. 2013;4:S147–S158. [PMC free article] [PubMed] [Google Scholar]
- Hesse S, Mach H, Fröhlich S, et al. An early botulinum toxin A treatment in subacute stroke patients may prevent a disabling finger flexor stiffness six months later: a randomized controlled trial. Clin Rehabil. 2012;26:237–245. doi: 10.1177/0269215511421355. [DOI] [PubMed] [Google Scholar]
- Lance JW. The control of muscle tone, reflexes and movement: Robert Wartenberg lecture. Neurology. 1980;30:1303–1313. doi: 10.1212/wnl.30.12.1303. [DOI] [PubMed] [Google Scholar]
- Li S, Francisco GE. New insights into the pathophysiology of post-stroke spasticity. Front Hum Neurosci. 2015;9:192. doi: 10.3389/fnhum.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann M, Albanese A, Heinen F, et al. Safety and efficacy of botulinum toxin type A following long-term use. Eur J Neurol. 2006;13:35–40. doi: 10.1111/j.1468-1331.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Naumann M, Jankovic J. Safety of botulinum toxin type A: a systematic review and meta-analysis. Curr Med Res Opin. 2004;20:981–990. doi: 10.1185/030079904125003962. [DOI] [PubMed] [Google Scholar]
- Opheim A, Danielsson A, Murphy M, et al. Early prediction of long-term upper limb spasticity after stroke: part of the SALGOT study. Neurology. 2015;85:873–880. doi: 10.1212/WNL.0000000000001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolucci S, Smania N. Improving the quality of life of stroke survivors: what to do next? The Italian action for the implementation of a Poststroke Checklist. Eur J Phys Rehabil Med. 2015;51:233–235. [PubMed] [Google Scholar]
- Philp I, Brainin M, Walker MF, et al. Development of a poststroke checklist to standardize follow-up care for stroke survivors. J Stroke Cerebrovasc Dis. 2013;22:e173–e180. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Picelli A, Bonetti P, Fontana C, et al. Accuracy of botulinum toxin type A injection into the gastrocnemius muscle of adults with spastic equinus: manual needle placement and electrical stimulation guidance compared using ultrasonography. J Rehabil Med. 2012a;44:450–452. doi: 10.2340/16501977-0970. [DOI] [PubMed] [Google Scholar]
- Picelli A, Lobba D, Midiri A, et al. Botulinum toxin injection into the forearm muscles for wrist and fingers spastic overactivity in adults with chronic stroke: a randomized controlled trial comparing three injection techniques. Clin Rehabil. 2014a;28:232–242. doi: 10.1177/0269215513497735. [DOI] [PubMed] [Google Scholar]
- Picelli A, Roncari L, Baldessarelli S, et al. Accuracy of botulinum toxin type A injection into the forearm muscles of chronic stroke patients with spastic flexed wrist and clenched fist: manual needle placement evaluated using ultrasonography. J Rehabil Med. 2014b;46:1042–1045. doi: 10.2340/16501977-1871. [DOI] [PubMed] [Google Scholar]
- Picelli A, Tamburin S, Bonetti P, et al. Botulinum toxin type A injection into the gastrocnemius muscle for spastic equinus in adults with stroke: a randomized controlled trial comparing manual needle placement, electrical stimulation and ultrasonography-guided injection techniques. Am J Phys Med Rehabil. 2012b;91:957–964. doi: 10.1097/PHM.0b013e318269d7f3. [DOI] [PubMed] [Google Scholar]
- Picelli A, Tamburin S, Dambruoso F, et al. Topical distribution of initial paresis of the limbs to predict clinically relevant spasticity after ischemic stroke: a retrospective cohort study. Eur J Phys Rehabil Med. 2014c;50:489–494. [PubMed] [Google Scholar]
- Picelli A, Tamburin S, Gajofatto F, et al. Association between severe upper limb spasticity and brain lesion location in stroke patients. Biomed Res Int. 2014d;2014;162754 doi: 10.1155/2014/162754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales RL, Kong KH, Goh KJ, et al. Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2012;26:812–821. doi: 10.1177/1545968311430824. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Gracies JM, Graham HK, et al. Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1691–1698. doi: 10.1212/01.wnl.0000311391.00944.c4. [DOI] [PubMed] [Google Scholar]
- Smania N, Colosimo C, Bentivoglio AR, et al. Use of botulinum toxin type A in the management of patients with neurological disorders: a national survey. Funct Neurol. 2013;28:253–258. [PMC free article] [PubMed] [Google Scholar]
- Smania N, Picelli A, Munari D, et al. Rehabilitation procedures in the management of spasticity. Eur J Phys Rehabil Med. 2010;46:423–438. [PubMed] [Google Scholar]
- Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–1232. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]
- Urban PP, Wolf T, Uebele M, et al. Occurrence and clinical predictors of spasticity after ischemic stroke. Stroke. 2010;41:2016–2020. doi: 10.1161/STROKEAHA.110.581991. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. FDA gives update on botulinum toxin safety warnings; established names of drugs changed. 2010. [Accessed 05 May 2016]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm175013.htm.
- Ward AB. A literature review of the pathophysiology and onset of post-stroke spasticity. Eur J Neurol. 2012;19:21–27. doi: 10.1111/j.1468-1331.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- Wissel J, Manack A, Brainin M. Toward an epidemiology of post-stroke spasticity. Neurology. 2013;80:S13–S19. doi: 10.1212/WNL.0b013e3182762448. [DOI] [PubMed] [Google Scholar]
- Wissel J, Verrier M, Simpson DM, et al. Post-stroke spasticity: predictors of early development and considerations for therapeutic intervention. PM R. 2015;7:60–67. doi: 10.1016/j.pmrj.2014.08.946. [DOI] [PubMed] [Google Scholar]