Summary
Adhesive dentistry has undergone great progress in the last decades. In light of minimal-invasive dentistry, this new approach promotes a more conservative cavity design, which relies on the effectiveness of current enamel-dentine adhesives. Adhesive dentistry began in 1955 by Buonocore on the benefits of acid etching. With changing technologies, dental adhesives have evolved from no-etch to total-etch (4th and 5th generation) to self-etch (6th, 7th and 8th generation) systems. Currently, bonding to dental substrates is based on three different strategies: 1) etch-and-rinse, 2) self-etch and 3) resin-modified glass-ionomer approach as possessing the unique properties of self-adherence to the tooth tissue. More recently, a new family of dentin adhesives has been introduced (universal or multi-mode adhesives), which may be used either as etch-and-rinse or as self-etch adhesives.
The purpose of this article is to review the literature on the current knowledge for each adhesive system according to their classification that have been advocated by many authorities in most operative/restorative procedures. As noted by several valuable studies that have contributed to understanding of bonding to various substrates helps clinicians to choose the appropriate dentin bonding agents for optimal clinical outcomes.
Keywords: dental bonding agents, smear layer, adhesive systems, self-etch, etch-and-rinse
Introduction
The development and regular use of adhesive materials has begun to revolutionize many aspects of restorative and preventive dentistry. Attitudes towards cavity preparation are altering since, with adhesive materials, it is no longer necessary to prepare the cavity to provide mechanical retention through such features as dovetails, grooves, undercuts, sharp internal angles in order to retain the filling (1). These techniques are, therefore, responsible for the conservation of large quantities of sound tooth substance, which would otherwise be victim to the dental bur. Microleakage a major dental problem, which is probably responsible for many cases of secondary caries, may be reduced or eliminated. These adhesive are therefore critical for the success of aesthetic materials restorative in modern dentistry.
Dental adhesives are solutions of resin monomers that make the resin dental substrate interaction achievable (2). Adhesive systems are composed of monomers with both hydrophilic groups and hydrophobic groups. The former enhance wettability to the dental hard tissues, while the latter allow the interaction and co-polymerization with the restorative material (3). The chemical composition of adhesives also includes curing initiators, inhibitors or stabilizers, solvents and, in some cases, inorganic fillers (3). However, it is necessary to consider the anatomy of tooth. In particular, composition and structure of two main tissues, enamel and dentine, need to be examined in order to understand how they influence adhesive bonds. Details of the composition of these tissues are shown in Table 1. The mineralized part of the tooth is a complex structure made of different hard tissues, which have a quite distinct ultra-morphology and composition. Enamel is composed of a hard solid crystalline structure-hydroxyapatite (HAp) with strong intermolecular forces, high-energy surface, besides water and organic material. Dentin is a biological composite of HAp that envelops collagen. Dentin is intrinsically humid, and less hard than enamel, with low intermolecular forces and low-energy surfaces. The dentin is different from enamel, as it has smear layer, organic contents and presence of fluid inside the dentinal tubules. In addition, the density of dentinal tubules varies with dentinal depth and, as well as the water content of dentin, is lowest in superficial dentin and highest in deep dentin. In superficial dentin, which contains fewer tubules, the permeation of resin into intertubular dentin will be responsible for most of the bond strength. In deep dentin, dentinal tubules are more in number: the intratubular permeability of resins will be responsible for higher bond strength (4). Dentin is also a substrate that undergoes change with age in an asymmetrical physiological aging process, leading to an increase of dentin thickness and decrease in dentin permeability (5). Furthermore, sclerotic and carious dentin suffers structural changes that result in a higher mineralization and a consequently reduced permeability (5). Unlike dentin, enamel can be dried easily: so bonding process to enamel is different from that of dentin.
Table 1.
Composition of tooth tissues.
| Components | Enamel | Dentine |
|---|---|---|
| Inorganic phase (mainly hydroxyapatite) (%) | 94–96 | 50–70 |
| Calcium phosphate ratio | 1.64 | 1.56 |
| Organic phase (mainly collagen ) (%) | 4–5 | 20–30 |
| Water (%) | 1–4 | 10–20 |
History and Evolution
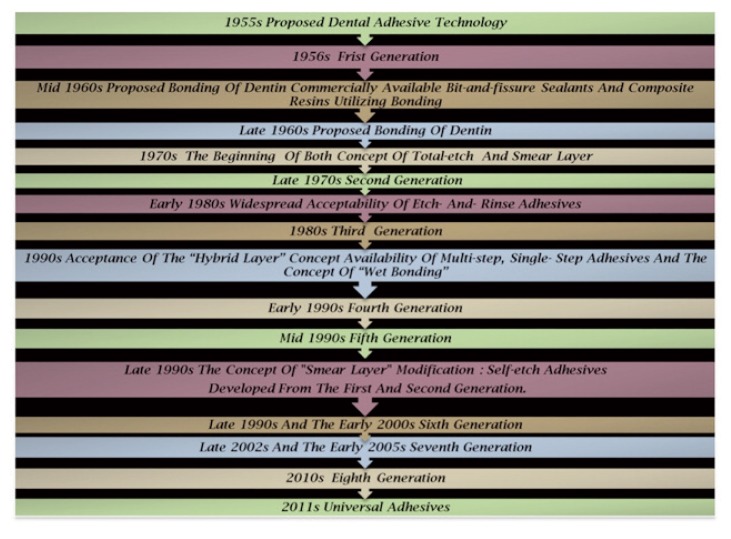
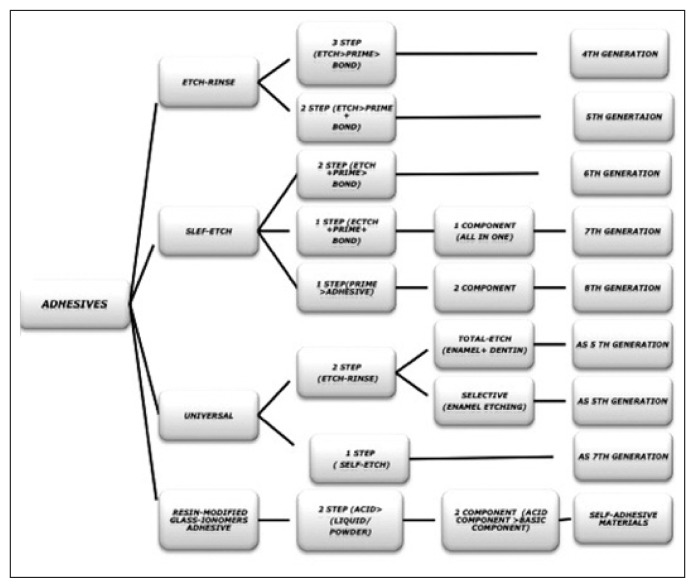
The history of dental adhesives started as early as 1949, when Dr. Hagger, a Swiss chemist who worked for DeTrey/Amalgamated Dental Company, applied the patent for the first dental adhesive: only dentin was initial substrate for bonding not the enamel. Hagger patented a “Cavity Seal” material to be used in combination with the chemically curing resin “Sevriton”, in 1951. This product contained an adhesive called glycerolphosphoric acid dimethacrylate, which was polymerized using a sulfinic acid initiator, later known as “Sevriton Cavity Seal”. This adhesive rely on acidic monomers capable of etching and interacting on a molecular level with tooth surfaces in order to form physical/chemical bonds between the restoration and the tooth. Hagger’s concept was soon adopted by other investigators and different generations of dental adhesives evolved thereafter, despite the fact it was the first time that bonding to tooth structure became commercially available through the formation of an interface very similar to what is called today the hybrid layer (6). In 1952, it was postulated by Mclean and Kramer, that this material, “Sevriton Cavity Seal”, chemically bonded to tooth structure (7). This was the first report of changes in dentin promoted by an acidic monomer and may be considered to be the precursor of the hybrid layer concept (7). That concept is obvious in the development of newer generation of dentin adhesive. In 1954, Buonocore conducted successfully his first experiments on adhesion to enamel trough acid etching and he focused on altering the enamel surface to obtain a bond with filling material. Besides his groundbreaking research, in 1955 he described using 85% phosphoric acid to alter the enamel surface that could provide a surface suitable for bonding with risen and also to improve the retention of acrylic resin to pit-and-fissures (8). The mechanism of acid-etch enhanced adhesion was not published until 1968 (9), when Buonocore, Matsui and Gwinnett discussed the effect of phosphoric acid conditioning, which produced “prism-like” tags of resin materials that penetrated enamel surfaces. These resin tags were not seen in unconditioned enamel. The effect of phosphoric acid on enamel resulting in increased adhesion was now part of the dental literature, but it would be many years later that this principle would be widely accepted. This was the pioneering research of Minimally Invasive Dentistry (10). Enamel conditioning with phosphoric acid results in the formation of microporosities where resin penetrates to form “prism-like” resin tags. This yields an enamel bonding predominantly micromechanical (11). While the same concept applied to dentin in 1958 remained problematic, due to the use of strictly hydrophobic resins. As well, the high polymerization shrinkage of acrylic filling materials gave Buonocore’s invention only little impact on Restorative Dentistry at this time. The advent of composite materials with reduced polymerization shrinkage gave the necessary input to finally enter the era of “Adhesive Dentistry”. By the mid 1960S, the first commercially available pit-and-fissure sealants and composite resin materials utilizing this new adhesive technology were used clinically. Buonocore theorized that risen tags filling the defects created by the etchant were responsible for enamel adhesion, and by the late 1960s, he also proposed that bonding to dentin was possible (11). Since then, dental adhesive has been developed that provide numerically higher bond strength and more substantive bonded interfaces to both enamel and dentin. In the 1970s, for the first time, the concept of smear layer that blocked adhesion to dentin, as identification by Eick, using the scanning electron microscope (SEM) (12), and simultaneously, total-etch concept were being used. By the 1980s, etch-and-rinse adhesive had gained widespread acceptability. Nakabayashi, in 1982 (13), was the first to demonstrate true hybrid layer formation, and also who named this new biocomposite by name of hybrid layer. Moreover, he demonstrated that resin could infiltrate into acid-etched dentin to form a new structure composed of a resin-matrix reinforced by collagen fibrils. At the same time, hybrid layer was considered as the main bonding mechanism of bonding agents. This was best observed by transmission electron microscopy, but was later demonstrated by scanning electron microscopy following argon ion beam etching (14). In the early 1990s, the introduction of the three-step total-etch adhesive system represented a revolution in adhesive dentistry. Once dentin is etched with phosphoric acid and the etchant is rinsed off, hydrophilic primers are used before applying a uniform layer of hydrophobic resin to complete hybridization. However, two-step total-etch adhesive systems and two-step self-etch adhesives were introduced into the market in the late 1990s (Fig. 1). Whereas original simple bonding agents evolved to multi-step systems, recent development focuses on simplification of the application procedure in order to abate technique sensitivity and reduce manipulation time (Fig. 2).
Figure 1.
The Evolution of Bonding Adhesives.
Figure 2.
Adhesives by generations.
Smear layer
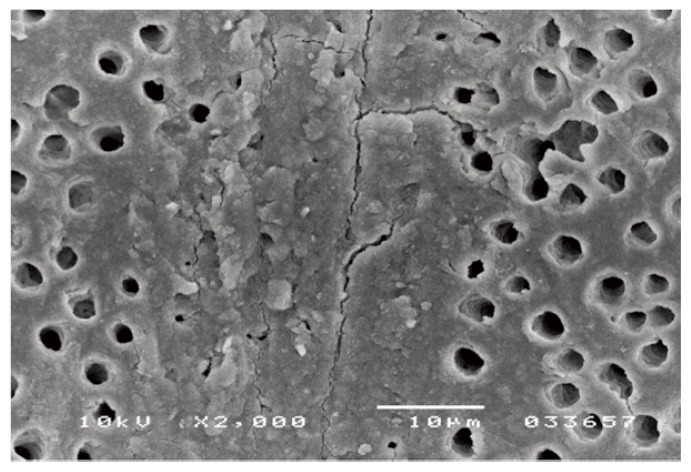
Cavity preparation alters the uppermost layer of tooth tissue, covering the tooth surface with a 1.0 μm layer of cutting debris, called smear layer (15) (Fig. 3). However, the orifices of the dentin tubules are obstructed by debris tags which may extend into the tubule to a depth of 1–10 mm is known as smear plugs. These smear plugs are contiguous with smear layer consisting of shattered and crushed hydroxyapatite, as well as fragmented and denatured collage that should not be underestimated. The thickness and morphology of the smear layer to the underlying dentine is related to the cavity preparations, while its composition has the characteristics of the tissue that was cut (these may also be contaminated by bacteria and saliva). In clinical conditions, a smear layer behaves as a true physical barrier, reducing dentinal permeability by 86% (16). In order to overcome this smear layer obstacle, a certain degree of etching is required before chemical bonding to the dentin surface regarding to the bond strength and durability of adhesion to dental hard tissues. Early non acidic adhesives failed enough to establish a bond with the underlying intact dentin. There are basically two options to overcome low bond strengths due to smear layer: the removal of the smear layer prior to bonding following an etch-and rinse procedure, or the use of bonding agents that can penetrate beyond the smear layer while incorporating it following a self-etch approach. In case of total-etch adhesive systems, the smear layer is essentially dissolved with phosphoric acid (H3PO4) and subsequently washed away during the rinsing step. With self-etching systems, various acidic primers are used to modify, disrupt, and/or solubilize the smear layer and, although the remnants are not washed away as with total-etch systems, still permit direct adhesive interaction with the dentin substrate. For both approaches, micromechanical interlocking is the basic mechanism of adhesion to enamel and dentin.
Figure 3.
SEM: micrograph of smear layer.
Variables in adhesive dental bonding system
Many resin adhesive systems and types have been developed to achieve a durable bond to dental tissues. Further complication are associated with the heterogeneity of tooth structure and composition, the hydrophilicity of the exposed dentine surface, the features of the dental substrate after cavity preparation and the characteristics of the adhesive itself, such as its physicochemical properties and its strategy of interaction with enamel and dentine (3, 17). Despite the major difference in the manner of etching between etch-and-rinse and self-etch adhesives, the other fundamental steps for adhesion, namely the ‘priming’ and actual ‘bonding’ phase, can be either separate or combined. Dental bonding systems are resin blends that possess both hydrophilic and hydrophobic properties by considering the hydrophilic groups enhance the wettability to the dental hard tissues; however, the hydrophobic groups interact and copolymerize with the restorative material and are thus called amphiphilic (18). In other words, adhesives are compounds containing both hydrophilic and hydrophobic monomers. The major difference between hydrophilic and hydrophobic adhesives is the chemistry of their monomers and solvents. The monomers most used in adhesive system are the hydroxylethyl methacrylate (HEMA) and the Bisphenol glycidyl methacrylate (bis-GMA). The first one, HEMA, is totally miscible in water and serves as an excellent polymerizable wetting agent for dental adhesives. Bis-GMA, instead, is the main monomer used in most dental composites and many adhesives, is much more hydrophobic and will only absorb about 3% water by weight into its structure when polymerized (19). A mixture of the two has intermediate characteristics and serves as a useful adhesive for the tooth. In order to enhance the wetting, spreading and penetration of the polymerizable monomers into the dentin substrate, solvents are always added to the mixture as “thinning” agents. These solvents are typically water, ethyl alcohol, butyl alcohol or acetone. The first three are very hydrophilic and thus enhance the interaction of the monomers with surface water, while acetone is good at displacing water from within the dentin. However, any solvent not displaced during the placement procedure, such as by drying appropriately, will be incorporated into the bonding layer and may serve as a weakening contaminant. The monomers present in dental adhesives are similar to those used in dental composite restoratives, thus ensuring that there will be strong interaction between the adhesive and the overlying composite.
Although adhesion is established and predictable clinical procedure, acid etching of dentin has always concerned both clinical and researchers, as critical and definite factor for the quality of adhesion. Moreover, inadvertent over-drying of etched dentin after acid rinsing substantially increases the risk of collapse of collagen mesh, which restricts the diffusivity of resin monomers throughout the intertubular dentin. De Goes et al. (19) recommended to brush out the excess water with a cotton pellet, a disposable brush, or a tissue paper. In the same fashion, over-wet conditions also results in lower bond strengths due to dilution of the adhesive. In addition, excessive etching of dentin may produce weak bonding due to the possibility that the resin monomers may not be able to penetrate into the open dentinal tubules and diffuse across the hydrated demineralized collagen network as deep as the etchant agent and allows fluid movement in the dentinal tubules. This movement of fluid pulls on the odontoblastic process and the patient experiences it as pain or postoperative sensitive. Thus, this lack of penetration leaves behind non-impregnated or poorly infiltrated, unsupported areas at the base of hybrid layer, which are more prone to micro-and nano-leakage, collagen hydrolysis and degradation of the interface over time. The literature and manufacture established the time for etching enamel and dentin that should be 15–30 s respectively, in order to obtain adequate bond performance. All of these molds can affect bond strength, physical properties of the cured resin composite and stress generated during resin polymerization. Also, the large, flat surfaces used in most laboratory bonding studies may overestimate the actual clinical bond strengths achieved.
Configuration or “C-factor”
The cavity configuration, or C-factor, was introduced by Prof. Carol Davidson and his colleagues in 1980s. The configuration factor (C-factor) is the ratio of bonded surface of the restoration to the unbonded surfaces (20). The C-factor can be used to predict which restorations are most likely to exhibit bond failures between the resin and the tooth. According to Feilzer et al., restorations with a C-factor less than one are more likely to survive polymerization contraction stresses and remain bonded to the tooth. This may be a problem, because Class I preparations have a mean C-factor of 4.03 and Class II preparations have a mean C-factor of 1.85 (21). The negative effect of C-factor is supported by He et al. (22), who reported that bulk filling a cavity with a C-factor of five produced the lowest bond strength: more microleakage has been reported as the C-factor increases. An in vivo study has also reported that the resin-dentin interdiffusion zone was detached from the overlying resin in restorations with a C-factor of five (23). Consequently, the higher the value of C-factor, the greater is the polymerization shrinkage. Therefore, three-dimensional tooth preparations (Class I) have the highest (most unfavorable) C-factor and thus are at more risk to the effects of polymerization shrinkage. C-factor plays a significant role when tooth preparation extends up to the root surface causing a V-shaped gap formation between the composite and root surface due to polymerization shrinkage.
Actuality and Classification of Contemporary Adhesives
Dentin bonding agent can be defined as “a thin layer of resin applied between the conditioned dentin and resin matrix of composite”. Over the years, there have been numerous classifications of dentin bonding agents that have been advocated by many authorities. Some of them are based on generation, the number of clinical steps and on the modern adhesive strategy.
Classification by generation
The concept of generation was used because of the complexity of bonding agents, the variety of classifications refers to when and in what order this type of adhesive was developed by the dental industry. Adhesive dentistry began in 1955 by Buonocore on the benefits of acid-etching. With changing technologies, dental adhesives have evolved from no-etch to total-etch (4th and 5th generation) to self-etch (6th, 7th and 8th generation) systems (24) and the details of these are shown in Table 2. Each generation has attempted to reduce the number of bottles involved in the process, to minimize the number of procedural steps, to provide faster application techniques and to offer improved chemistry to facilitate stronger bonding (Tab. 2).
Table 2.
Classification of dental bonding systems by generations.
| Generation | Number of steps | Surface pre-treatment | Components | Shear bond strength (MPa) |
|---|---|---|---|---|
| 1st | 2 | Enamel etch | 2 | 2 |
| 2nd | 2 | Enamel etch | 2 | 5 |
| 3nd | 3 | Dentine conditioning | 2–3 | 12–15 |
| 4th | 3 | Total etch | 3 | 25 |
| 5th | 2 | Total etch | 2 | 25 |
| 6th | 1 | Self-etch adhesive | 2 | 20 |
| 7th | 1 | Self-etch adhesive | 1 | 25 |
| 8th | 1 | Self-etch adhesive | 1 | Over 30 |
First Generation
The first generation bonding systems were published by Buonocore in 1956, who demonstrated that use of glycerophosphoric acid dimethacrylate (NPG-GMA) containing resin would bond to acid etched dentin (25). These bonding agents were designed for ionic bonding to hydroxyapatite or for covalent bonding (hydrogen bonding) to collagen. However, immersion in water would greatly reduce this bond. After nine years, Bowen used a coupling agent to overcome this problem (26). He addressed this issue using that acted as NPG-GMA a primer or adhesion promoter between enamel/dentin and resin materials by chelating with surface calcium, where one end would bond to dentin, and other would polymerize with composite resin (26). Overall, this generation leads to very poor clinical results as well as low bond strength in the 1–3 MPa range (27).
Second Generation
The second generation of dentin bonding agents were introduced in the late 1970s, and sought to improve the coupling agents that were utilized in the first generation of adhesives. The 2nd generation of dentin adhesives primarily used polymerizable phosphates added to bis-GMA resins to promote bonding to the calcium in mineralized tooth structure (27, 28). Bonding mechanism involves formation of ionic bond between calcium and chlorophosphate groups. This ionic bond would rapidly degrade in water submersion (again analogous to saliva) and even the water within the dentin itself, and cause debonding and/or micoleakage (27). The smear layer was still not removed, and this contributed to the relatively weak and unreliable bond strengths of this second generation (27). The smear layer is really a smooth layer of inorganic debris that remains on the prepared dentin surface as a result of tooth preparation with rotary instruments (the drill). This generation of bonding agents is no longer used, due mainly to failed attempts to bond with a loosely bond smear layer. Bond strength: 4–6 Mpa (29).
Third Generation
In the late 1970s and early 1980s, third generation dentin bonding agents were presented. The third generation bonding systems introduced a very important change: the acid etching of the dentin in an effort to modify or partially remove the smear layer (27). This opened the dentin tubules and allowed a primer to be placed after the acid was completely rinsed away. While this method achieved a greater bond, it was considered controversial in dentistry as the feeling existed that dentin ought not to be etched. After the primer was added, an unfilled resin was placed on both dentin and enamel. The weak link with this generation was the unfilled resins that simply did not penetrate the smear layer effectively according to Tao et al. in 1988 (30).
Fourth Generation
In 1980s and 1990s, fourth generation dentin bonding agents were introduced. The fourth generation materials was the first to achieved complete removal of smear layer (27) and still considered as the golden standard in dentin bonding. In this generation, the three primary components (etchant, primer and bonding) are typically packaged in separate containers and applied sequentially. The concept of total-etch technique and moist dentinal hallmarks of the 4th generation systems (27, 31), where dentin and enamel are etched at the same time with phosphoric acid (H3PO3) for a period of 15–20 s (32). However, the surface must be left moist “wet bonding”, in order to avoid collagen collapse. The application of a hydrophilic primer solution can infiltrate the exposed collagen network forming the hybrid layer (27, 33). The hybrid layer is formed by the resin infiltrated surface layer on dentin and enamel. The goal of ideal hybridization is to give high bond strengths and a dentin seal (13). Bond strengths for these adhesives were in the low- to mid-20 MPa range and significantly reduced margin leakage compared to earlier systems (8). This system was very technique sensitive and required an exacting technique of controlled etching with acid on enamel and dentin, followed by two or more components on both enamel and dentin. These systems are very effective when used correctly, have good long-term clinical track record, and are the most versatile of all the adhesive categories, because they can be used for virtually any bonding protocol (direct, indirect, self-cure, dual-cure or light-cure). These systems are still the standards by which the newer systems are judged. However, these systems can be very confusing and time consuming with so many bottles and application steps. Because of the complexity of multiple bottles and steps, dentists began requesting a simplified adhesive system.
Fifth Generation
In the 1990s and in the ongoing decade, the fifth generation bonding systems sought to simplified the process of fourth generation adhesion by reducing the clinical steps which results in reduced working time. These are distinguished by being “one step” or “one bottle” system. In addition, an improved way was needed to prevent collagen collapse of demineralized dentin and to minimize if not totally eliminate, postoperative sensitivity (17, 27, 34). So the most common method of simplification is “one bottle system” combined the primer and adhesive into one solution to be applied on enamel and dentin simultaneously with 35 to 37% phosphoric acid for 15–20 s. This single bottle, etch-and-rinse adhesive type shows the same mechanical interlocking with etched dentin occurs by means of resin tags, adhesive lateral branches and hybrid layer formation and shows high bond strength values to dentin with marginal seal in enamel (33). These kinds of adhesives systems may be more susceptible to water degradation over time than the fourth generation. This is because the polymerized primer of the “one bottle system” tends to be hydrophilic in nature. However, when using the fourth generation, the hydrophilic primer is covered by a more hydrophobic resin, making it less susceptible to water sorption. Not all 5th generation adhesives are compatible with dual and self-cured or core materials. The lower PH of the Oxygen-inhibited layer, or the monomers in some simplified products, are too acidic and thereby de-activate the tertiary amine in chemical-cured composites. As well as the same in regards to the number of applications (unfilled need more applications), so it is critical to follow the manufacturer’s directions.
Several long term studies indicate that 5th generation dental adhesive achieve high clinical bond strengths. In addition, the resin-dentin bond is prone to water degradation, 5th generation adhesives are more prone to water degradation than 4th generation dental adhesive. Representative dentin bond strength is 3 to 25 MPa.
Sixth Generation
The sixth generation bonding systems introduced in the latter part of the 1990s and the early 2000s also known as the “self-etching primers”, were a dramatic leap forward in technology. The sixth generation bonding systems sought to eliminate the etching step, or to include it chemically in one of the other steps: (self-etching primer + adhesive) acidic primer applied to tooth first, followed by adhesive or (self-etching adhesive) two bottles or unit dose containing acidic primer and adhesive; a drop of each liquid is mixed and applied to the tooth. It is recommended that the components are mixed together immediately before use. The mixture of hydrophilic and hydrophobic resin components is then applied to the tooth substrate (35). Evidently, these bonding systems are characterized by the possibility of achieving a proper bond to enamel and dentin using only one solution (27). The biggest advantage of the sixth generation is that their efficacy appears to be less dependent on the hydration state of the dentin than the total-etch systems (33). Unfortunately, the first evaluations of these new systems showed a sufficient bond to conditioned dentin while the bond with enamel was less effective. This may be due to the fact that the sixth generation systems are composed of an acidic solution that cannot be kept in place, must be refreshed continuously and have a pH that is not enough to properly etch enamel (36). In order to overcome this problem, it is recommended to etch enamel first with the traditional phosphoric acid prior to using it. However, those utilizing this technique should take care to confine the phosphoric acid solely to the enamel. Additional etching of the dentin with phosphoric acid could create an “over-etch” situation where the demineralization zone is too deep for subsequently placed primers to completely penetrate (33). While data indicates that 6th generation adhesives will adhere well to dentin (41 MPa at 24 hours), the bond to enamel is at least 25% weak to enamel then both the 4th and 5th generation adhesives in pooled data studies. Several respected clinicians have utilized 6th generation adhesives for bonding to dentin after selectively etching the enamel.
Seventh Generation
The seventh generation bonding systems was introduced in late 1999 and early 2005. The seventh generation or one-bottle self-etching system represents the latest simplification of adhesive systems. With these systems, all the ingredients required for bonding are placed in and delivered from a single bottle (33, 37). This greatly simplifies the bonding protocol as the claim was that could be achieved consistent bond strengths while completely eliminating the errors that could normally be introduced by the dentist or dental assistant who had to mix the separate components with other more complicated systems. However, incorporating and placing all of the chemistry required for a viable adhesive system into a single bottle, and having it remain stable over a reasonable period of time, poses a significant challenge (33). These inherently acidic systems tend to have a significant amount of water in their formulations and may be prone to hydrolysis and chemical breakdown (37, 38). Furthermore, once placed and polymerized, they are generally more hydrophilic than two-step self-etching systems; this condition makes them more prone to water sorption, limits the depth of resin infiltration into the tooth and creates some voids (39). The advantage of this generation was not any mixing required and the bond strengths were consistent. However, the seventh generation adhesives have proven to have the lowest initial and long term bond strengths of any adhesive on the market today that may be considers as disadvantage. Seventh generation adhesives involve the application of etch, primer, and adhesive which have already been mixed, followed by light curing the tooth. Seventh generation adhesives are “all-in-one” (40) if there has ever been such a thing. The clinical and scientific data on these adhesives proves that they are hydrophilic and degrade more rapidly. In addition, the chemistry mast be acidic, as etch is involved in this liquid, and this has been shown to adversely react with the composite initiator systems.
Eighth Generation
In 2010, voco America introduced voco futurabond DC as 8th generation bonding agent, which contains nanosized fillers (41). In the new agents, the addition of nano-fillers with an average particle size of 12 nm increases the penetration of resin monomers and the hybrid layer thickness, which in turn improves the mechanical properties of the bonding systems (42, 43). Nano-bonding agents are solutions of nano-fillers, which produce better enamel and dentin bond strength, stress absorption, and longer shelf life (24). It has been observed that filled bonding agents produced higher in vitro bond strength. These new agent from self-etch generations have an acidic hydrophilic monomers and can be easily used on the etched enamel after contamination with saliva or moisture (44). Based on the manufacturer, nano-particles acting as crosslinks, will reduced the dimensional changes (42, 43). The type of nano-fillers and the method that these particles are incorporated affect the adhesive viscosity and penetration ability of the resin monomers into collagen fibers spaces (43). Nano-fillers, with dimensions larger than 15–20 nm or a content of more than 1.0 percent by weight, both can increase the viscosity of the adhesives, and may cause accumulation of the fillers over the top of the moistured surface. These clusters can act as flaws which may induce cracks and cause a decrease in the bond strength (43).
Classification by mechanism of adhesion/clinical step
At this stage it was proposed a classification of bonding systems, which reflects their essential mode of use, rather than historical development:
Three-steps: involving etch, prime and bond. These bonding systems are supplied as three bottles, one each from etchant, primer and bonding agent. These are the most complicated to use in the clinic, but result in highest bond strengths (17) and greatest durability.
Two-steps 1: here the steps are etch, then finally prime and bond in a single coating. Bonding systems of this type employ substances in two bottles, one consisting of etchant, and the other of the combined prime and bond formulation.
Two-steps 2: for these systems, the two steps are etching and priming combined followed by bonding. It uses two bottles of components, the first containing a self-etching primer and the second the bonding agent. The self-etching primer modifies the smear layer on the surface of the dentine, and incorporates the products in the coating layer.
One-step: this uses a single bottle containing a formulation that blends a self-etching primer and bonding agent. Clinically, this is the easiest to use, and bond strengths are generally reported to be acceptable, despite the simplicity of bonding operation (45).
In order to understand the hybrid layer formation using total etch technique and the self etch technique, it is necessary to understand the components of bonding systems that consist of three main components: 1) etchant, 2) primer and 3) bonding resin:
Etchant: in total-etch technique the etchant used is 35–37% phosphoric acid. It prepares enamel and dentin to receive the primer. It creates microporosities, up to 7.5 microns which helps to create the resin tag formation and thereby results in micro mechanical bonding. The etchant in self-etch bonding agents is typically an acidic monomer that also serves as the primer.
Primer: the primer is composed of hydrophilic monomers usually carried in a water-soluble solvent (acetone, ethanol, water) to promote good flow and penetration into hydrophilic dentin, which can influence the resulting bond strength. Self-etch bonding agents utilize primers that are acidic monomers.
Dentin bonding agent (or Dentin Adhesive): can be defined as a thin layer of (usually unfilled) resin applied between the conditioned dentin and resin matrix of a composite. The adhesive promotes bonding between enamel or dentin and resin composite restorative material or resin cement. Adhesives act as a link between the hydrophilic resin primer and the hydrophobic resin composite. Proper curing is required to provide good retention and sealing. Seventh generation bonding agents utilize primer-adhesives that are acidic monomers.
Fillers: recently nanofillers have been added ranging from 0.5% to 40% by weight in the 8th generation adhesive systems. Fillers control handling and may improve strength. Fillers may increase film thickness of the adhesive layer.
Solvent: solvents include acetone, ethanol and water. The solvent affects the evaporation rate on the tray and in the mouth. Acetone evaporates quickly and requires the shortest drying time in the mouth. Ethanol evaporates more slowly and requires moderate drying time. Water evaporates very slowly and requires longest drying time. Bonding agents should be dispensed immediately before use to prevent premature evaporation of the solvent.
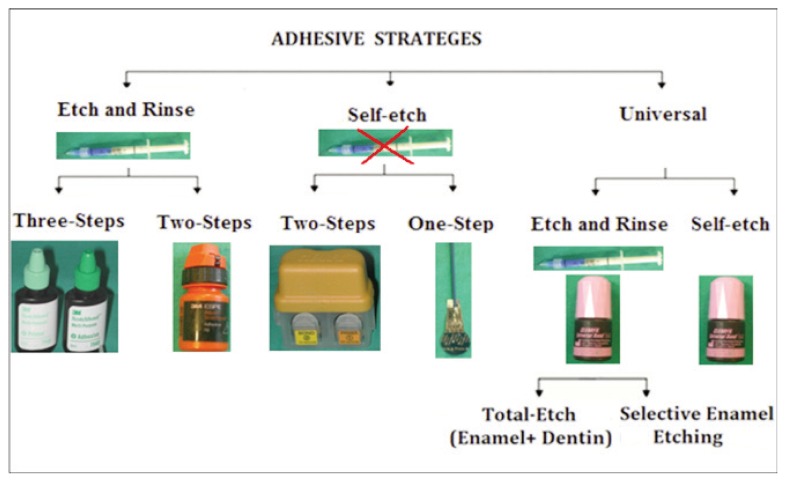
In current times, development of new products is occurring at an unprecedented rate. Dentin adhesives are currently available as three-step, two-step and single-step systems, depending on how the three cardinal steps of etching, priming and bonding to tooth substrate are accomplished or simplified (46). Moreover, they also considered the number of clinical steps required to apply the adhesives: 1. one-step adhesives that modify the smear layer; 2. two-step adhesives that: a) modify the smear layer; b) dissolve the smear layer; c) eliminate the smear layer; 3. three-step adhesives that eliminate the smear layer. However, the classification based on the adhesive strategy was proposed; three adhesion mechanisms are currently used by modern adhesive systems: 1. etch-and-rinse adhesives; 2. self-etching adhesives; 3. glass ionomer adhesives and resin-modified glass ionomers (19), which differ significantly in the manner they deal with tooth tissue (17). Considering the differences in professional judgment and manufacturers’ instructions regarding the selection of the adhesive strategy and the number of steps that give the dentist the opportunity to decide which bonding agents and techniques to utilize for different clinical treatment (Fig. 4; Tabs. 3–7).
Figure 4.
Modern adhesive strategies.
Table 3.
List of bonding agents available of 4th generation.
| Generation | Brand name | Manufacturer | Polymerisation |
|---|---|---|---|
| 4th generation Three-steps Etch-Rinse | All-Bond 2 | Bisco Schaumburg, IL, USA | Dual cured |
| All-Bond 3 | Bisco Schaumburg, IL, USA | Light cured, Dual | |
| Clearfil Liner Bond | Kuraray (Kurashiki, Japan) | Light or self cured | |
| Scotchbond Multi-Purpose | (3M ESPE, St. Paul, Minn. USA) | Light cured | |
| Adper Scotchbond Multi Purpose | (3M ESPE, St. Paul, Minn. USA) | Light cured, Dual | |
| Plus | |||
| Optibond Dual Cure | (Kerr, Orange, CA, USA) | Light cured | |
| Optibond FL | (Kerr, Orange, CA, USA) | Light cured | |
| Permagen | Light cured | ||
| Syntac Classic | (Ultradent Prod Inc, Utah, USA) | Light cured | |
| Denthesive | (Ivoclar-Vivadent, Schann, Liechtenstein) | Light cured | |
| Gluma Solid Bond | (Heraeus Kulzer, Wehrheim Germany) | Light cured | |
| EBS | (Heraeus Kulzer Hanau, Germany) | Light cured | |
| Gluma CPS | ESPE (now 3M ESPE; Seefeld, Germany) | Light cured | |
| Permaquik | Bayer (Heraeus-Kulzer; Leverkusen, Germany) | Self cured | |
| Amalgabond | Kerr (Ultradent) | Light cured | |
| Cmf | Parkell, Farmingdale, NY | Light cured | |
| FL Bond | Saremco, Rebstein, Switzerland) | Light cured | |
| ProBond | (Shofu Inc. Kyoto, Japan) | Light cured, Dual | |
| Bond-it | (Dentsply Caulk) | Light cured, Dual | |
| Ecusit-Primer/Mono | Pentron Corporation, Wallingford, CT, USA | Light cured | |
| Solobond Plus | DMG, Hamburg, Germany | Light cured | |
| Luxa bond total etch | VOCO, Cuxhaven, Germany DMG America | Light cured, Dual |
Table 7.
List of bonding agents available of Universal generation.
| Generation | Brand name | Manufacturer | Polymerisation |
|---|---|---|---|
| Multi-mode or Universal | All-Bond Universal | Bisco (Inc., Schaumburg, IL, USA) | Light cured, Dual |
| Prime&Bond Elect | Dentsply Caulk (Milford, DE, USA) | Light cured | |
| Xeno Select | Dentsply Caulk (Milford, DE, USA) | Light cured | |
| AdheSE Universal | Ivoclar Vivadent (Schaan, Principality of Liechtenstein) | Light cured | |
| G-aenial Bond | GC America (Alsip, IL, USA) | Light cured | |
| Clearfil Universal Bond | Kuraray (Tokyo, Japan) | Light cured, Self cured | |
| Scotchbond Universal Adhesive | 3M ESPE (St. Paul, MN, USA) | Light cured | |
| Futurabond U | Voco (Cuxhaven, Germany) | Light cured |
Etch and Rinse
Etch-and-rinse adhesive systems are the oldest of the multi-generation evolution of resin bonding systems. The three-steps total-etch adhesive systems were introduced in early 1990s (47), that involve acid-etching, priming and application of a separate adhesive. Each of the three-steps can accomplish multiple tasks ending with sealing the bonded interface with a relatively hydrophobic adhesive layer. Consequentially, an inter-diffusion layer is formed that called hybrid layer. Etch-and-rinse adhesives are characterized by an initial etching step, followed by a compulsory rinsing procedure which is responsible for the complete removal of smear layer and smear plugs. On enamel, acid-etching selectively dissolves the enamel rods, creating macro-and micro porosities which are readily penetrated, even by ordinary hydrophobic bonding agents, by capillary attraction (48). Upon polymerization, this micromechanical interlocking of tiny resin tags within the acid-etched enamel surface still provides the best achievable bond to the dental substrate (49). Dentin adhesion is more challenging than enamel adhesion due to dentin composition, rendering the etch-and-rinse strategy a highly sensitive technique (50). Concurrently, acid-etching promotes dentine demineralization over a depth of 3–5 lm, thereby exposing a scaffold of collagen fibrils that is nearly totally depleted of hydroxyapatite (23). The following step consists of the application of a primer containing specific monomers with hydrophilic properties, such as 2-Hydroxy ethyl meth-acrylate (HEMA), dissolved in organic solvents like acetone, ethanol or water. While HEMA is responsible for improving the wettability and promoting the re-expansion of the collagen network, the solvents are able to displace water from the dentine surface, thus preparing the collagen network for the subsequent adhesive resin infiltration (51). In the bonding step, a solvent-free adhesive resin is applied on the prepared surface, leading to the penetration of hydrophobic monomers not only into the inter-fibrilar spaces of the collagen network but also into dentine tubules. After infiltration, these monomers are polymerized in situ, resulting in the formation of a hybrid layer, which in combination with the presence of resin tags inside dentine tubules provides micromechanical retention to the composite restoration (52). From the traditional three-step etch-and-rinse adhesives, simplified two-step adhesives have been developed that combine the primer and the adhesive resin into one single solution. These simplified adhesives present a reduced ability to infiltrate the demineralized dentine substrate, thereby producing suboptimal hybridization when compared to their three-step counterparts (53). Moreover, the hydrophilic nature of such adhesives render them more prone to water sorption and consequently more susceptible to the effects of hydrolytic degradation. The solvent present in such adhesives is also more difficult to evaporate, frequently remaining entrapped within the adhesive layer after polymerization (54). The etch-and-rinse technique is considered to be critical and highly sensitive, because the over-dried dentin causes both demineralized collagen fibers to collapse and low monomer diffusion among the fibers, hampering the formation of a functionally suitable hybrid layer (HL), however the sensitivity is mostly related to the etching step itself and to the ostensibly antagonistic role of water in the bonding protocol. In ‘over-wet’ conditions, seems to cause phase separation between the hydrophobic and hydrophilic components of the adhesive, resulting in the formation of blister- and globule-like voids at the resindentine interface (55). In addition, the excessive presence of humidity may result in incomplete monomer polymerization and water adsorption in the HL. These effects can decrease the mechanical quality of the HL formed, causing its early degradation (56). However the conditions of over-dry and over-wet remains a major concern and difficult to standardize; must be considered not only extrinsic, but also intrinsic sources of humidity when an adhesive procedure is clinically performed. Therefore, the surface should be gently dried until the etched enamel presents its white-frosted appearance and dentine loses its shine and turns dull (57). Although etch-and-rinse adhesives are still the gold standard for dental adhesion and the oldest of the marketed adhesives, it seem to be incapable of preventing nanoleakage, (58) despite their satisfactory long-term clinical performance (50). Although occurring even in the absence of interfacial gaps, nanoleakage seems to play a negative role in bonding, especially in terms of durability (59). Thereby, the current trend is to develop simplified self-etching materials (60).
Self-etch
Self-etching systems were introduced to control the sensitivity to humidity of the etch-and-rinse technique as well as to simplify the clinical procedures of adhesive application, reducing clinical time (61). The self-etch adhesive systems are classified based on the number of clinical application steps: two-steps or one-step adhesives. The basic composition of self-etch primers and self-etch adhesive systems an aqueous solution of acidic functional monomers, with a pH relatively higher than that of phosphoric acid etchants. Therefore, self-etching adhesives have been classified according to their acidity: as strong (pH≤1), intermediate (pH=1.5), and mild (pH≥2) (62) Mild self-etch adhesives demineralize dentin only superficially leaving hydroxyapatite crystals around the collagen fibrils available for possible chemical interaction. Usually, the smear plug is not completely removed from the dentine tubule. As a result, a shallow hybrid layer is formed with submicron measures (63), as do the ultra-mild self-etch adhesives (64); on the contrary, strong self-etch adhesives demineralize dentin comparably to etch-and-rinse adhesives. The mild self-etch adhesives are assumed to cause less post-operative pain, as they use the smear layer as bonding substrate, leaving residual smear plugs that cause less dentinal fluid flow than etch-and-rinse adhesives. The role of water is to provide the medium for ionization and action of these acidic resin monomers. Self-etch adhesive systems also contain HEMA (2-hydroxyethyl-methacrylate) hydrophilic monomer, because of its low molecular weight HEMA acts as a co-solvent, minimizing phase separation and increasing the miscibility of hydrophobic and hydrophilic components into the solution and to increase the wettability of dentin surface (65). Bi or multi-functional monomers are added to provide strength to the cross-linking formed from monomeric matrix (3). Because self-etch adhesive systems do not require a separate acid conditioning step as they contain acidic monomers that simultaneously ‘condition’ and ‘prime’ the dental substrate (66), they are considered as simplified adhesive materials. Possibly, self-etching systems alter the “smear layer” that covers the dentin after tooth bur preparation, creating a thin HL of 0.5–1.2 mm thickness (67). For this system, the created tags are short (16 mm) and narrow. However, due to low acidity, the presence of a “smear layer” or “smear plugs” obliterates the tubule orifices is common after adhesive procedures, limiting hybridization of the peritubular dentin and resin tag formation. In spite of forming a thin HL, this system exhibits a chemical bond to the dentin substrate. Furthermore, self-etch dentin adhesive claimed to minimize post-operative hypersensitivity, because residual smear plugs are left which expose less dentinal tubules and causes less dentinal fluid flow than etch-and-rinse bonds, but the disadvantage is an insufficient enamel etching ability resultant from their less acidity and less injurious to the dental substrate than etch-and-rinse adhesives (68). Thus, it is very important to use these dentin adhesives properly in various clinical situations. On the basis of the steps of application, they can be categorized as: a two-steps “self-etch primers” (SEP) that is mostly solvent-free and a one-step “self-etch adhesives” (SEA) depending on whether a self-etching primer and adhesive resin are separately provided or are combined into one single solution. Two-step self-etching adhesive systems (SEA) require the use of two separate components: the first bottle containing primer and acid and the second bottle containing hydrophobic bond resin. The self-etching primer (SEP) used to condition the dental substrate, followed by the application of a hydrophobic bonding resin (69). The self-etching primer are aqueous acidic solutions containing various vinyl monomers (acidic, hydrophilic and hydrophobic monomers) which can simultaneously etch and infiltrate dental tissues, then photopolymerize with the bonding resin, thus forming a bond between the dental substrate and the restorative material applied after wards. Single-step self-etch adhesives that combine the functions of a self-etching primer and a bonding agent have been developed. One-step adhesives can be further subdivided into ‘two-component’ and ‘single-component’ one-step self-etch adhesives. By separating ‘active’ ingredients (like the functional monomer from water), two-component self-etch adhesives theoretically possess a longer shelf life, but additional and adequate mixing of both components is needed. The single-component one step adhesives can be considered as the only true ‘one-bottle’ or ‘all-in-one’ adhesives, as they combine ‘conditioning’, ‘priming’ and ‘application of the adhesive resin’, and do not require mixing (69). This kind of adhesive system combines acidic functional monomers, hydrophilic monomers, hydrophobic monomers, fillers, water and various solvent (acetone, ethanol, buthanol) and resin component, photo-inhibitors for bonding in a single solution. They are so-called as 7th generation dentin adhesive and undoubtedly the most convenient. The use of water as a solvent is indispensable for single-step self-etch adhesives to ensure the ionization of the acidic functional monomers, and the organic solvents are added to facilitate mixing of the hydrophilic and hydrophobic components (69). The presence of water and acidic functional monomers may compromise the bonding durability of single-step self-etch adhesives. However, the main disadvantages of one-step self-etch adhesives is related to their excessive hydrophilicity that makes the adhesive layer more prone to attract water from the intrinsically moist substrate (39). Due to such increased water affinity, these adhesives have been reported to act as semi-permeable membranes, even after polymerization, allowing water movement from the substrate throughout the adhesive layer (46). As a consequence, small droplets can be found at the transition between the adhesive layer and the lining composite, especially when polymerization of the latter is delayed. Besides promoting a decrease in bond strength between composite and substrate (70), such permeability of the adhesive layer seems to contribute to the hydrolysis of resin polymers and the consequent degradation of tooth-resin bond over time (71). In addition, acetone has a so-called “water-chasing” effect (72), thus it can infiltrate rapidly into the exposed dentinal tubules. However, its vapor pressure is much higher than that of other solvents like ethanol or water, and the adhesive may not infiltrate sufficiently in some situations. It was observed that the poor performance of self-etch adhesives could depend upon shallow resin tag penetration produced by the self-etching process, an inefficient curing caused by their acidic nature, or solvent retention and phase separation phenomena due to the coexistence of both hydrophilic and hydrophobic moieties in the same product (73). Most single-step dentin adhesives are very hydrophilic so that they can interact with underlying dentin. However, it may form water permeable adhesive layer, thus compromising bonding performance (74). To overcome this problem, All-Bond Universal contains minimum amount of ethanol and water as their solvent.
Universal adhesive systems
One of the most recent novelties, in adhesive dentistry, was the introduction of universal adhesives, that have been used since 2011 in clinical practice. These new products are known as “multi-mode” or “multi-purpose” adhesives because they may be used as self-etch (SE) adhesives, etch-and-rinse (ER) adhesives, or as SE adhesives on dentin and ER adhesives on enamel (a technique commonly referred to as “selective enamel etching”) (75, 76). This versatile new adhesion philosophy advocates the use of the simplest option of each strategy, that is, one-step self-etch (SE) or two-step etch-and-rinse (ER) (77), using the same single bottle of adhesive solution which is definitely much more challenging to dental substrates of different natures (i.e., sound, carious, sclerotic dentin, as well as enamel) (78). Beforehand etching enamel with phosphoric acid is often recommended, in particular when bonding to unground enamel. Indeed, the priming and bonding components can be separated or combined, resulting in three steps or two steps for etch-and-rinse systems, and two steps or one step for self-etch adhesives. Contemplating these two bonding strategies, adequate bonding to dentin can be completely achieved with either etch-and-rinse or self-etch adhesives; however, at enamel, the etch-and-rinse approach using phosphoric acid remains the preferred choice (79, 80). In relation to the application mode, self-etch adhesive systems reduce the possibility of iatrogenic induced clinical mis-manipulation during acid conditioning, rinsing and drying, which may occur when etch-and-rinse systems are used (81). On the other hand, some drawbacks may be listed for these SE materials. Unfortunately, one of the main drawbacks from applying SE adhesives to dentin and enamel is their inability to etch enamel to the same depth that phosphoric acid does, which is likely responsible for the higher rates of marginal discoloration in the enamel margins of cervical restoration due to their lower acidity. Thereby the degradation of SE was attributed to its acidic content, which increases the hydrophilicity of the adhesive layer and leads to water uptake and plasticization (82). So the long-term performance of simplified one-step adhesives is inferior in terms of bond durability (60, 83), in particular when compared to the gold-standard three-step etch-and-rinse approach. To overcome the weakness of previous generations of single-step self-etch adhesives, universal adhesives have been developed that allow for application of the adhesive with phosphoric acid pre-etching in the total etch or selective-etch approaches in order to achieve a durable bond to enamel and has been accepted by showing good results in vitro (84) and in vivo studies (85–87). Despite the similarities between adhesives, the composition of universal adhesive differs from the current SE systems by the incorporation of monomers that are capable of producing chemical and micromechanical bond adhesion to the dental substrates (75, 76). Its composition is an important factor to be taken account, since most of these adhesive contain specific carboxylate and/or phosphate monomers that bond ionically to calcium found in hydroxyapatite (Ca10[PO4]6[OH]2) (88, 89), that could be influence the bonding effectiveness (77). For example, Methacryloyloxydecyl Dihydrogen phosphate (MDP) is a functional monomer found in certain new adhesives, but not for older-generation bonding agents. This is a hydrophilic monomer with mild-etching properties. MDP is one of the monomers that enable a universal adhesive to be used with any etching techniques. Stable MDP-calcium salts are formed during this reaction and deposited in self-assembled nano-layers of varying degrees and quality depending on the adhesive system (90, 91). It also helps promote strong adhesion to the tooth surface via formation of non-soluble Ca2 salts. Furthermore, it contains biphenyl dimethacrylate (BPDM), dipentaerythritol pentaacrylate phosphoric acid ester (PEN-TA) (92) and polyalkenoic acid copolymer may enhance adhesion to tooth structures and have been part of the composition of different materials for decades. This may be important in terms of durability, as water sorption and hydrolytic breakdown of the adhesive interface over time has been implicated as one of the primary causes of bond failure (93, 68). Additionally, the matrix of universal is based on a combination of monomers of hydrophilic (hydroxyethul methacrylate /HEMA) hydrophobic (decandiol dimethacrylite /D3MA) and intermediate (bis-GMA) nature. This combination of properties allows universal adhesives to create a bridge over the gap between the hydrophilic tooth substrate and hydrophobic resin restorative, under a variety of surface conditions. Moreover, some universal adhesives may contain silane in their formulation, potentially eliminating the silanization step when bonding to glass ceramics or resin composites, for instance. Nevertheless, it is known that simplified materials are associated with lower in vitro bond strength results and poorer in vivo longevity of restorations, (94, 95). These findings are probably a result of the complex formulation of simplified adhesives and their high content of solvents, which may impair complete solvent volatilization and consequently lead to poorer adhesive polymerization (96). This multi-approach capability enables the clinician to apply the adhesive with the so-called selective enamel etching technique that combines the advantages of the etch-and-rinse technique on enamel, with the simplified self-etch approach on dentine with additional chemical bonding on remnant carbonated apatite crystallites in those bonding substrates. Therefore, the universal adhesives have much broader applications than 7th generation systems. Additionally, manufacturers typically state that universal adhesives can be used for the placement of both direct and indirect restorations and are compatible with self-cure, light-cure and dual-cure resin-based cements and bonds to metals, zirconia, porcelain and composite. While, the manufacturers of some universal adhesives still recommend the use of separate “activator” and dedicated primers to optimize bond strength to substrates such as porcelain and zirconia. Thus, it appears, at least in certain situations and with some products, that universal adhesives actually consist of two bottles, or require the use of an additional activator, or have chemistries that must be mixed prior to use, or bond most optimally to porcelain and zirconia with separately applied and dedicated primers, or are not compatible with a total-etch protocol. Further, there is an advantage in having an adhesive that can operate on these two procedures since it allows the dentist to choose his procedure according to the clinical case in order to optimize the final result. For instance, when the restoration requires strong bonding to enamel or in case of sclerotic dentin, it may be advisable to apply prior etching. The etching step can be modulated according to the length of time the phosphoric acid gel is applied prior to rinsing. On the other hand, it may be preferable to benefit completely from the self-etch path way, when dealing with cases confronting difficult access, limited time or poor patient compliance in very young patients.
Conclusions
Increasing demands for aesthetic restorative treatments have led to recent advances in dentistry, developing adhesive integrated materials (such as adhesive systems and composites) and techniques aimed at restoring the natural tooth appearance, especially in the anterior segment (97). The major requirement of adhesive aesthetic materials is the ability to achieve an excellent color matching with the natural teeth and the maintenance of the optical properties over time. The goals for esthetic dental restorations are to obtain morphologic, optical and biologic result miming natural enamel and dentine. This color matching is performed in order to obtain harmony with the surrounding anatomical structures (98).
Further the evolution of these materials and techniques has recently took steps forward and succeed in preserving teeth instead of extracting them. Most of these improvements were evident in conservative dentistry and in particular, adhesive dentistry (99).
This review about adhesive dentistry describes all the “generations” and types of adhesive product designs that have been introduced during the last 30 years. Since the introduction of the acid etched into clinical practice, various dentin bonding agents were developed to improved the quality of adhesives and composites restoration. The manufactures have been ongoing progress in the development of new dentin adhesive aiming to simplify the process, attempted to improve clinical results correlates to their stability over time and to their bond strength performance consequently lead to improve their effect on the durability of the resin bond. The new adhesive systems also can be attributed to their ability to decrease or eliminate postoperative sensitivity, improve marginal seal, reduce microleakage and enhance the flow of resin into fissure. The development of functional monomers with strong and stabile chemical affinity to hydroxyapatite is without doubt a valuable direction to continue for improvement of dental adhesion. Furthermore, long-term ageing also requires evaluation of its effect in establishing a long-term success of composite restoration.
Table 4.
List of bonding agents available of 5th generation.
| Generation | Brand name | Manufacturer | Polymerisation |
|---|---|---|---|
| 5th Generation Two-steps Etch-Rinse | Admira Bond | Voco, (Cuxhaven, Germany) | Light cured |
| Solobond M | Voco, (Cuxhaven, Germany) | Light cured | |
| Polibond | Voco, (Cuxhaven, Germany) | Dual cured | |
| Excite | Ivoclar Vivadent (Schaan, Lichtenstein) | Light cured | |
| Excite DSC | Ivoclar Vivadent (Schaan, Lichtenstein) | Dual cured | |
| ExciTE F | Ivoclar Vivadent (Schaan, Lichtenstein) | Light cured | |
| Gluma 2000 | Bayer, (now Heraeus-Kulzer; Leverkusen, Germany) | Light cured | |
| Gluma Comfort Bond | Heraeus Kulzer, Hanau, Germany | Light cured | |
| Gluma One Bond | Heraeus Kulzer, Hanau, Germany | Light cured | |
| One-Coat Bond | Coltène Whaledent (Altstätten, Switzerland) | Light cured | |
| Optibond Solo Plus | Kerr (Orange, Calif. USA) | Light cured | |
| Optibond SoloPlus Dual cure | Kerr (Orange, Calif. USA) | Dual cured | |
| Prime&Bond 2.0 | Dentsply-Detrey (Konstanz, Germany) | Light cured | |
| Prime&Bond 2.1 | Dentsply-Detrey (Konstanz, Germany) | Light cured | |
| Prime&Bond NT | Dentsply-Detrey (Konstanz, Germany) | Dual cured | |
| XP Bond | Dentsply-Detrey (Konstanz, Germany) | Self cured | |
| Stae | Southern Dental Industries (Victoria, Australia) | Light cured | |
| Syntac Single-Component | Ivoclar Vivadent (Schaan, Liechtenstein) | Light cured | |
| One Step | Bisco Inc., Schaumburg, IL, USA | Light cured | |
| One-Step Plus | Bisco Inc., Schaumburg, IL, USA | Light cured | |
| Adper Single Bond Plus, (Adper | 3M ESPE, St. Paul, MN,USA | Light cured | |
| Single Bond 2) | 3M ESPE (Seefeld, Germany) | ||
| Scotchbond 1 (Single Bond) | Kuraray (Osaka, Japan) | Light cured | |
| Clearfil Liner Bond 2 | Kuraray (Osaka, Japan) | Light cured | |
| Clearfil SE | Kuraray Medical Inc, Tokyo, Japan | Dual cured | |
| Clearfil Photobond | Kuraray Medical Inc, Tokyo, Japan | Self cured | |
| Clearfil New Bond | Pentron Corporation, Wallingford, CT, USA | Light cured | |
| Bond-1 | Sun Medical Co, Shiga, Japan | Dual cured | |
| Superbond C&B | Bisco Schaumburg, IL, USA | Self cured | |
| All bond plus | Bisco Schaumburg, IL, USA | Light cured |
Table 5.
List of bonding agents available of 6th generation.
| Generation | Brand name | Manufacturer | Polymerisation |
|---|---|---|---|
| 6th Generation Two-steps Self-Etch | ART Bond | Coltene (Alstatten, Switzerland) | Light cured |
| PUB 3 | Denstply (Konstanz, Germany) | Light cured | |
| Clearfil SE | Kuraray (Tokyo, Japan) | Light cured | |
| Clearfil Protect Bond | Kuraray (Osaka, Japan) | Light cured | |
| Denthesive 2 | Heraeus Kulzer (Wehrheim, Germany) | Light cured | |
| Tyrian SPE | Bisco (Schaumburg, IL, USA) | Light cured | |
| Adhe SE | Ivoclar Vivadent (Schaan, Liechtenstein) | Dual cured | |
| Adper Scotchbond SE self-etch | 3M ESPE (St. Paul, MN, USA) | Light cured | |
| FL bond II | Shofu Dental | Light cured | |
| Clearfill Liner bond 2V | Kuraray (Tokyo, Japan) | Dual cured | |
| Contax | DMG America | Dual cured | |
| Nanobond | Pentron Clinical | Dual cured | |
| Clearfil S3 Bond | Kuraray (Osaka, Japan) | Light cured | |
| G Bond | GC Corp (Tokyo, Japan | Light cured | |
| AQ Bond plus | Sun Medicals | Light cured | |
| Hybrid Bond | Vivadent (Schann, Liechtenstein) | Light cured | |
| All Bond SE | Bisco (Inc., Schaumburg, IL, USA) | Light cured | |
| iBond Gluma inside | Heraeus Kulzer (Hanau, Germany) | Light cured | |
| Fluoro bond Shake One | Shofu, (Tokyo, Japan) | Light cured | |
| One up Bond F+ | Tokuyama Corp, (Tokyo, Japan) | Light cured | |
| PSA Dyract | Dentsply, (Konstanz, Germany) | Light cured | |
| Xeno III | Dentsply, (Sankin) | Light cured | |
| Prompt Adper Prompt L-Pop | 3M ESPE (St. Paul, Minn. USA) | Light cured | |
| L-Pop | 3M ESPE (St. Paul, Minn. USA) | Light cured | |
| Brush and bond | Parkell | Light cured |
Table 6.
List of bonding agents available of 7th and 8th generation.
| Generation | Brand name | Manufacturer | Polymerisation |
|---|---|---|---|
| 7/8th Generation One-step Self-Etch | One Coat 7.0 | Coltène/Whaledent (AG, Altstätten, Switzerland) | Light cured |
| Xeno IV | Dentsply Caulk (Milford, DE, USA) | Light cured | |
| AdheSE One F (no mix) | Ivoclar Vivadent, (Schaan, Principality of Liechtenstein) | Light cured | |
| G-BOND | GC America (Alsip, IL, USA) | Light cured | |
| OptiBond All-In-One | Kerr (Orange, CA, USA) | Light cured | |
| Clearfil S3 Bond Plus | Kuraray (Tokyo, Japan) | Light cured | |
| Adper Easy one | 3M ESPE (St. Paul, Minn. USA) | Light cured | |
| Bond force (no mix) | Tokuyama Dental | Light cured | |
| Clearfill DC bond | Kuraray (Tokyo, Japan) | Dual cured | |
| Xeno IV DC | Dentsply Caulk (Milford, DE, USA) | Dual cured | |
| Futura bond DC | Voco (Germany) | Dual cured |
References
- 1.Vaidyanathan TK, Vaidyanathan J. Review Recent Advances in the Theory and Mechanism of Adhesive Resin Bonding to Dentin: A Critical Review. Inc. J Biomed Mater Res Part B: Appl Biomater. 2009;88:558–578. doi: 10.1002/jbm.b.31253. [DOI] [PubMed] [Google Scholar]
- 2.Perdigão J. New developments in dental adhesion. Dent Clin North Am. 2007;51:333–357. doi: 10.1016/j.cden.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Van Landuyt KL, Snauwaert J, De munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–3785. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 4.Susin AH, Vasconcellos WA, Saad JR, Oliveira Junior OB. Tensile bond strength of self-etching versus total-etching adhesive systems under different dentinal substrate conditions. Braz Oral Res. 2007;21(1):81–86. doi: 10.1590/s1806-83242007000100014. [DOI] [PubMed] [Google Scholar]
- 5.Perdigão J. Dentin bonding-variables related to the clinical situation and the substrate treatment. Dent Mater. 2010;26:e24–37. doi: 10.1016/j.dental.2009.11.149. [DOI] [PubMed] [Google Scholar]
- 6.Söderholm KJ. Dental adhesives how it all started and later evolved. J Adhes Dent. 2007;9(2):227–230. [PubMed] [Google Scholar]
- 7.Mclean JW, Kramer IRH. A clinical and pathological evaluation of a sulphinic acid-activated resin for use in restorative dentistry. Br Dent J. 1952;93:255–269. 291–293. [Google Scholar]
- 8.Buonocore M. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34:849–853. doi: 10.1177/00220345550340060801. [DOI] [PubMed] [Google Scholar]
- 9.Buonocore M, Marsui A, Gwinnett AJ. Penetration of resin dental materials into enamel surfaces with reference to bonding. Arch Oral Biol. 1968;13(1):61–70. doi: 10.1016/0003-9969(68)90037-x. [DOI] [PubMed] [Google Scholar]
- 10.Sezinando A. Looking for the ideal adhesive-A review. Rev port Estomatol Med Dent Cir Maxilofac. 2014;(4):194–206. [Google Scholar]
- 11.Swift E, Jr, Perdigao J, Heymann H. Bonding to enamel and dentin: a brief history and state of the art. Quintessence Int. 1995;26:95–110. [PubMed] [Google Scholar]
- 12.Eick J, Wilko R, Anderson C, Sorensen S. Scanning electron microscopy of cut tooth surfaces and identification of debris by use of the electron microprobe. J Dent Res. 1970;49:1359–1368. doi: 10.1177/00220345700490063601. [DOI] [PubMed] [Google Scholar]
- 13.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265–273. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 14.Inokoshi S, Hosoda H, Harnirattisai C, Shimida Y, Tatsumi T. A study on the resin-impregnated layer of dentin. Part I. A comparative study on the decalcified and undecalcified section and the application of argon ion beam etching to disclose the resin- impregnated layer of dentin. Jpn J conserve Dent. 1990;33:427–442. [Google Scholar]
- 15.Bowen RL, Eick JD, Henderson DA, Anderson DW. Smear layer: removal and bonding considerations. Oper Dent Suppl. 1984;3:30–34. [PubMed] [Google Scholar]
- 16.Price RB, Dérand T, Andreou P, Murphy D. The effect of two configuration factors, time, and thermal cycling on resin to dentin bond strengths. Biomaterials. 2003;24(6):1013–1021. doi: 10.1016/s0142-9612(02)00441-6. [DOI] [PubMed] [Google Scholar]
- 17.De Munck J, Van Landuyt K, Peumans M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118–132. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 18.Breschi L, Mazzoni A, De Stefano D, Ferrari M. Adhesion to intraradicular dentin: a review. J Adhes Sci Technol. 2009;7:1053e83. [Google Scholar]
- 19.Sideridou I, Tserki V, Papanastasiou G. Study of water sorption, solubilità and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials. 2003;24:655–665. doi: 10.1016/s0142-9612(02)00380-0. [DOI] [PubMed] [Google Scholar]
- 20.Feilzer AJ, de Gee AJ, Davidson CL. Setting stress in composite resin in relation to configuration of the restoration. J Dentl Researc. 1987;66(11):1636–1639. doi: 10.1177/00220345870660110601. [DOI] [PubMed] [Google Scholar]
- 21.De la Macorra JC, Gomez-Fernandez S. Quantification of the configuration factor in Class I and II cavities and simulated cervical erosions. European Journal of Prosthodontic Restorative Dentistry. 1996;4(1):29–33. [PubMed] [Google Scholar]
- 22.He Z, Shimada Y, Tagami J. The effects of cavity size and incremental technique on micro-tensile bond strength of resin composite in Class I cavities. Dental Materials. 2007;23(5):533–538. doi: 10.1016/j.dental.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Perdigão J, Lambrechts P, Van Meerbeek B, Braem M, Yildiz E, Yucel T, Vanherle G. The interaction of adhesive systems with human dentin. Am J Dent. 1996;9(4):167–173. [PubMed] [Google Scholar]
- 24.Joseph P, Yadav C, Satheesh K, Rahna R. Comparative evaluation of the bonding efficacy of sixth, seventh and eight generation bonding agents: An in vitro study. Int Res J Pharm. 2013;4(9):143–147. [Google Scholar]
- 25.Buonocore M, Wileman W, Brudevold F. A report on a risen composition capable of bonding to human dentin surfaces. J Dent Res. 1956;35:846–851. doi: 10.1177/00220345560350060401. [DOI] [PubMed] [Google Scholar]
- 26.Bowen RL. Adhesive bonding of various materials to hard tooth tissues II. Bonding to dentin promoted by a surface-active comonomer. J Dent Res. 1965;44:895–890. doi: 10.1177/00220345650440052401. [DOI] [PubMed] [Google Scholar]
- 27.Kugel G, Ferrari M. The science of bonding: from first to sixth generation. JADA. 2000;13:20–25. doi: 10.14219/jada.archive.2000.0398. [DOI] [PubMed] [Google Scholar]
- 28.American Dental Association Council on Dental Materials. Instruments and equipment. Dentin bonding systems: an update. JADA. 1987;114:91–95. [PubMed] [Google Scholar]
- 29.Broome JC, Duke ES, Norling BK. Shear bond strengths of composite resins with three different adhesives [Abstract] J Dent Res. 1985;64:244. [Google Scholar]
- 30.Tao L, Pashley DH, Boyd L. The effect of different types of smear layers on dentin and enamel bond strengths. Dent Mater. 1988;4:208–216. doi: 10.1016/s0109-5641(88)80066-6. [DOI] [PubMed] [Google Scholar]
- 31.Kanca J. A method for bonding to tooth structure using phosphoric acid as a dentin-enamel conditioner. Quintessence Int. 1991;22:285–290. [PubMed] [Google Scholar]
- 32.Tay FR, Gwinnett AJ, Wei SHY. Structural evidence of a sealed tissue interface with Total etch wet bonding technique, in vivo. J Dent Res. 1994;73:629–636. doi: 10.1177/00220345940730030801. [DOI] [PubMed] [Google Scholar]
- 33.Alex G. Adhesive considerations in the placement of direct composite restorations. Compend. 2008;1(1):20–25. [Google Scholar]
- 34.Leinfelder KF. Dentin adhesives for the twenty-first century. Dent Clin North Am. 2001;45(1):1–6. [PubMed] [Google Scholar]
- 35.Pashly EL, Agee K, Pashly DH, Tay F. Effect of one versus two applications of an unfilled, all-in-one adhesive on dentine bonding. J Dent. 2002;30:83–90. doi: 10.1016/s0300-5712(02)00002-7. [DOI] [PubMed] [Google Scholar]
- 36.Fabianelli A, Vichi A, Kugel G, Ferrari M. Influence of self-etching-priming bonding systems on sealing ability of Class II restorations: leakage and SEM evaluations. Paper presented at annual meeting of the International Association for Dental Research; Washington, D.C. 2000 April 6. [Google Scholar]
- 37.Mozner N, Salz U, Zimmermann J. Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater. 2005;21:895–910. doi: 10.1016/j.dental.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama N, Tay FR, Fujita K. Hydrolysis of functional monomers in single-bottle self-etching primer-correlation of 13C NMR and TEM findings. J Dent Res. 2006;85:422–426. doi: 10.1177/154405910608500505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tay FR, Pashly DH. Have dentin adhesives become too hydrophilic. Can Dent Assoc. 2003;69:726–731. [PubMed] [Google Scholar]
- 40.Yaseen SM, Subba Reddy VV. Comparative evaluation of shear bond strength of two self-etching adhesive(sixth and seventh generation)on dentin of primary and permanent teeth: An in vitro study. J Indian Soc Pedod Prev Dent. 2009 Jan-Mar;27(1):33–38. doi: 10.4103/0970-4388.50814. [DOI] [PubMed] [Google Scholar]
- 41.Pashley DH, Tay FR. Aggressiveness of contemporary self-etching adhesives. Part II: Etching effects on ungroud enamel. Dent Master. 2001;17(5):430–444. doi: 10.1016/s0109-5641(00)00104-4. [DOI] [PubMed] [Google Scholar]
- 42.Başaran G, Ozer T, Devecioğlu Kama J. Comparison of a recently developed nanofiller self-etching primer adhesive with other self-etching primers and conventional acid etching. Eur J Orthod. 2009 Jun;31(3):271–275. doi: 10.1093/ejo/cjn103. [DOI] [PubMed] [Google Scholar]
- 43.Kasraei SH, Atai M, Khamverdi Z, Khalegh Nejad S. Effect of nanofiller addition to an experimental dentin adhesive on microtensile bond strength to human dentin. J Dent (Tehran) 2009;6(2):91–96. [Google Scholar]
- 44.Karami Nogourani M, Javadi Nejad Sh, Homayunzadeh M. Sealant Microleakage in Saliva-Contaminated Enamel: Comparison between three adhesive systems. J Dent Sch. 2010;27(4):197–204. [Google Scholar]
- 45.Yazici AR, Celik C, Ozgünaltay G, Dayangaç B. Bond strength of different adhesive systems to dental hard tissues. Oper Dent. 2007;32(2):166–172. doi: 10.2341/06-49. [DOI] [PubMed] [Google Scholar]
- 46.Tay F, Pashley D, Suh B, Carvalho R, Itthagarun A. Single step adhesives are permeable membranes. J Dent. 2002;30:371–382. doi: 10.1016/s0300-5712(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 47.Deliperi S, Bardwell DN, Wegley C. Restoration interface microleakage using one total-etch and three self-etch adhesives. Oper Dent. 2007;32:179–184. doi: 10.2341/06-54. [DOI] [PubMed] [Google Scholar]
- 48.Gwinnett AJ, Matsui A. A study of enamel adhesives. The physical relationship between enamel and adhesive. Arch Oral Biol. 1967;12:1615–1620. doi: 10.1016/0003-9969(67)90195-1. [DOI] [PubMed] [Google Scholar]
- 49.Van Meerbeek B, De Munck J, Mattar D, Van Landuyt K, Lambrechts P. Microtensile bond strengths of an etch and rinse and self-etch adhesive to enamel and dentin as a function of surface treatment. Oper Dent. 2003;28:647–660. [PubMed] [Google Scholar]
- 50.Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005;21:864–881. doi: 10.1016/j.dental.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Carvalho RM, Mendonca JS, Santiago SL, et al. Effects of HEMA/solvent combinations on bond strength to dentin. J Dent Res. 2003;82:597–601. doi: 10.1177/154405910308200805. [DOI] [PubMed] [Google Scholar]
- 52.Van Meerbeek B, Dhem A, Goret-Nicaise M, Braem M, Lambrechts P, Vanherle G. Comparative SEM and TEM examination of the ultrastructure of the resin-dentin interdiffusion zone. J Dent Res. 1993;72:495–501. doi: 10.1177/00220345930720020501. [DOI] [PubMed] [Google Scholar]
- 53.Finger WJ, Balkenhol M. Practitioner variability effects on dentin bonding with an acetone-based one-bottle adhesive. J Adhes Dent. 1999;1:311–314. [PubMed] [Google Scholar]
- 54.Van Meerbeek B, Van Landuyt K, De Munck J, et al. Technique-sensitivity of contemporary adhesives. Dent Mater J. 2005;24:1–13. doi: 10.4012/dmj.24.1. [DOI] [PubMed] [Google Scholar]
- 55.Tay FR, Gwinnett JA, Wei SH. Micromorphological spectrum from overdrying to overwetting acid-conditioned dentin in water-free acetone-based, single-bottle primer / adhesives. Dent Mater. 1996;12:236–244. doi: 10.1016/s0109-5641(96)80029-7. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto M, Tay FR, Svizero NR, Gee AJ, Feilzer AJ, Sano H, et al. The effects of common errors on sealing ability of total-etch adhesives. Dent Mater. 2006;22:560–568. doi: 10.1016/j.dental.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Van Meerbeek B, Yoshida Y, Lambrechts P, et al. A TEM study of two water-based adhesive systems bonded to dry and wet dentin. J Dent Res. 1998;77:50–59. doi: 10.1177/00220345980770010501. [DOI] [PubMed] [Google Scholar]
- 58.Van Meerbeek B. The “myth” of nanoleakage. J Adhes Dent. 2007;9:491–492. [PubMed] [Google Scholar]
- 59.Pioch T, Staehle HJ, Duschner H, Garcia-Godoy F. Nanoleakage at the composite-dentin interface: a review. Am J Dent. 2001;14:252–258. [PubMed] [Google Scholar]
- 60.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dental Materials. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Sundfeld RH, Valentino TA, Alexandre RS, Briso ALF, Sun-defeld MLMM. Hybrid layer thickness and resin tag length of a self-etching adhesive bondedto sound dentin. J Dent. 2005;33:675–681. doi: 10.1016/j.jdent.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 62.De Munck J, Vargas M, Iracki J, Van Landuyt K, Poitevin A, Lambrechts P, Van Meerbeek B. One-day bonding effectiveness of new self-etch adhesives to burcut enamel and dentin. Oper Dent. 2005;30(1):39–49. [PubMed] [Google Scholar]
- 63.Kenshima S, Francci C, Reis A, Dourado Loguercio A, Eloy Rodrigues Filho L. Conditioning effect on dentin, resin tags and hybrid layer of different acidity self-etch adhesives applied to thick and thin smear layer. J Dent. 2006;34(10):775–783. doi: 10.1016/j.jdent.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Koshiro K, Sharanbir KS, Inoue S, Ikeda T, Sano H. New concept of resin-dentin interfacial adhesion: The nanointeraction zone. Journal of Biomedical Materials Research Part B. 2006;77(2):401–408. doi: 10.1002/jbm.b.30450. [DOI] [PubMed] [Google Scholar]
- 65.Van Landuyt KL, De Munck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, et al. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005;84:183–188. doi: 10.1177/154405910508400214. [DOI] [PubMed] [Google Scholar]
- 66.Peumans M, De Munck J, Van Landuyt KL, Poitevin A, Lambrechts P, Van Meerbeek B. Eight-year clinical evalution of a two-step self-etch adhesive with and without selective enamel etching. Dent Mater. 2010;26:1176–1184. doi: 10.1016/j.dental.2010.08.190. [DOI] [PubMed] [Google Scholar]
- 67.Reis A, Grandi V, Carlotto L, Bortoli G, Patzlaff R, Accorinte MLR, et al. Effect of smear layer thickness and acidity of self-etching solutions on early and long-term bond strength to dentin. J Dent. 2005;33:549–559. doi: 10.1016/j.jdent.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto M, Ito S, Tay FR, et al. Fluid movement across the resin-dentin interface during and after bonding. J Dent Res. 2004;83:843–848. doi: 10.1177/154405910408301104. [DOI] [PubMed] [Google Scholar]
- 69.Yeniad B, Albayrak AZ, Olcum NC, Avci D. Synthesis and photopolymerizations of new phosphonated monomers for dental applications. J Polym Sci Pt Polym Chem. 2008;46:2290–2299. [Google Scholar]
- 70.Van Landuyt KL, Snauwaert J, Peumans M, De Munck J, Lambrechts P, Van Meerbeek B. The role of HEMA in one-step self etch adhesives. Dent Mater. 2008;24:1412–1419. doi: 10.1016/j.dental.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 71.Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003;24:3795–3803. doi: 10.1016/s0142-9612(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 72.Reis AF, Giannini M, Pereira PN. Influence of water-storage time on the sorption and solubility behavior of current adhesives and primer/adhesive mixtures. Oper Dent. 2007;32:53–59. doi: 10.2341/06-13. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Spencer P. Continuing etching of an all in- one adhesive in wet dentin tubules. J Dent Research. 2005;84(4):350–354. doi: 10.1177/154405910508400411. [DOI] [PubMed] [Google Scholar]
- 74.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. Journal of Biomedical Materials Research. 2002;62(3):447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 75.Hanabusa M, Mine A, Kuboki T, Momoi Y, Van Ende A, Van Meerbeek B, De Munck J. Bonding effectiveness of a new “multi-mode” adhesive to enamel and dentine. J Dent. 2012;40(6):475–484. doi: 10.1016/j.jdent.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 76.Perdigao J, Sezinando A, Monteiro PC. Laboratory bonding ability of a multi-purpose dentin adhesive. Amer J Dent. 2012;25(3):153–158. [PubMed] [Google Scholar]
- 77.Munoz MA, Luque I, Hass V, Reis A, Loguercio AD, Bombarda NH. Immediate bonding properties of universal adhesives to dentine. J Dent. 2013;41(5):404–411. doi: 10.1016/j.jdent.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dental Materials. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 79.Erickson RL, Barkmeier WW, Kimmes NS. Bond strength of self-etch adhesives to pre-etched enamel. Dental Materials. 2009;25:1187–1194. doi: 10.1016/j.dental.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Rotta M, Bresciani P, Moura SK, Grande RH, Hilgert LA, Baratieri LN, et al. Effects of phosphoric acid pretreatment and substitution of bonding resin on bonding effectiveness of self-etching systems to enamel. The Journal of Adhesive Dentistry. 2007;9:537–545. [PubMed] [Google Scholar]
- 81.Boillagguet S, Gysi P, Wataha JC, Ciucchi B, Cattani M, Godin CH, et al. Bond strength of composite to dentin using conventional, one step, and self-etching adhesive systems. Journal of Dentistry. 2001;29:55–61. doi: 10.1016/s0300-5712(00)00049-x. [DOI] [PubMed] [Google Scholar]
- 82.Van Landuyt KL, De Munck J, Mine A, Cardoso MV, Peumans M, Van Meerbeek B. Filler debonding & subhybrid layer failures in self-etch adhesives. J Dent Res. 2010;89(10):1045–1050. doi: 10.1177/0022034510375285. [DOI] [PubMed] [Google Scholar]
- 83.De Munck J, Mine A, Poitevin A, Van Ende A, Cardoso MV, Van Landuyt KL, et al. Metaanalytic review of parameters involved in dentin bonding. Journal of Dental Research. 2012;91:351–357. doi: 10.1177/0022034511431251. [DOI] [PubMed] [Google Scholar]
- 84.Munoz MA, Luque-Martinez I, Malaquias P, Hass V. In vitro longevity of bonding properties of Universal Adhesives to dentin. Operative Dentistry. 2015:40–41. doi: 10.2341/14-055-L. [DOI] [PubMed] [Google Scholar]
- 85.Peumans M, De Munck J, Van Landuty KL, Poitevin A, Lambrechts P, Van Meerbeek B. Eight-year clinical evaluation of a 2-step self-etch adhesive with and without selective enamel etching. Dental Materials. 2010;26(12):1176–1184. doi: 10.1016/j.dental.2010.08.190. [DOI] [PubMed] [Google Scholar]
- 86.Frankenberger R, Lohbauer U, Roggendorf MJ, Naumann M, Taschner M. Selective enamel etching reconsidered: better than etch-and-rinse and self-etch. J Adhes Dent. 2008;10:339–344. [PubMed] [Google Scholar]
- 87.Erickson RL, Barkmeier WW, Latta MA. The role of etching in bonding to enamel: a comparison of self-etching and etch-and-rinse adhesive systems, Dent. Mater. 2009;25:1459–1467. doi: 10.1016/j.dental.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 88.Fukegawa D, Hayakawa S, Yoshida Y, et al. Chemical interaction of phosphoric acid ester with hydroxyapatite. J Dent Res. 2006;85(10):941–944. doi: 10.1177/154405910608501014. [DOI] [PubMed] [Google Scholar]
- 89.Van Landuyt KL, Yoshida Y, Hirata I, et al. Influence of the chemical structure of functional monomers on their adhesive performance. J Dent Res. 2008;87(8):757–761. doi: 10.1177/154405910808700804. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida Y, Yoshihara K, Nagaoka N, et al. Self-assembled Nano-layering at the Adhesive interface. J Dent Res. 2012;91(4):376–381. doi: 10.1177/0022034512437375. [DOI] [PubMed] [Google Scholar]
- 91.Yoshihara K, Yoshida Y, Hayakawa S, et al. Novel fluorocarbon functional monomer for dental bonding. J Dent Res. 2014;93(2):189–194. doi: 10.1177/0022034513514447. [DOI] [PubMed] [Google Scholar]
- 92.Tay FR, Pashley DH. Aggressiveness of contemporary self-etching systems. I: depth of penetration beyond dentin smear layers. Dent Mater. 2001;17:296–308. doi: 10.1016/s0109-5641(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 93.De Munck J, Van Meerbeek B, Yoshida Y, et al. Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res. 2003;82(2):136–140. doi: 10.1177/154405910308200212. [DOI] [PubMed] [Google Scholar]
- 94.Munoz MA, Sezinando A, Luque-Martinez I, Szesz AL, Reis A, Loguercio AD, Bombaeda NH, Perdigao J. Influence of a hydrophobic resin coating on the bonding efficacy of three of universal adhesives. J Dent. 2014;42:595–602. doi: 10.1016/j.jdent.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 95.Tuncer D, Yazici AR, Ozgunaltay G, Dayangac B. Clinical evaluation of different adhesives used in the restoration of non-carious cervical lesions: 24-month results. Aust Dent J. 2013;58:94–100. doi: 10.1111/adj.12028. [DOI] [PubMed] [Google Scholar]
- 96.Yoshihara K, Nagaoka N, Sonoda A, Maruo Y, Makita Y, Okihara T. Effectiveness and stability of silane coupling agent incorporated in ‘universal’ adhesives. Dent Mater. 2016;32(10):1218–1225. doi: 10.1016/j.dental.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Migliau G, Besharat LK, Sofan AAA, Sofan EAA, Romeo U. Endo-restorative treatment of a severly discolored upper incisor: resolution of the “aesthetic” problem through Componeer veneering System. Annali di Stomatologia. 2015;VI(3–4):113–118. doi: 10.11138/ads/2015.6.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Migliau G, Piccoli L, Besharat LK, Romeo U. Benchmarking matching color in composite restorations. Annali di Stomatologia. 2016;VII(1–2):29–37. doi: 10.11138/ads/2016.7.1.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Migliau G, Piccoli L, Besharat LK, Di Carlo S, Pompa G. Evaluation of over-etching technique in the endodontically treated tooth restoration. Annali di Stomatologia. 2015;VI(1):10–14. [PMC free article] [PubMed] [Google Scholar]