Abstract
Background
We assessed sociodemographic and clinical factors that are associated with survival among infants with congenital diaphragmatic hernia (CDH).
Methods
Using data from the Metropolitan Atlanta Congenital Defects Program, we ascertained 150 infants born with CDH between 1979 and 2003 and followed via linkage with state vital records and the National Death Index. Kaplan–Meier survival probabilities and adjusted hazard ratios (HRs) were calculated for socioeconomic and clinical characteristics.
Results
Survival increased from 40 to 62% over the study period. White infants born before 1988 were 2.9 times less likely to survive than those born after 1988. Black infants’ survival did not show significant improvement after 1988. White infants’ survival was not significantly affected by poverty, whereas black infants born in higher levels of poverty were 2.7 times less likely to survive than black infants born in lower levels of neighborhood poverty. White infants with multiple major birth defects were 2.6 times less likely to survive than those with CDH alone. The presence of multiple defects was not significantly associated with survival among black infants.
Conclusions
Survival among infants and children with CDH has improved over time among whites, but not among blacks. Poverty is associated with lower survival among blacks, but not among whites. The presence of multiple defects is associated with lower survival among whites, but not among blacks. The differential effects of poverty and race should be taken into account when studying disparities in health outcomes.
Keywords: birth defects, congenital diaphragmatic hernia, population surveillance, poverty, race, survival
Introduction
Birth defects are the leading cause of infant mortality in the United States, contributing to 20% of infant deaths (Matthews et al., 2015). Among infants with birth defects, congenital diaphragmatic hernia (CDH) accounts for the largest number of inhospital neonatal deaths in the United States (Centers for Disease Control and Prevention, 2007). CDH occurs when the diaphragm fails to close during development. Intestinal organs herniate into the fetal chest cavity, resulting in severe pulmonary hypoplasia and pulmonary hypertension. A recent measure of prevalence of CDH in the United States is 6.6/10,000 live births (Mai et al., 2012), with survival rates in the first year reaching 68.7% (Wang et al., 2015).
The pathophysiology of CDH has led to the search for prognostic factors for survival that fall along biologic and clinical factors. The factors previously implicated in mortality among infants with CDH include the presence of major anomalies (e.g., neural tube defects, structural brain anomalies, congenital heart defects, gastrointestinal tract atresias, renal agenesis/dysgenesis, structural renal anomalies, and omphalocele) or a syndrome (e.g., trisomies, structural chromosomal anomalies, Cornelia de Lange syndrome, and Fryns syndrome) in addition to CDH (Harrison et al., 1978; Reynolds et al., 1984; Ssemakula et al., 1997; Yang et al., 2006), preterm birth (Reynolds et al., 1984; Levison et al., 2006; Stevens et al., 2009), low birthweight (Reynolds et al., 1984; Yang et al., 2006; Samangaya et al., 2012), right-sided CDH (Reynolds et al., 1984), and prenatal diagnosis (Harrison et al., 1978; Ssemakula et al., 1997; Yang et al., 2006; Stevens et al., 2009; Lazar et al., 2011; Samangaya et al., 2012). Prenatal measures of lung volume, such as observed lung-to-head ratio, observed-to-expected lung-to-head ratio, and total lung volume, are the most used predictors of severity and survival (Aly et al., 2010; Sola et al., 2010; Badillo and Gingalewski, 2014). Infants whose condition was tenuous enough to warrant the use of extracorporeal membrane oxygenation had higher mortality rates (Ssemakula et al., 1997; Neff et al., 2007).
It is well documented that socioeconomic factors, such as race and poverty, contribute to pediatric health disparities, necessitating research approaches that examine both biologic and social elements of child health outcomes (Cheng et al., 2015). Race has been found to be a factor in CDH survival rates (Aly et al., 2010; Sola et al., 2010), but there is less information on the effect of socioeconomic status (SES) on survival. In this study, we are able to include SES as a predictive factor on CDH survival. By applying survival analysis, we can take into account multiple causes of death and examine disparities in CDH survival based on clinical factors, SES, and race.
Materials and Methods
POPULATION
We identified live-born infants included in the Metropolitan Atlanta Congenital Defects Program (MACDP) who were diagnosed with CDH (Centers for Disease Control and Prevention/BPA code 756.610) and born between January 1, 1979, and December 31, 2003. The MACDP is a population-based birth defects surveillance program that was established in 1967 for active monitoring of birth defects among live births and fetuses born to women living in five counties of metropolitan Atlanta, Georgia, in the United States. The program collects demographic information and clinical data about structural birth defects among live-born and stillborn infants at ≥20 weeks gestation or ≥500 gm birthweight. Since 1994, the program has also ascertained pregnancies with birth defects from prenatal diagnostic sites so as to include all pregnancies electively terminated for a birth defect at any gestational age. The MACDP works in partnership with the Georgia Department of Public Health and has been approved by the institutional review board of the Centers for Disease Control and Prevention. Additional information about the MACDP has been published previously (Correa et al., 2007).
All case records of live-born infants with CDH were reviewed by a clinical geneticist (author S.K.S.). We excluded children with known chromosomal anomalies or syndromes with CDH. We also excluded children with isolated diaphragmatic paralysis or eventration because the treatment modalities and underlying causes of mortality are different among these than among children with CDH. The CDH-affected children were classified as left-sided or right-sided, isolated (the only major defect was CDH), or multiple (with at least one other major structural birth defect). Morgagni hernias were not included in the analysis. Death data among infants and children with CDH were initially identified using abstracted hospital records for MACDP data collection and vital records from the state of Georgia. To capture data for deaths that might have occurred outside of Georgia, all MACDP live-born CDH case records were linked with the National Death Index from January 1, 1979, through December 31, 2006. Details on the linkage process have been described previously (Wentworth et al., 1983; Centers for Disease Control and Prevention, 2000). For case records with no recorded death, survival was censored at the end of the study period (December 31, 2006).
STUDY VARIABLES
We assessed the significance of demographic and clinical variables on survival probability. The demographic factors included maternal race, infant gender, maternal age, and neighborhood poverty. The cutoff value for maternal age (≤28 years and >28 years) was based on the median value. Race was derived from medical records and was classified as white, black, or other. Information on ethnicity was not collected in early years of surveillance and, therefore, was not considered in this study. Race was treated as a marker for differential experiences and exposures rather than as a factor inherent in that person (Jones, 2001). Neighborhood poverty levels were assigned based on census tract of mother’s residence at delivery. This method was previously described and has been shown to be a valid proxy for individual SES (Krieger et al., 2003). To assign poverty levels, we used 1980 census data for children with CDH born from 1979 to 1984, 1990 census data for children born from 1985 to 1994, and 2000 census data for children born from 1995 to 2003. Low neighborhood poverty is defined as greater than 10% of the people in the neighborhood living below the federally defined poverty level. All cases were assigned a bivariate measure of geocoded neighborhood poverty, <10% and ≥10%. We analyzed clinical characteristics, including low birth weight (<2500 gm and ≥2500 gm), gestational age (<37 weeks and ≥37 weeks), presence of multiple birth defects (isolated vs. multiple), and CDH sidedness (left vs. right).
Abstracted records did not necessarily include all interventions applied in the clinical care of the infant with CDH, therefore, we were not able to classify infants by actual procedures received. To approximate treatment, we divided our birth cohort into birth subcohorts defined by intervention eras that varied in CDH management, such as timing of surgical repair and mechanical treatment, such as high-frequency ventilation and extracorporeal membrane oxygenation. After conducting a survey of interventions in the CDH literature, we determined there were three intervention eras: (1) before 1988 (immediate surgical repair); (2) 1988 to 1995 (preoperative stabilization and surgical delay); and (3) 1996 or later (addition of lung-sparing strategies) (Clark et al., 1998; Frenckner et al., 2007; Logan et al., 2007; Stevens et al., 2009; Hoffman et al., 2010). We subsequently found no statistical difference between eras 2 and 3, and that the survival curves for these eras violated the proportional hazard assumption; therefore, for the determination of hazard ratios (HRs), we divided infants by birth cohorts defined by two treatment periods: born <1988 (immediate surgical repair) and born ≥1988 (surgical delay, stabilization, and lung-sparing strategies).
STATISTICAL ANALYSIS
We estimated the survival probability of children with CDH using the Kaplan–Meier product-limit method (Allison, 1995). We used the log-rank test to examine the variation in survival by birth cohort and other possible prognostic factors. Variables with a p-value < 0.05, and variables theoretically relevant to survival (regardless of p-value), were then analyzed by estimating hazard ratios using a Cox proportional hazards model (Allison, 1995). Cox proportional HRs and 95% confidence intervals (CIs) were determined for individual variables, and then adjusted HRs and CIs were calculated in a multivariable proportional hazards model. The assumption of proportionality was tested using PROC PHREG in SAS version 9.2 (SAS, Cary, NC). The variables for race and preterm birth were removed from the model because of the strong association with the neighborhood poverty and low birth-weight variables, respectively (χ2 p < 0.0001). In the final predictive model, the variables for sidedness and low birthweight were removed because they were not statistically significant. Race was associated with poverty, and the literature supports a role for race in survival from birth defects (Wang et al., 2015). Therefore, the final model was stratified by white and black race to explore if prognostic factors differed by race; the number of infants classified as other (n = 14; Table 1) was too small to include that racial group in this analysis (Correa et al., 2007).
TABLE 1.
Overall Survival for Infants and Children with Congenital Diaphragmatic Hernia by Demographic and Clinical Characteristics, Atlanta, GA, Born 1979 to 2003
| Characteristics | Births, no. (%) | Deaths, no. (%) | Overall survival, % (95% CI) | Log-rank test p-valuea |
|---|---|---|---|---|
| TOTAL | 150 | 63 | ||
| Treatment era | 0.019 | |||
| Before 1988 | 37 (24.7) | 22 (59.5) | 40.5 (23.4–57.6) | |
| 1988 and after | 113 (75.3) | 41 (36.2) | 58.3 (46.0–70.6) | |
| Race | 0.109 | |||
| White | 84 (56.0) | 30 (35.7) | 62.5 (51.2–73.8) | |
| Black | 47 (31.3) | 26 (55.3) | 44.6 (30.3–58.8) | |
| Other | 14 (9.3) | 5 (35.7) | 64.3 (39.2–89.4) | |
| Infant’s gender | 0.883 | |||
| Male | 86 (57.3) | 35 (40.7) | 56.7 (45.0–68.4) | |
| Female | 63 (42.0) | 27 (42.9) | 57.0 (44.8–69.3) | |
| Maternal age, yearsb | 0.382 | |||
| ≤28 | 75 (50.0) | 35 (46.7) | 63.5 (52.5–74.5) | |
| >28 | 74 (49.3) | 28 (37.8) | 59.0 (45.7–72.3) | |
| Neighborhood poverty | 0.010 | |||
| <10% | 94 (62.7) | 32 (34.0) | 63.2 (52.0–74.4) | |
| ≥10% | 56 (37.3) | 31 (55.4) | 42.4 (28.4–56.4) | |
| Gestational age, weeks | 0.914 | |||
| ≥37 | 116 (77.3) | 49 (42.2) | 55.9 (46.0–65.9) | |
| <37 | 30 (20.0) | 13 (43.3) | 55.0 (36.4–73.6) | |
| Birthweight, grams | 0.267 | |||
| ≥2500 | 122 (81.3) | 47 (39.5) | 58.7 (48.9–68.5) | |
| <2500 | 27 (18.0) | 15 (55.6) | 43.8 (24.8–62.8) | |
| Presence of multiple birth defects | 0.003 | |||
| No | 113 (75.3) | 40 (35.4) | 62.0 (52.0–72.0) | |
| Yes | 37 (24.7) | 23 (62.2) | 37.4 (21.7–53.2) | |
| CDH sidedness | 0.298 | |||
| Left | 102 (68.0) | 39 (38.2) | 60.9 (51.1–70.7) | |
| Right | 33 (22.0) | 16 (48.5) | 44.0 (22.3–65.7) |
Significant p-values are shown in bold font.
Maternal age determined by cutoff point of median age.
Cases with missing variable values were not included in the table.
95% CI, 95% confidence interval; CDH, congenital diaphragmatic hernia.
Results
We identified 150 infants with CDH that met our inclusion criteria. There were 61 deaths among the 150 infants and children with CDH for an overall fatality rate of 41%. Characteristics of the CDH study population are shown in Table 1. The log-rank tests of equality for all variables are shown in Table 1. Based on the log-rank test, three variables were assessed in model building: treatment period (p = 0.019), neighborhood poverty (p = 0.010), and presence of multiple birth defects (p = 0.003).
HRs for the statistically significant variables are shown in Table 2. HRs were first obtained for variables in a univariate model. In the univariate model, infants born before 1988 were 1.9 times more likely to die than infants born after 1988 (p = 0.013). Infants born to mothers who lived in neighborhoods in which 10% or more of the families lived below the federal poverty level had higher probability of mortality than those who were born to mothers who lived in low poverty neighborhoods (HR, 1.8; 95% CI, 1.1–3.0). Infants born with multiple birth defects had higher probability of mortality than those infants born with isolated CDH (HR, 2.0; 95% CI, 1.2–3.5). When the variables were analyzed in a multivariable model and adjusted for the other significant variables, all variables remained statistically significant.
TABLE 2.
Unadjusted and Adjusteda Hazard Ratios and 95% Confidence Intervals for Infants and Children with Congenital Diaphragmatic Hernia, Atlanta, Georgia, Born 1979 to 2003 with Vital Status Follow-up Through 2006
| Characteristics | Unadjusted HR (95% CI) n = 150 |
AHR (95% CI) n = 150 |
AHR (95% CI) white n = 84 |
AHR (95% CI) black n = 47 |
|---|---|---|---|---|
| Treatment era | ||||
| <1988 | 1.94 (1.25–3.27) | 2.11 (1.25–3.57) | 2.86 (1.38–5.90) | 1.17 (0.45–3.04) |
| ≥1988 | 1.00 | 1.00 | 1.00 | 1.00 |
| Neighborhood povertyb | ||||
| ≥10% | 1.83 (1.11–3.00) | 1.72 (1.04–2.84) | 1.11 (0.44–2.78) | 2.67 (1.05–6.81) |
| <10% | 1.00 | 1.00 | 1.00 | 1.00 |
| Presence of multiple birth defects | ||||
| CDH + other defects | 2.08 (1.24–3.48) | 2.06 (1.22–3.49) | 2.59 (1.20–5.57) | 1.40 (0.61–3.21) |
| CDH only | 1.00 | 1.00 | 1.00 | 1.00 |
Adjusted for treatment era, neighborhood poverty, and presence of multiple birth defects.
Neighborhood poverty refers to the percentage of residents in a neighborhood living below the federal poverty level. The sample was divided at the 10% point to allow for adequate cases for statistical analysis. Areas where 20% of residents live below poverty are considered areas of high concentration of poverty (https://www.census.gov/population/socdemo/statbriefs/povarea.html). White children in this study did not live in areas of high poverty.
Significant ratios are bolded.
HR, hazard ratio; 95% CI, 95% confidence interval; AHR, adjusted hazard ratio; CDH, congenital diaphragmatic hernia.
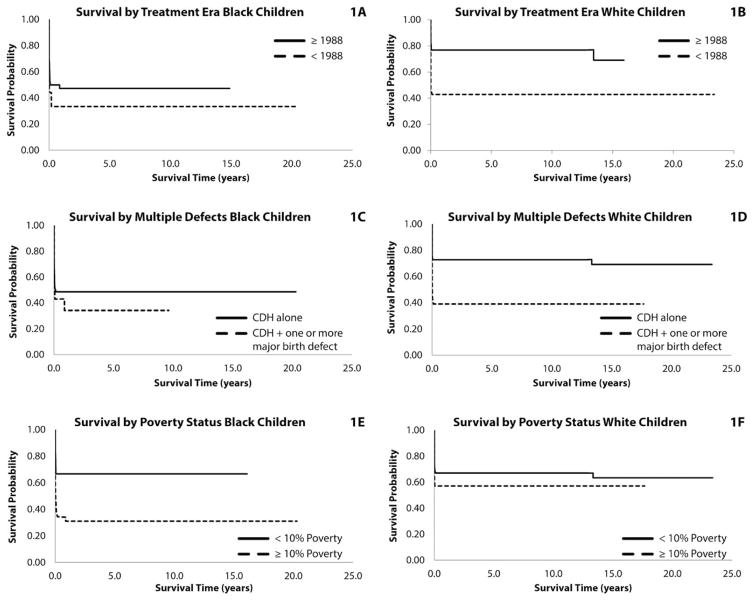
The adjusted HRs and 95% CIs for treatment period, neighborhood poverty, and presence of multiple birth defects, stratified by race, are shown in Table 2, and the counts by race and variable are shown in Table 3. The Kaplan–Meier survival curves for these variables, stratified by race, are shown in Figure 1. Survival for black infants improved over time, but not significantly. As shown in Figure 1A, B, survival for both black and white infants was poor before 1988. Survival for white infants, however, improved significantly after 1988. White infants born before 1988 were 2.9 times more likely to die than infants born in 1988 and later. Survival for black infants, however, did not improve significantly for those born in 1988 and later (Table 2).
TABLE 3.
Counts of Infants and Children Included in Stratified Survival Analysis
| Characteristics | White N = 84 |
Black N = 47 |
|---|---|---|
| Treatment era | ||
| <1988 | 28 | 9 |
| ≥1988 | 56 | 38 |
| Neighborhood poverty | ||
| ≥10% | 14 | 29 |
| <10% | 70 | 18 |
| Presence of multiple birth defects | ||
| CDH + other defects | 18 | 14 |
| CDH only | 66 | 33 |
CDH, congenital diaphragmatic hernia.
FIGURE 1.
(A) Congenital diaphragmatic hernia (CDH) survival in black children by era, ≤1988/>1988. Survival probability of black children with congenital diaphragmatic hernia, Atlanta, GA, born 1979 to 2003 by treatment era. Treatment eras are before 1988 (routine immediate surgical repair), and post-1988 (preoperative stabilization, delayed surgical repair, and addition of lung-sparing strategies). Adjusted hazard ratio (AHR), 1.17; confidence interval (CI), 0.45–3.04). (B) CDH survival in white children by era, ≤1988/>1988. Survival probability of white children with congenital diaphragmatic hernia, Atlanta, GA, born 1979 to 2003 by treatment era. Treatment eras are before 1988 (routine immediate surgical repair), and post-1988 (preoperative stabilization, delayed surgical repair, and addition of lung sparing strategies). AHR, 2.86; CI, 1.38–5.90). (C) CDH survival in black children by presence of multiple defects, CDH + other defects/CDH alone. Survival probability of black children with congenital diaphragmatic hernia, Atlanta, GA, born 1979 to 2003 by presence or absence of multiple defects. Categories are CDH alone and CDH plus one or more major birth defects. AHR, 1.40; CI, 0.61–3.21. (D) CDH survival in white children by presence of multiple defects, CDH + other defects/CDH alone. Survival probability of white children with congenital diaphragmatic hernia, Atlanta, GA, born 1979 to 2003 by presence or absence of multiple defects. Categories are CDH alone and CDH plus one or more major birth defects. AHR, 2.59; CI, 1.20–5.57. (E) CDH survival in black children by percent of residents living below federal poverty level, <10%/≥10%. Survival probability of black children with congenital diaphragmatic hernia, Atlanta, GA, born 1979 to 2003 by neighborhood poverty level. Categories are <10% neighborhood residents live below federal poverty level and ≥10% neighborhood residents live below the federal poverty level. AHR, 2.67; CI, 1.05–6.81. (F) CDH survival in white children by percent of residents living below federal poverty level, <10%/≥10%. Survival probability of white children with congenital diaphragmatic hernia, Atlanta, GA, born 1979 to 2003 by neighborhood poverty level. Categories are <10% neighborhood residents live below the federal poverty level and ≥10% neighborhood residents live below the federal poverty level. AHR, 1.11; CI, 0.44–2.78.
White infants with multiple birth defects were 2.6 times more likely to die than white infants with isolated CDH (p = 0.015; Table 2). The presence of multiple birth defects did not affect survival for black infants (p = 0.406). As shown in Figure 1C, D, survival for black infants was poor, regardless of the presence of multiple birth defects. Survival for white infants with multiple birth defects was comparable with black infants who either did or did not have multiple birth defects.
Black infants born to mothers who lived in higher poverty neighborhoods were 2.7 times more likely to die than black infants born in low poverty (p = 0.040; Table 2). The survival of white infants was not affected by the level of neighborhood poverty (p = 0.783). Black infants born to mothers who lived in low poverty neighborhoods had a survival comparable with white infants who were either born in low or high poverty neighborhoods (Fig. 1E, F).
Discussion
Survival for live-born infants with CDH in the metropolitan Atlanta area has improved significantly over the study time period of 1979 to 2006, increasing from 40.5 to 61.9%, with an overall long-term survival rate of 60% at 28 years of follow-up. These findings approximate the results reported from a U.K. survival analysis for the years 1985 to 2003, which found an overall survival rate of 57.1% at 20 years (Tennant et al., 2010). We found that increased survival was associated with treatment period after 1988 (birth cohort), and decreased survival was associated with presence of multiple birth defects and neighborhood poverty ≥10%. A previous study using data from the MACDP found that the percentage of infants with CDH who survived to 1 year of age increased from 19% (years 1968–1971) to about 54% (years 1996–1999) (Dott et al., 2003). The impact of multiple birth defects is in agreement with previous mortality studies (Skari et al., 2000; Stege et al., 2003; Ontario Congenital Anomalies Study Group, 2004; Logan et al., 2007).
The MACDP surveillance data did not collect detailed information on specific clinical interventions; therefore, we operated under an informed assumption that multiple treatment strategies occur concurrently, and that at particular times certain protocols will be considered “standard of care.” In our survival analysis, infants born 1988 and later had significantly improved survival compared with infants born before 1988. The changes in standard of care for CDH likely account for the dramatic improvement in survival in the post-1988 era. However, because clinical interventions might have occurred in concert, teasing out the primary modality(ies) responsible for improved survival is not possible.
The percentage of deaths differed for black and white children with CDH in our study, although race was not a statistically significant variable in the univariate analysis. Nor did a previous MACDP survival study of CDH find a significant difference in mortality by race (Dott et al., 2003). Although the differences in survival are not statistically significant in our study, they demonstrate a pattern of increasing CDH survival disparity between black and white individuals in this particular study population. Other birth defect survival analyses have shown a significant difference in survival by race (Petrini et al., 1997; Malcoe et al., 1999; Siffel et al., 2003; Frenckner et al., 2007; Stevens et al., 2009), and this suggested that race deserved further consideration in our survival analysis. Moreover, we found that race was significantly associated with poverty. Upon stratifying by race, we discovered that the predictive factors differed significantly. White infants’ survival significantly improved over time; however, survival did not improve for black infants across time periods. It is noteworthy that the survival rates for both black and white infants were comparable in the pre-1988 birth cohort. This is a metropolitan population and all infants born in this population potentially had access to tertiary care hospitals; further studies would need to be conducted to assess aspects of access to care, timing of care, types of treatments administered, and severity of cases.
Focusing strictly on aspects of care, however, overlooks the compelling interplay of race and poverty and their effects on CDH survival. White infants’ survival was not significantly affected by neighborhood poverty, but black infants born to mothers living in higher poverty neighborhoods had a 2.7-fold lower survival than black infants from less impoverished neighborhoods. Poverty’s effect on the survival of infants with CDH is not surprising because other studies have described the effect of poverty on child survival. Even though overall rates of childhood mortality are declining, children born into poverty have not fully benefited from the improved survival (Singh and Kogan, 2007). Other studies, such as an analysis of the Kids’ Inpatient Database, have demonstrated better survival for patients with CDH with private insurance (Sola et al., 2010), another indication of the impact of poverty and class status on survival, separate from race. The Kids’ Inpatient Database study also showed increased survival for white children with CDH.
The presence of multiple major birth defects remained a consistent predictor of the risk of mortality from CDH. Upon stratification by race, only white infants with multiple birth defects were at significantly increased risk of mortality compared with white infants with isolated CDH, adjusting for treatment period and neighborhood poverty. The risk from multiple major birth defects for black infants was slightly elevated, but was not statistically significant, adjusting for treatment period and neighborhood poverty. The results for black infants differ from those reported by Wang et al. (2011). In that study, the presence of multiple birth defects increased mortality associated with CDH. We stratified our analysis by race to address potential confounding with poverty; our findings suggest that, for black infants, the effect of poverty on survival outweighs the impact of treatment period and the presence of multiple birth defects. White infant survival was affected by both the benefits of treatment advances over time and the deleterious impact of multiple birth defects; however, the impact of poverty on survival was negligible.
This study had several strengths. First, this was a population-based study, not a selective clinical trial or clinic-based cohort that could introduce bias in case ascertainment. Second, MACDP uses active multisite case ascertainment at birth hospitals, pediatric hospitals, and prenatal offices, with a high level of detail and data quality. Third, our analysis covered a 26-year period of case follow-up. Fourth, we used multiple sources for death ascertainment. Last, we included a measure of neighborhood poverty to assess the socioeconomic factors associated with survival for CDH.
Our study is not without limitations. We assumed that children for whom we had no available date of death were still alive at the end of the study period, but our use of multiple data sources should limit the possibility of missed deaths. We do not have an assessment of the impact of prenatal diagnosis and termination of pregnancy, which could affect the measure of true survival rates. Severe cases of CDH are typically the ones detected by prenatal diagnosis (Lazar et al., 2011; Samangaya et al., 2012) and a previous study using the MACDP found that 15 to 18% of prenatally diagnosed CDH cases were terminated (Cragan and Gilboa, 2009). Therefore, termination of more severe cases of CDH could contribute to an improved survival rate, especially in recent eras with improved and more broadly utilized prenatal detection. Third, the treatment period variable does not refer specifically to any one intervention, but encompasses the overall changes in intervention that may have led to increased survival.
CONCLUSION
In summary, this study demonstrates that overall survival for CDH has improved over time, but with noted differences by race. Factors with a significant negative impact on survival are the presence of multiple birth defects and neighborhood poverty levels, but these had differential effects by race. Our findings highlight that studies need to take into consideration the differential effects of poverty and race on survival probabilities. Race and SES, which are related, affect health outcomes differently (Williams, 2002; Williams et al., 2009). Teasing out these differences can provide insight into the social factors that contribute to health disparities.
Acknowledgments
Funding source: No external funding was received for this study.
We extend our sincere gratitude to all MACDP staff members for their conscientious and skilled data collection and cleaning efforts. The authors have no financial relationships relevant to this article to disclose, nor do they have conflicts of interest to disclose. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Allison PD. Survival analysis using the SAS system: a practical guide. Cary, NC: SAS Institute; 1995. [Google Scholar]
- Aly H, Bianco–Batlles D, Mohamed MA, Hammad TA. Mortality in infants with congenital diaphragmatic hernia: a study of the United States National Database. J Perinatol. 2010;30:553–557. doi: 10.1038/jp.2009.194. [DOI] [PubMed] [Google Scholar]
- Badillo A, Gingalewski C. Congenital diaphragmatic hernia: treatment and outcomes. Semin Perinatol. 2014;38:92–96. doi: 10.1053/j.semperi.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Death Index user manual. Hyattsville, MD: National Center for Health Statistics; 2000. [Google Scholar]
- Centers for Disease Control and Prevention. Hospital stays, hospital charges, and in-hospital deaths among infants with selected birth defects--United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56:25–29. [PubMed] [Google Scholar]
- Cheng TL, Goodman E Committee on Pediatric Research. Race, ethnicity, and socioeconomic status in research on child health. Pediatrics. 2015;135:e225–e237. doi: 10.1542/peds.2014-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RH, Hardin WD, Jr, Hirschl RB, et al. Current surgical management of congenital diaphragmatic hernia: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 1998;33:1004–1009. doi: 10.1016/s0022-3468(98)90522-x. [DOI] [PubMed] [Google Scholar]
- Correa A, Cragan JD, Kucik JE, et al. Reporting birth defects surveillance data 1968–2003. Birth Defects Res A Clin Mol Teratol. 2007;79:66–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- Cragan JD, Gilboa SM. Including prenatal diagnoses in birth defects monitoring: experience of the Metropolitan Atlanta Congenital Defects Program. Birth Defects Res A Clin Mol Teratol. 2009;85:20–29. doi: 10.1002/bdra.20508. [DOI] [PubMed] [Google Scholar]
- Dott MM, Wong LY, Rasmussen SA. Population-based study of congenital diaphragmatic hernia: risk factors and survival in Metropolitan Atlanta, 1968–1999. Birth Defects Res A Clin Mol Teratol. 2003;67:261–267. doi: 10.1002/bdra.10039. [DOI] [PubMed] [Google Scholar]
- Frenckner BP, Lally PA, Hintz SR, et al. Prenatal diagnosis of congenital diaphragmatic hernia: how should the babies be delivered? J Pediatr Surg. 2007;42:1533–1538. doi: 10.1016/j.jpedsurg.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Harrison MR, Bjordal RI, Langmark F, Knutrud O. Congenital diaphragmatic hernia: the hidden mortality. J Pediatr Surg. 1978;13:227–230. doi: 10.1016/s0022-3468(78)80391-1. [DOI] [PubMed] [Google Scholar]
- Hoffman SB, Massaro AN, Gingalewski C, Short BL. Predictors of survival in congenital diaphragmatic hernia patients requiring extracorporeal membrane oxygenation: CNMC 15-year experience. J Perinatol. 2010;30:546–552. doi: 10.1038/jp.2009.193. [DOI] [PubMed] [Google Scholar]
- Jones CP. Invited commentary: “race,” racism, and the practice of epidemiology. Am J Epidemiol. 2001;154:299–304. doi: 10.1093/aje/154.4.299. discussion 305–306. [DOI] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, et al. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: the Public Health Disparities Geocoding Project (US) J Epidemiol Community Health. 2003;57:186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar DA, Cass DL, Rodriguez MA, et al. Impact of prenatal evaluation and protocol-based perinatal management on congenital diaphragmatic hernia outcomes. J Pediatr Surg. 2011;46:808–813. doi: 10.1016/j.jpedsurg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Levison J, Halliday R, Holland AJ, et al. A population-based study of congenital diaphragmatic hernia outcome in New South Wales and the Australian Capital Territory, Australia, 1992–2001. J Pediatr Surg. 2006;41:1049–1053. doi: 10.1016/j.jpedsurg.2006.01.073. [DOI] [PubMed] [Google Scholar]
- Logan JW, Rice HE, Goldberg RN, Cotten CM. Congenital diaphragmatic hernia: a systematic review and summary of best-evidence practice strategies. J Perinatol. 2007;27:535–549. doi: 10.1038/sj.jp.7211794. [DOI] [PubMed] [Google Scholar]
- Mai CT, Riehle–Colarusso T, O’Halloran A, et al. Selected birth defects data from population-based birth defects surveillance programs in the United States, 2005–2009: featuring critical congenital heart defects targeted for pulse oximetry screening. Birth Defects Res A Clin Mol Teratol. 2012;94:970–983. doi: 10.1002/bdra.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcoe LH, Shaw GM, Lammer EJ, Herman AA. The effect of congenital anomalies on mortality risk in white and black infants. Am J Public Health. 1999;89:887–892. doi: 10.2105/ajph.89.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1–30. [PubMed] [Google Scholar]
- Neff KW, Kilian AK, Schaible T, et al. Prediction of mortality and need for neonatal extracorporeal membrane oxygenation in fetuses with congenital diaphragmatic hernia: logistic regression analysis based on MRI fetal lung volume measurements. AJR Am J Roentgenol. 2007;189:1307–1311. doi: 10.2214/AJR.07.2434. [DOI] [PubMed] [Google Scholar]
- Petrini J, Damus K, Johnston RB., Jr An overview of infant mortality and birth defects in the United States. Teratology. 1997;56:8–10. doi: 10.1002/(SICI)1096-9926(199707/08)56:1/2<8::AID-TERA3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Luck SR, Lappen R. The “critical” neonate with diaphragmatic hernia: a 21-year perspective. J Pediatr Surg. 1984;19:364–369. doi: 10.1016/s0022-3468(84)80254-7. [DOI] [PubMed] [Google Scholar]
- Samangaya RA, Choudhri S, Murphy F, et al. Outcomes of congenital diaphragmatic hernia: a 12-year experience. Prenat Diagn. 2012;32:523–529. doi: 10.1002/pd.3841. [DOI] [PubMed] [Google Scholar]
- Siffel C, Wong LY, Olney RS, Correa A. Survival of infants diagnosed with encephalocele in Atlanta, 1979–98. Paediatr Perinat Epidemiol. 2003;17:40–48. doi: 10.1046/j.1365-3016.2003.00471.x. [DOI] [PubMed] [Google Scholar]
- Singh GK, Kogan MD. Widening socioeconomic disparities in US childhood mortality, 1969–2000. Am J Public Health. 2007;97:1658–1665. doi: 10.2105/AJPH.2006.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skari H, Bjornland K, Haugen G, et al. Congenital diaphragmatic hernia: a meta-analysis of mortality factors. J Pediatr Surg. 2000;35:1187–1197. doi: 10.1053/jpsu.2000.8725. [DOI] [PubMed] [Google Scholar]
- Sola JE, Bronson SN, Cheung MC, et al. Survival disparities in newborns with congenital diaphragmatic hernia: a national perspective. J Pediatr Surg. 2010;45:1336–1342. doi: 10.1016/j.jpedsurg.2010.02.105. [DOI] [PubMed] [Google Scholar]
- Ssemakula N, Stewart DL, Goldsmith LJ, et al. Survival of patients with congenital diaphragmatic hernia during the ECMO era: an 11-year experiment. J Pediatr Surg. 1997;32:1683–1689. doi: 10.1016/s0022-3468(97)90506-6. [DOI] [PubMed] [Google Scholar]
- Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112(3 Pt 1):532–535. doi: 10.1542/peds.112.3.532. [DOI] [PubMed] [Google Scholar]
- Stevens TP, van Wijngaarden E, Ackerman KG, et al. Timing of delivery and survival rates for infants with prenatal diagnoses of congenital diaphragmatic hernia. Pediatrics. 2009;123:494–502. doi: 10.1542/peds.2008-0528. [DOI] [PubMed] [Google Scholar]
- Tennant PW, Pearce MS, Bythell M, Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet. 2010;375:649–656. doi: 10.1016/S0140-6736(09)61922-X. [DOI] [PubMed] [Google Scholar]
- Ontario Congenital Anomalies Study Group. Apparent truth about congenital diaphragmatic hernia: a population-based database is needed to establish benchmarking for clinical outcomes for CDH. J Pediatr Surg. 2004;39:661–665. doi: 10.1016/j.jpedsurg.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hu J, Druschel C, Kirby RS. Twenty-five-year survival of children with birth defects in New York State: a population-based study. Birth Defects Res A Clin Mol Teratol. 2011;91:995–1003. doi: 10.1002/bdra.22858. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu G, Canfield MA, et al. Racial/ethnic differences in survival of United States children with birth defects: a population-based study. J Pediatr. 2015;166:819–826. doi: 10.1016/j.jpeds.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth DN, Neaton JD, Rasmussen WL. An evaluation of the Social Security Administration master beneficiary record file and the National Death Index in the ascertainment of vital status. Am J Public Health. 1983;73:1270–1274. doi: 10.2105/ajph.73.11.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR. Racial/ethnic variations in women’s health: the social embeddedness of health. Am J Public Health. 2002;92:588–597. doi: 10.2105/ajph.92.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123(Suppl 3):S174–S184. doi: 10.1542/peds.2008-2233H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Carmichael SL, Harris JA, Shaw GM. Epidemiologic characteristics of congenital diaphragmatic hernia among 2.5 million California births, 1989–1997. Birth Defects Res A Clin Mol Teratol. 2006;76:170–174. doi: 10.1002/bdra.20230. [DOI] [PubMed] [Google Scholar]