Abstract
Stiff-knee gait is characterized by diminished and delayed knee flexion during swing. Rectus femoris transfer surgery, a common treatment for stiff-knee gait, is often recommended when a patient exhibits prolonged activity of the rectus femoris muscle during swing. Treatment outcomes are inconsistent, in part, due to limited understanding of the biomechanical factors contributing to stiff-knee gait. This study used a combination of gait analysis and dynamic simulation to examine how activity of the rectus femoris during swing, and prior to swing, contribute to knee flexion. A group of muscle-actuated dynamic simulations was created that accurately reproduced the gait dynamics of ten subjects with stiff-knee gait. These simulations were used to examine the effects of rectus femoris activity on knee motion by eliminating rectus femoris activity during preswing and separately during early swing. The increase in peak knee flexion by eliminating rectus femoris activity during preswing (7.5 ± 3.1°) was significantly greater on average (paired t-test, p = 0.035) than during early swing (4.7 ± 3.6°). These results suggest that preswing rectus femoris activity is at least as influential as early swing activity in limiting the knee flexion of persons with stiff-knee gait. In evaluating rectus femoris activity for treatment of stiff-knee gait, preswing as well as early swing activity should be examined.
Introduction
Stiff-knee gait is a debilitating consequence of cerebral palsy characterized by diminished knee motion and delayed peak knee flexion during swing. Each year, three out of every 1,000 children manifest one or more of the symptoms of cerebral palsy (CDC, 2004). The ambulatory types of cerebral palsy account for 48-79% of all cases (Stanley et al., 2000). Stiff-knee gait is one of the most common gait abnormalities in ambulatory children with cerebral palsy (Wren et al., 2005). Many individuals with stiff-knee gait frequently trip or perform inefficient compensatory movements due to inadequate toe clearance (Sutherland and Davids, 1993).
Distal transfer of the rectus femoris is a common surgical treatment for stiff-knee gait (Gage et al., 1987; Perry, 1987). Though increased vasti and decreased iliopsoas activity have been identified as potential causes of stiff-knee gait (Goldberg et al., 2004), the limited knee flexion is usually attributed to abnormal prolongation of rectus femoris activity into early swing phase (Gage et al., 1987; Perry, 1987; Sutherland and Davids, 1993; Sutherland et al., 1975; Sutherland et al., 1990; Waters et al., 1979). Rectus femoris transfer surgery is intended to decrease the muscle's ability to extend the knee while preserving its ability to generate hip flexion moment (Asakawa et al., 2002; Gage et al., 1987; Perry, 1987), which promotes knee flexion (Asakawa et al., 2002; Kerrigan et al., 1998; Piazza and Delp, 1996). Several studies have reported that rectus femoris transfer typically improves knee flexion (Gage et al., 1987; Õunpuu et al., 1993a; Õunpuu et al., 1993b; Rethlefsen et al., 1999; Sutherland et al., 1990). However, less positive outcomes related to swing phase peak knee flexion have more recently been reported in some patients (Yngve et al., 2002).
Outcomes of surgical treatments for stiff-knee gait are inconsistent, in part, due to insufficient understanding of the biomechanical factors contributing to stiff-knee gait. Although rectus femoris transfer is thought to improve knee flexion by decreasing knee extension moment, Goldberg et al. (2003) found that many subjects with stiff-knee gait did not walk with abnormally large knee extension moments during early swing, but they walked with abnormally low knee flexion velocity at toe-off. Goldberg et al. (2006) subsequently reported that many subjects with stiff-knee gait walked with abnormally large knee extension moments during double support, which were correlated with low knee flexion velocity at toe-off. Moreover, most subjects with favorable outcomes following surgery walked with decreased knee extension moments during double support and corresponding increased knee flexion velocities at toe-off (Goldberg et al., 2006). These results suggest that knee extension moment, which is influenced by rectus femoris activity, prior to toe-off, rather than after toe-off, may be a more prevalent contributor to stiff-knee gait than previously thought. A better understanding of when rectus femoris activity contributes to stiff-knee gait is necessary to refine clinical indications for rectus femoris transfer surgery.
This study used dynamic simulation, in combination with gait analysis, to evaluate the relative importance of preswing (i.e., the period immediately prior to toe-off) rectus femoris activity as a biomechanical factor contributing to diminished knee flexion in subjects with stiff-knee gait. We hypothesized that rectus femoris activity during preswing has a greater impact on peak knee flexion than rectus femoris activity during early swing (i.e., the period from toe-off to peak knee flexion) in subjects with stiff-knee gait. We tested this hypothesis by simulating the elimination of rectus femoris activity during preswing and separately during early swing for a group of ten subjects with cerebral palsy walking with stiff-knee gait and computing the resulting changes in knee flexion. Identifying the function of rectus femoris activity during preswing and early swing in subjects with stiff-knee gait contributes to our understanding of this gait abnormality and provides insights needed to improve treatment planning.
Methods
The subjects in this study underwent gait analysis at Connecticut Children's Medical Center in Hartford, CT. Gait analysis data, including three-dimensional joint angles, ground reaction forces and moments, and surface electromyographic (EMG) recordings from preamplifier electrodes, were collected as a routine part of treatment planning. Our inclusion criteria (Goldberg et al., 2006) required that each subject (i) subsequently underwent rectus femoris transfer surgery as a correctional treatment for stiff-knee gait, (ii) was between 6 and 17 years of age prior to surgery, (iii) had not undergone a selective dorsal rhizotomy, and (iv) walked without orthoses or other assistance. Ten subjects were identified and categorized as exhibiting stiff-knee gait in at least one limb preoperatively (Table 1).
Table 1.
Descriptive values for 10 subjects with stiff-knee gait and 15 able-bodied subjects.
| Subject | Rectus femoris electromyography deviations | Age (years) | Mass (kg) | Knee ROM from toe-off to peak knee flexion (°) | Knee ROM total (°) | Peak knee flexion (°) | Timing of peak knee flexion (% swing)) | Double support |
Preswing |
Early swing |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (s) | Duration (s) | Average knee extension moment (Nm/kg) | Duration (s) | Average knee extension moment (Nm/kg) | ||||||||
| 1 | Prolonged loading response burst, brief shutdown at toe-off, excessive firing in swing | 12.8 | 38.1 | 6.9 | 26.5 | 35.7 | 37.8 | 0.117 | 0.072 | 0.194 | 0.072 | 0.054 |
| 2 | Continuous activity | 10.3 | 26.9 | 17.5 | 29.3 | 40.7 | 39.4 | 0.090 | 0.144 | 0.296 | 0.144 | 0.040 |
| 3 | Prolonged loading response burst, inappropriate firing in swing | 10.8 | 29.3 | 14.6 | 36.3 | 45.2 | 42.9 | 0.103 | 0.141 | 0.399 | 0.141 | 0.017 |
| 4 | Continuous activity | 9.12 | 32.2 | 9.0 | 20.9 | 45.9 | 45.4 | 0.084 | 0.126 | 0.378 | 0.126 | 0.077 |
| 5 | Continuous activity | 12.5 | 36.5 | 6.0 | 11.8 | 49.2 | 38.9 | 0.144 | 0.167 | 0.688 | 0.167 | 0.056 |
| 6 | Prolonged loading response burst, continuous activity in swing | 6.84 | 21.8 | 11.8 | 32.1 | 50.7 | 40.7 | 0.146 | 0.156 | 0.396 | 0.156 | 0.017 |
| 7 | Prolonged loading response burst, inappropriate firing in initial and mid swing | 8.23 | 22.2 | 17.9 | 44.6 | 51.3 | 40.4 | 0.072 | 0.120 | 0.126 | 0.120 | 0.015 |
| 8 | Continuous activity | 15.6 | 53.9 | 5.1 | 15.2 | 53.1 | 51.9 | 0.160 | 0.160 | 0.688 | 0.160 | 0.037 |
| 9 | Continuous activity | 10.8 | 28.6 | 18.5 | 25.8 | 54.4 | 48.3 | 0.100 | 0.141 | 0.293 | 0.141 | 0.008 |
| 10 | Continuous activity | 12.8 | 30.8 | 16.4 | 41.6 | 64.0 | 41.7 | 0.122 | 0.113 | 0.395 | 0.113 | 0.053 |
| Able-bodied* | None | 10.5 (2.9) | 35.2 (13.6) | 31.0 (4.3) | 59.5 (6.8) | 65.8 (5.3) | 33.0 (3.1) | 0.066 (0.014) | 0.135| (0.018) | 0.185 (0.080) | 0.135 (0.018) | 0.031 (0.016) |
Able-bodied subject values are given as average (standard deviation).
Four gait parameters (Goldberg et al., 2006) were used to determine whether a subject walked with stiff-knee gait: peak knee flexion in swing phase (e.g., Gage et al., 1987; Sutherland et al., 1990), knee range of motion in early swing (Goldberg et al., 2003), total knee range of motion (e.g., Gage et al., 1987; Õunpuu et al., 1993), and timing of peak knee flexion during swing phase (e.g., Sutherland et al., 1990; Õunpuu et al., 1993). A limb was classified as “stiff” if three or more of these measures were more than two standard deviations below (or above in the case of the timing measure) the average control value. Control data were collected from 15 able-bodied subjects of approximately the same average age, height, and weight as the subjects with cerebral palsy (Table 1). Surface EMG data were not used to include or exclude subjects from this study. However, all of the subjects with stiff-knee gait did exhibit abnormal rectus femoris activity (Table 1). All subjects gave informed consent for the collection of their gait data. Mutual institutional approval was obtained for retrospective analysis of these data. The data analysis included the creation of subject-specific dynamic simulations.
A three-dimensional, full-body musculoskeletal model with 21 degrees of freedom and 92 muscle-tendon actuators formed the foundation of each simulation (Fig. 1). The position and orientation of the pelvis relative to ground was defined with 6 degrees of freedom. The head, arms, and torso were represented as a rigid segment connected with the pelvis by a ball-and-socket joint (Anderson and Pandy, 1999). The remaining lower extremity joints were modeled as follows: each hip as a ball-and-socket joint, each knee as a planar joint with tibiofemoral and patellofemoral translational constraints as a function of knee flexion (Delp et al., 1990), and each ankle and subtalar joints as revolute joints (Inman, 1976). Each muscle-tendon actuator was modeled as a Hill-type muscle in series with tendon based on musculotendon parameters from Delp et al. (1990). The musculoskeletal model and corresponding dynamic simulation code were produced using SIMM and the Dynamics Pipeline (Delp and Loan, 2000) along with SD/FAST (Parametric Technology Corporation, Waltham, MA). The musculoskeletal model was used in conjunction with gait analysis data to create subject-specific dynamic simulations.
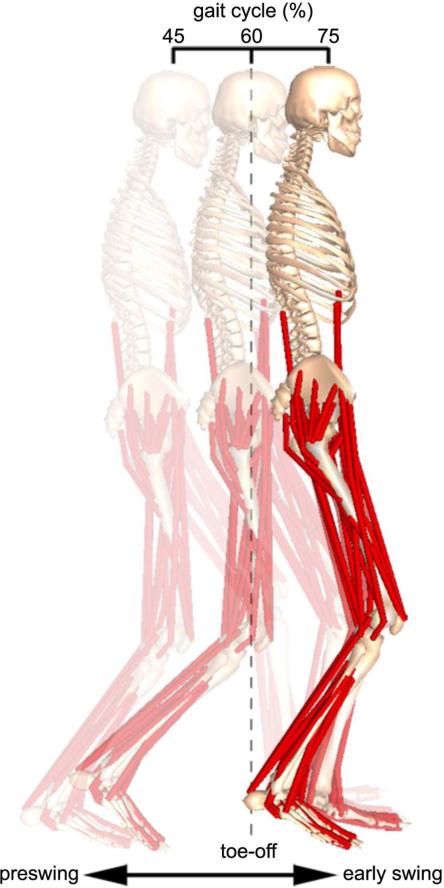
Figure 1.
Muscle-actuated dynamic simulation of a subject's gait during the period of preswing through early swing. A three-dimensional, full-body musculoskeletal model with 21 degrees of freedom and 92 muscle-tendon actuators was used in conjunction with the subject's gait analysis data to create each subject-specific simulation. The dynamic simulation is shown at the initiation of preswing (left), just following toe-off (center), and at the termination of early swing (right). Each subject-specific simulation was used to conduct simulation experiments to evaluate the relative importance of preswing rectus femoris activity as a biomechanical factor contributing to the subject's diminished knee flexion.
A muscle-actuated dynamic simulation of each subject was created using a four-step process. First, the musculoskeletal model was scaled to represent the experimentally measured size of each subject. Second, inverse kinematics analysis was utilized to obtain values of generalized coordinates for the model that closely matched the experimentally measured kinematics of each subject. Third, a residual elimination algorithm (Thelen and Anderson, 2005) was applied to achieve dynamic consistency between the model's motions and the experimentally measured ground reactions of each subject, by adjusting pelvis translations and back rotations. Fourth, computed muscle control (Thelen et al., 2003) was implemented to determine an optimal set of muscle activities that produced forward simulations and that were generally consistent with the experimentally measured kinematics and EMG patterns of each subject. Constraints were placed on the muscle activity of each simulation based on the recorded EMG. For example, when activity was recorded for rectus femoris during early swing, the simulated rectus femoris was required to have activity during this time as well. This four-step process was used to create simulations for each subject's preoperative gait during the period of preswing through peak knee flexion in swing. The simulated joint angles reproduced the subjects’ measured hip, knee, and ankle angles within 3°. The subject-specific dynamic simulations were used to conduct subsequent simulation experiments.
The simulation of each subject was altered to examine the effects of rectus femoris activity on knee motion. In particular, the activity of rectus femoris was eliminated during preswing and separately during early swing, creating two new simulations per subject, to determine the muscle's relative importance to peak knee flexion for each case (Fig. 2). By observing the changes in peak knee flexion between the new and unperturbed simulations, the muscle's contribution to knee motion was inferred for that period of time in which its activity was eliminated. For these simulation experiments, preswing was defined to be the period of the gait cycle before toe-off equal in length of time to early swing. Early swing was defined to be the period of the gait cycle from toe-off to peak knee flexion. Equal lengths of time were chosen for preswing and early swing to remove any intrasubject variability which would weight the effects of each period by a percentage of simulation time. On average, the duration of preswing was 20 ms longer than double support.
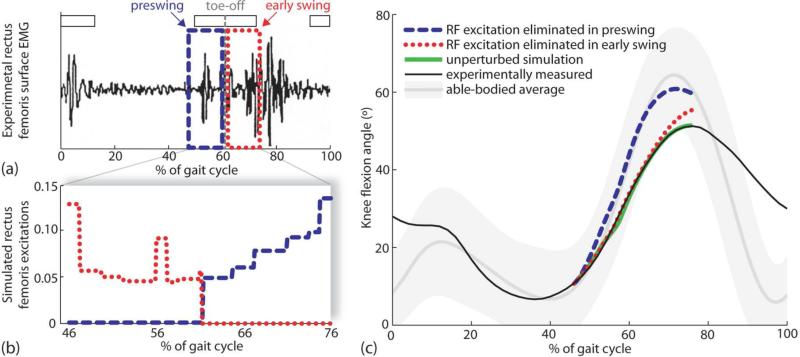
Figure 2.
Example (subject 7) of methods used to determine increase in peak knee flexion when rectus femoris activity was eliminated during preswing and separately during early swing. (a) Rectus femoris surface EMG of a subject with stiff-knee gait was recorded over an entire gait cycle. Normal rectus femoris EMG timing is indicated by horizontal white bars (Bleck, 1987). Toe-off is indicated by a vertical dashed line at 61% of the gait cycle. Two time periods were selected for analysis: early swing (i.e., period from toe-off to peak knee flexion) and preswing (i.e., period before toe-off equal in duration to early swing). (b) Two simulation experiments were conducted by eliminating rectus femoris activity during preswing (dashed line) and separately during early swing (dotted line) to determine the muscle's effect on peak knee flexion. (c) Simulated changes in knee flexion angles were different when rectus femoris activity was eliminated during preswing (dashed line) or early swing (dotted line). The unperturbed simulation (thick solid line) and experimentally measured (thin solid line) knee angles are shown for comparison. Normal knee flexion (shaded line) and two standard deviations of the normal curve (shaded region) are shown as well.
We evaluated our hypothesis regarding the relative importance of preswing and early swing rectus femoris activity by conducting a paired t-test at the 0.05 significance level. A one-tailed test was used due to a priori expectation about directionality (i.e., rectus femoris activity during preswing has a greater impact on peak knee flexion than rectus femoris activity during early swing in subjects with stiff-knee gait). The null hypothesis was that the difference in peak knee flexion change between the preswing and the early swing simulations was zero. The test was performed against the right-tailed alternative hypothesis that peak knee flexion increased more, on average, in the simulations when rectus femoris activity was eliminated during preswing than during early swing.
Results
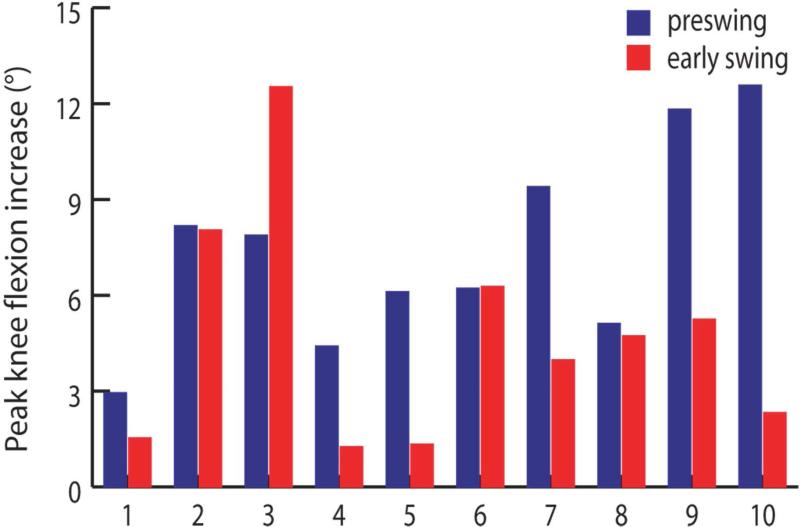
Peak knee flexion increased more (p = 0.035), on average, when rectus femoris activity was eliminated during preswing than during early swing in our simulations (Fig. 3). Peak knee flexion increased 7.5±3.1° when activity was eliminated during preswing and 4.7±3.6° when eliminated during early swing. Peak knee flexion increased more for the preswing case than the early swing case in the majority of subject simulations. For six subjects (1, 4, 5, 7, 9, and 10), the increase in peak knee flexion was 90% higher or more for the preswing case than for the early swing case. For three subjects (2, 6, and 8), the increase in peak knee flexion was similar (within 10%) for the preswing case and the early swing case. For the remaining subject (3), the increase in peak knee flexion was substantially lower (37%) for the preswing case than for the early swing case.
Figure 3.
Increase in peak knee flexion caused by eliminating rectus femoris activity during preswing and separately during early swing in simulations of ten subjects with stiff-knee gait (ordered by increasing unperturbed peak knee flexion). The increase was determined by eliminating rectus femoris activity in a forward dynamic simulation and computing the change in peak knee flexion compared with the unperturbed value (Fig. 2). The changes in simulated knee motion give insight into the biomechanical contribution of rectus femoris for that period of time in which its activity was eliminated.
Discussion
Rectus femoris transfer surgery is often performed to treat stiff-knee gait when a patient exhibits prolonged activity of the rectus femoris into early swing. However, Goldberg et al. (2006) suggested that rectus femoris activity prior to toe-off may also contribute to stiff-knee gait by causing abnormally large knee extension moments and corresponding low knee flexion velocity at toe-off. Our results confirm that preswing rectus femoris activity is at least as important as early swing activity and, for some subjects with stiff-knee gait, may limit knee flexion more than activity in early swing. In evaluating rectus femoris activity for treatment of stiff-knee gait, preswing and early swing EMG should be examined.
There are several possible biomechanical explanations why preswing rectus femoris activity may limit knee flexion more than early swing activity. First, impaired motor control may cause varying levels of preswing and early swing rectus femoris activity (Fig. 4). In fact, preswing and early swing EMG activity of the rectus femoris varies considerably in children with stiff-knee gait (Miller et al., 1997). Excessive preswing activity (Fig. 4a) may result in above normal muscle force that limits knee flexion. Second, the delay between muscle excitation and muscle force generation suggests that preswing rectus femoris activity may cause forces that persist into early swing phase and limit knee flexion. This delay in electromechanical coupling has been reported to be between 30-100 ms (Cavanagh and Komi, 1979), which is roughly 25-75% of the duration of preswing or early swing for the subjects in this study. Third, musculoskeletal geometry and multibody dynamics may cause varying magnitudes of preswing and early swing muscle forces that produce joint motion. The transmission of muscle force to joint motion depends on the muscle's moment arm, which varies during movement. In fact, rectus femoris has large potential during double support to decrease peak knee flexion velocity (Goldberg et al., 2004). Preswing activity may result in a potentially large knee extension moment that limits knee flexion.
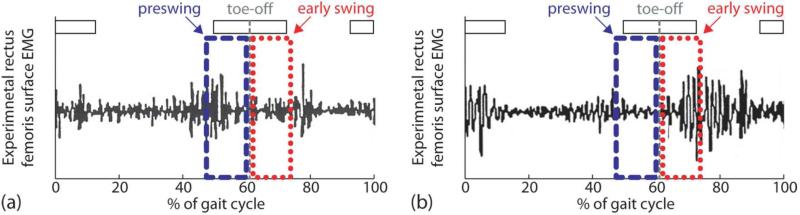
Figure 4.
Two subjects with varying levels of preswing and early swing rectus femoris activity. (a) Subject 8 had more rectus femoris activity in preswing compared to early swing. (b) Subject 3 had less rectus femoris activity in preswing compared to early swing.
There are several possible reasons why simulated increases in peak knee flexion varied across subjects. First, EMG patterns and simulated muscle activity varied across subjects. Constraints were placed on the muscle activity of each simulation based on the recorded EMG. As a result, elimination of high rectus femoris activity led to large simulated increases in peak knee flexion. For example, subject 3 had more activity in early swing compared to preswing (Fig. 4b); consequently, peak knee flexion increased more when rectus femoris activity was eliminated during early swing than during preswing (Fig. 3). The presence of high vasti activity in lieu of rectus femoris activity may have attenuated the simulated increases in peak knee flexion. Second, joint motion and body mass properties varied across subjects and these can affect the change in knee motion caused by rectus femoris activity. For example, improper positioning of the foot before toe-off may dramatically decrease the ankle power required for proper knee flexion. Third, the duration of the simulation times varied across subjects and long simulations may have produced large changes in knee flexion. Given two simulations differing only in length of time, a longer simulation eliminating rectus femoris activity increases peak knee flexion more than a shorter simulation because the inhibitory knee extension moment, in part due to rectus femoris muscle force, is reduced for a longer period of time.
By carefully defining the duration of preswing to equal the duration of early swing, the intra-subject results were not contaminated by the effects of a long perturbation being compared with those of a short perturbation. The period of double support is defined by vertical ground reaction force measurements. Knee flexion motion along with ground reactions define early swing. There was significant variation between double support and early swing durations for each subject (Table 1). For example, double support was roughly 54 ms shorter than early swing for subject 2. If we had not controlled the duration over which we perturbed RF activity in the simulations, then this difference would have allowed the early swing perturbations to affect the model motion for 60% more time than double support perturbations. For this reason, preswing was defined to be the period before toe-off equal in length of time to early swing.
The simulated elimination of rectus femoris activity was not intended to represent the activity of able-bodied control subjects. Although rectus femoris activity for an individual subject is repeatable, there are significant differences across subjects (Arsenault et al., 1986). In some cases using surface electrodes, a bi-phasic pattern (i.e., one main burst during swing-to-stance transition and the second main burst during stance-to-swing transition) can be observed (Arsenault et al., 1986, Murray et al, 1984, Shiavi, 1985). In other cases using fine wire electrodes, no muscle activity or a brief, weak burst is observed during stance-to-swing transition (Perry, 1987). In a study with both types of electrodes, cross-talk from the underlying vasti contaminated the EMG of rectus femoris recorded with surface electrodes (Nene et al., 2004). Rather than simulating rectus femoris activity of able-bodied control subjects, the simulations in this study allowed the muscle's contribution (i.e., importance) to knee flexion to be determined for that period of time in which its activity was eliminated.
The muscle-actuated simulations of stiff-knee gait developed in this study had several limitations. First, the model used in this study was scaled to represent the size and mass properties of each subject, but not individual impairments (e.g., skeletal deformities, muscle contractures, and spasticity). Second, the simulations did not explicitly model arm motions, which may have minimally affected the motions of other body segments. Third, the model utilized muscle parameters representative of an able-bodied adult, whereas the subjects in this study were children with neuromuscular abnormalities. Fourth, the forces produced by muscles in our simulations may not have accurately represented the forces generated by individual subjects even though the net joint moments were representative of each subject. Although the increases in peak knee flexion reported may change if we made different modeling assumptions, our conclusions regarding the relative importance of preswing and early swing activity would be unlikely to change significantly because the same assumptions would be simulated across both time periods.
Our finding that preswing rectus femoris activity is an important biomechanical factor contributing to diminished knee flexion in subjects with stiff-knee gait is consistent with the findings of others. Several studies have shown that swing-phase initial conditions are important in generating knee flexion during normal gait with (Piazza and Delp, 1996) and without (Mochon and McMahon, 1980; Mena et al., 1981) muscle activity. More recently, Goldberg et al., 2003 demonstrated the importance of swing-phase initial conditions in stiff-knee gait. Our finding supports these studies because preswing muscle forces generate initial conditions for swing phase (e.g., knee flexion velocity at toe-off). In particular, excessive rectus femoris force during double support has the potential to decrease knee flexion velocity at toe-off in normal gait (Goldberg et al., 2004). Some studies have reported subjects with stiff-knee gait exhibit a below normal knee flexion velocity at toe-off (Granata et al., 2000; Goldberg et al., 2003) and others have simulated the proportional relationship between knee flexion velocity at toe-off and swing-phase knee flexion for normal gait (Piazza and Delp, 1996) and stiff-knee gait (Goldberg et al., 2003). Our finding of the cause-effect relationship between preswing rectus femoris activity and swing-phase knee flexion is consistent with these studies as well. The current work demonstrates the impact of rectus femoris activity on swing-phase knee flexion and provides a direct comparison of preswing and early swing importance for a number of subjects with stiff-knee gait.
Many subjects with stiff-knee gait walked with excessive knee flexion in stance phase (e.g., crouch gait). This results in larger than normal knee extension moments during double support. Large knee moments generated by the knee extensors are necessary to support the body (McNee et al., 2004), but diminish knee flexion velocity at toe-off and reduce peak knee flexion (Goldberg et al., 2006). Recent analyses (Goldberg et al., 2006) suggest improvements in stiff-knee gait are associated with sufficient decreases in excessive knee extension moments during double support and corresponding increases in knee flexion velocity at toe-off. Further analyses are necessary to determine if the correction of excessive knee flexion in stance may diminish the excessive knee extension moments in double support. If so, excessive rectus femoris excitation may no longer be necessary for body support. Correcting excessive knee flexion in stance may increase knee flexion in swing.
The combination of gait analysis and dynamic simulation in this study identified the importance of preswing rectus femoris activity in stiff-knee gait. This result indicates that excessive preswing rectus femoris activity is a biomechanical factor contributing to diminished knee flexion in subjects with stiff-knee gait. While gait analysis tools alone are useful for characterizing stiff-knee gait, dynamic simulation provides an additional, valuable tool for investigating its underlying biomechanical causes and the mechanisms leading to improvement following treatment.
Acknowledgements
The authors are grateful to Clay Anderson, Darryl Thelen, May Liu, Saryn Goldberg, and the staff of the Center for Motion Analysis at the Connecticut Children's Medical Center. This work was supported by an NSF Graduate Fellowship, NIH R01 HD046814, and NIH Roadmap for Medical Research U54 GM072970.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson FC, Pandy MG. A dynamic optimization solution for vertical jumping in three dimensions. Computer Methods in Biomechanics and Biomedical Engineering. 1999;2:201–231. doi: 10.1080/10255849908907988. [DOI] [PubMed] [Google Scholar]
- Arsenault AB, Winter DA, Marteniuk RG. Is there a ‘normal’ profile of EMG activity in gait? Medical & Biological Engineering & Computing. 1986;24:337–343. doi: 10.1007/BF02442685. [DOI] [PubMed] [Google Scholar]
- Asakawa DS, Blemker SS, Gold GE, Delp SL. In vivo motion of the rectus femoris muscle after tendon transfer surgery. Journal of Biomechanics. 2002;35:1029–1037. doi: 10.1016/s0021-9290(02)00048-9. [DOI] [PubMed] [Google Scholar]
- Bleck EE. Orthopaedic management in cerebral palsy. MacKeith Press; Oxford: 1987. p. 78. [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. European Journal of Applied Physiology and Occupational Physiology. 1979;42:159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment. Morbidity and Mortality Weekly Report. 2004;53:57–59. [PubMed] [Google Scholar]
- Delp SL, Loan JP. A computational framework for simulation and analysis of human and animal movement. IEEE Computing in Science and Engineering. 2000;2:46–55. [Google Scholar]
- Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Transactions on Biomedical Engineering. 1990;37:757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- Gage JR, Perry J, Hicks RR, Koop S, Werntz JR. Rectus femoris transfer to improve knee function of children with cerebral palsy. Developmental Medicine and Child Neurology. 1987;29:159–166. doi: 10.1111/j.1469-8749.1987.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Granata KP, Abel MF, Damiano DL. Joint angular velocity in spastic gait and the influence of muscle-tendon lengthening. Journal of Bone and Joint Surgery A. 2000;82:174–186. doi: 10.2106/00004623-200002000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Anderson FC, Pandy MG, Delp SL. Muscles that influence knee flexion velocity in double support: implications for stiff-knee gait. Journal of Biomechanics. 2004;37:1189–1196. doi: 10.1016/j.jbiomech.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Õunpuu S, Arnold AS, Gage JR, Delp SL. Kinematic and kinetic factors that correlate with improved knee flexion following treatment for stiff-knee gait. Journal of Biomechanics. 2006;39:689–698. doi: 10.1016/j.jbiomech.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Õunpuu S, Delp SL. The importance of swing-phase initial conditions in stiff-knee gait. Journal of Biomechanics. 2003;36:1111–1116. doi: 10.1016/s0021-9290(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Inman VT. The Joints of the Ankle. Williams and Wilkins Co.; Baltimore, MD.: 1976. [Google Scholar]
- Kerrigan DC, Roth RS, Riley PO. The modeling of adult spastic paretic stiff-legged gait swing period based on actual kinematic data. Gait & Posture. 1998;7:117–124. doi: 10.1016/s0966-6362(97)00040-4. [DOI] [PubMed] [Google Scholar]
- McNee AE, Shortland AP, Eve LC, Robinson RO, Gough M. Lower limb extensor moments in children with spastic diplegic cerebral palsy. Gait & Posture. 2004;20:171–176. doi: 10.1016/j.gaitpost.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Mena D, Mansour JM, Simon SR. Analysis and synthesis of human swing leg motion during gait and its clinical applications. Journal of Biomechanics. 1981;14:823–832. doi: 10.1016/0021-9290(81)90010-5. [DOI] [PubMed] [Google Scholar]
- Miller F, Dias RC, Lipton GE, Albarracin JP, Dabney KW, Castagno P. The effect of rectus EMG patterns on the outcome of rectus femoris transfers. Journal of Pediatric Orthopaedics. 1997;17:603–607. doi: 10.1097/00004694-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Mochon S, McMahon TA. Ballistic walking. Journal of Biomechanics. 1980;13:49–57. doi: 10.1016/0021-9290(80)90007-x. [DOI] [PubMed] [Google Scholar]
- Murray MP, Mollinger LA, Gardner GM, Sepic SB. Kinematic and EMG patterns during slow, free, and fast walking. Journal of Orthopaedic Research. 1984;2:272–280. doi: 10.1002/jor.1100020309. [DOI] [PubMed] [Google Scholar]
- Nene A, Byrne C, Hermens H. Is rectus femoris really a part of quadriceps? assessment of rectus femoris function during gait in able-bodied adults. Gait & Posture. 2004;20:1–13. doi: 10.1016/S0966-6362(03)00074-2. [DOI] [PubMed] [Google Scholar]
- Õunpuu S, Muik E, Davis RB, Gage JR, DeLuca PA. Rectus femoris surgery in children with cerebral palsy. Part I: The effect of rectus femoris transfer location on knee motion. Journal of Pediatric Orthopaedics. 1993;13:325–330. doi: 10.1097/01241398-199305000-00010. [DOI] [PubMed] [Google Scholar]
- Õunpuu S, Muik E, Davis RB, Gage JR, DeLuca PA. Rectus femoris surgery in children with cerebral palsy. Part II: A comparison between the effect of transfer and release of the distal rectus femoris on knee motion. Journal of Pediatric Orthopaedics. 1993;13:331–335. doi: 10.1097/01241398-199305000-00011. [DOI] [PubMed] [Google Scholar]
- Perry J. Distal rectus femoris transfer. Developmental Medicine and Child Neurology. 1987;29:153–158. doi: 10.1111/j.1469-8749.1987.tb02130.x. [DOI] [PubMed] [Google Scholar]
- Piazza SJ, Delp SL. The influence of muscles on knee flexion during the swing phase of gait. Journal of Biomechanics. 1996;29:723–733. doi: 10.1016/0021-9290(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Rethlefsen S, Tolo VT, Reynolds RA, Kay R. Outcome of hamstring lengthening and distal rectus femoris transfer surgery. Journal of Pediatric Orthopaedics. 1999;8:75–79. [PubMed] [Google Scholar]
- Shiavi R. Electromyographic patterns in adult locomotion: a comprehensive review. Journal of Rehabilitation Research and Development. 1985;22:85–98. doi: 10.1682/jrrd.1985.07.0085. [DOI] [PubMed] [Google Scholar]
- Stanley F, Blair E, Alberman E. Cerebral Palsies: Epidemiology and Causal Pathways. MacKeith Press; London: 2000. pp. 22–48. [Google Scholar]
- Sutherland DH, Davids JR. Common gait abnormalities of the knee in cerebral palsy. Clinical Orthopaedics. 1993;288:139–147. [PubMed] [Google Scholar]
- Sutherland DH, Larsen JL, Mann R. Rectus femoris release in selected patients with cerebral palsy. Developmental Medicine and Child Neurology. 1975;17:26–34. doi: 10.1111/j.1469-8749.1975.tb04953.x. [DOI] [PubMed] [Google Scholar]
- Sutherland DH, Santi M, Abel MF. Treatment of stiff-knee gait in cerebral palsy: a comparison by gait analysis of distal rectus femoris transfer versus proximal rectus release. Journal of Pediatric Orthopaedics. 1990;10:433–441. [PubMed] [Google Scholar]
- Thelen DG, Anderson FC, Delp SL. Generating dynamic simulations of movement using computed muscle control. Journal of Biomechanics. 2003;36:321–328. doi: 10.1016/s0021-9290(02)00432-3. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Anderson FC. Using computed muscle control to generate forward dynamic simulations of human walking from experimental data. Journal of Biomechanics. 2006;39:1107–1115. doi: 10.1016/j.jbiomech.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Waters RL, Garland DE, Perry J. Stiff-legged gait in hemiplegia: surgical correction. Journal of Bone & Joint Surgery. 1979;61-A:927–933. [PubMed] [Google Scholar]
- Wren TAL, Rethlefsen S, Kay RM. Prevalence of specific gait abnormalities in children with cerebral palsy: influence of cerebral palsy subtype, age, and previous surgery. Journal of Pediatric Orthopaedics. 2004;25:79–83. doi: 10.1097/00004694-200501000-00018. [DOI] [PubMed] [Google Scholar]
- Yamaguchi GT, Zajac FE. A planar model of the knee joint to characterize the knee extensor mechanism. Journal of Biomechanics. 1989;22:1–10. doi: 10.1016/0021-9290(89)90179-6. [DOI] [PubMed] [Google Scholar]
- Yngve DA, Scarborough N, Goode B, Haynes R. Rectus and hamstring surgery in cerebral palsy: a gait analysis study of results by functional ambulation level. Journal of Pediatric Orthopaedics. 2002;22:672–676. [PubMed] [Google Scholar]