Abstract
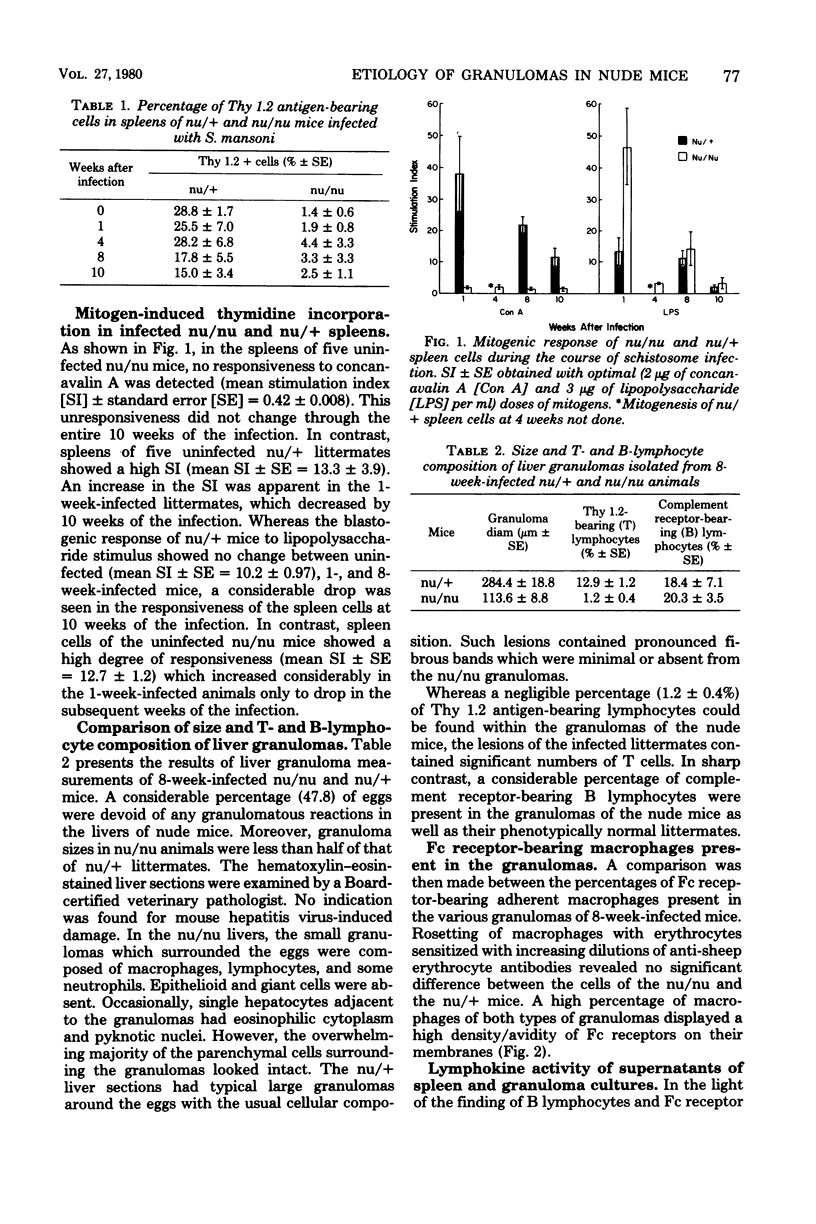
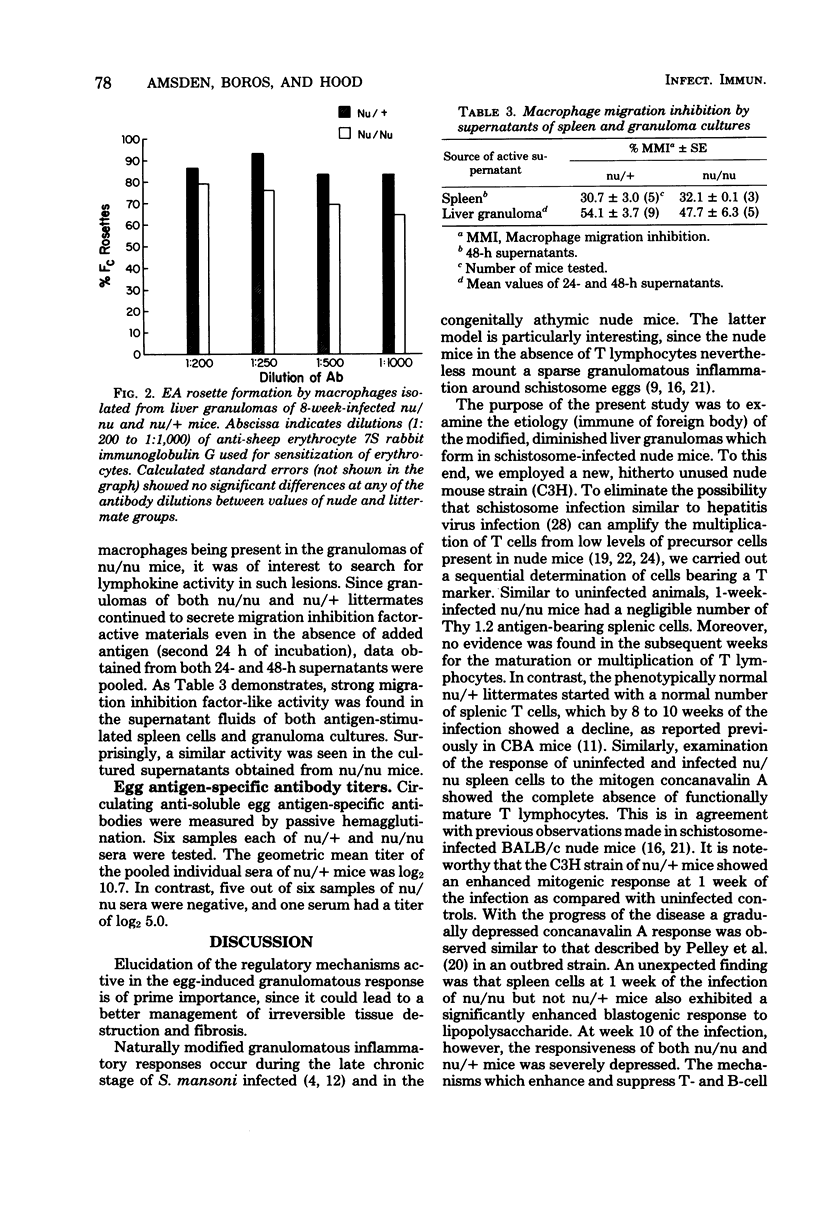
The effect of schistosome infection on the presence and maturation of splenic T lymphocytes in C3H/HeN nu/nu and nu/+ mice was examined. Spleens of uninfected nu/nu mice contained very low numbers (u to 2%) of T lymphocytes. This percentage did not increase throughout the 10 weeks of the infection. Spleens of uninfected nu/+ littermates contained 28.8% T cells, which decreased to 15.0% by week 10 of the infection. Similarly, whereas spleen cells of normal or infected nu/nu mice were nonresponsive to concanavalin A, the initial high response of nu/+ mice gradually diminished. Both nu/nu and nu/+ spleen cells responded well to lipopolysaccharide initially, but by 10 weeks their responsiveness declined. Sera of five infected nu/nu mice contained no antibodies to egg antigens, and one had a low titer (log2 5.0). In contrast, the mean titer of sera from six nu/+ mice was log2 10.7 Nu/+ mice had typical florid lesions, but nu/nu mice mounted sparse granulomatous reactions around eggs in the liver without evidence for hepatocellular damage. Dispersed liver granulomas of nu/nu mice contained 1.2% T and 20.3% B lymphocytes. Lesions of nu/+ mice contained 12.9% T and 18.4% B cells. Eighty percent of the macrophages from nu/nu and nu/+ granulomas displayed high density/avidity Fc receptors. Production of migration inhibition factor-active lymphokine by liver granulomas and spleens of schistosome-infected nu/nu mice is suggestive of the immune role of B cells in the granulomatous inflammation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsden A. F., Boros D. L. Fc-receptor-bearing macrophages isolated from hypersensitivity and foreign-body granulomas. Delineation of macrophage dynamics, fc receptor density/avidity and specificity. Am J Pathol. 1979 Aug;96(2):457–476. [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Mannik M. The macrophage receptor for IgG: number and affinity of binding sites. J Immunol. 1973 Jun;110(6):1455–1463. [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S., Pelley R. P. The secretion of migration inhibitory factor by intact schistosome egg granulomas maintained in vitro. Nature. 1973 Nov 23;246(5430):224–226. doi: 10.1038/246224a0. [DOI] [PubMed] [Google Scholar]
- Buchanan R. D., Fine D. P., Colley D. G. Schistosoma mansoni infection in mice depleted of thymus-dependent lymphocytes. II. Pathology and altered pathogenesis. Am J Pathol. 1973 May;71(2):207–218. [PMC free article] [PubMed] [Google Scholar]
- Byram J. E., Doenhoff M. J., Musallam R., Brink L. H., von Lichtenberg F. Schistosoma mansoni infections in T-cell deprived mice, and the ameliorating effect of administering homologous chronic infection serum. II. Pathology. Am J Trop Med Hyg. 1979 Mar;28(2):274–285. doi: 10.4269/ajtmh.1979.28.274. [DOI] [PubMed] [Google Scholar]
- Byram J. E., von Lichtenberg F. Altered schistosome granuloma formation in nude mice. Am J Trop Med Hyg. 1977 Sep;26(5 Pt 1):944–956. doi: 10.4269/ajtmh.1977.26.944. [DOI] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Population dynamics of T and B lymphocytes in the lymphoid organs, circulation, and granulomas of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1979 Mar;28(2):291–299. doi: 10.4269/ajtmh.1979.28.291. [DOI] [PubMed] [Google Scholar]
- Colley D. G. Immune responses to a soluble schistosomal egg antigen preparation during chronic primary infection with Schistosoma mansoni. J Immunol. 1975 Jul;115(1):150–156. [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. II. Thymectomy. Am J Pathol. 1967 Nov;51(5):757–767. [PMC free article] [PubMed] [Google Scholar]
- Greene B. M., Colley D. G. Eosinophils and immune mechanisms. III. Production of the lymphokine eosinophil stimulation promoter by mouse T lymphocytes. J Immunol. 1976 Apr;116(4):1078–1083. [PubMed] [Google Scholar]
- Hsu C. K., Hsu S. H., Whitney R. A., Jr, Hansen C. T. Immunopathology of schistosomiasis in athymic mice. Nature. 1976 Jul 29;262(5567):397–399. doi: 10.1038/262397a0. [DOI] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophils and immune mechanisms: production of the lymphokine eosinophil stimulation promoter (ESP) in vitro by isolated intact granulomas. J Reticuloendothel Soc. 1975 Nov;18(5):283–293. [PubMed] [Google Scholar]
- Jenssen H. L., Eckert R., Werner H., Köhler H., Pasternak G., Friemel H. Lymphokines of T and B cells. I. Demonstration that T and B cells of tumor antigensensitised mice produce different lymphokines changing surface charge of target cells. Oncology. 1977;34(4):159–163. doi: 10.1159/000225213. [DOI] [PubMed] [Google Scholar]
- Loor F., Roelants G. E. High frequency of T lineage lymphocytes in nude mouse spleen. Nature. 1974 Sep 20;251(5472):229–230. doi: 10.1038/251229a0. [DOI] [PubMed] [Google Scholar]
- Pelley R. P., Ruffier J. J., Warren K. S. Suppressive effect of a chronic helminth infection, schistosomiasis mansoni, on the in vitro responses of spleen and lymph node cells to the T cell mitogens phytohemagglutinin and concanavalin A. Infect Immun. 1976 Apr;13(4):1176–1183. doi: 10.1128/iai.13.4.1176-1183.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. M., DiConza J. J., Gold J. A., Reid W. A. Schistosomiasis in the congenitally athymic (nude) mouse. I. Thymic dependency of eosinophilia, granuloma formation, and host morbidity. J Immunol. 1977 Feb;118(2):594–599. [PubMed] [Google Scholar]
- Pritchard H., Micklem H. S. Haemopoietic stem cells and progenitors of functional T-lymphocytes in the bone marrow of 'nude' mice. Clin Exp Immunol. 1973 Aug;14(4):597–607. [PMC free article] [PubMed] [Google Scholar]
- Rabellino E. M., Metcalf D. Receptors for C3 and IgG on macrophage, neutrophil and eosinophil colony cells grown in vitro. J Immunol. 1975 Sep;115(3):688–692. [PubMed] [Google Scholar]
- Raff M. C. Theta-bearing lymphocytes in nude mice. Nature. 1973 Dec 7;246(5432):350–351. doi: 10.1038/246350a0. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Macrophage heterogeneity in receptor activity: the activation of macrophage Fc receptor function in vivo and in vitro. J Immunol. 1975 Mar;114(3):976–981. [PubMed] [Google Scholar]
- Rocklin R. E., MacDermott R. P., Chess L., Schlossman S. F., David J. R. Studies on mediator production by highly purified human T and B lymphocytes. J Exp Med. 1974 Nov 1;140(5):1303–1316. doi: 10.1084/jem.140.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E. J., Steerenberg P. A. Intestinal phase of Trichinella spiralis in congenitally athymic (nude) mice. J Parasitol. 1974 Dec;60(6):1056–1057. [PubMed] [Google Scholar]
- SCHLESINGER M. IMMUNE LYSIS OF THYMUS AND SPLEEN CELLS OF EMBRYONIC AND NEONATAL MICE. J Immunol. 1965 Mar;94:358–364. [PubMed] [Google Scholar]
- Schedi M. P., Goldstein G., Boyce E. A. Differentiation of T cells in nude mice. Science. 1975 Dec 19;190(4220):1211–1213. [PubMed] [Google Scholar]
- Sugane K., Kasahara T., Shioiri-Nakano K. Release of migration inhibitory factor from mouse T and B cells activated by insoluble phytomitogens. Jpn J Exp Med. 1975 Feb;45(1):19–24. [PubMed] [Google Scholar]
- Unkeless J. C., Eisen H. N. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975 Dec 1;142(6):1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. S. Separate Fc-receptors for immunoglogulins IgG2a and IgG2b on an established cell line of mouse macrophages. J Immunol. 1976 Apr;116(4):911–914. [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S. Hepatosplenic schistosomiasis mansoni: an immunologic disease. Bull N Y Acad Med. 1975 Apr;51(4):545–550. [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Sonozaki H., Cohen S. The production of migration inhibition factor by B and T cells of the guinea pig. J Exp Med. 1973 Oct 1;138(4):784–797. doi: 10.1084/jem.138.4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]


