Abstract
To investigate the effects of Kluyveromyces marxianus on immune responses, intestinal structure and microbiota in broilers, 840 1-d-old broiler chicks were randomly divided into seven groups (eight replicates) and were fed basal diets without or with 0.25, 0.50, 1.0, 1.5, 2.0, and 2.5 g/kg of K. marxianus (2.0×1010 CFU/g). Serum and intestine samples were collected at 21 d of age. The results showed that increasing K. marxianus addition linearly reduced feed conversion ratio but linearly elevated relative thymus weight, as well as quadratically increased serum lysozyme and IgG levels, with the medium dose (1.0 g/kg) being the most effective. The ratio of villus height to crypt depth of jejunum and ileum, ileal villus height and sucrase activity, as well as the mRNA expression of ileal mucin-2, claudin-1 and sodium glucose cotransporter 1 linearly responded to the increasing K. marxianus addition. Supplemental K. marxianus at low (0.5 g/kg), medium (1.5 g/kg) and high (2.5 g/kg) dose all decreased the abundance of phylum Cyanobacteria, increased the abundance of phylum Firmicutes and genus Lactobacillus in ileum. The high dose of K. marxianus addition also reduced the abundance of order Rickettsiales and Pseudomonadales along with species Acinetobacter junii. Ileal bacterial communities between K. marxianus-treated and untreated groups formed distinctly different clusters. In summary, K. marxianus supplementation benefits feed efficiency and immune function, as well as intestinal structure in broilers, which might be attributed to the improved ileal microbial structure. Supplemental K. marxianus at high dose (2.5 g/kg) was more effective for feed efficiency and intestinal health of broilers, while the innate immunity was optimized at a medium dose (1.0 g/kg).
Introduction
Yeast probiotic (mainly the Saccharomyces cerevisiae) had been widely used in animal production, as it was indicated to improve growth performance, immune responses, and intestinal health in animals [1–4]. This might be due to its prebiotic function and interaction with specific immune cells of host by the cell wall components [1–4]. However, there is paucity of information regarding the effects of other yeast species in animals. Kluyveromyces marxianus, a food-grade probiotic, have been approved by Chinese Ministry of Health and European Union. K. marxianus had gained increasing attention on the commercial production field because of its unique physiological properties such as high growth rate, active metabolic functions, high thermostability and high content of mannan in the cell wall in comparison with S. cerevisiae [5–7], based on which K. marxianus addition might be helpful for growth performance and physiological function of animals. Experiments in ruminants have evidenced that K. marxianus addition had ability to improve feed efficiency and enhance some digestive enzymes activities [8, 9]. In an in vitro study, it was suggested that K. marxianus had immunomodulatory properties as well as a protective role for intestinal barrier function [10–13]. However, it was unknown whether dietary K. marxianus supplementation could be helpful for immune responses and intestinal structure in chickens.
Gut microbiota has major impact on the bioavailability and bioactivity of dietary components, playing important roles in host nutritional, physiological and protective functions [14, 15]. It is necessary to decipher the content and diversity of intestinal microbial community in order to understand and exploit gut microbiota and the potential influence of its manipulation on performance and health of host. Besides, an understanding and a description of gut microbiota are critical for the development of new feed additives and the appropriate manipulation of diet to improve growth performance and health status in chickens [16]. It was suggested that the improvement of growth performance, immune function and intestinal health in chickens could be linked with the modification of intestinal microbial structure [17–20]. In vitro, K. marxianus was identified as a functional probiotic with associated antibacterial properties and plays a role in modifying microbial composition [10–13]. Nevertheless, it is unknown whether dietary K. marxianus supplementation can improve intestinal microbial structure in animals, which might subsequently benefit the immune function and intestinal structure of host. Therefore, the present study was conducted to investigate the effects of K. marxianus supplementation on growth performance, immune responses and intestinal structure in broiler chickens. In addition, we aimed to assess the shifts in intestinal microbial community structure induced by dietary treatment to explain the possible beneficial effects of K. marxianus addition on broilers.
Materials and methods
Birds and experimental design
The experimental animal protocol for this study was approved by the Animal Care and Use Committee of China Agricultural University. A total of 840 one-day-old female Arbor Acre broiler chicks were randomly allocated into 7 treatment groups with 8 replicates of each. Each replicate pen involving 15 birds. Initial body weights were similar across all the replicates. Birds received basal diets in mash form without or with 0.25, 0.50, 1.0, 1.5, 2.0, 2.5 g/kg K. marxianus (K. marxianus FIM-1, 2.0×1010 CFU/g) throughout the trial period. The product was provided by Shanghai Engineering Research Center of Industrial Microorganisms (China), and has been deposited in China General Microbiological Culture Collection Center (CGMCC) with a reference number of 10621. The composition of basal diets are shown in Table 1. All birds were raised in wired three-level battery cages (100 cm long × 80 cm wide × 40 cm high/cage). Feed and fresh water were available ad libitum. The lighting schedule was 20 h light and 4 h dark throughout the experiment. The room temperature was controlled with heaters and gradually reduced from 35°C on d 1 to 24°C on d 21 and then kept roughly constant. Birds were vaccinated using combined Newcastle disease virus (NDV) and infectious bronchitis virus on d 7 through intranasal and intraocular administration, and on d 21 via oral administration.
Table 1. Composition and nutrient levels of diets (g/kg).
| Ingredients | Stage | |
|---|---|---|
| 1−21 d | 22−35 d | |
| Maize | 559.7 | 613.7 |
| Soybean meal (43%, crude protein) | 376.3 | 318.8 |
| Soybean oil | 23.7 | 31.3 |
| Limestone | 12.7 | 12.1 |
| Sodium chloride | 3.5 | 3.5 |
| Dicalcium phosphate | 17.5 | 14.9 |
| Choline chloride (50%) | 2.0 | 2.0 |
| DL-Methionine (98%) | 2.0 | 1.2 |
| L-Lysine·HCl (99%) | 0.2 | 0.1 |
| Antioxidant | 0.2 | 0.2 |
| Multivitamin1 | 0.2 | 0.2 |
| Multimineral2 | 2.0 | 2.0 |
| Yeast3 | +/- | +/- |
| Nutrient levels | ||
| Metabolic Energy (MJ/kg) | 12.14 | 12.56 |
| Crude Protein | 210.0 | 190.0 |
| Available Phosphorus | 4.5 | 4.0 |
| Calcium | 10.0 | 9.0 |
| Lysine | 11.5 | 10.0 |
| Methionine | 5.0 | 4.0 |
1 Supplied per kg of diet: retinyl acetate, 24 mg; cholecalciferol, 6 mg; menadione, 2.65 mg; thiamin, 2 mg; riboflavin, 6 mg; cyanocobalamin, 0.025 mg; α-tocopherol acetate, 20 mg; biotin, 0.0325 mg; folic acid, 1.25 mg; pantothenic acid, 12 mg; niacin, 50 mg.
2 Supplied per kg of diet: Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0.15 mg; I, 0.35 mg.
3 Yeast (Kluyveromyces marxianus, the measured value of clump count was 1.5×1010 CFU/g) was substituted for the same amount of maize.
Sample collection and procedure
Birds were randomly selected from each replicate pen (8 birds per group) on d 21 for samples collection. Individual blood samples were taken aseptically from the wing vein. Serum samples were separated by centrifugation of blood at 3000 rpm for 10 min at 4°C and stored at −30°C until analysis. After blood collection, these birds were slaughtered through cervical dislocation. Spleen, thymus and bursa from each bird were excised and weighed to determine the relative weight of immune organs, which were expressed as the ratio of organ weight (g) to body weight (kg). Mid-segments of jejunum and ileum were harvested and cut into two sections: one of which was fixed in 4% paraformaldehyde solution for morphology measurement, and the other was put into liquid nitrogen and kept at −80°C for quantification of gene expression. Meanwhile, digesta and mucosa from the remainder intestine were collected and quick-freezed using liquid nitrogen, followed by storage at −80°C until further analysis.
Performance measurement
Body weight and feed intake were recorded for each replicate at 21 and 35 d of age. Average body weight (ABW) at 21 and 35 d of age, along with average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) during the grower period (1−21 d) and the overall period (1−35 d) were calculated.
Biochemical assay of serum and intestinal mucosa
Activity of serum lysozyme was determined colorimetrically under the instruction of a commercial kit (Jiancheng Biotechnology Institute, Nanjing, China). Concentrations of serum IgG, IgM, and IgA as well as intestinal secretory IgA (sIgA) were assayed by double-antibody sandwich ELISA using commercial kits (Bethyl Laboratories Inc., Montgomery, TX, USA) according to the manufacturer′s instructions. Intestinal alkaline phosphatase (ALP) activity was measured by colorimetric assay using a corresponding diagnostic kit (Jiancheng Biotechnology Institute, Nanjing, China) under the manufacturer′s protocols. Activity of intestinal disaccharidase was determined as described previously [21]. The results of above–mentioned intestinal indicies were normalised by total protein content, which was determined using BCA protein quantitation kits (CWBiotech Co. Ltd, Beijing, China).
Intestinal morphological analysis
The 2-μm cross–sections of jejunal and ileal tissues were obtained after staining with hematoxylin-eosin using standard paraffin-embedding procedures. For each section, ten representative intact villi were selected for morphology examination using Leica DMI6000B light microscope equipped with an image-processing software (Leica application suite V4.2). Villus height (VH) was determined from the tip of villus to the junction of villus and crypt, crypt depth was defined as the depth of emboly between adjacent villi, villus height to crypt depth ratio (VCR) was calculated. The mean value of these ten values represents the final value of each sample.
Quantification of mRNA expression of intestinal genes
Total RNA were extracted from the ileum using Trizol Reagent (Invitrogen biotechnology Inc., Carlsbad, USA). Extracted RNA was dissolved in RNase-free water and quantified using Nanodrop spectrophotometer (ND-2000 UV-Vis; Thermo Scientific Inc., USA). RNA Purity was verified by determining the absorbance ratio at 260:280 nm. RNA integrity was evaluated on the basis of the spectral curve [22]. The cDNA samples were obtained by reverse transcription of total RNA using PrimeScriptTM RT reagent kit with gDNA Eraser (Takara Biotechnology Inc., Osaka, Japan) and were kept at –20°C until analyzed. Real-time PCR for measuring intestinal genes expression was carried out using SYBR® Premix Ex TaqTM (Tli RNaseH Plus) (Takara Biotechnology Inc., Osaka, Japan) in ABI 7500 Real Time PCR Systems (Applied Biosystems, Foster City, California, USA). The expression of β-actin was used as an internal control to normalise the amount of initial RNA for each sample. The reaction volume of 20 μl mixture contained 10 μl SYBR® Premix Ex Taq (Tli RNaseH Plus), 0.4 μl ROX Reference Dye, 0.4 μl of each forward and reverse primer, 6.8 μl dilution and 2 μl cDNA template. Primer sequences for the target and reference genes are shown in Table 2. The protocol for all these genes were 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s. All measurements were carried out in duplicate. PCR efficiency for each gene was figured out according to the slope of cDNA relative standard curve that was generated using pooled samples. The efficiency values between the reference and target genes were consistent. The abundance of β-actin mRNA was not influenced by dietary treatment. Specificity of PCR products was evaluated by the analysis of melting curve. The results of relative mRNA expression of intestinal genes were calculated using the 2-ΔΔCt method [23].
Table 2. Primers used in the real-time PCR.
| Genes1 | Primer sequence2 (5′-3′) | Accession no. |
|---|---|---|
| β-Actin | F: GAGAAATTGTGCGTGACATCA | L08165 |
| R: CCTGAACCTCTCATTGCCA | ||
| Mucin-2 | F: TTCATGATGCCTGCTCTTGTG | XM_421035 |
| R: CCTGAGCCTTGGTACATTCTTGT | ||
| Claudin-1 | F: CATACTCCTGGGTCTGGTTGGT | AY750897.1 |
| R: GACAGCCATCCGCATCTTCT | ||
| SGLT1 | F: GATGTGCGGATACCTGAAGC | AJ236903 |
| R: AGGGATGCCAACATGACTGA | ||
| PepT1 | F: TACGCATACTGTCACCATCA | AY029615 |
| R: TCCTGAGAACGGACTGTAAT | ||
| L-FABP | F: GAAGGGTAAGGACATCAA | NM_204192 |
| R: TCGGTCACGGATTTCAGC |
1SGLT1, sodium glucose cotransporter 1; PepT1, H+-dependent peptide transporter 1
L-FABP, liver fatty acid-binding protein.
2 F, forward; R, reverse.
Pyrosequencing of ileal microbiota
DNA samples were extracted from ileal digesta using QIAamp DNA Stool Mini Kits (Qiagen Inc., Hilden, Germany) according to the manufacturer′s instructions. The concentration and quality of DNA samples were checked with gel electrophoresis and a Nanodrop 2000 spectrophotometer (Thermo Scientific Inc., Waltham, USA). Bacterial 16S rDNA sequences spanning the variable regions V3–V4 were amplified using primer 515 F (5′-GTG CCA GCM GCC GCG GTA A-3′) and 806 R (5′-GGA CTA CHV GGG TWT CTA AT-3′). All PCR were carried out in 30-μl reaction volumes containing 15 μl of Phusion High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA, USA), 0.2 μM forward and reverse primers, and 10 ng template DNA. Amplification by PCR consisted of the following: initial denaturation at 98°C for 1 min, followed by 30 cycles at 98°C for 10 s, 50°C for 30 s, and 72°C for 30 s, and a final extension step at 72°C for 5 min. PCR products were detected by 2% agarose gel electrophoresis and purified with QIAquick Gel Extraction Kit (Qiagen Inc., Hilden, Germany). A library was constructed using TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, USA) and detected by Qubit and q-PCR quantification. Pyrosequencing for 16S rDNA was carried out on the Illumina HiSeq2500 PE250 platform (Illumina, San Diego, USA). All of the procedures were conducted by Novogene Bioinformatics Technology Co. Ltd. (Beijing, China). Sample reads were assembled using Mothur software. Clustering of filtered sequences into operational taxonomic units (OTUs) was achieved using Uparse at 97% sequence identity. Taxonomic classification at different taxonomic levels of these OTU sequences was performed by comparing sequences to the GreenGene database. Qiime software and Python scripts were used for the analysis of microbial diversity. The Unifrac approach was used to estimate pairwise distances between samples and to establish beta diversity, which was visualized by principal component analysis (PCA) and clustering analysis.
Statistical analysis
Data were presented as mean ± standard error of the mean (SEM). Pens were used as the experimental unit for growth performance parameters, whereas an individual bird served as the experimental unit for other parameters. The polynomial regression analysis (SPSS 18.0) was used to test the linear and quadratic nature of the response to the additive dosage of K. marxianus. Significance was defined as P < 0.05 and 0.05 < P < 0.10 was considered to be a tendency towards significance.
Results
Growth performance
Dietary K. marxianus supplementation had no effect (P > 0.05) on ABW of birds at 21 or 35 d of age (Table 3), as well as ADG and ADFI during grower period and the overall period. However, with the increase of K. marxianus addition, FCR during grower period and the overall period exhibited positive linear response (P < 0.05), with greater levels (2.0 and 2.5 g/kg) being more effective.
Table 3. Effects of Kluyveromyces marxianus on growth performance1 of broilers.
| Dose (g/kg) | 21 d | 1−21 d | 35 d | 1−35 d | ||||
|---|---|---|---|---|---|---|---|---|
| ABW | ADG | ADFI | FCR | ABW | ADG | ADFI | FCR | |
| 0 | 673 | 29.95 | 42.09 | 1.406 | 1683 | 46.89 | 77.14 | 1.645 |
| 0.25 | 693 | 30.85 | 43.25 | 1.403 | 1723 | 47.33 | 77.83 | 1.645 |
| 0.50 | 697 | 31.11 | 43.32 | 1.393 | 1734 | 47.92 | 78.17 | 1.632 |
| 1.0 | 678 | 30.18 | 41.43 | 1.373 | 1695 | 47.06 | 76.05 | 1.617 |
| 1.5 | 715 | 31.96 | 43.96 | 1.377 | 1760 | 48.91 | 78.90 | 1.614 |
| 2.0 | 688 | 30.67 | 41.80 | 1.364 | 1724 | 47.40 | 76.25 | 1.609 |
| 2.5 | 699 | 31.17 | 42.60 | 1.367 | 1703 | 47.50 | 76.25 | 1.606 |
| SEM | 4.5 | 0.211 | 0.255 | 0.0046 | 7.7 | 0.232 | 0.425 | 0.0065 |
| P-value | ||||||||
| Linear | 0.196 | 0.204 | 0.753 | 0.001 | 0.547 | 0.445 | 0.346 | 0.024 |
| Quadratic | 0.299 | 0.318 | 0.890 | 0.002 | 0.182 | 0.330 | 0.518 | 0.064 |
n = 8 replicates per group.
1ABW, average body weight (g); ADG, average daily gain (g); ADFI, average daily feed intake (g); FCR, feed conversion ratio.
Relative weight of immune organs and serum parameters
There were no changes (P > 0.05) in the relative weight of spleen and bursa, as well as serum IgA and IgM levels in response to K. marxianus addition (Table 4). However, there was a linear increase (P < 0.05) in the relative thymus weight responded to the increasing K. marxianus addition. Responses to increasing K. marxianus addition on serum lysozyme and IgG levels were quadratic (P < 0.05), with the medium dose (1.0 g/kg) being the most effective.
Table 4. Effects of Kluyveromyces marxianus on the relative weight1 of immune organs and serum parameters of broilers (21 d of age).
| Dose (g/kg) |
Spleen | Bursa | Thymus | IgG mg/mL |
IgA mg/mL |
IgM mg/mL |
Lysozyme U/mL |
|---|---|---|---|---|---|---|---|
| 0 | 0.92 | 2.12 | 2.68 | 1.65 | 0.38 | 0.30 | 171.24 |
| 0.25 | 0.83 | 2.15 | 2.68 | 1.85 | 0.44 | 0.33 | 194.57 |
| 0.50 | 0.80 | 2.04 | 2.76 | 2.53 | 0.33 | 0.36 | 185.62 |
| 1.0 | 0.86 | 1.99 | 2.90 | 3.14 | 0.40 | 0.34 | 204.97 |
| 1.5 | 0.82 | 1.94 | 2.88 | 2.46 | 0.41 | 0.23 | 204.57 |
| 2.0 | 0.79 | 1.96 | 2.98 | 2.31 | 0.39 | 0.24 | 202.51 |
| 2.5 | 0.87 | 2.22 | 3.42 | 2.89 | 0.45 | 0.27 | 185.90 |
| SEM | 0.022 | 0.059 | 0.069 | 0.144 | 0.024 | 0.019 | 3.632 |
| P-value | |||||||
| Linear | 0.602 | 0.923 | 0.001 | 0.044 | 0.507 | 0.103 | 0.228 |
| Quadratic | 0.483 | 0.344 | 0.003 | 0.040 | 0.705 | 0.334 | 0.016 |
n = 8 replicates per group.
1 Relative weight of immune organs was expressed as the ratio of organ weight (g) to body weight (kg).
Intestinal biochemical parameters and morphological structure
K. marxianus supplementation had no influence (P > 0.05) on sIgA content, as well as ALP and maltase activities in the jejunum and ileum (Table 5), but exerted positive linear effect (P < 0.05) on ileal sucrase activity. No difference (P > 0.05) was noted in jejunal or ileal crypt depth of broilers after K. marxianus supplementation (Table 6). However, with the increase of K. marxianus addition, the VCR of jejunum and ileum coupled with ileal VH linearly increased (P < 0.05).
Table 5. Effects of Kluyveromyces marxianus on intestinal biochemical indicies1 of broilers (21 d of age).
| Dose (g/kg) |
Jejunum | Ileum | ||||||
|---|---|---|---|---|---|---|---|---|
| sIgA | AKP | Suc | Mal | sIgA | AKP | Suc | Mal | |
| 0 | 3.02 | 0.87 | 136.78 | 295.59 | 5.36 | 0.68 | 166.97 | 242.86 |
| 0.25 | 3.35 | 1.01 | 154.72 | 294.18 | 5.00 | 0.71 | 174.62 | 232.40 |
| 0.50 | 2.89 | 0.86 | 148.89 | 334.73 | 4.05 | 0.61 | 195.19 | 216.91 |
| 1.0 | 2.60 | 1.18 | 143.59 | 299.85 | 5.64 | 0.61 | 191.43 | 237.95 |
| 1.5 | 3.37 | 0.96 | 136.87 | 290.13 | 5.88 | 0.63 | 206.98 | 267.28 |
| 2.0 | 3.82 | 1.13 | 129.41 | 292.23 | 6.11 | 0.62 | 198.64 | 251.95 |
| 2.5 | 3.26 | 0.90 | 154.50 | 279.11 | 5.57 | 0.66 | 208.67 | 219.54 |
| SEM | 0.179 | 0.040 | 4.517 | 5.890 | 0.216 | 0.014 | 5.345 | 5.709 |
| P-value | ||||||||
| Linear | 0.332 | 0.550 | 0.848 | 0.153 | 0.087 | 0.347 | 0.018 | 0.765 |
| Quadratic | 0.612 | 0.195 | 0.796 | 0.237 | 0.223 | 0.165 | 0.040 | 0.407 |
n = 8 replicates per group.
1 sIgA, secretory Ig A (mg/g protein); AKP, alkaline phosphatase (U /mg protein); Suc, sucrase (U/mg protein); Mal, maltase (U/mg protein).
Table 6. Effects of Kluyveromyces marxianus on intestinal morphology1 of broilers (21 d of age).
| Dose (g/kg) |
Jejunum | Ileum | ||||
|---|---|---|---|---|---|---|
| VH | CD | VCR | VH | CD | VCR | |
| 0 | 938.16 | 140.57 | 7.03 | 574.59 | 97.37 | 6.30 |
| 0.25 | 912.11 | 145.67 | 6.72 | 613.58 | 106.01 | 6.08 |
| 0.50 | 895.90 | 131.04 | 7.20 | 595.66 | 99.03 | 6.23 |
| 1.0 | 975.95 | 142.51 | 7.39 | 621.17 | 106.91 | 6.24 |
| 1.5 | 1014.85 | 147.69 | 7.33 | 666.40 | 104.74 | 6.89 |
| 2.0 | 1022.13 | 140.05 | 7.72 | 643.50 | 95.25 | 7.14 |
| 2.5 | 981.88 | 133.02 | 7.71 | 659.47 | 109.27 | 6.55 |
| SEM | 23.258 | 3.497 | 0.149 | 12.763 | 2.269 | 0.131 |
| P-value | ||||||
| Linear | 0.137 | 0.745 | 0.043 | 0.033 | 0.604 | 0.042 |
| Quadratic | 0.289 | 0.768 | 0.131 | 0.085 | 0.874 | 0.111 |
n = 8 replicates per group.
1 VH, villus height (μm); CD, crypt depth (μm); VCR, the ratio of VH:CD.
Relative mRNA expression of intestinal genes
K. marxianus supplementation did not affect (P > 0.05) the relative expression of ileal liver fatty acid-binding protein (L-FABP) and H+-dependent peptide transporter 1 (PepT1) (Table 7), but linearly increased (P < 0.05) the expression of mucin-2, claudin-1, and sodium glucose cotransporter-1 (SGLT-1) in the ileum.
Table 7. Effects of Kluyveromyces marxianus on the relative mRNA expression of ileal genes1 of broilers (21 d of age).
| Dose (g/kg) |
Mucin-2 | Claudin-1 | L-FABP | PepT-1 | SGLT-1 |
|---|---|---|---|---|---|
| 0 | 1.03 | 1.06 | 1.11 | 1.17 | 0.95 |
| 0.25 | 0.96 | 0.91 | 1.19 | 0.96 | 1.05 |
| 0.50 | 0.93 | 1.14 | 0.91 | 1.30 | 0.93 |
| 1.0 | 1.21 | 1.09 | 1.12 | 1.09 | 1.07 |
| 1.5 | 1.20 | 1.07 | 0.97 | 0.97 | 1.04 |
| 2.0 | 1.11 | 1.24 | 0.96 | 0.98 | 1.15 |
| 2.5 | 1.26 | 1.49 | 1.46 | 1.19 | 1.21 |
| SEM | 0.032 | 0.076 | 0.091 | 0.105 | 0.038 |
| P-value | |||||
| Linear | 0.003 | 0.006 | 0.652 | 0.886 | 0.038 |
| Quadratic | 0.011 | 0.004 | 0.427 | 0.928 | 0.105 |
n = 8 replicates per group.
1 L-FABP, liver fatty acid-binding protein; PepT-1, H+-dependent peptide transporter 1; SGLT-1, sodium glucose cotransporter 1.
Microbial community structure of ileum
The 16S rDNA sequencing was used to analyze the ileal microbial community structure of birds in the control group and K. marxianus-treated groups at three different doses (0.5, 1.5, 2.5 g/kg). Rarefaction curves for the observed OTUs approach a plateau, indicating that sequencing depth was sufficient for the coverage of all OTUs present in ileal samples (S1 Fig). Good′s coverage indice, used to estimate the percentage of total bacterial OTUs represented in a sample, were over 99%, suggesting the 16S rDNA results from each library represented the majority of bacteria in broiler ileum. Notably, K. marxianus supplementation did not influence alpha diversity (Shannon, Simpson, ACE, and Chao1 indexes) of ileal microbiota (S1 Table).
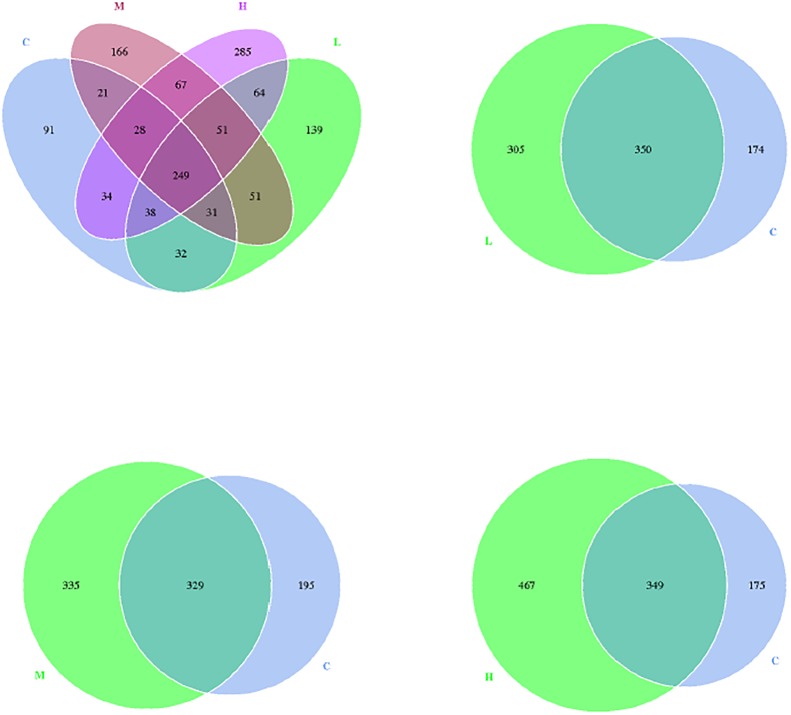
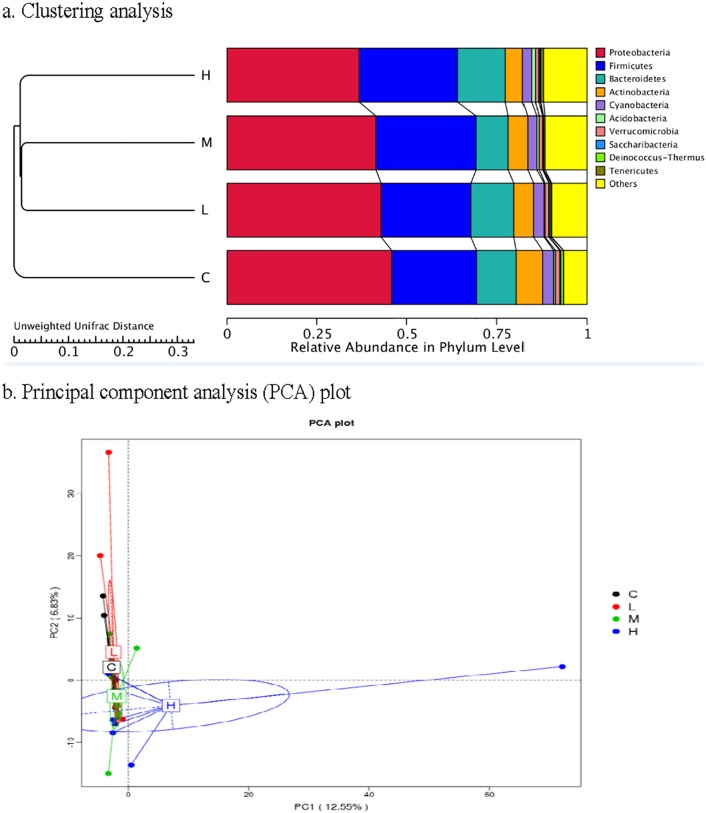
The beta diversity analysis was used to estimate the similarity among different groups. The prevailing occurrence of a common core of shared taxa is exhibited in detail by the overlapping Venn diagrams in Fig 1. The low-dose treatment and the control groups shared 350 bacterial OTUs, while the medium-dose treatment and control groups shared 329 bacterial OTUs, and 349 OTUs were shared by the high-dose treatment and control groups. There were 305, 335 and 467 unique OTUs of ileal microbiota in the low-, medium- and high-dose groups, respectively. The clustering analysis revealed short Unifrac distances among these three K. marxianus groups and long branches separating the samples from the control and K. marxianus-treated groups (Fig 2A). The dissimilarity in the ileal microbial community was greatest between the control and high-dose groups. Similarly, principal component analysis (PCA) defined groups where the samples from the control and high-dose groups occupied distinct positions (Fig 2B).
Fig 1. Venn graph for ileal microbiota of broilers at 21 d of age (n 8).
Each circle represents a group of samples. The numbers of common bacterial operational taxonomic units (OTUs) are displayed in the overlapping section between different circles, while the numbers in the non-overlapping section between different circles represent the number of their respectively unique OTUs. C, control group; L, low-dose (0.5 g/kg) group with K. marxianus; M, medium-dose (1.5 g/kg) group with K. marxianus; H, high-dose (2.5 g/kg) group with K. marxianus.
Fig 2. Similarity analysis of ileal microbiota between the control and Kluyveromyces marxianus-treated birds (n 8).
a. Clustering analysis: the left is UPGMA (unweighted pair-group method with arithmetic mean) clustering tree structure, while the relative abundance of main taxa at phylum taxonomic level is displayed on the right. b. PCA: abscissa represents the first principal component, ordinate represents the second principal component, and the percentage represents the contribution of the principal component to the sample difference. C, control group; L, low-dose (0.5 g/kg) group with K. marxianus; M, medium-dose (1.5 g/kg) group with K. marxianus; H, high-dose (2.5 g/kg) group with K. marxianus.
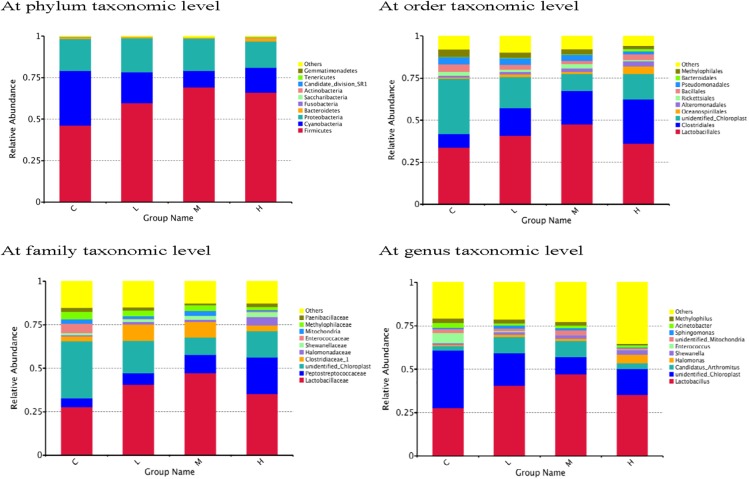
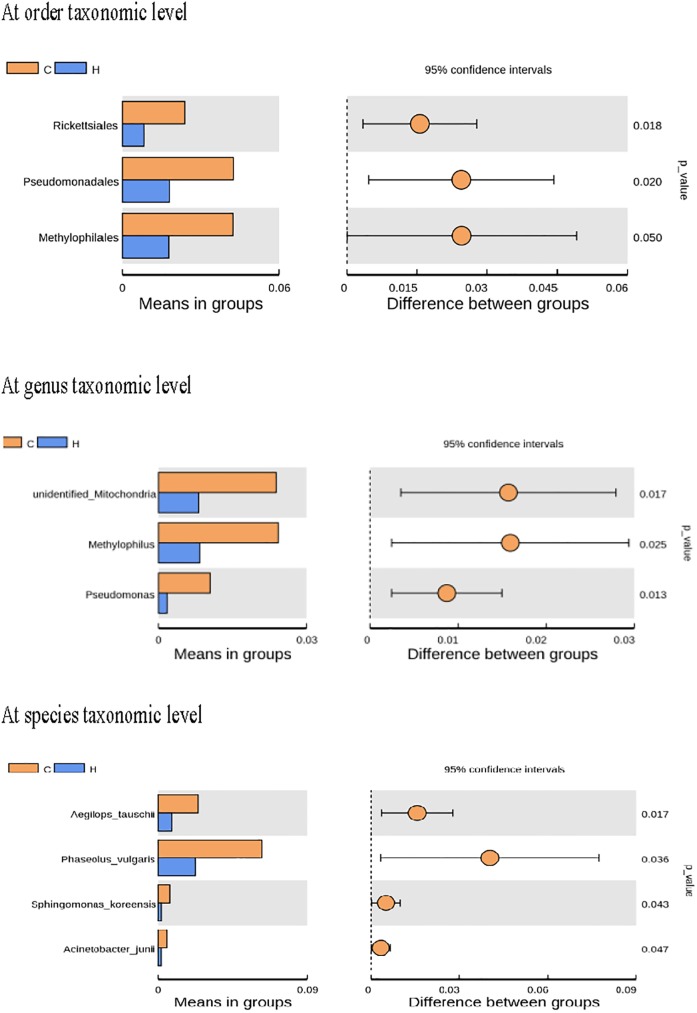
The dominant phyla in the ileum across all the groups were the Firmicutes, Cyanobacteria and Proteobacteria, together accounting for more than 90% of the total sequences (Fig 3). Birds supplemented with K. marxianus at these three levels had higher relative abundance of Firmicutes and lower relative abundance of Cyanobacteria in the ileal microbiota. However, the abundance of Proteobateria was flat among all the groups. Order level microbiota analysis revealed that the ileal microbiota was dominated by Lactobacillales and Clostridiales, the latter of which was increased in K. marxianus-treated groups as compared to the control group. Family level microbiota analysis revealed that the ileal microbiota was dominated by Lactobacteriaceae and Peptostreptococcaceae, which were increased after K. marxianus treatment. Besides, the Halomonadaceae abundance was increased especially in the high-dose group relative to control group. At genus level, an increase in the abundance of Lactobacillus was also detected in response to K. marxianus addition. Further analysis revealed that high-dose group had lower abundance of Rickettsiales, Pseudomonadales (Pseudomonas genus in particular), Methylophilale (Methylophilus genus in particular), Acinetobacter junii, Sphingomonas koreensis, Phaseolus vulgaris and Aegilops tauschii in the ileum than those in the control group (Fig 4).
Fig 3. Effects of Kluyveromyces marxianus on the relative abundance of main taxa (top ten) in the ileum at different taxonomic levels of broilers (n 8).
C, control group; L, low-dose (0.5 g/kg) group with K. marxianus; M, medium-dose (1.5 g/kg) group with K. marxianus; H, high-dose (2.5 g/kg) group with K. marxianus.
Fig 4. Differentiated species between the control and high-dose group with Kluyveromyces marxianus (n 8).
Mean proportions, 95% confidence intervals and P values are represented for each taxon for the two groups of birds. T-tests were used when comparing the relative abundance of individual taxa between different groups. C, control group; H, high-dose (2.5 g/kg) group with K. marxianus.
Discussion
Live yeast (mainly the S. cerevisiae) supplementation has been reported to improve the growth performance in animals [1–4]. However, little information is available regarding the effects of K. marxianus addition in chickens. Similar to the study in ruminants [8], we found that increasing K. marxianus addition linearly reduced FCR during grower and the overall period of birds, revealing a role for K. marxianus addition, especially at high dose (2.0 and 2.5 g/kg), in improving the feed efficiency in broiler chickens.
Determination the relative weight of immune organs including bursa of Fabricius, thymus and spleen is a common method for evaluation of immune status in chickens. Increased weight of immune organs in developing healthy animals indicated an increased immunological organ development, which could be beneficial for the immune function of chickens [24]. A previous study indicated that live yeast (S. boulardii) addition increased the relative weight of thymus and bursa in broilers [25]. Similarly, this study showed that relative weight of thymus was linearly increased by increasing K. marxianus addition, indicating that K. marxianus may have led to improved thymus development and subsequently benefit the immunity of broilers. Immunoglobulins bind to antigenic epitopes are critical for humoral immunity. Lysozyme that produced by the phagocytes can disintegrate the polysaccharide walls of Gram-positive and negative bacteria, which is important in innate immune response [26, 27]. Beneficial effects of live yeast (S. cerevisiae) addition on serum immunoglobulins and lysozyme were observed in animals due to its interaction with specific immune cells of host [25, 26, 28, 29]. In this study, serum IgG level and lysozyme activity quadratically responded to increasing K. marxianus addition and peaked at the dose of 1.0 g/kg, indicating a stimulation of immune system by K. marxianus addition especially at a medium dose, thereby providing protection against pathogen invasion in broiler chickens.
Mucosal immunity against infection is mainly mediated by the action of sIgA, which can block the connection between pathogens and epithelium, thereby protecting the intestinal epithelium from pathogenic microorganisms and enteric toxins [30]. Recent studies have shown that live yeast (S. cerevisiae or S. boulardii) addition increased intestinal sIgA level in broiler chickens [25, 31]. However, in this study, K. marxianus addition did not obviously influence the jejunal and ileal sIgA content, which implied different physiological roles of K. marxianus from other yeast species. Intestinal brush border enzymes that can be classified structurally as intrinsic and extrinsic to the membrane are important for nutrients absorption and gut barrier function [32]. ALP is an intrinsic enzyme in brush border, and serves as an indicator of functional and mature enterocytes in the gut [33]. Disaccharidase is an extrinsic enzyme, which participates in digestion and absorption of nutrients and can also function as a marker for evaluating intestinal mucosal integrity [21]. Few studies were available regarding the effect of live yeast on brush border enzymes in animals. In the present study, though K. marxianus addition did not influence intestinal ALP activity, ileal sucrase activity was linearly increased by increasing addition of K. marxianus. This implied that K. marxianus addition could be, to a degree, helpful for intestinal absorption and integrity of broilers. In support of this view, we also observed an improved intestinal morphology in broilers supplemented with K. marxianus, as evidenced by the linear increase in VCR of jejunum and ileum and ileal VH in response to K. marxianus addition. This was similar to some previous studies, in which live yeast (S. cerevisiae and S. boulardii) addition improved intestinal morphological structure in both pigs and broilers [1, 2, 25]. As an interface between the host and the environment, intestinal villi perform special functions to maintain intestinal absorption and integrity [21, 34]. Accordingly, the improved intestinal morphology indicated an enhancement of intestinal barrier and absorption capacity of broilers fed with K. marxianus, which might result in better feed efficiency.
The mucus layer is the first line of defense encountered by intestinal bacteria. Mucin-2 is the primary mucin gene in the mucus layer in the small intestine [35]. Studies pertaining to the effect of live yeast on intestinal mucin profile are sparse. In the present study, increasing K. marxianus addition linearly increased ileal mucin-2 gene expression, suggesting the idea of an enhanced mucosal barrier function after K. marxianus addition especially at high dose (2.5 g/kg). This might contribute to the improvement of intestinal morphological structure because of the less bacterial stimulation, and could be beneficial for intestinal immunity since mucin also helps to localize sIgA to the gut epithelium [36]. Another essential component of intestinal barriers are tight junctions (TJs), which create a protective barrier that aids in absorption while preventing translocation of harmful molecules [37]. TJs are comprised of several unique proteins, such as claudin-1 that plays a key role in the action of TJs [38]. This study revealed a linear increase in claudin-1 expression in response to increasing K. marxianus addition, which was similar to the report of Rajput et al. [25], who found that live yeast (S. boulardii) addition increased TJs proteins expression of broilers. Increased TJs protein expression could result in an enhancement of intestinal barrier function [39], which subsequently reduce the diffusion of macromolecules such as bacterial toxin and pathogens from intestinal lumen into blood circulation, thereby promoting the intestinal and systemic health of host. Nutrient transporters that loaded in the brush border membrane of intestine are greatly important for nutrients absorption. L-FABP that expressed in liver and intestine is responsible for the metabolism and intracellular transportation of lipids [40]. PepT-1 participates in the transportation of peptides into epithelial cells [41]. SGLT-1 mediates Na+-dependent glucose absorption across the luminal membrane of animal enterocytes, which is the major route for the utilisation of dietary sugars [42]. It was reported that probiotic addition increased some types of intestinal nutrient transporters in broilers [43]. An in vitro study revealed that S. boulardii treatment did not markedly enhance H+-dependent peptide absorption but promoted Na+-dependent glucose absorption of porcine intestinal epithelium [44]. Herein, we found that increasing K. marxianus addition linearly increased ileal SGLT-1 expression, which suggested an enhancement of glucose (energy) absorption of birds supplemented with K. marxianus especially at high dose and may subsequently benefit the growth performance. Therefore, the enhanced intestinal absorption, as characterized by the improved intestinal morphology coupled with the elevated SGLT-1 expression, could also be related to the improved feed efficiency of broilers after K. marxianus addition.
Gut microbiota contribute to maintenance of the normal physiological function of intestine, providing a series of beneficial effects on their hosts, such as nutrient digestion, immune function, and protection from invasive pathogens [14–17]. In the present study, we found a high similarity in the ileal microbial community among the broilers treated with different doses of K. marxianus through PCA and clustering analysis. In contrast, there was a distinct difference between these K. marxianus-treated and control groups, suggesting that supplemental K. marxianus, especially at high dose (2.5 g/kg) shifted intestinal microbial composition of broilers. This was supported by Venn diagram analysis, which revealed the largest number of unique bacterial OTUs in the ileal microbiota of birds fed with high-dose of K. marxianus. The taxonomical composition analysis revealed an enrichment of the phylum Firmicutes and a reduction of the phylum Cyanobacteria in the ileum after K. marxianus addition. Firmicutes play important roles in polysaccharide decomposition and consequently contribute to the utilisation of dietary energy and intestinal health [45–47]. Also, the reduced feed conversion ratio of broilers was accompanied by the increased abundance of Firmicutes [17, 48], which implied a positive correlation between Firmicutes abundance and feed efficiency. Cyanobacteria can produce cyanotoxins that induce oxidative stress and apoptosis of somatic cells and pose a danger to human health after accumulation through the food chain and drinking water [49, 50]. Therefore, the increase in Firmicutes and the reduction of Cyanobacteria in the ileum could be related to the improved feed efficiency and intestinal structure of broilers after K. marxianus addition. At the order level, increases in the abundance of Clostridiales and Lactobacillales (Lactobacillus genus in particular) were observed after K. marxianus addition. Clostridiales was indicated to be associated with the degradation of cellulose in gut [51]. This might be also beneficial for dietary energy utilisation and consequently contribute to the improved feed efficiency of broilers. Lactobacillus, as a typical probiotic bacterium, have been reported to modulate immune responses in animals [52, 53]. It could thus be speculated that the increased abundance of Lactobacillus as a result of K. marxianus addition may also be involved with the improved innate immunity of broilers. Among the bacterial species with low abundance, we also found that K. marxianus addition at high dose decreased the abundance of Rickettsiales, Pseudomonadales and Acinetobacter junii, which are defined as pernicious gut microorganisms that induce a variety of disorders of host [54–57]. Thus, it could be assumed that there was a connection between the reduced harmful germs in gut and the improved intestinal health of birds after K. marxianus addition at high dose.
Conclusions
Dietary K. marxianus supplementation was beneficial for feed efficiency, host immunity, and intestinal structure of broiler chickens. The host immune-related indices (serum IgG and lysozyme levels) of broilers optimized at medium dose (1.0 g/kg) of K. marxianus addition. However, the responses to K. marxianus addition on feed conversion ratio and intestinal structure were linearly. Supplemental K. marxianus especially at high dose (2.5 g/kg) improved intestinal microbial structure, which may contribute to the improved feed efficiency and intestinal structure of broiler chickens.
Supporting information
(a) Rarefaction curves calculated at the lowest subsample size of 30000 sequences per sample, show the effects of sequencing efforts on the observed number of OTUs at 97% sequence similarity. (b) Good’s coverage indices. C, control group; L, low-dose (0.5 g/kg) group with K. marxianus; M, medium-dose (1.5 g/kg) group with K. marxianus; H, high-dose (2.5 g/kg) group with K. marxianus.
(DOCX)
(DOCX)
Acknowledgments
This work was financially supported by Shanghai Engineering Research Center of Industrial Microorganisms, College of Life Sciences, Fudan University (Shanghai, China) and China Agricultural Research System program (CARS-42). The authors received no specific funding for this work. The authors thank Yuanyuan Wu, Yunshuang Yue, Dafei Yin, and Yuanyang Dong (China Agricultural University, Beijing, China) for their assistance in the process of animals feeding and samples collection. The authors have declared that no competing interests exist.
Abbreviations
- ABW
average body weight
- ADG
average daily gain
- ADFI
average daily feed intake
- FCR
feed conversion ratio
- VH
villus height
- VCR
villus height to crypt depth ratio
- SEM
standard error of the mean
- sIgA
secretory immunoglobulin A
- ALP
alkaline phosphatase
- SGLT1
sodium glucose cotransporter 1
- PepT1
H+-dependent peptide transporter 1
- L-FABP
liver fatty acid-binding protein
- OTUs
operational taxonomic units
- PCA
Principal component analysis
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was financially supported by Shanghai Engineering Research Center of Industrial Microorganisms, College of Life Sciences, Fudan University (Shanghai, China) and China Agricultural Research System program (CARS-42). The authors received no specific funding for this work.
References
- 1.Bontempo V, Di Giancamillo A, Savoini G, Dell'Orto V, Domeneghini C. Live yeast dietary supplementation acts upon intestinal morpho-functional aspects and growth in weanling piglets. Anim Feed Sci Tech. 2006; 129(3–4): 224–236. [Google Scholar]
- 2.Haldar S, Ghosha TK, Toshiwatia, Bedford MR. Effects of yeast (Saccharomyces cerevisiae) and yeast protein concentrate on production performance of broiler chickens exposed to heat stress and challenged with Salmonella enteritidis. Anim Feed Sci Tech. 2011; 168(1–2): 61–71. [Google Scholar]
- 3.Jang YD, Kang KW, Piao LG, Jeong T.S., Auclair E., Jonvel S., et al. Effects of live yeast supplementation to gestation and lactation diets on reproductive performance, immunological parameters and milk composition in sows. Livest Sci. 2013; 152(2–3): 167–173. [Google Scholar]
- 4.Heugten EV, Funderburke DW, Dorton KL. Growth performance, nutrient digestibility, and fecal microflora in weanling pigs fed live yeast. J Anim Sci. 2003; 81(4): 1004–1012. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TH, Fleet GH, Rogers RL. Composition of the cell walls of several yeast species. Appl Microbiol Biot. 1998; 50(2): 206–212. [DOI] [PubMed] [Google Scholar]
- 6.Lane MM, Burke N, Karreman R, Wolfe KH, O'Byrne CP, Morrissey JP. Physiological and metabolic diversity in the yeast Kluyveromyces marxianus. Antonie van Leeuwenhoek. 2011; 100(4): 507–519. doi: 10.1007/s10482-011-9606-x [DOI] [PubMed] [Google Scholar]
- 7.Lane MM, Morrissey JP. Kluyveromyces marxianus: a yeast emerging from its sister’s shadow. Fungal Biol Rev. 2010; 24(1–2): 17–26. [Google Scholar]
- 8.Tripathi MK, Karim SA. Effect of individual and mixed live yeast culture feeding on growth performance, nutrient utilization and microbial crude protein synthesis in lambs. Anim Feed Sci Tech. 2010; 155(2): 163–171. [Google Scholar]
- 9.Tripathi MK, Karim SA. Effect of yeast cultures supplementation on live weight change, rumen fermentation, ciliate protozoa population, microbial hydrolytic enzymes status and slaughtering performance of growing lamb. Livest Sci. 2011; 135(1): 17–25. [Google Scholar]
- 10.Kourelis A, Kotzamanidis C, Litopoulou-Tzanetaki E, Papaconstantinou J, Tzanetakis N, Yiangou M. Immunostimulatory activity of potential probiotic yeast strains in the dorsal air pouch system and the gut mucosa. J Appl Microbiol. 2010; 109(1): 260–271. doi: 10.1111/j.1365-2672.2009.04651.x [DOI] [PubMed] [Google Scholar]
- 11.Romanin D, Serradell M, Maciel DG, Lausada N, Garrote GL, Rumbo M. Down-regulation of intestinal epithelial innate response by probiotic yeasts isolated from kefir. Int J Food Microbiol. 2010; 140(2–3): 102–108. doi: 10.1016/j.ijfoodmicro.2010.04.014 [DOI] [PubMed] [Google Scholar]
- 12.Maccaferri S, Klinder A, Brigidi P, Cavina P, Costabile A. Potential probiotic kluyveromyces marxianus b0399 modulates the immune response in caco-2 cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system. Appl Environ Microb. 2012; 78(4): 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolla PA, Carasi P, Bolla ML, De Antoni GL, Serradell Mde L. Protective effect of a mixture of kefir-isolated lactic acid bacteria and yeasts in a hamster model of Clostridium difficile infection. Anaerobe. 2013; 21(6): 28–33. [DOI] [PubMed] [Google Scholar]
- 14.Susan EP, Paul WO, Catherine S, Ross RP, Fitzgerald GF. Intestinal microbiota, diet and health. Br J Nutr. 2014; 111(3): 387–402. doi: 10.1017/S0007114513002560 [DOI] [PubMed] [Google Scholar]
- 15.Lecomte V, Kaakoush NO, Maloney CA, Raipuria M, Huinao KD, Mitchell HM, et al. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS ONE. 2015; 10 (5): e0126931 doi: 10.1371/journal.pone.0126931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiharto S. Role of nutraceuticals in gut health and growth performance of poultry. J Saudi Soc Agric Sci. 2016; 15(2): 99–111. [Google Scholar]
- 17.Singh KM, Shah T, Deshpande S, Jakhesara SJ, Koringa PG, Rank DN, et al. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol Biol Rep. 2012; 39(12): 10595–10602. doi: 10.1007/s11033-012-1947-7 [DOI] [PubMed] [Google Scholar]
- 18.De-Maesschalck C, Eeckhaut V, Maertens L, De Lange L, Marchal L, Nezer C, et al. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl Environ Microbiol. 2015; 81(17): 5880–5888. doi: 10.1128/AEM.01616-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torok VA, Hughes RJ, Mikkelsen LL, Perez-Maldonado R, Balding K, MacAlpine R, et al. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl Environ Microbiol. 2011; 77(17): 5868–5878. doi: 10.1128/AEM.00165-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelakis E, Raoult D. The increase of Lactobacillus species in the gut flora of newborn broiler chicks and ducks is associated with weight gain. PLoS ONE. 2010; 5(5): e10463 doi: 10.1371/journal.pone.0010463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamb-Rosteski JM, Kalischuk LD, Inglis GD, Buret AG. Epidermal growth factor inhibits Campylobacter jejuni induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect Immun. 2008; 76(8): 3390–3398. doi: 10.1128/IAI.01698-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006; 27(2–3): 126–139. doi: 10.1016/j.mam.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001; 25(4): 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 24.Heckert RA, Estevez I, Russekcohen E, Pettitriley R. Effects of density and perch availability on the immune status of broilers. Poult Sci. 2002; 81(4): 451–457. [DOI] [PubMed] [Google Scholar]
- 25.Rajput IR, Li LY, Xin X,Wu BB, Juan ZL, Cui ZW, et al. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult Sci. 2013; 92(4): 956–965. doi: 10.3382/ps.2012-02845 [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Zhang HJ, Yu SH, Wu SG, Yoon I, Quigley J, et al. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult Sci. 2008; 87(7): 1377–1384. doi: 10.3382/ps.2007-00418 [DOI] [PubMed] [Google Scholar]
- 27.Nash JA, Ballard TNS, Weaver TE, Akinbi HT. The peptidoglycan-degrading property of lysozyme is not required for bactericidal activity in vivo. J Immunol. 2006; 177(1): 519–526. [DOI] [PubMed] [Google Scholar]
- 28.Milewski S, Wojcik R, Zaleska B, Malaczewska J, Tanski Z, Siwicki AK. Effect of Saccharomyces cerevisiae dried yeast on the meat performance traits and selected indicators of humoral immunity in lambs. Acta Vet Brno. 2013; 82(2): 147–151. [Google Scholar]
- 29.Zanello G, Meurens F, Serreau D, Chevaleyre C, Melo S, Berri M, et al. Effects of dietary yeast strains on immunoglobulin in colostrum and milk of sows. Vet Immunol Immunop. 2013; 152(1–2): 20–27. [DOI] [PubMed] [Google Scholar]
- 30.Mantis NJ, Rol N, Corthesy B. Secretory IgA′s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011; 4(6): 603–611. doi: 10.1038/mi.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J, Zhang HJ, Wu SG, Yu SH, Yoon I, Moore D, et al. Effect of Saccharomyces cerevisiae fermentation product on immune functions of broilers challenged with Eimeria tenella. Poult Sci. 2009; 88(10): 2141–2151. doi: 10.3382/ps.2009-00151 [DOI] [PubMed] [Google Scholar]
- 32.Usha NSP, Krishnapura S. Beneficial influence of dietary spices on the ultrastructure and fluidity of the intestinal brush border in rats. Br J Nutr. 2010; 104(1): 31–39. doi: 10.1017/S0007114510000334 [DOI] [PubMed] [Google Scholar]
- 33.Fasina YO, Garlich JD, Classen HL, Ferket PR, Havenstein GB, Grimes JL, et al. Response of turkey poults to soybean lectin levels typically encountered in commercial diets. 1. Effect on growth and nutrient digestibility. Poult Sci. 2004; 83(9): 1559–1571. [DOI] [PubMed] [Google Scholar]
- 34.McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001; 3(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 35.Hecht G. Innate mechanisms of epithelial host defense: spotlight on intestine. Am J Physiol-Cell Ph. 1999; 277(3): C351–C358. [DOI] [PubMed] [Google Scholar]
- 36.Rogier EW, Frantz AL, Bruno MEC, Kaetzel CS. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens. 2014; 3(2): 390–403. doi: 10.3390/pathogens3020390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballard ST, Hunter JH, Taylor AE. Regulation of tight junction permeability during nutrient absorption across the intestinal epithelium. Annu Rev Nutr. 1995; 15(1): 35–55. [DOI] [PubMed] [Google Scholar]
- 38.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002; 156(6): 1099–1111. doi: 10.1083/jcb.200110122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goyer M, Loiselet A, Bon F, L'Ollivier C, Laue M, Holland G, et al. Intestinal cell tight junctions limit invasion of candida albicans through active penetration and endocytosis in the early stages of the interaction of the fungus with the intestinal barrier. PLoS ONE. 2016; 11(3): e0149159 doi: 10.1371/journal.pone.0149159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ockner RK, Ho WKL, Poppenhausen RB, Ho WK. Binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science. 1972; 177(4043): 56–58. [DOI] [PubMed] [Google Scholar]
- 41.Leibach FH, Ganapathy V. Peptide transporters in the intestine and the kidney. Annu Rev Nutr. 1996; 16(1): 99–119. [DOI] [PubMed] [Google Scholar]
- 42.Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Ionescu C, et al. Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate. Br J Nutr 2010; 104(5): 647–655. doi: 10.1017/S0007114510000954 [DOI] [PubMed] [Google Scholar]
- 43.Jahromi MF, Altaher YW, Shokryazdan P, Ebrahimi R, Ebrahimi M, Idrus Z, et al. Dietary supplementation of a mixture of Lactobacillus strains enhances performance of broiler chickens raised under heat stress conditions. Int J Biometeorol. 2016; 60(7): 1099–1110. doi: 10.1007/s00484-015-1103-x [DOI] [PubMed] [Google Scholar]
- 44.Breves G, Walter C, Burmester M, Schroder B. In vitro studies on the effects of Saccharomyces boulardii and Bacillus cereus var. toyoi on nutrient transport in pig jejunum. J Anim Physiol An N. 2000. 84(1–2): 9–20. [Google Scholar]
- 45.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006; 444(7122): 1022–1023. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 46.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased apacity for energy harvest. Nature. 2006; 444(7122): 1027–1031. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 47.Wang LL, Hatem A, Catalyurek UV, Morrison M, Yu ZT. Metagenomic insights into the carbohydrate-active enzymes carried by the microorganisms adhering to solid digesta in the rumen of cows. PLoS ONE. 2013; 8(11): e78507 doi: 10.1371/journal.pone.0078507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torok VA, Ophel-Keller K, Loo M, Hughes RJ. Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism. Appl Environ Microbiol. 2008; 74(3): 783–791. doi: 10.1128/AEM.01384-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Funari E, Testai E. Human health risk assessment related to cyanotoxins exposure. Crit Rev Toxicol. 2008; 38(2): 97–125. doi: 10.1080/10408440701749454 [DOI] [PubMed] [Google Scholar]
- 50.Rymuszka A, Sieroslawska A, Bownik A, Skowroński T. Microcystin-LR modulates selected immune parameters and induces necrosis/apoptosis of carp leucocytes. Environ Toxicol Chem. 2010; 29(3): 569–574. doi: 10.1002/etc.87 [DOI] [PubMed] [Google Scholar]
- 51.Zhu LF, Wu Q, Dai JY, Zhang SN, Wei FW. Evidence of cellulose metabolism by the giant panda gut microbiome. PNAS. 2011; 108(43): 17714–17719. doi: 10.1073/pnas.1017956108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angelakis E, Raoult D. The increase of Lactobacillus species in the gut flora of newborn broiler chicks and ducks is associated with weight gain. PLoS ONE. 2010; 5(5): e10463 doi: 10.1371/journal.pone.0010463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlasova AN, Chattha KS, Kandasamy S, Liu Z, Esseili M, Shao LL, et al. Lactobacilli and Bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs. PLoS ONE. 2013; 8(10): e76962 doi: 10.1371/journal.pone.0076962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valbuena G, Walker DH. Infection of the endothelium by members of the order Rickettsiales. Thromb Haemostasis. 2009; 102(6): 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broniek G, Langwinska-Wosko E, Szaflik J, Wroblewska M. Acinetobacter junii as an aetiological agent of corneal ulcer. Infection. 2014; 42(6): 1051–1053. doi: 10.1007/s15010-014-0647-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greig SL, Scott LJ. Intravenous minocycline: A review in acinetobacter infections. Drugs. 2016; 76(15): 1467–1476. doi: 10.1007/s40265-016-0636-6 [DOI] [PubMed] [Google Scholar]
- 57.Wagner S, Sommer R, Hinsberge S, Lu C, Hartmann RW, Empting M, et al. Novel strategies for the treatment of Pseudomonas Aeruginosa infections. J Med Chem. 2016; 59(13): 5929–5969. doi: 10.1021/acs.jmedchem.5b01698 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Rarefaction curves calculated at the lowest subsample size of 30000 sequences per sample, show the effects of sequencing efforts on the observed number of OTUs at 97% sequence similarity. (b) Good’s coverage indices. C, control group; L, low-dose (0.5 g/kg) group with K. marxianus; M, medium-dose (1.5 g/kg) group with K. marxianus; H, high-dose (2.5 g/kg) group with K. marxianus.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.