Abstract
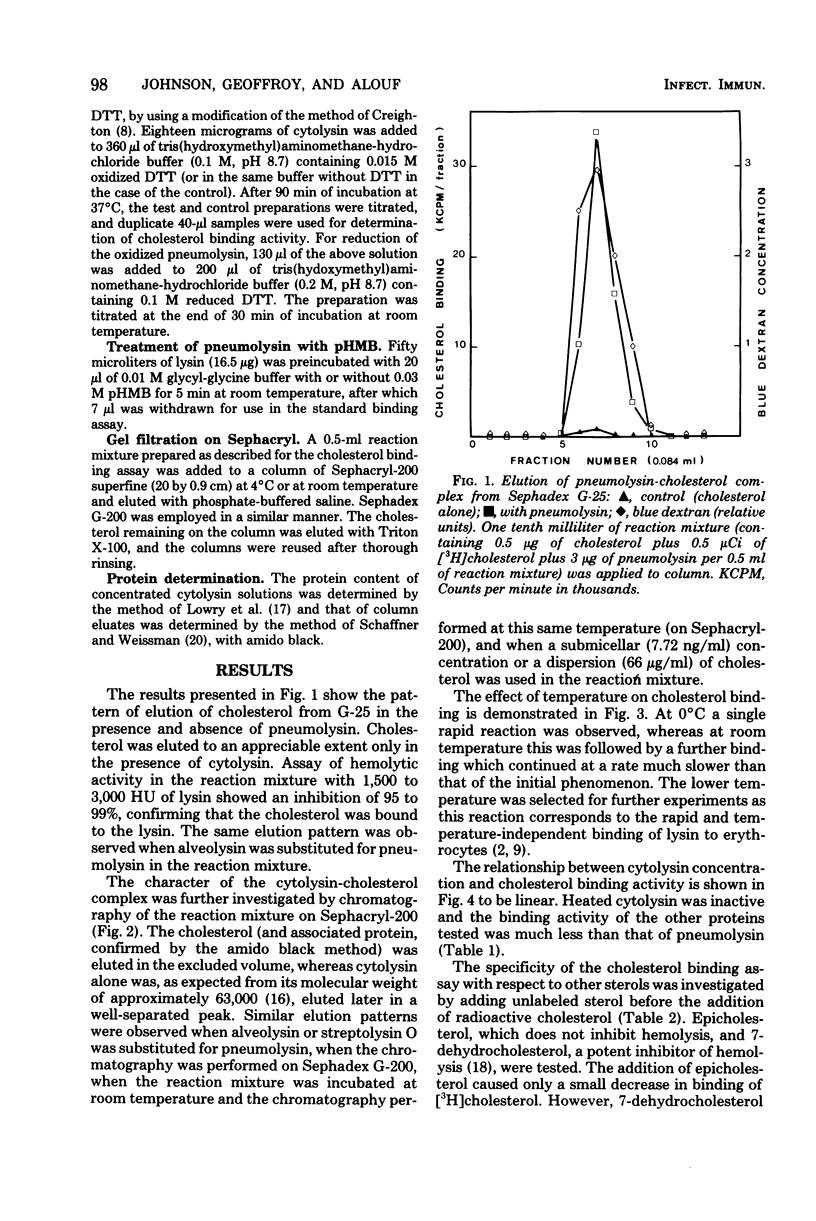
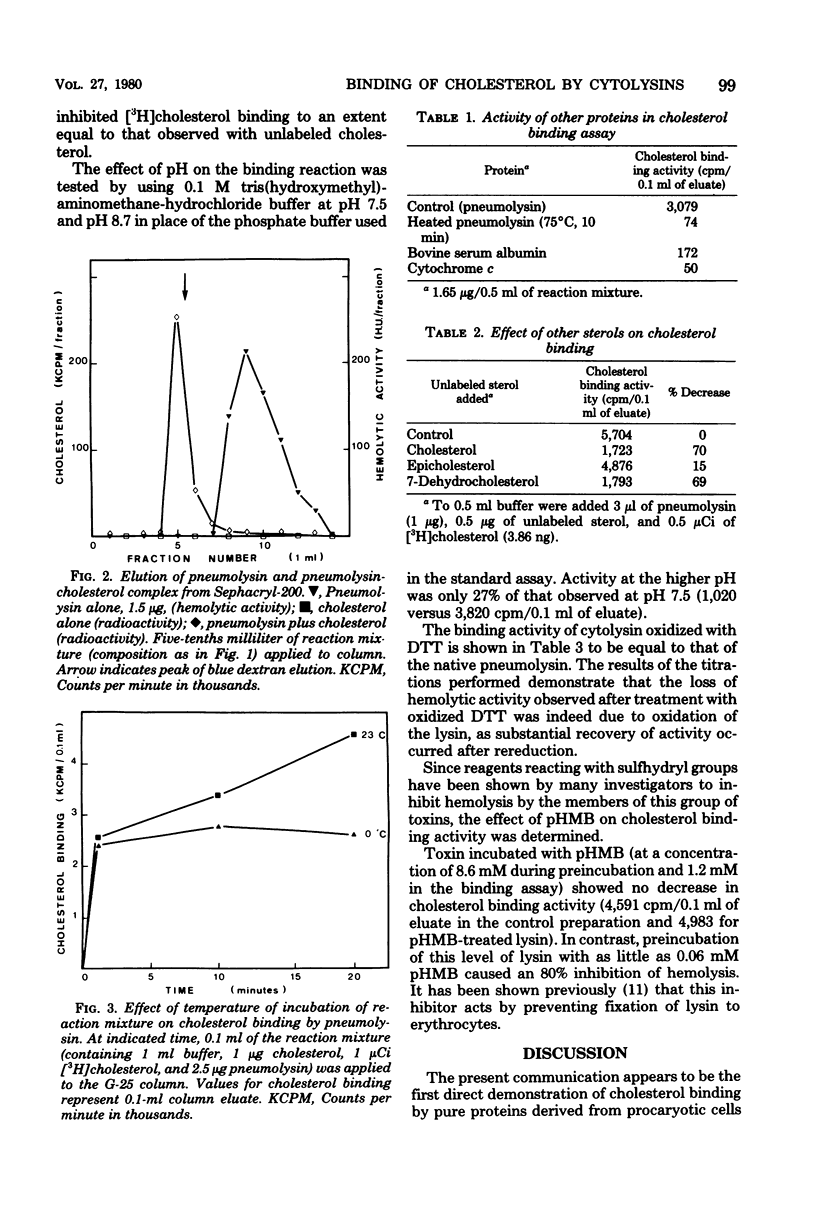
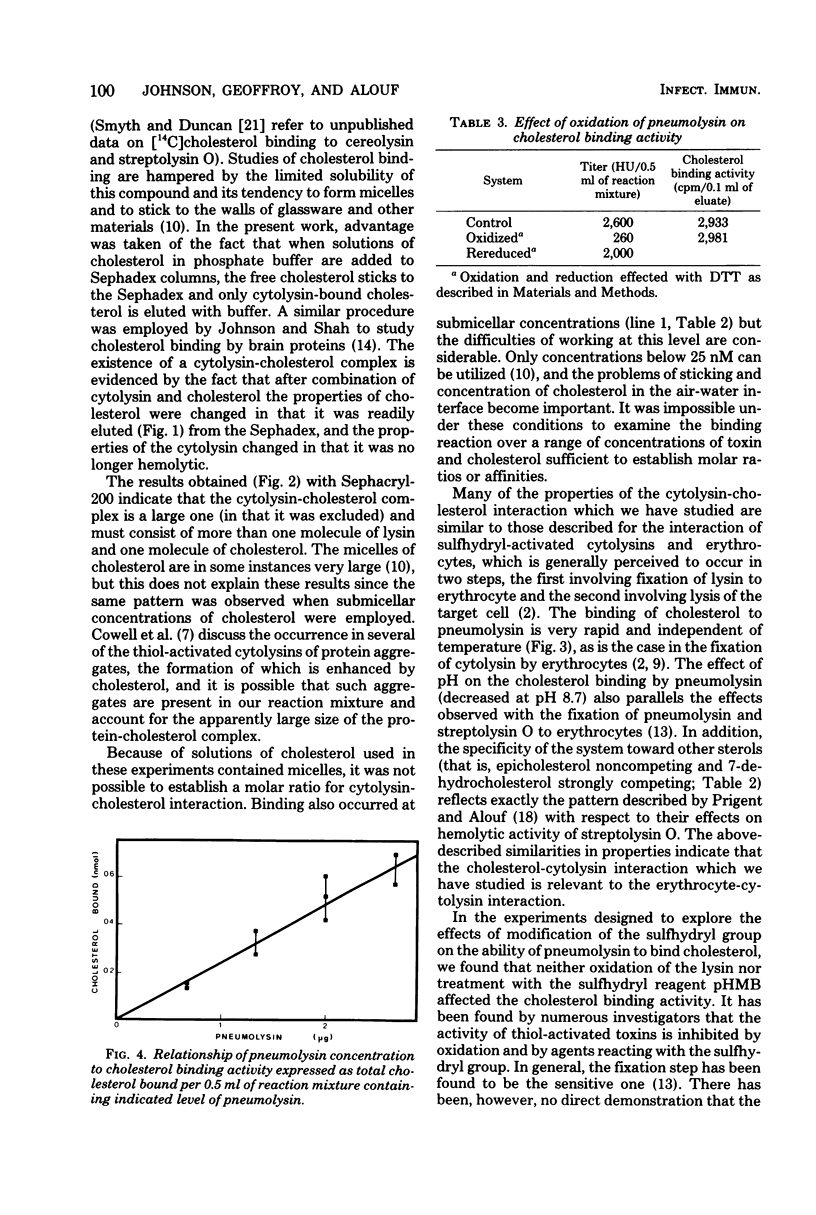
The binding of cholesterol by pneumolysin, alveolysin, and streptolysin O has been demonstrated. The properties of the cytolysin-cholesterol interaction parallel those of cytolysin-erythrocyte interaction in that the reaction is rapid, temperature independent, decreased at elevated pH, and shows the same specificity with respect to other related sterols. However, oxidized or p-hydroxymercuribenzoate-treated thoxin showed no decrease in cholesterol-binding activity, whereas the ability of cytolysin to bind to erythrocytes was modified by such treatment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E., Kiredjian M., Geoffroy C. Purification de l'hémolysine thiol-dépendante extracellulaire de Bacillus alvei. Biochimie. 1977;59(3):329–336. doi: 10.1016/s0300-9084(77)80150-8. [DOI] [PubMed] [Google Scholar]
- Alouf J. E., Raynaud M. Action de la streptolysine O sur les membranes cellulaires. I. Fixation sur la membrane érythrocytaire. Ann Inst Pasteur (Paris) 1968 Jun;114(6):812–827. [PubMed] [Google Scholar]
- Cowell J. L., Bernheimer A. W. Role of cholesterol in the action of cereolysin on membranes. Arch Biochem Biophys. 1978 Oct;190(2):603–610. doi: 10.1016/0003-9861(78)90316-8. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Kim K. S., Bernheimer A. W. Alteration by cereolysin of the structure of cholesterol-containing membranes. Biochim Biophys Acta. 1978 Feb 21;507(2):230–241. doi: 10.1016/0005-2736(78)90419-4. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Renaturation of the reduced bovine pancreatic trypsin inhibitor. J Mol Biol. 1974 Aug 15;87(3):563–577. doi: 10.1016/0022-2836(74)90104-1. [DOI] [PubMed] [Google Scholar]
- Fehrenbach F. J., Eibl H. Interaction of streptolysin-O with natural and artificial membranes. Z Naturforsch C. 1977 Jan-Feb;32(1-2):101–109. doi: 10.1515/znc-1977-1-217. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Reynolds J. A. Self-association of cholesterol in aqueous solution. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2313–2316. doi: 10.1073/pnas.70.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Allen J. H. The role of cytolysin in pneumococcal ocular infection. Am J Ophthalmol. 1975 Sep;80(3 Pt 2):518–521. doi: 10.1016/0002-9394(75)90219-6. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Aultman K. S. Studies on the mechanism of action of oxygen-labile haemolysins. J Gen Microbiol. 1977 Aug;101(2):237–241. doi: 10.1099/00221287-101-2-237. [DOI] [PubMed] [Google Scholar]
- Johnson M. K. Properties of purified pneumococcal hemolysin. Infect Immun. 1972 Nov;6(5):755–760. doi: 10.1128/iai.6.5.755-760.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Shah S. N. Binding of squalene, lanosterol, desmosterol, and cholesterol to proteins in brain and liver 105,000 g supernatant fractions: evidence for specific binding sites. Lipids. 1976 Sep;11(9):645–651. doi: 10.1007/BF02532881. [DOI] [PubMed] [Google Scholar]
- Kanbayashi Y., Hotta M., Koyama J. Kinetic study on streptolysin O. J Biochem. 1972 Feb;71(2):227–237. doi: 10.1093/oxfordjournals.jbchem.a129759. [DOI] [PubMed] [Google Scholar]
- Kreger A. S., Bernheimer A. W. Physical behavior of pneumolysin. J Bacteriol. 1969 Apr;98(1):306–307. doi: 10.1128/jb.98.1.306-307.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Prigent D., Alouf J. E. Interaction of steptolysin O with sterols. Biochim Biophys Acta. 1976 Aug 16;443(2):288–300. doi: 10.1016/0005-2736(76)90511-3. [DOI] [PubMed] [Google Scholar]
- Prigent D., Geoffroy C., Alouf J. E. Purification de la streptolysine O par chromatographie covalente sur gel de thiol-agarose. C R Acad Sci Hebd Seances Acad Sci D. 1978 Oct 23;287(10):951–954. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]


