Abstract
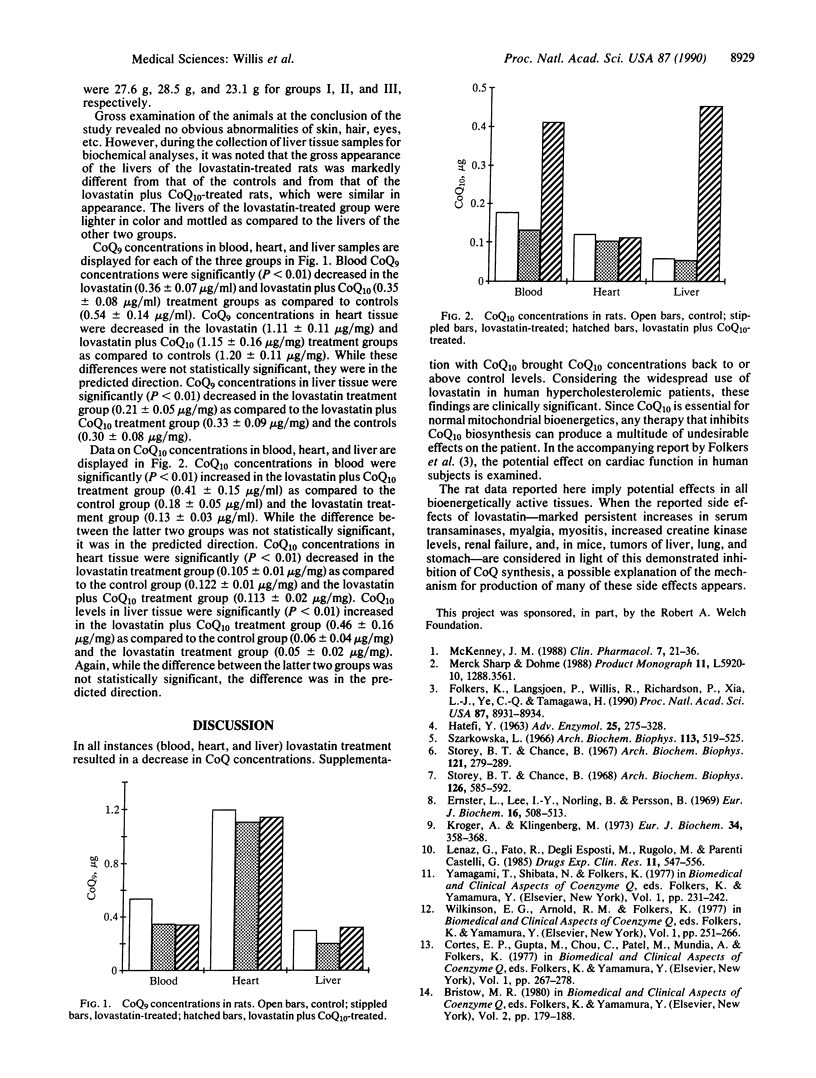
Lovastatin is used for the treatment of hypercholesterolemia. It functions by inhibiting the enzyme, 3-hydroxy-3-methylglutaryl-coenzyme A reductase (EC 1.1.1.34), that is required for the conversion of 3-hydroxy-3-methylglutaryl-coenzyme A to mevalonic acid. Since biosynthesis of both cholesterol and coenzyme Q (CoQ) requires mevalonic acid as a precursor, it was considered that lovastatin therapy would also result in a lowering of cellular CoQ levels. This study was conducted to determine whether lovastatin treatment does decrease CoQ levels and whether such decreases can be prevented by CoQ supplementation. Forty-five adult male Holtzman rats were randomly assigned to one of three treatment groups. Controls were fed ground laboratory rat chow ad libitum. The other two groups were fed ground laboratory rat chow containing 400 mg of lovastatin per kg of diet ad libitum. One of the lovastatin-fed groups received CoQ10 (15 mg per kg of body weight) daily via stomach intubation. After 4 weeks, samples of heart, liver, and blood were analyzed for CoQ concentrations. Results indicated that CoQ concentrations in all tissues analyzed were decreased in lovastatin-treated rats. Lovastatin-treated animals that were supplemented with CoQ10 had blood, heart, and liver CoQ10 concentrations that approximated or exceeded those of control animals. It is concluded that lovastatin does indeed lower tissue concentrations of CoQ and that a return to normal can be achieved by supplementation with CoQ.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Folkers K., Langsjoen P., Willis R., Richardson P., Xia L. J., Ye C. Q., Tamagawa H. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8931–8934. doi: 10.1073/pnas.87.22.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATEFI Y. COENZYME Q (UBIQUINONE). Adv Enzymol Relat Areas Mol Biol. 1963;25:275–328. doi: 10.1002/9780470122709.ch5. [DOI] [PubMed] [Google Scholar]
- Komorowski J., Muratsu K., Nara Y., Willis R., Folkers K. Significance of biological parameters of human blood levels of CoQ10. Biofactors. 1988 Jan;1(1):67–69. [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem. 1973 Apr;34(2):358–368. doi: 10.1111/j.1432-1033.1973.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Langsjoen P. H., Vadhanavikit S., Folkers K. Effective treatment with coenzyme Q10 of patients with chronic myocardial disease. Drugs Exp Clin Res. 1985;11(8):577–579. [PubMed] [Google Scholar]
- Lenaz G., Fato R., Degli Esposti M., Rugolo M., Parenti Castelli G. The essentiality of coenzyme Q for bioenergetics and clinical medicine. Drugs Exp Clin Res. 1985;11(8):547–556. [PubMed] [Google Scholar]
- McKenney J. M. Lovastatin: a new cholesterol-lowering agent. Clin Pharm. 1988 Jan;7(1):21–36. [PubMed] [Google Scholar]
- Oda T. Effect of coenzyme Q10 on stress-induced cardiac dysfunction in paediatric patients with mitral valve prolapse: a study by stress echocardiography. Drugs Exp Clin Res. 1985;11(8):557–576. [PubMed] [Google Scholar]
- Rossi E., Norling B., Persson B., Ernster L. Studies with ubiquinone-depleted submitochondrial particles. Effects of extraction and reincorporation of ubiquinone on the kinetics of succinate dehydrogenase. Eur J Biochem. 1970 Nov;16(3):508–513. doi: 10.1111/j.1432-1033.1970.tb01110.x. [DOI] [PubMed] [Google Scholar]
- Solaini G., Landi L., Pasquali P., Rossi C. A. Protective effect of endogenous coenzyme Q on both lipid peroxidation and respiratory chain inactivation induced by an adriamycin-iron complex. Biochem Biophys Res Commun. 1987 Sep 15;147(2):572–580. doi: 10.1016/0006-291x(87)90969-7. [DOI] [PubMed] [Google Scholar]
- Storey B. T., Chance B. Rate of reduction of ubiquinone by NADH in electron transport particles. Arch Biochem Biophys. 1967 Aug;121(2):279–289. doi: 10.1016/0003-9861(67)90077-x. [DOI] [PubMed] [Google Scholar]
- Storey B. T. Rate of ubiquinone oxidation in electron transport particles reduced by succinate. Arch Biochem Biophys. 1968 Aug;126(2):585–592. doi: 10.1016/0003-9861(68)90445-1. [DOI] [PubMed] [Google Scholar]
- Szarkowska L. The restoration of DPNH oxidase activity by coenzyme Q (ubiquinone). Arch Biochem Biophys. 1966 Mar;113(3):519–525. doi: 10.1016/0003-9861(66)90228-1. [DOI] [PubMed] [Google Scholar]
- Vadhanavikit S., Morishita M., Duff G. A., Folkers K. Micro-analysis for coenzyme Q10 in endomyocardial biopsies of cardiac patients and data on bovine and canine hearts. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1165–1169. doi: 10.1016/s0006-291x(84)80255-7. [DOI] [PubMed] [Google Scholar]



