Abstract
As well as modulating integrin activation, a conserved NPxY motif in integrin cytoplasmic tails that binds the FERM domain-containing proteins kindlin and sorting nexin-17 plays pivotal roles in integrin recycling and degradation.
The ability of metazoan cells to sense and adhere to the insoluble extracellular matrix (ECM) that surrounds them is central to multicellular life. Integrins, the major family of ECM adhesion receptors responsible for this ability, are transmembrane αβ heterodimers that link the ECM to intracellular cytoskeletal and signaling networks. Integrins are thus integral to a range of essential processes, including cell migration, embryonic development, tissue formation, vasculogenesis, inflammatory and immune responses, and wound healing[1]. Like other cell-surface receptors, integrins can be regulated by controlling cell surface delivery, endocytosis, and subsequent recycling or degradation. Indeed, the importance of integrin internalization and recycling in a range of cellular processes is increasingly well appreciated[2,3]. However, a unique and defining feature of integrin regulation is integrin activation; the allosteric transition from conformations with relatively low-affinity to high-affinity for ECM ligands. This transition is controlled by the binding of the FERM (4.1, ezrin, radixin, moesin) domain from the protein talin to a membrane-proximal NPxY motif in the short cytoplasmic tail of the integrin β subunit[4]. More recently, human disease mutations and knockout studies have implicated a second family of FERM- domain proteins, the kindlins, in integrin activation[5–7]. Kindlins bind the membrane-distal β tail NPxY motif and its preceding threonines (Fig 1) and kindlin deficiency leads to defects in integrin activation and signaling. However, the molecular basis for kindlin function is not understood. In this issue, Margadant et al. [8] provide new insights into the differential roles of talin and kindlin in β1 integrin regulation and for the first time link kindlins to control of lysosomal degradation of integrins. Using alternate approaches, two other recent papers[9,10] also reveal a role for the kindlin-binding NPxY motif in determining whether integrins are lysosomally degraded or are recycled to the cell surface. However, these investigators find that binding of the FERM domain-containing protein sorting nexin-17 (SNX17) triggers recycling versus degradation. While many details remain to be elucidated, and discrepancies resolved, taken together all three new papers suggest that the dynamics of FERM domain binding to integrin NPxY motifs govern not only activation but also recycling and degradation. Understanding how this occurs, if and how the processes are linked, and how FERM binding and competition is regulated will be the next challenges.
Figure 1.
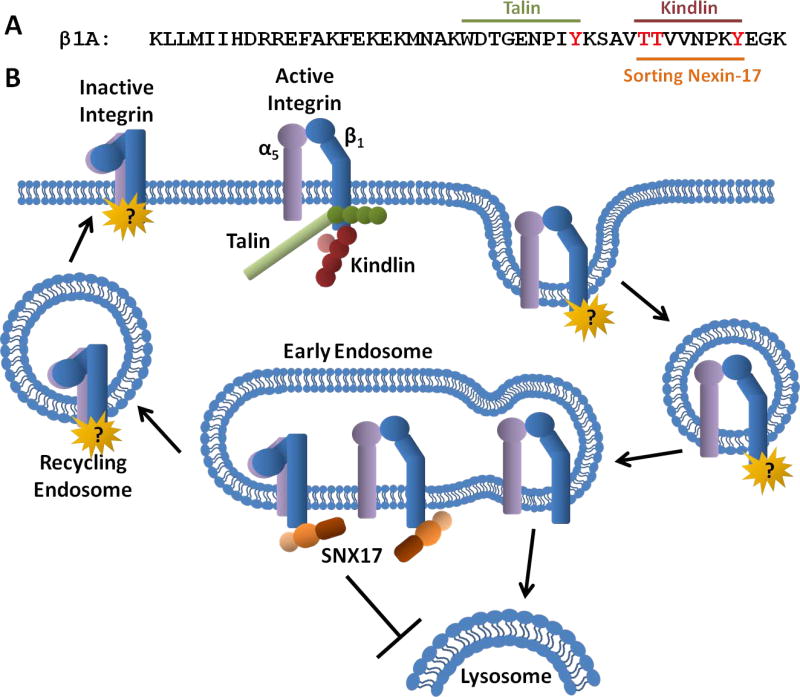
(A) Amino acid sequence of the cytoplasmic tail of human β1 integrin. Regions important for binding talin, kindlin and sorting nexin-17 (SNX17) are indicated while key residues mutated to disrupt binding are highlighted in red. (B) Model for regulation of integrin activation and trafficking. Binding of talin and kindlin leads to integrin activation at the plasma membrane. Internalization leads to dissociation of talin and kindlin while SNX17 binds integrins in early endosomes and, by an unknown mechanism, facilitates their recycling. Integrins unable to bind SNX17 are targeted for lysosomal degradation. Kindlin’s role in integrin trafficking remains controversial but kindlin and SNX17 can compete for binding to integrin and they do not colocalize in the same subcellular compartment. Whether other tail-binding proteins regulate additional steps in recycling has yet to be determined.
Margadant et al. investigated the roles of the talin- and kindlin-binding sites in β1 integrin activation and trafficking by reconstituting embryoid body-derived β1 integrin-null cells with wild-type β1 or with β1 containing tyrosine to alanine mutations in the membrane-proximal or membrane-distal NxxY motifs. As expected, the talin-binding mutant was defective in inducing cell scattering, cell migration, fibrillogenesis, fibrillar adhesion formation and integrin activation. Surprisingly, mutation of the distal tyrosine produced only modest inhibition of integrin activation and, at least in these cells, this tyrosine was dispensable for cell scattering, migration and fibrillogenesis. However, mutation of this distal tyrosine resulted in a dramatic drop in cell-surface expression of the mature β1 subunit due to enhanced lysosomal degradation of endocytosed integrin, suggesting that the distal tyrosine is important for protecting the integrin from sorting to the lysosome. To test whether this was due to interruption of kindlin binding, Margadant et al. knocked down kindlin and found that integrin activation was only modestly impaired, as they had seen for kindlin-binding defective integrins. However, α5β1 levels were greatly reduced, primarily due to increased lysosomal targeting and degradation. These data suggest that kindlin binding to integrin β1 tails somehow triggers recycling of internalized integrin rather than degradation, and may explain our earlier observation that over-expression of wild-type, but not integrin-binding defective, kindlin increases β1 surface expression[11]. A molecular mechanism by which kindlin binding protects integrins from degradation was not provided but the results of Böttcher et al. [9] and Steinberg et al. [10], published while Margadant et al. was under review, provide important new insights into control of integrin degradation.
Böttcher et al. generated knock-in β1 integrin mice containing alanine substitutions at the distal tyrosine or the preceding threonine motif (Fig 1). Both mutations inhibit kindlin binding and, consistent with the vital role of kindlins, resulted in peri-implantation lethality. Like Margadant et al., Böttcher and colleagues found that mutations in the kindlin-binding site induced increased degradation of β1 integrin, in addition to defects in activation and cell adhesion. They also confirmed that β1 surface expression is increased in cells that overexpress kindlin and reduced in cells that lack kindlin, supporting a role for kindlin in regulating integrin surface levels. However, unlike Margadant et al., they found that β1 integrin degradation is not altered in kindlin-deficient cells and instead they present data suggesting a novel role for kindlin in regulating integrin mRNA levels. Reasons for these discrepancies remain unresolved, but the apparent inability of kindlin to explain enhanced degradation of mutant integrins prompted Böttcher et al. to screen for other β tail binding proteins that protect integrins from degradation. Using mass spectrometry screening techniques, they identified SNX17 as a β1 tail-binding protein whose interaction is perturbed by mutations in the threonine motif. They further showed that the SNX17 FERM domain directly binds the kindlin-binding site in β1 integrin and that SNX17 and kindlin can compete for binding to integrin. This was of interest because sorting nexins are endosome-associated proteins and SNX17 has previously been shown to bind the cytoplasmic domains of vesicular cargoes resulting in their recycling to the plasma membrane[12]. Consistent with this, Böttcher et al., showed SNX17 colocalization with β1 integrins in early endosomes, but not in late endosomes or lysosomes, and that knockdown of SNX17 generates phenotypes that recapitulate those of the threonine β1 mutant; defective cell migration, decreased mature integrin, decreased integrin surface levels, and an increased lysosomal degradation rate of integrin. These phenotypes are rescued by re-expressing wild-type SNX17 but not an integrin- binding defective SNX17 mutant, confirming the importance of direct SNX17-integrin interactions in governing integrin recycling.
At the same time, another group independently reached a similar conclusion about the role of SNX17 in integrin recycling[10]. Steinberg et al., found that β1 and β5 integrins were among a panel of membrane proteins lost from the cell surface of SNX17 knockdown fibroblasts. They further showed that this was due to altered recycling but not altered internalization, and resulted in decreased focal adhesion size and increased cell migration, consistent with a shift from α5β1-mediated to αVβ3-mediated adhesions. Like Böttcher et al., they reported that without SNX17 binding the β1 membrane-distal NPxY motif, integrins are lysosomaly degraded. Collectively both papers[7, 8] establish that SNX17, through a FERM-domain meditated interaction with the membrane-distal integrin NPxY motif, regulates integrin recycling.
In summary, while the first β tail NPxY motif is central to integrin activation because of interactions with talin, it now seems that the second FERM domain-binding NPxY motif has more complex and variable functions. Kindlin binding at this site modulates integrin activation but the distal tyrosine mutation does not prevent activation in all settings [8] and in purified systems talin binding is sufficient to trigger activation[13]. However, the membrane-distal NPxY motif is required for binding events that dictate subcellular sorting of β1 integrins and it seems clear that binding of SNX-17, favors integrin recycling back to the plasma membrane from sorting endosomes rather than lysosomal degradation (Fig 1). Although there was no agreement on the activation state of integrins in early endosomes[8–10], if integrin activation influences recycling, kindlin might indirectly impact trafficking my affecting activation. Importantly, both the Steinberg and Böttcher papers report that kindlin and SNX17 are not found in the same subcellular compartments, and a model is proposed where integrin-kindlin interactions modulate activation at the plasma membrane but upon internalization kindlin dissociates and integrin-SNX17 binding on endosomes determines whether integrins are recycled or sent for lysosomal degradation. It is unclear whether direct competition between kindlin and SNX17 for binding to integrins influences this process or whether other factors lead to dissociation of kindlin from endocytosed integrins. FERM domains bind integrins via a PTB-like subdomain and we have shown that a wide range of PTB-domains can bind integrin β tails[14]. Therefore it will be of interest to determine whether other NPxY motif-binding FERM or PTB domain proteins contribute to other steps in integrin recycling and whether competition between various NPxY-binding partners governs integrin internalization and trafficking. How kindlin fits into this picture remains unclear and whether kindlins' effects on cell-surface integrin expression levels are due to sorting[8] or mRNA levels[9] or a combination of the two remains to be resolved. Regardless, the possibility that kindlin exerts some of its integrin-regulatory functions through trafficking must now be considered.
References
- 1.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valdembri D, Sandri C, Santambrogio M, Serini G. Regulation of integrins by conformation and traffic: it takes two to tango. Mol. Biosyst. 2011;7:2539–2546. doi: 10.1039/c1mb05066d. [DOI] [PubMed] [Google Scholar]
- 3.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 4.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouaouina M, Calderwood DA. Kindlins. Curr. Biol. 2011;21:R99–101. doi: 10.1016/j.cub.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 7.Malinin NL, Plow EF, Byzova TV. Kindlins in FERM adhesion. Blood. 2010;115:4011–4017. doi: 10.1182/blood-2009-10-239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margadant C, Kreft M, de Groot DJ, Norman JC, Sonnenberg A. Distinct Roles of Talin and Kindlin in Regulating Integrin alpha5beta1 Function and Trafficking. Curr. Biol. 2012 doi: 10.1016/j.cub.2012.06.060. [DOI] [PubMed] [Google Scholar]
- 9.Bottcher RT, Stremmel C, Meves A, Meyer H, Widmaier M, Tseng HY, Fassler R. Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail. Nat. Cell Biol. 2012;14:584–592. doi: 10.1038/ncb2501. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg F, Heesom KJ, Bass MD, Cullen PJ. SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J. Cell Biol. 2012;197:219–230. doi: 10.1083/jcb.201111121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harburger DS, Bouaouina M, Calderwood DA. Kindlin-1 and -2 directly bind the C- terminal region of β integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 2009;284:11485–11497. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat. Cell Biol. 2012;14:29–37. doi: 10.1038/ncb2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye F, Hu G, Taylor D, Ratnikov B, Bobkov AA, McLean MA, Sligar SG, Taylor KA, Ginsberg MH. Recreation of the terminal events in physiological integrin activation. J. Cell Biol. 2010 doi: 10.1083/jcb.200908045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calderwood DA, Fujioka Y, de Pereda JM, Garcia Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH. Integrin β cytoplasmic domain interactions with phosphotyrosine- binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci U S A. 2003;100:2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]