Abstract
Background
A high incidence of non-traumatic osteonecrosis has been reported in HIV-infected patients. We investigated the levels of D-dimer and C-reactive protein (CRP) in a cohort of HIV-infected adults with and without osteonecrosis of the femoral head.
Methods
43 HIV-infected patients with osteonecrosis of the femoral head and a comparison group of 50 HIV-infected patients with negative MR imaging of the hips and for whom serial plasma samples were available were included. D-dimer and CRP levels were measured prior to and at the time of diagnosis for osteonecrosis patients, at the time of negative MR imaging of the hips for controls, and ≥6 months later for both groups.
Results
Biomarker levels were elevated at the time of diagnosis in the osteonecrosis cohort compared with controls. Median D-dimer value was 0.32 µg/mL in the osteonecrosis group compared to <0.22 µg/mL in the control group (p=0.016). For CRP the corresponding values were 2.52 mg/L and 1.23 mg/L (p=0.003). Post-diagnosis, D-dimer and CRP levels were also elevated in the osteonecrosis patients compared to controls. Linear regression demonstrated a rise in D-dimer levels from pre-diagnosis to diagnosis in the osteonecrosis patients while CRP levels did not change significantly over time.
Conclusions
Compared to controls, patients who developed osteonecrosis had elevated levels of D-dimer and CRP at diagnosis. D-dimer levels increased while CRP levels did not change significantly from pre-diagnosis to diagnosis. These data suggest that patients with higher levels of inflammation are at an increased risk of osteonecrosis.
Keywords: HIV, osteonecrosis, D-dimer, C-reactive protein
Background
A high incidence of non-traumatic osteonecrosis has been reported in HIV-infected patients [1]. Although cases are frequently associated with well-established predisposing factors for osteonecrosis, including corticosteroid use, hyperlipidemia, and alcohol abuse, studies have also identified protease inhibitor use and history of advanced HIV disease as risk factors in this population [2–6]. While the pathogenesis is not well understood, it has been postulated that non-traumatic osteonecrosis develops as a result of intravascular coagulation followed by increased intraosseus venous pressure, reduced arterial flow, and hypoxic bone death. Supporting this, pro-coagulant abnormalities, both inherited and acquired, have been associated with increased risk of osteonecrosis [7, 8]. Our group had previously identified an association between positive anti-cardiolipin antibody and osteonecrosis in HIV-infected adults [6]. No differences were seen in lupus anticoagulant or protein S levels in this cohort compared with HIV-infected controls without osteonecrosis.
In HIV-infected patients, elevated levels of D-dimer, a fibrin degradation product, have recently been associated with increased risk of death and opportunistic infections [9, 10]. Levels of C-reactive protein (CRP), an acute phase protein with both pro-inflammatory and anti-inflammatory effects, are elevated in HIV-infected adults relative to HIV negative controls [11–13]. CRP levels correlate with HIV disease progression independent of CD4+ T-cell count and HIV viral load [14], and predict survival in HIV-infected individuals [13]. In this population, elevated CRP levels have been associated with traditional cardiovascular disease risk factors, including tobacco use and elevated cholesterol, as well as with increased visceral adipose tissue and lipodystrophy [15, 16]. The clinical implications of an elevated CRP and the optimal utilization of CRP levels in the evaluation of HIV infected adults have not yet been established in larger clinical trials, however.
Since coagulation and inflammation are thought to play a role in the development of osteonecrosis, we investigated the levels of D-dimer and CRP in longitudinal samples from a well-characterized cohort of HIV-infected adults with and without osteonecrosis of the femoral head.
Methods
HIV-infected patients with either asymptomatic or symptomatic osteonecrosis of the femoral head enrolled in studies at the NIH Clinical Center and for whom serial plasma samples were available were included in the study. Asymptomatic patients (n=17) were identified on MRI scanning performed as part of a previously reported prospective study [6], and symptomatic patients (n=26) with MRI confirmation were enrolled in a natural history study of osteonecrosis. Fifty HIV-infected patients with no evidence of osteonecrosis on MRI scanning, performed as part of the same study, and for whom serial plasma samples were available, served as the control group. The latter group had previously served as a control group in the analysis of coagulation abnormalities associated with osteonecrosis as noted above. Clinical and laboratory data have been previously published in reports on the prevalence, incidence, and natural history of osteonecrosis in HIV-infected patients [1, 6].
Concentrations of D-dimer in plasma samples frozen at −70°C were measured using the Sta Liatest D-Di immunoturbidimetric assay (Diagnostica Stago; detection limit of 0.22 µg/mL). Levels of CRP were determined by a highly sensitive immunonephelometry test (hsCRP, BN-II, Siemens Healthcare Diagnostics; detection limit of 0.17 mg/L). For the osteonecrosis cohort, samples were selected at the time of osteonecrosis diagnosis (+/− 2 months) and at time-points at least 6 months prior to and at least 6 months after the diagnosis time point. For control participants, samples were selected from the time of negative MRI imaging and 6 months later. Values below the detection limit of the assay were assigned a value of 0.21 µg/mL for D-dimer and of 0.16 mg/L for CRP.
Statistical Analysis
The primary objective was to compare D-dimer and CRP levels between patients with osteonecrosis and controls to look for an association between biomarker levels and osteonecrosis. D-dimer and CRP levels were compared at the time of MRI scan (diagnosis for osteonecrosis or documentation of normal femoral head for controls) between patients with osteonecrosis and controls using the Mann-Whitney U-statistic. To evaluate the relationship between biomarker levels over time and osteonecrosis, a line was fit to each subject’s biomarker values over time. The slopes of each line, which describe the changes over time, were compared between the osteonecrotic patients and controls using a Mann Whitney U-statistic as well as a linear regression model that adjusted for viral load and total CD4 counts.
Results
Stored plasma samples were available for 43 osteonecrosis patients, 17 with asymptomatic and 26 with symptomatic disease. Samples were available from a time-point prior to the diagnosis of osteonecrosis for 26 patients (median - 6 months, range -29 to -6 months), at the time of diagnosis for 35 patients (median 0 months, time of diagnosis, range -2 to 2 months), and after diagnosis for 38 patients (median 7 months, range 6 to 35 months). All 50 control patients had samples available from the time of their negative MRI imaging and 5–7 months later (median 6 months).
The demographic characteristics of the patients included in the study are similar to those of the total cohort published previously [1, 6]. Selected demographic and clinic parameters of the participants at the time of MRI imaging are summarized in Table 1. The osteonecrosis cohort differed from the controls in the known duration of HIV infection (11.7 years vs. 8.8 years, p=0.003) and CD4 T-cell count (465 cells/mm3 vs. 686 cells/mm3, p=0.008). No significant difference was seen in the percentage of patients receiving antiretroviral therapy (84% of the osteonecrosis cohort vs. 92% of the controls, p=0.82) or in the percentage of patients with suppressed viral loads (49% of the osteonecrosis cohort vs. 52% of the controls, p=0.83). Antiretroviral regimens were similar between the two groups. Reflecting our clinic population at the time, 40% of the osteonecrosis patients and 60% of the controls had received prior interleukin-2 therapy (p=0.05). Of note, 19 (38%) of the control patients had received IL-2 in the year preceding their sample time-points, with interval from IL-2 ranging from 30 to 268 days. Six (14%) osteonecrosis patients had received IL-2 proximal to a sample time-point, ranging from 50 to 280 days before. As previously reported, osteonecrosis patients had a higher frequency of anti-cardiolipin IgG antibody levels greater than 23 units (32% vs. 10%, p=0.01).
Table 1.
Demographic and clinical parameters for the osteonecrosis patients and controls.
| Osteonecrosis (n=43) |
Control (n=50) |
P-value | |
|---|---|---|---|
| Age in years, median (range) | 45.4 (20.8–61.8) | 41.4 (28.3–70.0) | 0.06 |
| Male sex, n (%) | 39 (91%) | 43 (86%) | 0.49 |
| Duration of HIV at time of MRI in years, median (range) | 11.7 (1.6–19.5) | 8.8 (0.4–16.4) | 0.003 |
| CD4+ T-cell count nadir, cells/mm3, median (range) | 143 (10–505) | 213 (11–494) | 0.42 |
| CD4+ T-cell count, cells/mm3, median (range) | 465 (12–1117) | 686 (71–1705) | 0.008 |
| CD4+ T-cell percentage, median (range) | 22 (1–51) | 33 (10–57) | 0.02 |
| HIV viral load, copies/mL, median (range) | <50 (<50–242,844) | <50 (<50–306,953) | 0.83 |
| Number with HIV viral load < 50 copies/mL (%) | 21 (49%) | 26 (52%) | 0.83 |
| Receiving combination antiretroviral therapy, n (%) | 36 (84%) | 46 (92%) | 0.82 |
| Nucleoside Reverse Transcriptase Inhibitor, n (%) | 36 (84%) | 46 (92%) | |
| Non-nucleoside Reverse Transcriptase Inhibitor, n (%) | 15 (35%) | 19 (38%) | |
| Protease Inhibitors, n (%) | 27 (63%) | 35 (70%) | |
| Integrase inhibitor (raltegravir), n (%) | 1 (2%) | 0 (0%) | |
| History of Interleukin-2 Therapy, n (%) | 17 (40%) | 30 (60%) | 0.05 |
| Anticardiolipin antibody (ACA) | |||
| Positive ACA IgM or IgG, n (%) | 22/37 (60%) | 21/49 (57%) | 0.08 |
| Positive ACA IgG, n (%) | 20/37 (54%) | 17/49 (34%) | 0.04 |
| ACA >23 Units, n (%) | 12/37 (32%) | 5/49 (10%) | 0.01 |
| Biomarker levels at time of diagnosis (osteonecrosis) or negative MRI (controls) | |||
| D-dimer (µg/mL), median (range) | 0.32 (<0.22–2.08) | <0.22 (<0.22–2.57) | 0.016 |
| CRP (mg/L), median (range) | 2.52 (0.21–42.7) | 1.23 (<0.17–24.4) | 0.003 |
| Biomarker levels at the time of diagnosis in persons with HIV viral load <50 copies/mL at the time of diagnosis (osteonecrosis, n=21) or negative MRI (controls, n=26) | |||
| D-dimer (µg/mL), median (range) | 0.32 (<0.22–1.97) | <0.22 (<0.22–2.57) | 0.09 |
| CRP (mg/L), median (range) | 2.52 (0.43–42.7) | 1.35 (<0.17–5.7) | 0.01 |
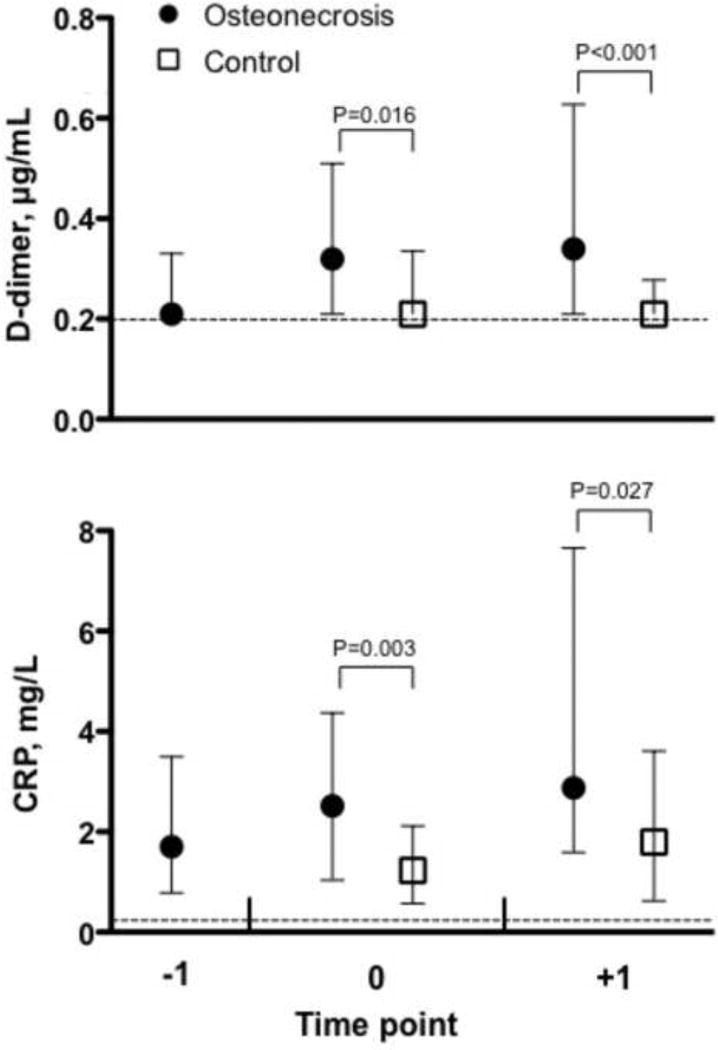
Both D-dimer and CRP levels were significantly elevated at the time of diagnosis in the osteonecrosis cohort compared with controls (Table 1). For D-dimer, the median value was 0.32 µg/mL in the osteonecrosis group compared to <0.22 µg/mL in the control group (p=0.016), and for CRP the corresponding values were 2.52 mg/L and 1.23 mg/L (p=0.003). D-dimer and CRP levels were also significantly elevated in the osteonecrosis patients compared with the controls in the post-diagnosis period (Figure 1). These relationships remained significant when adjustments were made for HIV viral load and anti-cardiolipin antibody status. When examining changes in D-dimer and CRP over time by group, no significant differences were observed. Linear regression using data from the osteonecrosis patients with samples available for at least two time points (n= 36) and all control patients (2 time points each), adjusted for HIV viral load at the first time-point, demonstrated that D-dimer levels rose from the pre-diagnosis to diagnosis for osteonecrosis patients, while remaining stable in the control cohort. CRP levels were stable over time (slopes were not different from zero) for both the osteonecrosis and control cohorts.
Figure 1.
D-dimer (top panel) and CRP (bottom panel) values for HIV-infected patients with osteonecrosis and controls. Medians with interquartile ranges are presented. For osteonecrosis patients, time point −1 represents data from before osteonecrosis diagnosis (6 to 29 months prior to diagnosis, n=26), time point 0 is at diagnosis (+/− 2 months, n=35) and time point +1 is after diagnosis (6 to 35 months after diagnosis, n=38). For control participants, time point 0 represents data from the time of negative MR imaging of the hips and time point +1 is 6 months later (n=50). The dashed line indicates the lower limit of detection for the assays.
No difference was seen in D-dimer or CRP levels between the asymptomatic and symptomatic cohorts (data not shown).
Discussion
This study demonstrates that the occurrence of osteonecrosis in HIV-infected patients is associated with increased levels of two biomarkers that have been associated with poor clinical outcomes in HIV-infected patients. Compared to controls, patients who developed osteonecrosis had elevated levels of D-dimer and CRP at and after diagnosis. In osteonecrosis patients with samples available prior to diagnosis, D-dimer levels increased while CRP remained unchanged over time, whereas no significant change was noted in either biomarker in the control cohort. These data suggest that elevations in D-dimer are associated with the development of osteonecrosis. CRP elevations, however, predate the development of osteonecrosis, suggesting that at-risk patients have persistently higher levels of chronic inflammation, potentially as a result of ongoing immune activation, compared to the control cohort.
HIV-infected patients who develop osteonecrosis often have traditional risk factors associated with the development of osteonecrosis in other populations, but appear to be at increased risk in the context of these risk factors. Given the findings of this study, one potential explanation is that the development of osteonecrosis is multifactorial, and that chronic inflammation, as evidenced by persistently higher levels of CRP, creates an environment in which, for example, use of corticosteroids results in a much greater risk of developing osteonecrosis. The direct role, if any, that chronic inflammation plays in the development of osteonecrosis cannot be determined from this study.
Non-traumatic osteonecrosis of the femoral head is pathologically characterized by venous hypertension and impaired venous circulation and is thought to be caused by intravascular coagulation and microcirculatory thrombosis, all of which result in compromised venous drainage. An association between thrombophilia and/or hypofibrinolysis with osteonecrosis in adults has been reported in numerous studies spanning more than 40 years. In 1961, Nilsson described a case of osteonecrosis of the hip associated with hypofibrinolysis [17]. Subsequently associations were described between PAI-1 levels and osteonecrosis [8, 18]. Depressed fibrinolysis was postulated to result in inadequate lysis of venous thrombi in bone, impaired bone venous circulation, and venous hypertension of bone.
Reports on D-dimer and CRP levels in patients with osteonecrosis are limited. In a recent report evaluating patients with idiopathic or corticosteroid-associated osteonecrosis, D-dimer levels were significantly higher in the latter, and tended to be higher in the former, compared to a similarly aged control group [19]. D-dimer levels were also significantly higher in Gaucher disease patients with osteonecrosis compared to those without osteonecrosis [20]. CRP levels were significantly elevated in a cohort of patients with non-traumatic osteonecrosis compared to age- and sex-matched controls [21]. However, a report of patients with severe acute respiratory syndrome (SARS) who subsequently developed osteonecrosis found that osteonecrosis patients differed from controls in levels of protein C, plasminogen activator inhibitor, plasminogen, and antithrombin III, but not in D-dimer levels [22]. In that study, samples were drawn after the diagnosis of osteonecrosis, and at least 6 months after treatment for SARS. [23]
Osteonecrosis thus appears to be another complication of HIV infection, like cardiovascular disease, that is a consequence of ongoing immune activation and inflammation. Whether immunosuppression contributes to the risk of osteonecrosis is uncertain; while the median CD4 count was lower in the osteonecrosis group, both groups had a relatively high median CD4 count, and the majority of patients in both groups had viral loads of <50 copies/ml. Biomarkers such as D-dimer and CRP can potentially identify HIV-infected patients at increased risk of osteonecrosis and other non-opportunistic complications, who may be good candidates for assessing therapeutic strategies that focus on reducing these non-opportunistic complications.
Acknowledgments
Financial support: This research was supported by the Intramural Research Programs of the NIH Clinical Center and the National Institute of Allergy and Infectious Diseases.
Footnotes
Potential conflicts of interest: all authors, no conflicts of interest
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health or the U.S. Department of Health and Human Services.
References
- 1.Morse CG, Mican JM, Jones EC, Joe GO, Rick ME, Formentini E, et al. The incidence and natural history of osteonecrosis in HIV-infected adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44:739–748. doi: 10.1086/511683. [DOI] [PubMed] [Google Scholar]
- 2.Allison GT, Bostrom MP, Glesby MJ. Osteonecrosis in HIV disease: epidemiology, etiologies, and clinical management. AIDS. 2003;17:1–9. doi: 10.1097/01.aids.0000042940.55529.93. [DOI] [PubMed] [Google Scholar]
- 3.Glesby MJ, Hoover DR, Vaamonde CM. Osteonecrosis in patients infected with human immunodeficiency virus: a case-control study. The Journal of infectious diseases. 2001;184:519–523. doi: 10.1086/322779. [DOI] [PubMed] [Google Scholar]
- 4.Hasse B, Ledergerber B, Egger M, Flepp M, Bachmann S, Bernasconi E, et al. Antiretroviral treatment and osteonecrosis in patients of the Swiss HIV Cohort Study: a nested case-control study. AIDS research and human retroviruses. 2004;20:909–915. doi: 10.1089/aid.2004.20.909. [DOI] [PubMed] [Google Scholar]
- 5.Martin K, Lawson-Ayayi S, Miremont-Salame G, Blaizeau MJ, Balestre E, Lacoste D, et al. Symptomatic bone disorders in HIV-infected patients: incidence in the Aquitaine cohort (1999–2002) HIV medicine. 2004;5:421–426. doi: 10.1111/j.1468-1293.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Masur H, Jones EC, Joe GO, Rick ME, Kelly GG, et al. High prevalence of osteonecrosis of the femoral head in HIV-infected adults. Annals of internal medicine. 2002;137:17–25. doi: 10.7326/0003-4819-137-1-200207020-00008. [DOI] [PubMed] [Google Scholar]
- 7.Glueck CJ, Freiberg RA, Fontaine RN, Tracy T, Wang P. Hypofibrinolysis, thrombophilia, osteonecrosis. Clinical orthopaedics and related research. 2001:19–33. doi: 10.1097/00003086-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Jones LC, Mont MA, Le TB, Petri M, Hungerford DS, Wang P, et al. Procoagulants and osteonecrosis. The Journal of rheumatology. 2003;30:783–791. [PubMed] [Google Scholar]
- 9.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS medicine. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D, et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. The Journal of infectious diseases. 2009;200:973–983. doi: 10.1086/605447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. Journal of acquired immune deficiency syndromes. 2008;48:142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boger MS, Shintani A, Redhage LA, Mitchell V, Haas DW, Morrow JD, et al. Highly sensitive C-reactive protein, body mass index, and serum lipids in HIV-infected persons receiving antiretroviral therapy: a longitudinal study. Journal of acquired immune deficiency syndromes. 2009;52:480–487. doi: 10.1097/qai.0b013e3181b939e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman JG, Goldwasser P, Holman S, DeHovitz J, Minkoff H. C-reactive protein is an independent predictor of mortality in women with HIV-1 infection. Journal of acquired immune deficiency syndromes. 2003;32:210–214. doi: 10.1097/00126334-200302010-00014. [DOI] [PubMed] [Google Scholar]
- 14.Lau B, Sharrett AR, Kingsley LA, Post W, Palella FJ, Visscher B, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Archives of internal medicine. 2006;166:64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 15.Masia M, Bernal E, Padilla S, Graells ML, Jarrin I, Almenar MV, et al. The role of C-reactive protein as a marker for cardiovascular risk associated with antiretroviral therapy in HIV-infected patients. Atherosclerosis. 2007;195:167–171. doi: 10.1016/j.atherosclerosis.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Samaras K, Gan SK, Peake PW, Carr A, Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity. 2009;17:53–59. doi: 10.1038/oby.2008.500. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson IM, Krook H, Sternby NH, Soderberg E, Soderstrom N. Severe thrombotic disease in a young man with bone marrow and skeletal changes and with a high content of an inhibitor in the fibrinolytic system. Acta medica Scandinavica. 1961;169:323–337. doi: 10.1111/j.0954-6820.1961.tb07838.x. [DOI] [PubMed] [Google Scholar]
- 18.Glueck CJ, Glueck HI, Mieczkowski L, Tracy T, Speirs J, Stroop D. Familial high plasminogen activator inhibitor with hypofibrinolysis, a new pathophysiologic cause of osteonecrosis? Thrombosis and haemostasis. 1993;69:460–465. [PubMed] [Google Scholar]
- 19.Cenni E, Fotia C, Rustemi E, Yuasa K, Caltavuturo G, Giunti A, et al. Idiopathic and secondary osteonecrosis of the femoral head show different thrombophilic changes and normal or higher levels of platelet growth factors. Acta orthopaedica. 2011;82:42–49. doi: 10.3109/17453674.2011.555368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shitrit D, Rudensky B, Zimran A, Elstein D. D-dimer assay in Gaucher disease: correlation with severity of bone and lung involvement. American journal of hematology. 2003;73:236–239. doi: 10.1002/ajh.10361. [DOI] [PubMed] [Google Scholar]
- 21.Shuai B, Shen L, Yang YP, Xie J, Shou ZX, Wei B. Low plasma adiponectin as a potential biomarker for osteonecrosis of the femoral head. The Journal of rheumatology. 2010;37:2151–2155. doi: 10.3899/jrheum.100342. [DOI] [PubMed] [Google Scholar]
- 22.Sun W, Li ZR, Shi ZC, Zhang NF, Zhang YC. Changes in coagulation and fibrinolysis of post-SARS osteonecrosis in a Chinese population. Int Orthop. 2006;30:143–146. doi: 10.1007/s00264-005-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter BO, Shen J, Kovacs JA, Davey RT, Rehm C, Lozier J, et al. Interleukin-2 cycling causes transient increases in high-sensitivity C-reactive protein and D-dimer that are not associated with plasma HIV-RNA levels. AIDS. 2009;23:2015–2019. doi: 10.1097/QAD.0b013e32832d72c6. [DOI] [PMC free article] [PubMed] [Google Scholar]