Abstract
We briefly summarize several new stimulation techniques. There are many new methods of human brain stimulation including modification of already known methods and brand-new methods. In this paper, we focus on theta burst stimulation (TBS), repetitive monophasic pulse stimulation, paired- and quadri-pulse stimulation, transcranial alternating current stimulation (tACS), paired associative stimulation, controllable pulse shape TMS (cTMS) and deep-brain TMS. For every method, we summarize the state of the art and discuss issues that remain to be addressed.
Introduction
In this paper, we briefly summarize several new stimulation techniques. There are many new methods of human brain stimulation including modification of already known methods and brand-new methods. In this paper, we focus on theta burst stimulation (TBS), repetitive monophasic pulse stimulation, paired- and quadri-pulse stimulation, transcranial alternating current stimulation (tACS), paired associative stimulation, controllable pulse shape TMS (cTMS) and deep-brain TMS. For every method, we summarize the state of the art and discuss issues that remain to be addressed.
Theta Burst Stimulation (TBS)
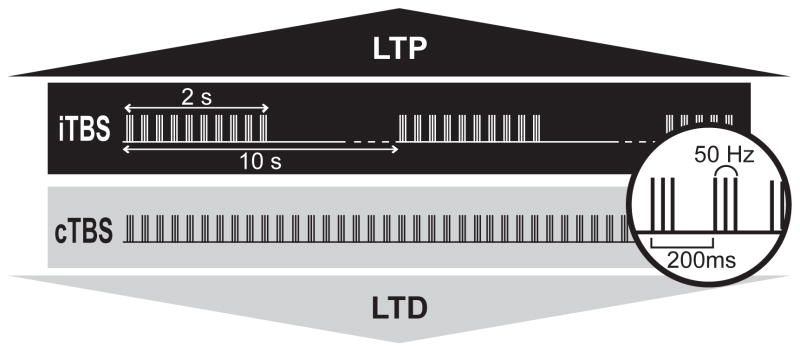
The theta burst pattern of rTMS was developed in 2004 based on the physiological pattern of neuronal firing found in the hippocampus of animal (1). The basic element of TBS contains a three-pulse burst at 50 Hz given every 200 ms (i.e. 5Hz). Using this basic pattern, two major TBS paradigms were developed: continuous theta burst stimulation (cTBS) and intermittent theta burst stimulation (iTBS) (Figure 1). The stimulus intensity required for TBS (80% of active motor threshold) is lower than that for other rTMS protocols. Active motor threshold (AMT) is defined as the minimum intensity of single pulse stimulation required to produce an MEP of greater than 200μV on more than five out of ten trials from the contralateral first dorsal interosseous muscle (FDI) while the subject is maintaining a voluntary contraction of about 20% of maximum in the FDI. With such low intensity, subjects receiving TBS seldom complain of any unpleasant feeling that is seen in the other rTMS stimulation. In addition, in comparison to other protocols, TBS can produce plasticity-like effects with a much shorter conditioning time.
Figure 1.
When bursts are given every 200 ms continuously (cTBS), an LTD-like effect induced. On the contrary, when 2-second trains of TBS are given with 8-second breaks in between (iTBS), an LTP-like effect is induced.
Continuous theta burst stimulation (cTBS)
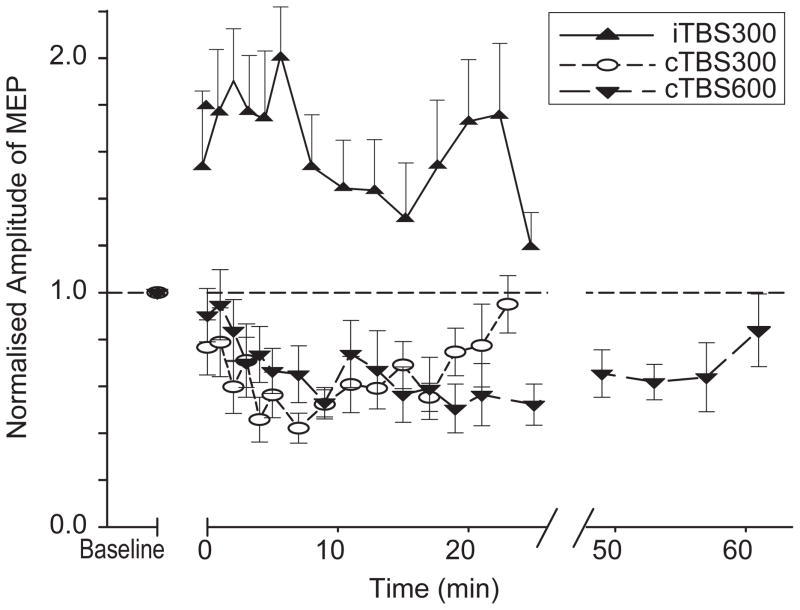
This paradigm was designed to induce a LTD-like effect, and consists of a continuous train of bursts without interruption. cTBS at an intensity of 80% AMT over the primary motor area produces significant inhibition of MEP size lasting for 20 or 60 minutes depending on if the stimulation is given for 20 seconds (300 pulses) or 40 seconds (600 pulses) (Figure 2) (2)(3)(4). cTBS given at a lower intensity (i.e. 60% AMT) produces no significant after effect (2).
Figure 2.
Twenty or 40 seconds of cTBS suppresses the size of MEPs for 20 or 60 minutes, respectively. In contrast, 190 seconds of iTBS enhances MEPs for around 20 minutes.
Intermittent theta burst stimulation (iTBS)
iTBS was developed to induce an LTP-like effect. This protocol consists of a train of ten bursts (lasting 2 seconds) given every 10 seconds for a total of 20 cycles. 190 seconds of iTBS at a stimulus intensity of 80% AMT over the primary motor area facilitates MEPs for about 20 minutes. (Figure 2) (2)(3)(4).
Muscle activity and TBS
Huang and colleagues have demonstrated that tonic contraction of the target muscle during cTBS or iTBS conditioning abolishes almost all of the after-effects of cTBS and iTBS (5). Similar contraction immediately after conditioning for one minute enhances the effect of iTBS, and converts the suppressive effect of cTBS into a facilitation effect (5). Contraction at 10 minutes after cTBS had no long lasting effect (5). These data imply that muscle activity during stimulation causes a serious problem with induction of any after-effect, and therefore should be minimized or best avoided. Likewise, muscle activity should also be avoided for at least a few minutes after the end of cTBS, if one is trying to induce an inhibitory effect. Nevertheless, if a facilitatory effect is desired, such muscle contraction may be beneficial. It is important to note that the stimulus intensity is referenced to the AMT. This implies that before TBS is applied, subjects are required to contract the target muscle for about 3–5 min, the time required to complete the assessment of AMT. Gentner and colleagues (6) have recently suggested that this preactivation might be crucial to produce the excitability depressing effect of cTBS when it is applied over 20 sec. They found that cTBS (20 sec) produced enhancement, instead of depression, of corticospinal excitability, unless it was conditioned by voluntary contraction of sufficient duration (5 min). A similar requirement for isometric contraction was not noted for cTBS of twice the duration (40 sec).
Issues that need to be addressed
TBS is a newly developed technique, and therefore there are several technical parameters (e.g. burst frequency, intervals between bursts, pause duration, stimulus intensity) that might be manipulated to enhance the effect of stimulation. At the present time, iTBS is less efficient than cTBS and the results of iTBS are usually weaker and less consistent compared with cTBS. Further improvement of the iTBS protocol is the focus of ongoing research. On the other hand, it is not uncommon to see a reduction in the amount of facilitation at around 7 and 9 min after iTBS (2, 3). It is not clear whether the dip is caused by a delayed inhibition occurring at around this time or if iTBS induces two phases of facilitation with different mechanisms. Further study of this may help us to understand more about the underlying mechanism of iTBS. Since the after effect of other plasticity induction protocols on conscious humans has never been studied minute by minute as has been done with TBS, it is not clear whether this reduction is unique to iTBS or not. Another issue that deserves further studies is the interaction between TBS and physiological activities.
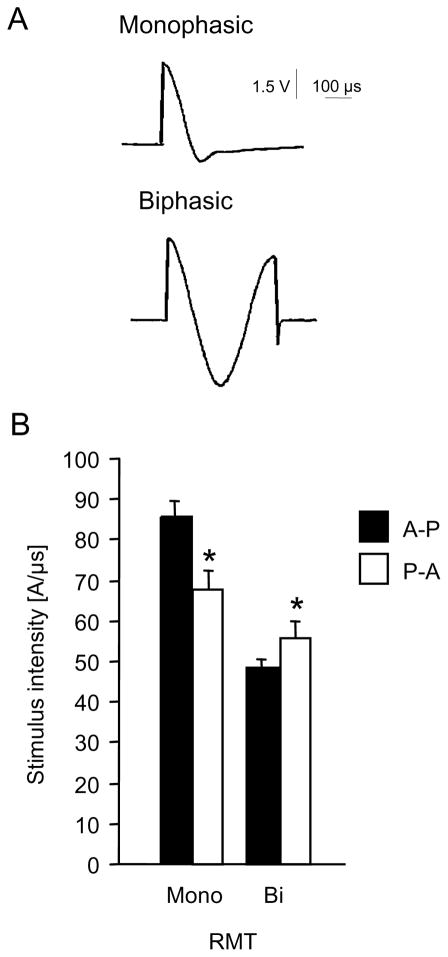
Influence of transcranial magnetic pulse shape and current direction (Figure 3)
Figure 3.
A, current induced in a probe coil of 1 cm diameter by different types of transcranial magnetic stimulators, recorded and stored by an oscilloscope. Upper part, waveform induced by a MagPro stimulator in the “monophasic” mode. Lower part, waveform induced in the “biphasic” mode.
B, motor threshold with the target muscle at rest (RMT) in 12 healthy subjects, mean +/− SE. Biphasic (bi) or monophac (mono) stimuli with an anterior (P-A) or posterior (A-P) initial current direction. Asterisks indicate significant post-hoc differences between current directions for a particular waveform. For all graphs the same Dantec MagPro stimulator and the same MC-B70 coil were used. Modified from Sommer et al. 2002 and 2006.
Since the very first days of transcranial magnetic stimulation (TMS) it was clear that the precentral gyrus is sensitive to the direction of the applied current. Turning the round coil centred at the vertex resulted in a switch of the side of the excited cortex which was much easier to excite and the motor threshold much lower if the current in the brain flowed anteriorly rather than posteriorly (7,8). With the advent of biphasic stimulators, the issue was lost out of sight, because for biphasic pulses the difference between anterior and posterior current orientation is much less pronounced than for monophasic pulses (9–11). Since biphasic pulses differed mainly in the second and third quarter cycles it was therefore suggested that they contribute essentially to the net differences in the effects of stimulation (12–14). Furthermore epidural recordings in vivo demonstrated a different D- and I-wave pattern for biphasic than for monophasic pulses, suggesting that either biphasic pulses stimulate interneurons at a different site than monophasic pulses, or that they activate a different subset of interneurons altogether (15).
The impact of pulse shape and direction on the effect of repetitive TMS (rTMS) is much less clear. For suprathreshold pulses, the MEP facilitation during fast repetitive rTMS (16) is clearly modified by pulse shape and current orientation: Biphasic high frequency stimuli of either direction induce a moderate facilitation; this can be further enhanced by monophasic pulses directed anteriorly in the motor cortex. By contrast, monophasic pulses directed posteriorly induce an inhibition, even seemingly paradoxically at high frequencies (17). For low frequencies the issue has not been studied in detail.
For subthreshold rTMS, low frequency rTMS shows a stronger MEP inhibition after monophasic posteriorly directed than after biphasic pulses of any direction (18, 19). For faster frequencies and for the current direction, the issue is still open.
For theta burst stimulation, biphasic pulses directed posteriorly in the motor cortex appear most promising, although the difference to other pulse types vanishes if the stimulus intensity is adjusted to the respective motor threshold (20).
In the visual cortex, the phosphene threshold is lower with latero-medial than with the opposite current orientation (21), and the scotoma induction is easier with monophasic than with biphasic pulses (22). By contrast, the conditioning pulse of the transcallosally mediated interhemispheric inhibition effect is not sensitive to current direction (23).
Conclusion and open questions
Current direction and pulse configuration differently influence the effectiveness of single-pulse TMS and rTMS. However, a clear physiological model or complete empirical data predicting the effect of all pulse shape parameters and their optimal values for a given application is not currently available. Understanding these relationships is important for both research and clinical uses of TMS. New TMS devices which would allow more flexible control of the pulse parameters could help clarify this issue (see discussion of cTMS below).
Paired pulse I-wave TMS
Repetitive paired-pulse TMS interventions have been described that target short-interval cortical inhibition (SICI) and intra-cortical facilitation (ICF), and these interventions can modulate both of these effects (24)(25)(26). An excitatory repetitive paired-pulse TMS intervention targeting inter-neuronal networks involved in the generation of high-frequency descending volleys known as indirect (I)-waves has also been proposed (27). I-waves have a periodicity of ~1.5ms, and are thought to result from trans-synaptic activation of corticospinal neurons via excitatory cortical interneurones or via recurrent activation (28). Paired TMS at I-wave periodicity can lead to a facilitatory interaction between the second pulse and the I-waves generated by the first (29)(30)(31) which increases the amplitude of the motor evoked potential (MEP) compared to that with paired pulses at non-I-wave intervals. The rationale for designing a TMS intervention around I-wave periodicity is that persistently activating facilitatory I-wave interactions might be a means for increasing the efficacy of trans-synaptic events involved in their generation. It has been argued that this approach might have an analog in spike-timing dependent models of synaptic plasticity (32).
The intervention, originally given the acronym iTMS to indicate its association with I-waves, consists of paired-pulse TMS at an inter-pulse interval (IPI) of 1.5ms. The pulses are of equal intensity and are adjusted so as to give a motor evoked potential of ~0.5–1mV when delivered as a pair (33)(34)(35)(27).
The original description of this technique used a 30-minute protocol, with paired TMS delivered every 5 seconds, and reported a substantial increase in MEP amplitude, but a short-lived aftereffect (27). More recently, a longer aftereffect has been described with a shorter duration of intervention and a lesser degree of MEP facilitation (15 minutes (33)).
Considerations other than the duration of the intervention are the intensity of stimulation, rate of presentation of stimuli, and the choice of IPI. These parameters remain to be systematically investigated. If I-wave networks are up-regulated through associative plasticity mechanisms, too strong a stimulus intensity may saturate the system and limit the effectiveness of the intervention. The low-frequency of rate of stimulation (0.2Hz) makes the intervention comfortable for the subject and allows for the possibility of monitoring excitability changes during the intervention (MEP amplitude to the paired pulse TMS) and of performing motor tasks in association with stimulation (33), however higher frequency rates may turn out to be more effective and time-efficient. The choice of IPI requires further investigation and could in principle be adjusted to match each individual’s I-wave peaks, although errors in the estimation of these peaks may limit the usefulness of this embellishment to the protocol.
Quadripulse stimulation
Here, we introduce a new protocol of repetitive transcranial magnetic stimulation (rTMS), which induces long-lasting plastic changes in the human primary motor cortex. The impetus for developing the new rTMS protocol, quadripulse stimulation (QPS) (37), is similar to that of repetitive paired pulse TMS (27, 37).
Since tetanic stimulation protocols are often used in animal experiments to induce robust LTP and the number of pulses per train in such tetanic stimulation protocol is known to be a very potent factor to influence the level of synaptic plasticity in the hippocampus (36), Hamada et al (37) presume that a greater enhancement of motor cortical excitability can be provoked by increasing the number of short interval pulses per train. In QPS, one train consisted of four monophasic pulses at the same intensity separated by 1.5 ms, repeatedly given at 0.2 Hz, whereas it consisted of paired pulses of equal intensity separated by 1.5 ms, repeatedly given at 0.2 Hz in PPS. It was found that QPS induced long-lasting locally restricted facilitation of motor cortical excitability for up to 75 min without affecting motor thresholds. This facilitation was considered to be a cortical event because responses to brain-stem stimulation were unchanged after QPS. Short-interval intracortical facilitation was enhanced after QPS, whereas short-interval intracortical inhibition was unaffected (38). These findings presumably indicate that QPS mainly enhances I-wave summations at the primary motor cortex and that a greater enhancement of motor cortical excitability can be provoked by increasing the number of pulses per train. QPS seems to be one of the promising methods for inducing plastic changes in the brain. It is unclear, however, if more repetitions and/or higher stimulation frequency would even be more effective.
Several issues to be addressed in the future
This method is at very early stage of development and many issues should be resolved in the future. One point of this method is using monophsic pulses (see Influence of transcranial magnetic pulse shape and current direction). What occurs when using the same protocol with biphasic pulses? The following parameters should be searched in the future. The interpulse interval, intertrain interval, pulse number of one train and so on.
Transcranial alternating current stimulation
The first systematic study to demonstrate interference with brain rhythms as seen in the electroencephalogram (EEG) by non-invasive modulation of motor cortex using weak transcranial alternating current (tACS) through the intact human scalp used electrode sizes and positions similar to those in transcranial direct current stimulation studies (tDCS) (39).
Motor evoked potentials revealed by transcranial magnetic stimulation, EEG-power and reaction times measured in a motor implicit learning task, were analysed in order to detect changes of cortical excitability after 2–7 min of AC stimulation superimposed and without direct current (DC) shift using 1, 10, 15, 30, 45 Hz of stimulation frequency over the primary motor cortex in altogether 48 healthy subjects. A marked decrease of MEP amplitude of up to 20%, and improved implicit motor learning was observed after 10 Hz AC stimulation only. If the anodal or cathodal DC stimulation was combined with 5, 10 and 15 Hz AC stimulation, the MEP amplitudes were increased after anodal 10 and 15 Hz stimulation. No significant changes in any of the analysed frequency bands of EEG after sinusoidal AC or DC stimulation were found. Although transcranial application of weak AC current may appear to be a tool for clinical research in diseases with altered EEG activity, the effects of this study were weak when compared with tDCS effects. This is probably due to the comparatively low amplitude of a maximum of 400 μA. Higher intensities led to a flickering sensation caused by retinal stimulation and was then not applied in this first study due to safety concerns with respect to seizure induction. Just as with rTMS or tDCS in the past future, studies will have to explore the safety limits of this new technique.
Paired associative stimulation
Paired associative stimulation (PAS) consists of low-frequency repetitive peripheral nerve stimulation combined with near-synchronous TMS over the contralateral target cortex. If 60–180 pairs are applied to the motor cortex, this protocol has been shown to induce amplitude changes of MEPs elicited in the resting target muscle. The relative timing of the two stimulus modalities determines the direction of amplitude changes. Shortening the interval between the median nerve stimulation and TMS applied to the optimal position for eliciting MEPs in the abductor pollicis brevis muscle from 25 ms (“PAS25”) to 10 ms (“PAS10”), changed the effect from facilitation to depression of APB MEP size while longer intervals did not induce any lasting excitability changes (40)(41). This observation suggests that PAS-induced plasticity in human motor cortex is governed by strict temporal rules. The dependence on the sequence of induced events resembles spike-timing dependent plasticity of associative LTP, a concept developed in animal studies. To induce reliable effects in the resting motor cortex, suprathreshold TMS-intensities are needed. When subjects perform voluntary contractions during application of the protocol, even subthreshold magnetic stimulation intensities may be sufficient to produce similar effects (42). Shorter inter-pair intervals may also enhance the efficacy of the protocol (43).
At present it remains an open question as to which degree PAS-induced excitability changes are confined to the cortical level (44)(40)(41) or may be accompanied by changes at subcortical locations (45). Within the cortex, upper layers are implicated by a number of studies utilizing different approaches (42)(46)(47); Di Lazzaro, personal communication). PAS-induced excitability changes appear to be rather synapse specific. This is suggested by the fact that they were maximal in the muscle representation receiving homotopical input by afferent stimulation and TMS (48)(49)(50)(51) that SICI remained unchanged after PAS25 (42)(50)(52) and that PAS did not alter the degree to which vibration of muscle bellies influenced the size of MEPs recorded from the same or adjacent muscles (53).
Enhancement of cortical excitability induced by PAS evolved rapidly (within 30 min), and was persistent (at least 30–60 min duration), yet reversible. The duration of effects may be extended to more than 24 h when subjects are under the influence of L-DOPA when treated with PAS (54). Neither of PAS25 or PAS10 led to a significant change of cortical excitability if subjects were pre-medicated with dextromethorphan, a blocker of NMDA receptors (41, 52). Because dextromethrophan blocks NMDA receptors, involvement of these receptors is implicated in PAS-induced plasticity. In addition, PAS10-induced depression of cortical excitability was blocked by pre-mediation with nimodipine, an L-type voltage-gated Ca-channel blocker. The efficacy of PAS to induce plasticity may be modified by several modulating neurotransmitters, such as serotonin, dopamine, norepinephrine and acetylcholine (55). The role of dopamine is also implicated in studies on Parkinsonian patients who show less-than-normal PAS-induced facilitation (56, 57) which can be restored by substitution of the dopaminergic deficit. Modulation of cortical elements by acetylcholine may underlie the dramatic modulation of PAS-induced plasticity by attention (58).
PAS-induced effects are variable between subjects (59). While some of this variability might depend on circadian factors (60), probably a substantial part arises from interindividual genetic variations (Missitzi, Classen et al., in preparation) or variations in the activation history of the PAS-recipient cortex. Training of ballistic (61) or dynamic (53) thumb abductions led to a temporary blockade of PAS-induced plasticity suggesting that prior activity may profoundly influence the efficacy of the PAS-protocol. This finding may also indicate that PAS-induced plasticity probes a functionally relevant cortical plasticity mechanism, one that is possibly related to acquisition of a motor skill and dexterity (62, 63).
Issues for future studies
Some observations suggest that PAS may generate cortical excitability changes with properties surprisingly similar to those of LTP as revealed by invasive animal studies. Future studies should aim to prove or refute the hypothesis of equivalence of mechanisms by studying PAS-protocols in animals. In humans, methodological studies should aim to address the source of variability between subjects and sessions. Likely, from these studies, it will be possible to develop modifications that may lead to robust specific regional excitability changes that may be therapeutically useful.
Controllable pulse shape TMS (cTMS)
CTMS Device Features
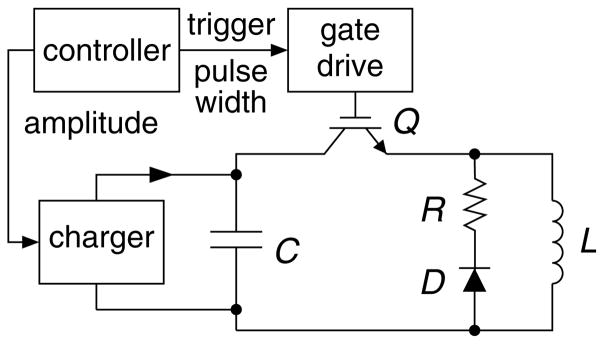
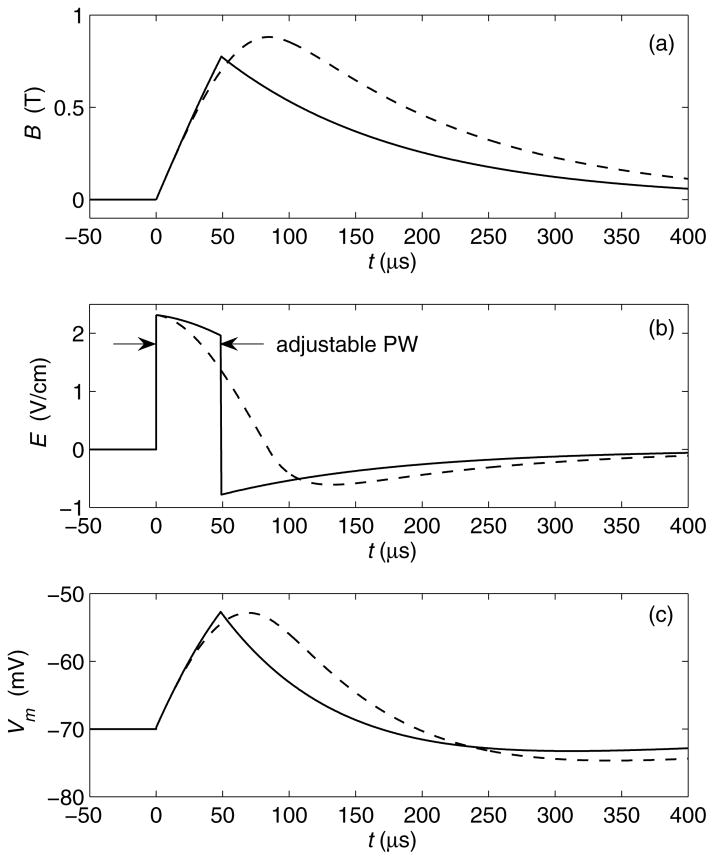
Conventional TMS devices induce cosine electric field pulses with very limited control over the pulse parameters. In contrast, controllable pulse shape TMS devices (cTMS) use a different electronic circuit topology to generate near-rectangular pulses with parameters that are adjustable over a wide continuous range. Fig. 4 shows the circuit topology of a low-frequency, monophasic cTMS device (64). Unlike conventional TMS devices which use thyristors to switch the coil current, this circuit deploys an insulated-gate bipolar transistor (IGBT) switch, Q, which allows the operator to control the pulse width (PW). Further, the energy storage capacitor, C, is larger than that in conventional TMS devices, enabling a wide range of PW adjustment and near-rectangular electric field pulses. Fig. 5 shows pulse waveforms generated by the topology in Fig. 4, in comparison to those of a conventional monophasic TMS device. The cTMS magnetic field rise is more linear, resulting in an approximately constant strength of the initial electric field phase. This near-rectangular pulse produces faster neuronal membrane potential change than the cosine pulse. Consequently, the energy necessary to depolarize a neuron and the coil heating are significantly reduced (64).
Fig. 4.
Circuit topology of low-frequency, monophasic cTMS device generating near-rectangular electric field pulses with adjustable pulse width.
Fig. 5.
Waveform comparison of monophasic cosine TMS (dashed line) and cTMS (solid line, generated by topology in Fig. 1). (a) Magnetic field B; (b) induced electric field E; (c) neuronal membrane potential Vm for membrane time constant of 150 μs.
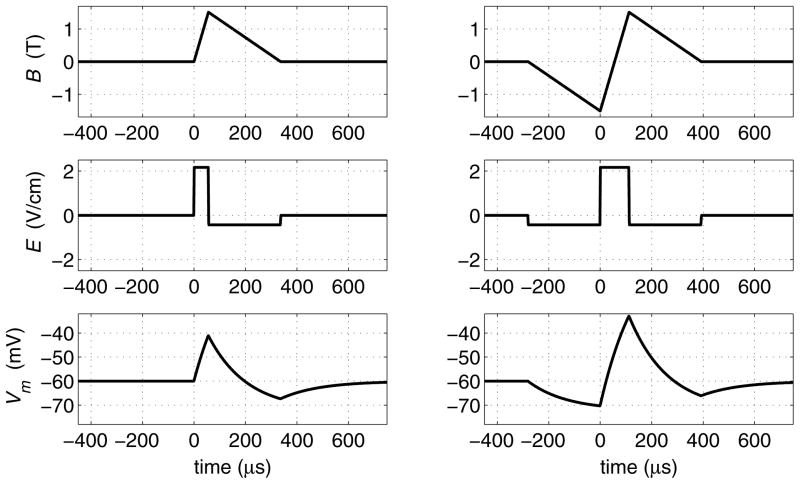
The cTMS topology from Fig. 4 can be enhanced to use an additional switch and energy storage capacitor to recycle the pulse energy and to allow independent control of the amplitudes of the positive and negative electric field phases, in addition to PW control. This enhanced topology can generate trains of both monophasic (Fig. 6, left) and biphasic (Fig. 6, right) magnetic pulses at frequencies up to 200 Hz (depending on the pulse parameter selection).
Figure 6.
Waveforms corresponding to monophasic (left) and biphasic (right) triangular magnetic pulses generated by cTMS device with energy recycling. Same waveform descriptions as in Fig. 5. Energy storage capacitors are assumed to be large.
CTMS Applications
Some examples of how cTMS technology could be used to study the effect of pulse shape parameters and optimize stimulation paradigms are listed below.
Strength-duration curve measurement
The PW adjustment feature of cTMS can be used to empirically derive the strength-duration response curves of neuronal populations (64, 65, 66). The strength-duration curve depends on the biophysical properties of the axonal membrane which can be altered by neurological and psychiatric diseases, or by pharmacology. Thus, strength-duration curve measurement could give insight into the effect of diseases and drugs on neuronal function.
RTMS optimization
The ability of cTMS devices to generate high-frequency trains of a wide range of pulse shapes could allow optimization of the neuromodulatory effect of rTMS. As discussed above, there is some evidence that rTMS with predominantly unipolar electric field pulses, like those induced by conventional monophasic devices, may have a stronger and longer lasting effect on neural excitability than the biphasic pulses generated by conventional rTMS stimulators (22)(18)(19)(17)(67)(68) (see Influence of transcranial magnetic pulse shape and current direction). Unlike existing monophasic machines, energy-recycling cTMS devices could generate long, high-frequency trains of predominantly unipolar pulses, enabling further study of the effectiveness of such stimulation paradigms.
Tolerability enhancement
The selection of pulse parameters may also affect scalp sensation relative to cortical stimulation (69). Thus, cTMS could be used to optimize the stimulus shape to improve the tolerability of TMS.
Coil heating reduction
The reduction in coil heating associated with the use of cTMS near-rectangular pulses (63) could significantly benefit high-frequency, high-power therapeutic applications such as rTMS and magnetic seizure therapy (MST).
TMS of deep brain regions
Conventional rTMS methods are not able to activate deep brain regions effectively because the induced electric field decreases rapidly as a function of the depth of the target structure [70–73]. Direct stimulation of deeper regions is feasible only at the expense of inducing high intensity in superficial cortical regions which might cause epileptic seizures and other undesired effects [74–77].
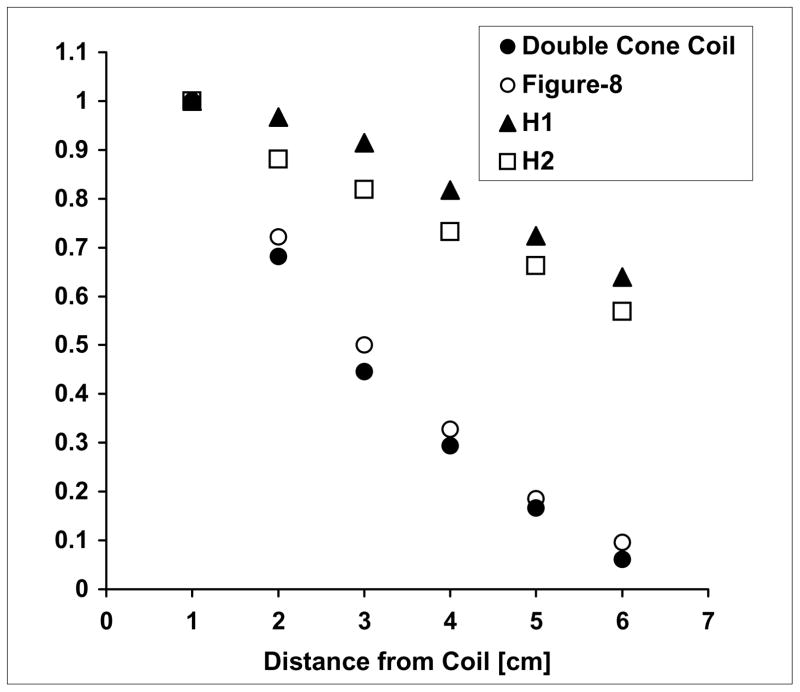
Generally, larger TMS coils have slower decay of the electric field in depth, but they are also less focal. H coils are designed to achieve effective stimulation of deeper neuronal regions by inducing spatial summation of the induced electric field and reducing the electric field attenuation as a function of distance, at the expense of reduced focality. Figure 7 shows the decay rate of the electric field induced by different coils, as a function of distance.
Fig. 7.
Decay of the electric with distance from various TMS coils. Phantom measurements of the electric field induced at each distance is calculated relative to the field induced 1 cm from the coil, in the ‘z’ (superior-inferior) direction. Data are presented for the H1-coil, H2-coil, double cone coil and figure-8 coil.
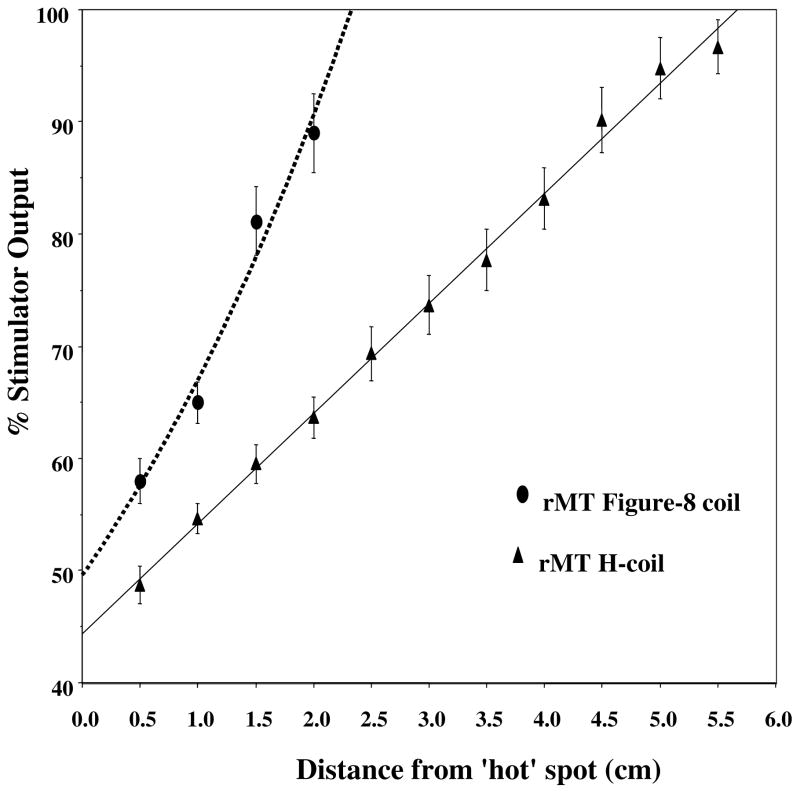
Figure 8 shows the intensity required for inducing APB activation with the standard figure-8 coil and with an H-coil, as a function of the coil distance from the ‘hot spot’ on the scalp (see details in reference 78).
Fig. 8.
Intensity needed for APB stimulation at different heights above the scalp. Resting motor threshold of the APB was measured at different distances above the ‘hot spot’ when using either the H-coil or the figure-8 coil. The % of stimulator power needed to reach the resting motor threshold vs. the distance of the coil from the ‘hot spot’ on the skull is plotted. The points represent means and standard deviations of 6 healthy volunteers.
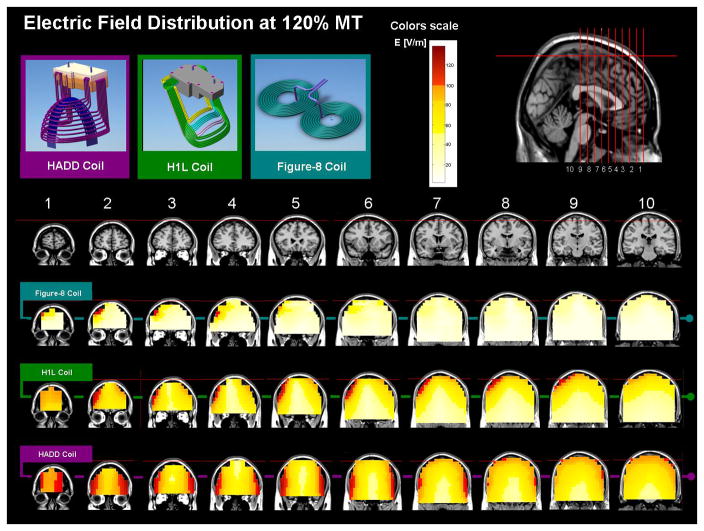
Figure 9 shows a 3D distribution of the electric field induced by a standard figure-8 coil and two H-coil versions when placed over the prefrontal cortex, using intensity of 120% of an average APB motor threshold. The H-coils can activate deeper brain areas and therefore expand the potential feasibility of TMS for research and treatment of various neurological and psychiatric disorders.
Fig. 9.
Maps of the electric field induced by three coils placed over the prefrontal cortex. The maps represent phantom brain measurements of the absolute electric field induced at each pixel. The red colors indicate field magnitude above the threshold for neuronal activation, which was set to 100 V/m. The field maps are adjusted for stimulator output required to obtain 120% of the threshold (120 V/m), at a depth of 1.5 cm. a. Field maps of a standard figure-8 coil. b. Field maps of the H1L coil, which was designed to activate lateral prefrontal regions in the left hemisphere. c. Field maps of the HAAD coil, which was designed to activate deep bilateral prefrontal regions.
Various versions of the deep TMS H-coil have been constructed based on several design principles. The first one is spatial summation of electric impulses. The induced electric field at a target deep brain region is obtained by optimal summation of electric fields induced by several coils or coil elements with common direction placed at different locations around the skull, which may be connected either in series or in parallel. The second principle is optimal orientation of stimulating coil elements. Neuronal activation occurs when the electric field magnitude reaches a certain threshold. This threshold depends on the orientation of the induced field. Physiological studies indicate that optimal activation occurs when the electric field is oriented parallel to the nerve fibre [ 9, 10, 79]. In the H-coils, since several coil elements are placed at different locations around the scalp, it is particularly important to guarantee optimal coordinated orientation of the various elements. The third principle, which is critical for reducing the attenuation of the electric field with distance from the coil, is minimization of non-tangential components. It has been shown [71–73, 85] that coil elements which are non-tangential to the surface induce accumulation of surface charge, which leads to a reduction of both the magnitude and the depth of penetration of the electric field. Hence, coil elements carrying currents non-tangential to the scalp have to be minimized and located far from the target deep brain region. The forth principle of H-coil design is remote placement of the return current paths. Coil elements carrying currents directed oppositely to the preferred direction (the return paths) should be located far from the deep brain target. These elements may be located either adjacent to distant head regions or far from the head, but limited in distance in order to avoid large non-tangential elements as explained above.
The principle of summation can be used temporally as well as spatially. Neuronal activation occurs when the trans-membrane potential at the target is depolarized to a critical level (threshold) [81–82]. This process depends on both the magnitude and the duration of the induced electric field, as demonstrated in the strength-duration curve [86, 65 ]. The synchronized discharges of several stimulators, or a multi-channels stimulator, can produce temporal summation of electric impulses from different spatial locations [87]. In this setup each channel or stimulator is discharged via a different coil at a different location around the scalp. This setup may be used with any TMS coil, but an H-coil design is preferable for activation of deep brain regions, allowing a combination of both spatial and temporal summation. The time delays between the various discharges can be controlled at the scale of microseconds. The use of multiple channels can improve the heating rate and power consumption, as well as the flexibility of controlling the spatial and temporal field distribution.
Some H-coil designs with slow electric field decay rate have been demonstrated to activate deep brain regions using mathematical simulations and phantom brain measurements [84, 86, 87]. The safety of the H-coil used over the prefrontal cortex at low and high frequencies (up to 20Hz) and the initial characterization of cognitive effects induced by such stimulation have been reported [88, 89].
Future issues to be studied
Future studies are required in order to characterize the H-coil design efficacy for various clinical applications. A thorough characterization of the neural response is required, in order to clarify its dependence on various parameters, including the shapes of the electric field pulses, the delay times between pulses and relative current polarity. Preliminary results suggest that the value of the trans-membrane potential threshold for neural activation may depend on the time course of the induced electric field. If these findings are confirmed, this may enable an increase the focality of activation of deep brain regions. Another subject for future studies is the application of paired [27, 35] or quadripulse stimulation [37] with a multi-channel system. Varying the relative amplitudes of the adjacent pulses may alter their effect on neural excitability [90]. This may provide additional means of modulating facilitation and/or suppression in superficial and deep-brain regions.
Contributor Information
Dr Ying-Zu Huang, Department of Neurology, Chang Gung Memorial Hospital and Chang Gung, University College of Medicine, Taipei, Taiwan.
Dr Martin Sommer, Department of Clinical Neurophysiology, University of Goettingen, Robert-Koch-Street 40, D - 37075 Goettingen, Germany, EU.
Dr Gary Thickbroom, Centre for Neuromuscular and Neurological disorders, University of Western Australia, Australia.
Dr Masashi Hamada, Department of Neurology, Graduate School of Medicine, the University of Tokyo, Tokyo, Japan.
Dr Alvaro Pascual-Leone, Berenson-Allen Center for Noninvasive Brain Stimulation, Beth Israel Deaconess Medical Center, Harvard Medical School.
Dr Walter Paulus, Department of Clinical Neurophysiology, University of Goettingen, Germany.
Dr Joseph Classen, Dept. Neurology, University of Wuerzburg, Wuerzburg, Germany.
Dr Angel V. Peterchev, Division of Brain Stimulation and Therapeutic Modulation, Department of Psychiatry, Columbia University, New York, USA.
Dr Abraham Zangen, Department of Neurobiology, Weizmann Institute of Science, Rehovot 76100, Israel.
Dr Yoshikazu Ugawa, Department of Neurology, School of Medicine, Fukushima Medical University, Fukushima, Japan.
References
- 1.Kandel ER, Spencer WA. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- 2.Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007a;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 4.Huang YZ, Rothwell JC. Theta burst stimulation. Transcranial brain stimulation for treatment of psychiatric disorders. In: Marcolin MA, Padberg F, editors. Advances in biological psychiatry. Karger; 2007. pp. 187–203. [Google Scholar]
- 5.Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of Physiological Activity on an NMDA-Dependent Form of Cortical Plasticity in Human. Cereb Cortex. 2007b doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- 6.Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of Human Corticospinal Excitability Induced by Magnetic Theta-burst Stimulation: Evidence of Rapid Polarity-Reversing Metaplasticity. Cerebral Cortex. 2007 doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- 7.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex [letter] Lancet. 1985;1(8437):1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 8.Chiappa KH, Cros D, Cohen D. Magnetic stimulation: Determination of coil current flow direction. Neurology. 1991;41:1154–1155. doi: 10.1212/wnl.41.7.1154. [DOI] [PubMed] [Google Scholar]
- 9.Niehaus L, Meyer BU, Weyh T. Influence of pulse configuration and direction of coil current on excitatory effects of magnetic motor cortex and nerve stimulation. Clin Neurophysiol. 2000;111(1):75–80. doi: 10.1016/s1388-2457(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 10.Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor threshold in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001;112:250–258. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- 11.Sommer M, Alfaro A, Rummel M, et al. Half sine, monophasic and biphasic transcranial magnetic stimulation of the human motor cortex. Clin Neurophysiol. 2006;117:838–844. doi: 10.1016/j.clinph.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Maccabee PJ, Nagaranjan SS, Amassian VE, et al. Influence of pulse sequence, polarity and amplitude on magnetic stimulation of human and porcine periheral nerve. J Physiol. 1998;513:571–585. doi: 10.1111/j.1469-7793.1998.571bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohning DE. Introduction and overview of TMS physics. In: George MS, Belmaker RH, editors. Transcranial magnetic stimulation in neuropsychiatry. Washington, D.C. London, England: American Psychiatric Press, Inc; 2000. pp. 13–44. [Google Scholar]
- 14.Corthout E, Barker AT, Cowey A. Transcranial magnetic stimulation Which part of the current waveform causes the stimulation? Exp Brain Res. 2001;141(1):128–132. doi: 10.1007/s002210100860. [DOI] [PubMed] [Google Scholar]
- 15.Di Lazzaro V, Oliviero A, Pilato F, et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115(2):255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 17.Tings T, Lang N, Tergau F, Paulus W, Sommer M. Orientation-specific fast rTMS maximizes corticospinal inhibition and facilitation. Exp Brain Res. 2005;124:323–333. doi: 10.1007/s00221-005-2253-6. [DOI] [PubMed] [Google Scholar]
- 18.Sommer M, Lang N, Tergau F, Paulus W. Neuronal tissue polarization induced by repetitive transcranial magnetic stimulation? Neuroreport. 2002;13:809–811. doi: 10.1097/00001756-200205070-00015. [DOI] [PubMed] [Google Scholar]
- 19.Arai N, Okabe S, Furubayashi T, Terao Y, Yuasa K, Ugawa Y. Comparison between short train, monophasic and biphasic repetitive transcranial magnetic stimulation (rTMS) of the human motor cortex. Clin Neurophysiol. 2005;116(3):605–613. doi: 10.1016/j.clinph.2004.09.020. Epub 2004 Nov 2005. [DOI] [PubMed] [Google Scholar]
- 20.Zafar N, Paulus W, Sommer M. Comparative assessment of best conventional with best theta burst repetitive transcranial magnetic stimulation protocols on human motor cortex excitability. Clin Neurophysiol. 2008;7:7. doi: 10.1016/j.clinph.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Kammer T, Beck S, Erb M, Grodd W. The influence of current direction on phosphene thresholds evoked by transcranial magnetic stimulation. Clin Neurophysiol. 2001;112(11):2015–2021. doi: 10.1016/s1388-2457(01)00673-3. [DOI] [PubMed] [Google Scholar]
- 22.Antal A, Kincses TZ, Nitsche MA, et al. Pulse configuration dependent effects of repetitive transcranial magnetic stimulation on visual perception. Neuroreport. 2002;13(17):1–5. doi: 10.1097/00001756-200212030-00013. [DOI] [PubMed] [Google Scholar]
- 23.Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89(3):1256–1264. doi: 10.1152/jn.00950.2002. Epub 2002 Oct 1230. [DOI] [PubMed] [Google Scholar]
- 24.Khedr EM, Gilio F, Rothwell J. Effects of low frequency and low intensity repetitive paired pulse stimulation of the primary motor cortex. Clin Neurophysiol. 2004;115:1259–1263. doi: 10.1016/j.clinph.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Sommer M, Kamm T, Tergau F, Ulm G, Paulus W. Repetitive paired-pulse transcranial magnetic stimulation affects corticospinal excitability and finger tapping in Parkinson’s disease. Clin Neurophysiol. 2002;113:944–950. doi: 10.1016/s1388-2457(02)00061-5. [DOI] [PubMed] [Google Scholar]
- 26.Sommer M, Tergau F, Wischer S, Paulus W. Paired-pulse repetitive transcranial magnetic stimulation of the human motor cortex. Exp Brain Res. 2001;139:465–472. doi: 10.1007/s002210100791. [DOI] [PubMed] [Google Scholar]
- 27.Thickbroom GW, Byrnes ML, Edwards DJ, Mastaglia FL. Repetitive paired-pulse TMS at I-wave periodicity markedly increases corticomotor excitability: A new technique for modulating synaptic plasticity. Clinical Neurophysiology. 2006;117(1):61–66. doi: 10.1016/j.clinph.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Ziemann U, Rothwell JC. I waves in motor cortex. Journal of Clinical Neurophysiology. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. Journal of Physiology. 2002;538:253–61. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalography & Clinical Neurophysiology. 1996;101:263–72. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- 31.Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. Journal of Physiology. 1998;511:181–90. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: Experimental framework and human models. Experimental Brain Research. 2007;180(4):583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- 33.Benwell NM, Mastaglia FL, Thickbroom GW. Paired-pulse rTMS at trans-synaptic intervals increases corticomotor excitability and reduces the rate of force loss during a fatiguing exercise of the hand. Experimental Brain Research. 2006;175(4):626–632. doi: 10.1007/s00221-006-0579-3. [DOI] [PubMed] [Google Scholar]
- 34.Di Lazzaro V, Thickbroom GW, Pilato F, Profice P, Dileone M, Mazzone P, Insola A, Ranieri F, Tonali PA, Rothwell JC. Direct demonstration of the effects of repetitive paired-pulse transcranial magnetic stimulation at I-wave periodicity. Clinical Neurophysiology. 2007;118(6):1193–1197. doi: 10.1016/j.clinph.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 35.Hamada M, Hanajima R, Terao Y, Arai N, Furubayashi T, Inomata-Terada S, Yugeta A, Matsumoto H, Shirota Y, Ugawa Y. Origin of facilitation in repetitive, 1.5ms interval, paired pulse transcranial magnetic stimulation (rPPS) of the human motor cortex. Clinical Neurophysiology. 2007a;118(7):1596–601. doi: 10.1016/j.clinph.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Nakao K, Matsuyama K, Matsuki N, Ikegaya Y. Amygdala stimulation modulates hippocampal synaptic plasticity. Proc Natl Acad Sci USA. 2004;101:14270–14275. doi: 10.1073/pnas.0405709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamada M, Hanajima R, Terao Y, et al. Quadro-pulse stimulation is more effective than paired-pulse stimulation for plasticity induction of the human motor cortex. Clinical Neurophysiology. 2007;118(12):2672–2682. doi: 10.1016/j.clinph.2007.09.062. [DOI] [PubMed] [Google Scholar]
- 38.Hamada M, Hanajima R, Terao Y, Furubayashi T, Ugawa Y. Plasticity induction of the human motor cortex by quadro-pulse transcranial magnetic stimulation. Annual Meetings of Society of Neuroscience; San Diego. 2007b. [Google Scholar]
- 39.Antal a, Boros K, Poreisz C, Chaieb L, Termey D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain stimulation. 2008;1:97–105. doi: 10.1016/j.brs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–84. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 41.Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, et al. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–45. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- 42.Kujirai K, Kujirai T, Sinkjaer T, Rothwell JC. Associative plasticity in human motor cortex during voluntary muscle contraction. J Neurophysiol. 2006;96:1337–46. doi: 10.1152/jn.01140.2005. [DOI] [PubMed] [Google Scholar]
- 43.Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant’Angelo A, Girlanda P, et al. Rapid-rate paired associative stimulation of the median nerve and motor cortex can produce long-lasting changes in motor cortical excitability in humans. J Physiol. 2006;575:657–70. doi: 10.1113/jphysiol.2006.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy FD, Norton JA, Gorassini MA. Role of sustained excitability of the leg motor cortex after transcranial magnetic stimulation in associative plasticity. J Neurophysiol. 2007;98:657–67. doi: 10.1152/jn.00197.2007. [DOI] [PubMed] [Google Scholar]
- 45.Meunier S, Russmann H, Simonetta-Moreau M, Hallett M. Changes in spinal excitability after PAS. J Neurophysiol. 2007;97:3131–5. doi: 10.1152/jn.01086.2006. [DOI] [PubMed] [Google Scholar]
- 46.Litvak V, Zeller D, Oostenveld R, Maris E, Cohen A, Schramm A, et al. LTP-like changes induced by paired associative stimulation of the primary somatosensory cortex in humans: source analysis and associated changes in behaviour. Eur J Neurosci. 2007;25:2862–74. doi: 10.1111/j.1460-9568.2007.05531.x. [DOI] [PubMed] [Google Scholar]
- 47.Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, et al. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–52. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, et al. Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain. 2003;126:2586–96. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- 49.Ridding MC, Taylor JL. Mechanisms of motor-evoked potential facilitation following prolonged dual peripheral and central stimulation in humans. J Physiol. 2001;537:623–31. doi: 10.1111/j.1469-7793.2001.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenkranz K, Rothwell JC. Differences between the effects of three plasticity inducing protocols on the organization of the human motor cortex. Eur J Neurosci. 2006;23:822–9. doi: 10.1111/j.1460-9568.2006.04605.x. [DOI] [PubMed] [Google Scholar]
- 51.Weise D, Schramm A, Stefan K, Wolters A, Reiners K, Naumann M, et al. The two sides of associative plasticity in writer’s cramp. Brain. 2006;129:2709–21. doi: 10.1093/brain/awl221. [DOI] [PubMed] [Google Scholar]
- 52.Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, et al. Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex. 2006;16:376–85. doi: 10.1093/cercor/bhi116. [DOI] [PubMed] [Google Scholar]
- 54.Kuo MF, Paulus W, Nitsche MA. Boosting focally-induced brain plasticity by dopamine. Cereb Cortex. 2008;18:648–51. doi: 10.1093/cercor/bhm098. [DOI] [PubMed] [Google Scholar]
- 55.Ziemann U, Meintzschel F, Korchounov A, Ilic TV. Pharmacological modulation of plasticity in the human motor cortex. Neurorehabil Neural Repair. 2006;20:243–51. doi: 10.1177/1545968306287154. [DOI] [PubMed] [Google Scholar]
- 56.Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain. 2006;129:1059–69. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- 57.Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, Ikeda A, et al. Altered plasticity of the human motor cortex in Parkinson’s disease. Ann Neurol. 2006;59:60–71. doi: 10.1002/ana.20692. [DOI] [PubMed] [Google Scholar]
- 58.Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- 59.Fratello F, Veniero D, Curcio G, Ferrara M, Marzano C, Moroni F, et al. Modulation of corticospinal excitability by paired associative stimulation: reproducibility of effects and intraindividual reliability. Clin Neurophysiol. 2006;117:2667–74. doi: 10.1016/j.clinph.2006.07.315. [DOI] [PubMed] [Google Scholar]
- 60.Sale MV, Ridding MC, Nordstrom MA. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res. 2007;181:615–26. doi: 10.1007/s00221-007-0960-x. [DOI] [PubMed] [Google Scholar]
- 61.Ziemann U, Ilic TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–72. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenkranz K, Kacar A, Rothwell JC. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci. 2007a;27:12058–66. doi: 10.1523/JNEUROSCI.2663-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenkranz K, Williamon A, Rothwell JC. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci. 2007b;27:5200–6. doi: 10.1523/JNEUROSCI.0836-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peterchev AV, Jalinous R, Lisanby SH. A transcranial magnetic stimulator inducing near-rectangular pulses with controllable pulse width (cTMS) IEEE Transactions on Biomedical Engineering. 2008;55:257–266. doi: 10.1109/TBME.2007.900540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barker AT, Garnham CW, Freeston IL. Magnetic nerve stimulation: the effect of waveform on efficiency, determination of neural membrane time constants and the measurement of stimulator output. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:227–237. [PubMed] [Google Scholar]
- 66.Panizza M, Nilsson J, Roth BJ, Basser PJ, Hallett M. Relevance of stimulus duration for activation of motor and sensory fibers: implications for the study of H-reflexes and magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1992;85:22–29. doi: 10.1016/0168-5597(92)90097-u. [DOI] [PubMed] [Google Scholar]
- 67.Arai N, Okabe S, Furubayashi T, Mochizuki H, Iwata NK, Hanajima R, Terao Y, Ugawa Y. Differences in after-effect between monophasic and biphasic high-frequency rTMS of the human motor cortex. Clinical Neurophysiology. 2007;118:2227–2233. doi: 10.1016/j.clinph.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Taylor JL, Loo CK. Stimulus waveform influences the efficacy of repetitive transcranial magnetic stimulation. Journal of Affective Disorders. 2007;97:271–276. doi: 10.1016/j.jad.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 69.Geddes LA. Optimal stimulus duration for extracranial cortical stimulation. Neurosurgery. 1987;20:94–99. doi: 10.1097/00006123-198701000-00023. [DOI] [PubMed] [Google Scholar]
- 70.Maccabee PJ, Eberle L, Amassian VE, Cracco RQ, Rudell A, Jayachandra M. Spatial distribution of the electric field induced in volume by round and figure ‘8’ magnetic coils: relevance to activation of sensory nerve fibers. Electroencephalogr Clin Neurophysiol. 1990;76:131–141. doi: 10.1016/0013-4694(90)90211-2. [DOI] [PubMed] [Google Scholar]
- 71.Tofts PS. The distribution of induced currents in magnetic stimulation of the brain. Phys Med Biol. 1990;35:1119–1128. doi: 10.1088/0031-9155/35/8/008. [DOI] [PubMed] [Google Scholar]
- 72.Tofts PS, Branston NM. The measurement of electric field, and the influence of surface charge, in magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:238–239. doi: 10.1016/0168-5597(91)90077-b. [DOI] [PubMed] [Google Scholar]
- 73.Eaton H. Electric field induced in a spherical volume conductor from arbitrary coils: application to magnetic stimulation and MEG. Med Biol Eng Comput. 1992;30:433–440. doi: 10.1007/BF02446182. [DOI] [PubMed] [Google Scholar]
- 74.Stokic DS, McKay WB, Scott L, Sherwood AM, Dimitrijevic MR. Intracortical inhibition of lower limb motor-evoked potentials after paired transcranial magnetic stimulation. Exp Brain Res. 1997;117:437–443. doi: 10.1007/s002210050238. [DOI] [PubMed] [Google Scholar]
- 75.Terao Y, Ugawa Y, Sakai K, Uesaka Y, Kanazawa I. Transcranial magnetic stimulation of the leg area of the motor cortex in humans. Acta Neurol Scand. 1994;89:378–383. doi: 10.1111/j.1600-0404.1994.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 76.Terao Y, Ugawa Y, Hanajima R, Machii K, Furubayashi T, Mochizuki H, Enomoto H, Shiio Y, Uesugi H, Iwata NK, Kanazawa I. Predominant activation of I1-waves from the leg motor area by transcranial magnetic stimulation. Brain Res. 2000;859:137–146. doi: 10.1016/s0006-8993(00)01975-2. [DOI] [PubMed] [Google Scholar]
- 77.Nadeem M, Thorlin T, Gandhi OP, Persson M. Computation of electric and magnetic stimulation in human head using the 3-D impedance method. IEEE Trans Biomed Eng. 2003;50:900–7. doi: 10.1109/TBME.2003.813548. [DOI] [PubMed] [Google Scholar]
- 78.Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol. 2005;116:775–779. doi: 10.1016/j.clinph.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 79.Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neuroph ysiol. 1992;9:132–136. [PubMed] [Google Scholar]
- 80.Durand D, Ferguson AS, Dalbasti T. Induced electric fields by magnetic stimulation in nonhomogeneous conducting media. IEEE Eng Med Biol Soc 11th Annu Int Conf; Seattle. 1989. pp. 1252–1253. [Google Scholar]
- 81.Roth BJ, Basser PJ. A model of the stimulation of a nerve fiber by electromagnetic radiation. IEEE Trans Biomed Eng. 1990;37:588–597. doi: 10.1109/10.55662. [DOI] [PubMed] [Google Scholar]
- 82.Basser PJ, Roth BJ. Stimulation of a myelinated nerve axon by electromagnetic induction. Med Biol Eng Comput. 1991;29:261–268. doi: 10.1007/BF02446708. [DOI] [PubMed] [Google Scholar]
- 83.Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-t. [DOI] [PubMed] [Google Scholar]
- 84.Pascual-Leone A, Cohen LG, Brasil-Neto JP, Hallett M. Non-invasive differentiation of motor cortical representation of hand muscles by mapping of optimal current directions. Electroencephalogr Clin Neurophysiol. 1994;93:42–48. doi: 10.1016/0168-5597(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 85.Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol. 2002;19:361–370. doi: 10.1097/00004691-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 86.Bourland JD, Nyenhuis JA, Noe WA, Schaefer JD, Foster KS, Geddes LA. Motor and sensory strength-duration curves for MRI gradient fields. Proc Int Soc Magn Reson Med 4th Sci Meet Exhibit; New York. 1996. p. 1724. [Google Scholar]
- 87.Roth Y, Padberg F, Zangen A. Transcranial Magnetic Stimulation of Deep Brain Regions: Principles and Methods. Transcranial Brain Stimulation for Treatment in Mental Disorders. In: Marcolin MA, Padberg F, editors. Adv Biol Psychiatr. Vol. 23. Basel: Karger; 2007. pp. 204–225. [Google Scholar]
- 88.Roth Y, Amir A, Levkovitz Y, Zangen A. Three-Dimensional Distribution of the Electric Field Induced in the Brain by Transcranial Magnetic Stimulation Using Figure-8 and Deep H-Coils. J Clin Neurophysiol. 2007;24:31–38. doi: 10.1097/WNP.0b013e31802fa393. [DOI] [PubMed] [Google Scholar]
- 89.Levkovitz Y, Roth Y, Harel EV, Braw Y, Sheer A, Zangen A. A randomized controlled feasibility and safety study of deep transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:2730–2744. doi: 10.1016/j.clinph.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 90.Koch G, Franca M, Mochizuki H, Marconi B, Caltagirone C, Rothwell JC. Interactions between pairs of transcranial magnetic stimuli over the human left dorsal premotor cortex differ from those seen in primary motor cortex. J Physiol. 2007;578:551–562. doi: 10.1113/jphysiol.2006.123562. [DOI] [PMC free article] [PubMed] [Google Scholar]