Abstract
Learning about potential threats is critical for survival. Learned fear responses are acquired either through direct experiences or indirectly through social transmission. Social fear learning (SFL), also known as vicarious fear learning, is a paradigm successfully used for studying the transmission of threat information between individuals. Animal and human studies have begun to elucidate the behavioral, neural and molecular mechanisms of SFL. Recent research suggests that social learning mechanisms underlie a wide range of adaptive and maladaptive phenomena, from supporting flexible avoidance in dynamic environments to intergenerational transmission of trauma and anxiety disorders. This review discusses recent advances in SFL studies and their implications for basic, social and clinical sciences.
Keywords: vicarious fear learning, observational fear learning, fear learning by proxy, fear contagion, emotional contagion, intergenerational transmission, empathy
Social regulation of fear
In the spring of 2015 a photograph of a four-year-old Syrian refugee girl went viral on social media. In response to a photojournalist pointing at her a telephoto lens of his camera, the girl raised her hands up as if surrendering at gunpoint. The photo captured the terror of war in her expression: in order to survive, the little child had to learn quickly from others about life-threatening dangers and how to behave when facing them.
Much progress has been made in recent decades in understanding the behavioral, neural and molecular mechanisms of fear and anxiety [1–3]. Whereas fear occurs in the presence of an immediate or imminent threat, anxiety is defined as an anticipatory state driven by a probable or remote and uncertain threat [1]. Most of what we know about the neurobiology of fear and anxiety, whether innate or acquired, comes from research studying threat or defense responses in isolation from their social context. In a typical experimental setting, threat responses that are conserved across species, and which constitute a part of the human experience of fear, are triggered through exposure to a harmful stimulus, such as an electric shock. Eliciting defense responses through pain or threat of pain has allowed researchers to study innate and learned fear responses, as well as their underlying brain correlates. In fear conditioning (FC), the most commonly used form of fear learning, pairing a naturally aversive event (the unconditioned stimulus, US) with a neutral stimulus (conditioned stimulus, CS) endows the CS with an ability to trigger threat responses. Thus, the FC paradigm enables the study of the formation and maintenance of directly learned aversions.
In social species, however, fear is often acquired indirectly through social transmission. Ample data from human and animal studies provide evidence that social cues modulate learning and extinction of learned fear [4–8]. Studies show that exposure to social cues signaling threat, such as the sight, sound or smell of a scared conspecific may trigger or potentiate fear responses [9–12], which is termed fear contagion [13, 14]. Signaling of fear by a conspecific, when paired with a CS, may serve as a US and reinforce the establishment of threat responses to this CS, a phenomenon referred to as vicarious fear learning, vicarious aversive conditioning, fear learning by-proxy, observational fear learning or social fear learning (SFL) [4]. Social cues can also signal safety. Indeed, the physical presence of a familiar conspecific may attenuate fear responses and impair fear learning in an individual that is subjected to FC, a phenomenon known as social buffering of fear [5]. The presence of a conspecific has also been shown to protect against the acquisition, and to augment the extinction, of conditioned fear through processes called immunization [15, 16] and vicarious fear extinction [8, 17], respectively. The social context thus plays a powerful role in regulating fear. Social factors have also modulatory effects on anxiety. Moreover, fear and anxiety may be transmitted in similar contexts [9, 18]. When citing studies reporting possible transmission of both fear and anxiety, or anxiety alone, we will refer to these findings as social fear/anxiety learning.
The goal of this review is to present a comprehensive framework for understanding the neurobiology of social fear learning across taxa. We will begin by selectively reviewing SFL paradigms, and discussing how this form of learning depends on the integration of systems of fear learning and social cognition. Then, the known neural mechanisms of SFL are reviewed, followed by a discussion about sex differences and developmental aspects of SFL. Finally, we will discuss SFL mechanisms in dysfunctional (clinical) fear and anxiety, and end on a call for future research.
How is social fear learning studied?
The variety of experimental models used to study SFL reflects the diversity of ways that information is transmitted between individuals in every-day life of human and non-human animals. Following Rachman’s [19] suggestion of three principle means of fear acquisition (directly through conditioning or indirectly through vicarious exposure or instruction), current research on SFL can be broadly categorized as either instructed or observational (or ‘vicarious’). In the instructed SFL paradigms, the participant is usually directly informed about the CS-US contingencies through verbal instructions. Some variants of instructed paradigms, often used in studies with children, provide instructions indirectly, in a form of a short story or a brief description of a potential threat [20]. The major advantages of instructed paradigms are their ecological validity (mimicking every-day life situations), easiness to manipulate in the experimental setting, and the opportunity to study complex emotions [21, 22]. The major disadvantage of the instructed approach is that it can be only used in human subjects, thus limiting comparisons across species. Instructed SFL paradigms may be solely applied or in conjunction with observational SFL.
In observational fear learning, which is the main focus of this article, the subject learns CS-US contingencies through the pairing of a CS with conspecific’s threat response, serving as the US [4]. Depending on the sensory modality of fear transmission, SFL can be classified as: 1) visual SFL, which relies on the images of the frightened conspecific [23–26]; 2) auditory SFL, that uses fear vocalizations of the conspecific [10]; and 3) olfactory SFL that uses the odor of the frightened conspecific [27]. In most experimental observational SFL protocols in non-human animals, all modalities are used in conjunction as the demonstrator is directly exposed to the observer [28, 29]. Some studies use a second-order observational SFL procedure [30], which is analogous to second-order classical FC [31]. Second-order SFL begins with standard observational fear learning procedure in which a neutral CS is paired with the demonstrator’s expression of fear, and then in the subsequent training session, this CS (now called the first-order CS or CS1) is paired with a novel cue (second-order CS or CS2) [30]. The most commonly used measures of learning acquired through observational SFL are changes in autonomic activity, such as skin conductance responses, SCR, which are tested mostly in human studies [23, 25, 32]; behavioral threat responses, such as immobility or freezing, which are used in animal research [26, 33]; and avoidance behavior tested both in humans and animals [27, 34–37]. The major advantages of the observational fear learning is that it can be used in pre- and non-linguistic organisms, can be easily controlled experimentally, and allows for cross-species comparisons (for an example of an observational fear learning procedure commonly used in human studies see Fig. 1; for behavioral paradigms used in rodent SFL studies see Fig. 2).
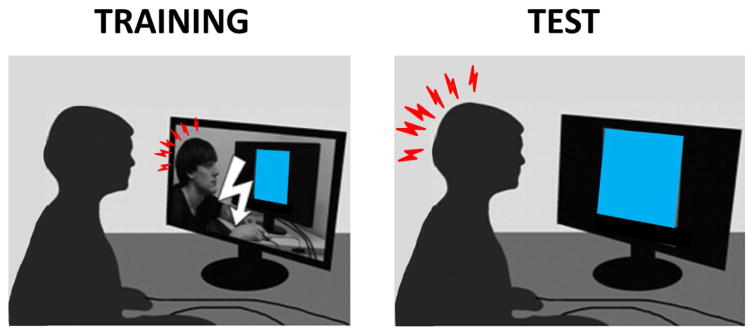
Figure 1. Observational fear learning procedure used in human studies.
During the training phase (left), an observer learns to fear a CS (blue rectangle) through watching on a screen a demonstrator receiving FC (electric shocks to the wrist paired with the CS). During the testing phase (right), the observer expresses fear during an exposure to the CS. (A white lightning bolt denotes an electric shock and red lightning bolts denote fear response).
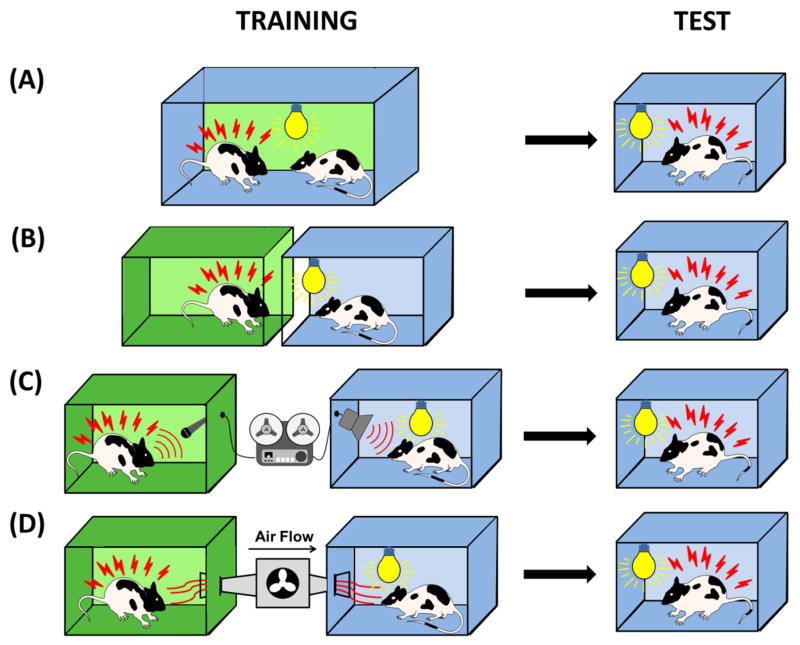
Figure 2. Behavioral paradigms used in SFL studies in rodents.
This figure depicts SFL behavioral paradigms used in rodent studies, with the left column showing the training and the right column showing the testing phase. In the most commonly used paradigm, during the training phase the observer (right) is placed together with a frightened demonstrator (left) and exposed to the CS (a shining light bulb for all experiments in our figure, although, various sensory modalities may be used) (A). In one variant of the SFL paradigm, the observer (right) and the demonstrator are physically isolated during training but are able to maintain a visual contact (B). In another variant of the SFL paradigm, the observer (right) is exposed to the prerecorded (or streamed life) vocalizations produced by the frightened demonstrator (C). In SFL studies relying on chemosignaling, the observer (right) and demonstrator (left) are physically isolated and the observer is exposed to the air delivered from the box with the frightened demonstrator (D).
Social cognition and SFL
There are many similarities between SFL and classical FC. However, in contrast to FC which is reinforced by direct physical harm, SFL relies solely on the transmission of social information, and should therefore be dependent on the perception and processing of this form of information. Animal data show that SFL is conditional upon an undisrupted social development and that social deprivation in juvenile period affects social transfer of fear in adulthood. For example, mice reared in social isolation displayed impaired observational fear learning as compared to mice reared in social pairs [38, 39], despite showing equivalent levels of classical FC [38]. A key question for research on SFL is therefore to describe the ways learning and social cognitive processes are integrated. Below we will discuss evidence from animal and human studies showing how SFL depends on the processing of social information.
Relatedness, similarity and familiarity
Animals are selective with regards to whom they learn from, and the result of SFL depends on both the characteristics of an individual animal and the situation that it is in. A growing body of research across animal species points at the importance of the specific relationship between individuals in social fear transmission. For example, although animals, from rodents to primates, can observationally acquire fears from unrelated conspecifics [4, 40], the strength of learning is enhanced by relatedness [28, 41, 42] and familiarity [43]. This suggests that threat cues emitted by a related, familiar individual are more salient, require reduced attentional resources to discriminate the identity of the demonstrator and/or are better recognized [42]. Similarly, in humans, learning through observing a conspecific’s distress is biased by group belonging. For example, fear information is more efficiently transmitted between individuals belonging to the same racial/ethnic (in-group) as compared to a different (out-group) [44]. A separate, but related, line of research examining the transmission of pain information in clinical settings reports enhancing effects of demonstrator-observer similarity [45]. This pattern of findings suggests that relatedness, similarity and familiarity increase empathetic response to a demonstrator’s distress, whereas being “out-group” decreases it. Relatedness and similarity also enhance the general or cue specific anxiolytic effects of the presence of another individual across animals [5, 44]. Interestingly, also in the appetitive learning domain, demonstrator-observer similarity augments vicarious responses in both monkeys [46] and humans [47].
Social dominance and attributed skill
Social hierarchy status and knowledge about the demonstrator have been identified by research as variables strongly influencing how animals use the information gleaned through observation [42, 48]. For example, primates are more likely to imitate behaviors modeled by a dominant and knowledgeable group member [46, 48]. SFL studies in rodents investigating the role of social hierarchy status found that group rank predicts vicarious learning of fear [42, 49]. In general, subordinate group members display better SFL than dominant animals [42, 49]. A human analog was reported by Selbing and colleagues [50] who found that a demonstrator described as skilled (versus unskilled) in avoiding aversive outcomes, facilitated avoidance learning in naïve observers. In humans, knowledge about the relative level of dominance of other individuals can be quickly acquired through observing the outcome of their dyadic confrontations. These observational learning experiences are then affecting subsequent learning from personal (FC) experiences about the same individuals whose images are used as CSs paired with the electric shock, so that conditioned threat responses are stronger to dominant versus submissive individuals [51].
Some data suggest that the effects of relatedness, similarity, familiarity and social hierarchy status on SFL can be cumulative [42]. The influence of these social biases on SFL might be an adaptive advantage that enables the acquisition of locally relevant knowledge about potential threats [52].
Emotional sharing and empathy
Apart from the cross-species learning advantage conferred by kinship, similarity, familiarity and social hierarchy status, emotional sharing and empathy social-emotional processes that have been shown to impact the transmission of emotional information. Based on studies in rodents showing that exposure to a frightened conspecific triggers an observer’s distress and fear, an argument has been made for the involvement of affective sharing/empathy as a critical factor in the transmission of fear information and the ensuing learning [41, 53]. This is consistent with a recent experiment in humans showing that a simple instruction manipulation that has been previously validated to enhance empathy with a target (encouraging the observer to pay close attention to a demonstrator’s discomfort during the application of “painful” electric shocks) augmented fear learning acquired through observing a demonstrator receiving electric shocks [54]. Research on empathy and pro-social behaviors in humans suggests that individuals expressing high levels of empathy learn pro-social behaviors faster [55]. It is plausible that the same relationship applies to SFL. In support of this, rodent studies reported that inbred gregarious mice strains better acquire SFL [40, 53]. Furthermore, similar to research on SFL, studies on empathy show that it is intimately linked to perceived similarity and relatedness [56]. So far, research on SFL has not differentiated the contribution of similarity from that of emotional sharing and empathy.
It is clear from the surveyed studies that social information shapes SFL. Yet, research has only begun to describe the specific impact of various social factors on SFL. Moreover, little if any research has addressed the more complex questions of how different social factors interactively affect SFL. In addition, it remains to be investigated how information intrinsic to the individual transmitting the information and specific situational/environmental demands interact. New knowledge about the mechanism underlying the influence of social cognition on SFL comes from studies on how social information interacts with threat processing. Below, we highlight a few important lines of research related to these questions and sketch a working neural model of SFL in human and non-humans.
Neural systems of SFL
Human and animal studies have consistently reported similarities between FC and SFL in terms of behavioral and neural processes [4]. Although, a number of brain structures are involved in FC, it is well-established that the acquisition, storage and expression of conditioned fear depend on the amygdala [1–3]. The lateral nucleus of the amygdala (LA) is a major site where inputs conveying information about the CS and US converge and synaptic plasticity underlying FC occurs [57]. Both human and animal SFL studies report that the acquisition and expression of SFL is associated with an increased amygdala activity [24, 25, 27, 29, 41; 58]. Rodent research demonstrates that pharmacological inactivation of the LA prevents acquisition of SFL [27, 41] indicating that a functional amygdala is necessary for SFL as it is for classical FC.
The converging evidence of behavioral and neural principles for FC and SFL highlights a core aversive learning network in the brain centered on the amygdala in interaction with regions of the anterior cingulate cortex (ACC). In FC, the ACC plays a role in controlling responses to threat and modulating fear learning [59]. Human and animal research shows an increased activity in the ACC during SFL [24, 49, 60, 61]. Recent studies in rodents found that pharmacological inactivation of the ACC or optogenetic inhibition of the ACC-amygdala projections prevented observational fear learning and left FC intact [41, 49, 62]. This suggests a critical role of the ACC in SFL but not in FC. However, studies in infant rats show that SFL may occur early in life before the ACC is mature and fully functional [27, 33]. This pattern of findings suggests that when the ACC is functional, it may be necessary for SFL. The ACC is a part of the affective pain processing system and receives projections from various sites, including the midline and intralaminar thalamic nuclei (MITN) which are part of the medial pain system [63–65]. Human and animal studies show that witnessing a conspecific that is distressed or in pain activates brain regions overlapping with the observer’s pain processing areas [33, 66, 67]. Rodent research shows that the acquisition of SFL is associated with an increased MITN activity [33] and pharmacological inactivation of the MITN prevents social transfer of fear [41]. Human and animal studies also suggest the role of other pain processing sites in SFL. For example, regions implicated by previous research in both nociceptive and empathic pain, such as the anterior insula (AI) or periaqueductal gray (PAG) [24, 33, 68]. Moreover, blocking the endogenous opioid system, which is known to relieve self-experienced pain, enhances observational learning through changes in activity within the amygdala, midline thalamus and the PAG [69].
There is considerable overlap of brain regions involved in FC and SFL, yet, the flow of information between these, and additional regions, differs. For example, a recent study examining both FC and observational fear learning in the same individuals, showed stronger connectivity between regions related to pain, such as the AI, and social cognition, such as the temporal parietal junction (TPJ), during observational learning as compared to FC [68]. Although the evidence supporting the involvement of the affective pain processing system in SFL is accumulating, it is still to be determined whether the information conveyed to the amygdala by the affective pain pathways reinforces SFL in a manner similar to the way the aversive US reinforces classical FC. Existing research shows that the information about social cues signaling threat may reach the amygdala through different sensory modalities and distinct pathways reflecting various modes of social fear transmission. For example, in rodents, SFL through 22 kHz stress vocalizations involves the auditory pathway and lesions or pharmacological inactivation of the medial geniculate nucleus disrupts social transmission of fear [10]. Similarly, SFL through the odor of a frightened conspecific engages olfactory and alarm pheromone processing pathways and the disruption of these pathways impairs SFL [27]. A recently published study in rats showed the critical role of the medial nucleus of the amygdala (Me), an established site underlying social recognition using olfactory cues in rodents, and the LA-Me connections in social fear learning [70]. Consistently, visual observational fear learning in humans is associated with activation of the visual system [25]. One of the major questions for future studies is to determine how neural systems for social cognition and threat processing interact in supporting SFL (for neural systems involved in SFL see: Fig. 3).
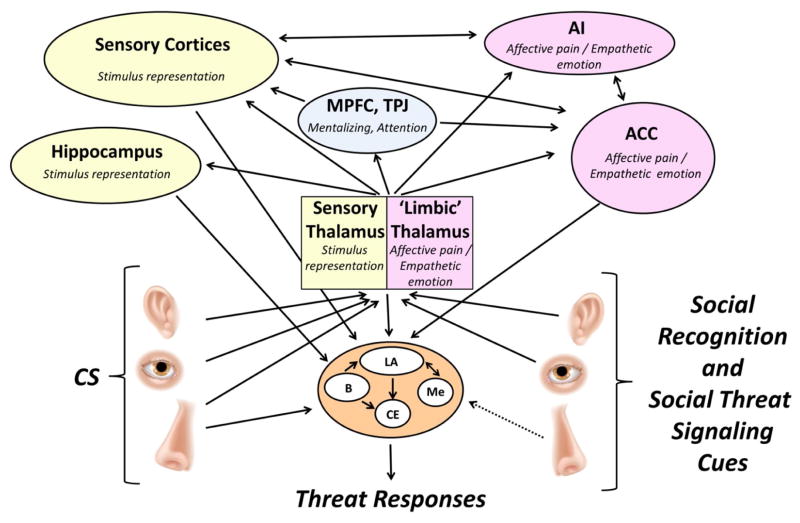
Figure 3. A model of neural systems of SFL.
The arrows describe the hypothetical flow of information between different functional brain regions that are most relevant to SFL. As in FC, the information about the CS and social cues signaling threat that is projected from the thalamus, hippocampus and cortical sites (or directly to the amygdala as in the case of olfactory cues) converges in the lateral nucleus of the amygdala (LA). The LA projects to the central nucleus of the amygdala (CE) that sends outputs to sites and systems directly controlling threat responses. Some information (e.g. about the context where learning occurs) reaches the LA through the basal nucleus of the amygdala (B) and some other cue representations project to the CE without the LA being involved. The medial nucleus of the amygdala (Me) mediates social behaviors and has bidirectional connections with the LA. The affective pain processing system, including midline nuclei of the thalamus (the ‘limbic’ thalamus), the anterior cingulate cortex (ACC) and the anterior insula (AI) are believed to process information about social cues signaling fear. The ACC-amygdala projections appear critical for the delivery of the information about social cues signaling threat to the amygdala. SFL is modified by medial prefrontal cortex (MPFC) which is responsible for interpretation of the other’s mental state and temporal-parietal junction (TPJ) that controls attention processes during learning. The dotted line represents a hypothetical alarm chemosignaling pathway that has been characterized in rodents but is mostly unknown in humans.
The Ontogeny of SFL
Rodent studies show that early in infancy, learning is biased towards attachment to a caregiver and FC is quiescent [71]. However, from birth on pups can acquire threat responses from their mother through SFL [27, 33]. The presence of a frightened mother activates the pup’s hypothalamus-pituitary-adrenal gland axis and causes a robust corticosterone rise, and an increased activity of the amygdala and several brain areas known to be involved in processing fear, stress and pain [27, 33]. Rodent studies demonstrate that SFL in infant rats occurs through chemosignalling pathways [27, 33]. It has not been determined whether alarm chemosignaling plays a role in human infants, although recent studies suggest that olfaction may mediate social fear transmission in adults [72, 73]. Interestingly, research shows that infant SFL may occur without any increased activation of the ACC or the insular cortex which are not fully functional until later in life [33].
The young child’s dependence on the caregiver and an associated unique sensitivity to the caregiver’s emotions suggest a special role of SFL in infancy and early childhood. This distinctive character of SFL in childhood may have an adaptive function which allows the offspring to learn early from parents about possible threats in the surrounding world [27, 74, 75). Indeed, human studies show that infants and young children exposed to novel stimuli paired with faces expressing fear learn to display fear to these stimuli [74, 75].
Social Learning and Transmission of Maladaptive Fears and Anxiety
Above, we highlighted various adaptive functions of SFL. Recent studies suggest that social learning also play an important role in the transmission of maladaptive fears and anxiety, such as those occurring in anxiety disorders and posttraumatic stress [9, 18, 20, 74–84]. In particular, clinical research shows that many phobias may be acquired through social transmission, either observational or instructed [85]. Intergenerational transmission of fear and anxiety may be explained by various mechanisms, including genetic and epigenetic mechanisms, environmental conditions, or gene-environment interactions [86–87]. A recent study on children-of-twins found that the association between parental and offspring anxiety was independent of genetic confounds, and likely depended on parental modeling of anxious behaviors and children’s social learning of anxiety [76]. Although fear and anxiety are distinct states [1], parent-child social transmission of each of these states requires a child to be uniquely sensitive to parental emotional expression. Indeed, experimental studies show that infants and young children quickly acquire parental fears [9, 77, 78, 82, 88]. In addition, recent studies show that clinical treatment of parental anxiety may prevent parent-child transmission of anxiety disorders and positive modeling may reverse vicariously learned anxious behaviors [89–92]. A child’s unique sensitivity to parental emotional states and the resultant parent-child social transmission of fear and anxiety may be explained by developmental factors, such as an early age of exposure, dependence on the caregiver, duration and intensity of exposure, as well as above discussed social factors, such as relatedness, similarity and familiarity. It is likely that social transmission of fear/anxiety may interact with other factors, such as genes in the development of maladaptive fears or anxiety. For example, a study investigating effects of functional polymorphism in the regulatory region (5-HTTLPR) of the human 5-HT transporter gene found that carriers of the short version of 5-HTTLPR comparing to carriers of the longer version displayed enhanced observational fear learning [93].
Concluding Remarks and Future Directions
Drawing on human and animal research, we have reviewed recent advances in social fear learning studies. We have presented evidence that social transmission of fear occurs through a variety of modes and engages various cognitive processes and distinct sensory modalities. A common feature characterizing all SFL experimental paradigms is a reliance on the integration of social cognition and fear learning processes. We have provided examples showing how SFL depends on social information and proposed a framework for understanding neural mechanisms of SFL. Future research should focus on the characterization of how social cognition and threat processing circuits interact in supporting SFL. Studies in rodents reported sex differences in levels of acquired threat responses following SFL; yet, the significance of these findings has to be investigated [29, 39]. Another line of research should address the role of altered SFL in rodent models of human disorders and diseases, such as autism and Alzheimer’s disease [70, 94]. Lastly, development of computational models and simulations of SFL will help to elucidate the workings of the underlying neural networks and will provide a mechanistic basis for understanding of the generation, maintenance and transmission of threat information [95].
Social transmission of fear plays significant evolutionary adaptive functions and occurs from infancy throughout the life of an individual. We have presented established and emerging evidence suggesting the importance of SFL mechanisms in dysfunctional fear and anxiety, especially in childhood and adolescence, as well as in other vulnerable populations, such as first responders or medical personnel frequently exposed to trauma. Understanding the neural and molecular mechanisms of SFL will pave the way to better prevention and treatment of socially transmitted maladaptive fears.
Trends Box.
Social learning of fear in humans requires integration of social cognition and fear learning mechanisms, and may occur through instruction or observation.
Studies have begun to characterize neural systems of social fear learning, highlighting the role of the amygdala and the brain affective pain processing system, including the anterior cingulate cortex.
Recent experiments suggest that avoidance responses acquired through social fear learning may play a role in the emergence and maintenance of habits and cultural traditions.
A unique sensitivity to the caregiver’s emotions and an early emergence of social fear learning allow infants to learn from their parents about potential environmental threats before the sensory and motor development enables them direct exploration of the surrounding environment.
In spite of its adaptive functions, recent preclinical and clinical studies suggest that social fear/anxiety learning mechanisms contribute to maladaptive fear and anxiety, such as anxiety disorders and vicarious or secondary trauma.
Outstanding Questions Box.
How and where are the conditioned stimulus – social signaling of threat contingencies encoded in the brain?
Are there common principles and processes governing social fear learning and the social transmission of safety, reward and disgust information?
Does physiological synchronization between the observer and the demonstrator affect (predict) learning outcome in social fear learning?
Does olfaction play a role in human social fear learning?
How do social fear/anxiety learning mechanisms interact with other heritable and environmental factors in anxiety disorders?
Acknowledgments
This work was supported by K08 MH014743-01A1, NARSAD Young Investigator Award from the Brain & Behavior Research Foundation and Todd Ouida Clinical Scholar Award in Childhood Anxiety & Depression to JD, and Knut and Alice Wallenberg Foundation (KAW 2014.0237) and an Independent Starting Grant (284366; Emotional Learning in Social Interaction project) from the European Research Council to AO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeDoux JE, Pine DS. Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. Am J Psychiatry. 2016;173(11):1083–1093. doi: 10.1176/appi.ajp.2016.16030353. [DOI] [PubMed] [Google Scholar]
- 2.McCullough KM, et al. Bridging the Gap: Towards a cell-type specific understanding of neural circuits underlying fear behaviors. Neurobiol Learn Mem. 2016;135:27–39. doi: 10.1016/j.nlm.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox AS, et al. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsson A, Phelps EA. Social learning of fear. Nat Neurosci. 2007;10:1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- 5.Gunnar MR, et al. Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Soc Neurosci. 2015;10:474–478. doi: 10.1080/17470919.2015.1070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiyokawa Y, et al. Social buffering reduces male rats’ behavioral and corticosterone responses to a conditioned stimulus. Horm Behav. 2014;65:114–118. doi: 10.1016/j.yhbeh.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Colnaghi L, et al. Social Involvement Modulates the Response to Novel and Adverse Life Events in Mice. PLoS One. 2016;11:e0163077. doi: 10.1371/journal.pone.0163077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golkar A, et al. Neural signals of vicarious extinction learning. Soc Cogn Affect Neurosci. 2016;11(10):1541–9. doi: 10.1093/scan/nsw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebowitz ER, et al. Avoidance moderates the association between mothers’ and children’s fears: findings from a novel motion-tracking behavioral assessment. Depress Anxiety. 2015;32:91–98. doi: 10.1002/da.22333. [DOI] [PubMed] [Google Scholar]
- 10.Kim EJ, et al. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One. 2010;5:e15077. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inagaki H, et al. Identification of a pheromone that increases anxiety in rats. Proc Natl Acad Sci U S A. 2014;111:18751–18756. doi: 10.1073/pnas.1414710112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brechbuhl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. 2008;321:1092–1095. doi: 10.1126/science.1160770. [DOI] [PubMed] [Google Scholar]
- 13.Keum S, Shin HS. Rodent models for studying empathy. Neurobiol Learn Mem. 2016;135:22–26. doi: 10.1016/j.nlm.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Dezecache G, Jacob P, Grezes J. Emotional contagion: its scope and limits. Trends Cogn Sci. 2015;19:297–299. doi: 10.1016/j.tics.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Golkar A, Olsson A. Immunization against social fear learning. J Exp Psychol Gen. 2016;145:665–671. doi: 10.1037/xge0000173. [DOI] [PubMed] [Google Scholar]
- 16.Mineka S, Cook M. Mechanisms involved in the observational conditioning of fear. J Exp Psychol Gen. 1993;122:23–38. doi: 10.1037//0096-3445.122.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Golkar A, et al. Other people as means to a safe end: vicarious extinction blocks the return of learned fear. Psychol Sci. 2013;24:2182–2190. doi: 10.1177/0956797613489890. [DOI] [PubMed] [Google Scholar]
- 18.de Rosnay M, et al. Transmission of social anxiety from mother to infant: an experimental study using a social referencing paradigm. Behav Res Ther. 2006;44:1165–1175. doi: 10.1016/j.brat.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Rachman S. The conditioning theory of fear-acquisition: a critical examination. Behav Res Ther. 1977;15:375–387. doi: 10.1016/0005-7967(77)90041-9. [DOI] [PubMed] [Google Scholar]
- 20.Remmerswaal D, et al. “Will a Cuscus bite you, if he shows his teeth?” Inducing a fear-related confirmation bias in children by providing verbal threat information to their mothers. J Anxiety Disord. 2010;24:540–546. doi: 10.1016/j.janxdis.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Atlas LY, et al. Instructed knowledge shapes feedback-driven aversive learning in striatum and orbitofrontal cortex, but not the amygdala. Elife. 2016;5:e15192. doi: 10.7554/eLife.15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nat Protoc. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haaker J, et al. Assessing social transmission of threats in humans using the observational fear conditioning procedure. Nat Protocols. 2017 doi: 10.1038/nprot.2017.027. in press. [DOI] [PubMed] [Google Scholar]
- 24.Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meffert H, et al. Prediction errors to emotional expressions: the roles of the amygdala in social referencing. Soc Cogn Affect Neurosci. 2015;10:537–544. doi: 10.1093/scan/nsu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon D, Shin HS. A mouse model for observational fear learning and the empathetic response. Curr Protoc Neurosci. 2011;Chapter 8(Unit 8):27. doi: 10.1002/0471142301.ns0827s57. [DOI] [PubMed] [Google Scholar]
- 27.Debiec J, Sullivan RM. Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc Natl Acad Sci U S A. 2014;111:12222–12227. doi: 10.1073/pnas.1316740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones CE, et al. Social transmission of Pavlovian fear: fear-conditioning by-proxy in related female rats. Anim Cogn. 2014;17:827–834. doi: 10.1007/s10071-013-0711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikosz M, et al. Sex differences in social modulation of learning in rats. Sci Rep. 2015;5:18114. doi: 10.1038/srep18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds G, Field AP, Askew C. Learning to fear a second-order stimulus following vicarious learning. Cogn Emot. 2015:1–8. doi: 10.1080/02699931.2015.1116978. [DOI] [PubMed] [Google Scholar]
- 31.Debiec J, et al. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci U S A. 2006;103(9):3428–33. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Q, Huang Y, Wang L. Left prefrontal activity reflects the ability of vicarious fear learning: a functional near-infrared spectroscopy study. Scientific World Journal. 2013;2013:652542. doi: 10.1155/2013/652542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang DJ, Debiec J. Neural correlates of the mother-to-infant social transmission of fear. J Neurosci Res. 2016;94:526–534. doi: 10.1002/jnr.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindstrom B, Olsson A. Mechanisms of social avoidance learning can explain the emergence of adaptive and arbitrary behavioral traditions in humans. J Exp Psychol Gen. 2015;144:688–703. doi: 10.1037/xge0000071. [DOI] [PubMed] [Google Scholar]
- 35.Masuda A, et al. Multisensory interaction mediates the social transmission of avoidance in rats: dissociation from social transmission of fear. Behav Brain Res. 2013;252:334–338. doi: 10.1016/j.bbr.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Masuda A, Aou S. Social transmission of avoidance behavior under situational change in learned and unlearned rats. PLoS One. 2009;4:e6794. doi: 10.1371/journal.pone.0006794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin AS. Social learning about predators: a review and prospectus. Learn Behav. 2004;32(1):131–40. doi: 10.3758/bf03196014. [DOI] [PubMed] [Google Scholar]
- 38.Yusufishaq S, Rosenkranz JA. Post-weaning social isolation impairs observational fear conditioning. Behav Brain Res. 2013;242:142–149. doi: 10.1016/j.bbr.2012.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panksepp JB, Lahvis GP. Differential influence of social versus isolate housing on vicarious fear learning in adolescent mice. Behav Neurosci. 2016;130:206–211. doi: 10.1037/bne0000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One. 2009;4:e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeon D, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kavaliers M, Colwell DD, Choleris E. Kinship, familiarity and social status modulate social learning about “micropredators” (biting flies) in deer mice. Behav Ecol Sociobiol. 2005;58:60–71. [Google Scholar]
- 43.Knapska E, et al. Social modulation of learning in rats. Learn Mem. 2010;17:35–42. doi: 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golkar A, Castro V, Olsson A. Social learning of fear and safety is determined by the demonstrator’s racial group. Biol Lett. 2015;11:20140817. doi: 10.1098/rsbl.2014.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goubert L, et al. Learning about pain from others: an observational learning account. J Pain. 2011;12:167–174. doi: 10.1016/j.jpain.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Chang SW, Winecoff AA, Platt ML. Vicarious reinforcement in rhesus macaques (macaca mulatta) Front Neurosci. 2011;5:27. doi: 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mobbs D, et al. A key role for similarity in vicarious reward. Science. 2009;324(5929):900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kendal R, et al. Chimpanzees copy dominant and knowledgeable individuals: implications for cultural diversity. Evol Hum Behav. 2015;36(1):65–72. doi: 10.1016/j.evolhumbehav.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones CE, Monfils MH. Dominance status predicts social fear transmission in laboratory rats. Anim Cogn. 2016;19(6):1051–1069. doi: 10.1007/s10071-016-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selbing I, Lindstrom B, Olsson A. Demonstrator skill modulates observational aversive learning. Cognition. 2014;133:128–139. doi: 10.1016/j.cognition.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Haaker J, Molapour T, Olsson A. Conditioned social dominance threat: observation of others’ social dominance biases threat learning. Soc Cogn Affect Neurosci. 2016;11(10):1627–37. doi: 10.1093/scan/nsw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laland KN. Social learning strategies. Learn Behav. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- 53.Keum S, et al. Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav. 2016;15:231–242. doi: 10.1111/gbb.12278. [DOI] [PubMed] [Google Scholar]
- 54.Olsson A, et al. Vicarious Fear Learning Depends on Empathic Appraisals and Trait Empathy. Psychol Sci. 2016;27:25–33. doi: 10.1177/0956797615604124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lockwood PL, et al. Neurocomputational mechanisms of prosocial learning and links to empathy. Proc Natl Acad Sci U S A. 2016;113(35):9763–8. doi: 10.1073/pnas.1603198113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaki J. Empathy: a motivated account. Psychol Bull. 2014;140(6):1608–47. doi: 10.1037/a0037679. [DOI] [PubMed] [Google Scholar]
- 57.Debiec J, LeDoux JE. The Amygdala Networks of Fear: From Animal Models to Human Psychopathology. In: McKay D, Abramowitz JS, Taylor S, Asmundson GJG, editors. Current Perspectives on the Anxiety Disorders: Implications for DSM-V and Beyond. Springer; 2009. pp. 107–126. [Google Scholar]
- 58.Hooker CI, et al. The influence of personality on neural mechanisms of observational fear and reward learning. Neuropsychologia. 2008;46(11):2709–24. doi: 10.1016/j.neuropsychologia.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bissiere S, et al. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biol Psychiatry. 2008;63(9):821–31. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim BS, et al. Differential regulation of observational fear and neural oscillations by serotonin and dopamine in the mouse anterior cingulate cortex. Psychopharmacology (Berl) 2014;231:4371–4381. doi: 10.1007/s00213-014-3581-7. [DOI] [PubMed] [Google Scholar]
- 61.Kim S, et al. Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proc Natl Acad Sci U S A. 2012;109:15497–15501. doi: 10.1073/pnas.1213903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allsop SA, et al. 2016 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2016. A cortico-amygdala circuit encodes observational fear learning. Program No. 456.16/JJJ14. Online. [Google Scholar]
- 63.Fuchs PN, et al. The anterior cingulate cortex and pain processing. Front Integr Neurosci. 2014;8:35. doi: 10.3389/fnint.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98(14):8077–82. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288(5472):1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 66.Betti V, Aglioti SM. Dynamic construction of the neural networks underpinning empathy for pain. Neurosci Biobehav Rev. 2016;63:191–206. doi: 10.1016/j.neubiorev.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Zaki J, et al. The Anatomy of Suffering: Understanding the Relationship between Nociceptive and Empathic Pain. Trends Cogn Sci. 2016;20(4):249–59. doi: 10.1016/j.tics.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindström B, Haaker J, Olsson A. Neural and computational underpinnings of social threat learning. Association for Psychological Science Annual Convention; New York. May 2015.2015. [Google Scholar]
- 69.Haaker J, Yi J, Olsson A. Influence of opioidergic neurotransmission on vicarious acquisition of fear. Seventh European Meeting on Human Fear Conditioning; Bochum. May 2015.2015. [Google Scholar]
- 70.Twining RC, et al. An intra-amygdala circuit specifically regulates social fear learning. Nat Neurosci. 2017;20(3):459–469. doi: 10.1038/nn.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Debiec J, Sullivan RM. The neurobiology of safety and threat learning in infancy. Neurobiol Learn Mem. 2016 doi: 10.1016/j.nlm.2016.10.015. http://dx.doi.org/10.1016/j.nlm.2016.10.015; [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 72.de Groot JH, Semin GR, Smeets MA. Chemical communication of fear: A case of male-female asymmetry. J Exp Psychol Gen. 2014;143(4):1515–25. doi: 10.1037/a0035950. [DOI] [PubMed] [Google Scholar]
- 73.Radulescu AR, Mujica-Parodi LR. Human gender differences in the perception of conspecific alarm chemosensory cues. PLoS One. 2013;8(7):e68485. doi: 10.1371/journal.pone.0068485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoehl S, Pauen S. Do infants associate spiders and snakes with fearful facial expressions? Evol Hum Behav 2016 [Google Scholar]
- 75.Askew C, Field AP. Vicarious learning and the development of fears in childhood. Behav Res Ther. 2007;45:2616–2627. doi: 10.1016/j.brat.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Eley TC, et al. The Intergenerational Transmission of Anxiety: A Children-of-Twins Study. Am J Psychiatry. 2015;172(7):630–7. doi: 10.1176/appi.ajp.2015.14070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aktar E, et al. The interplay between expressed parental anxiety and infant behavioural inhibition predicts infant avoidance in a social referencing paradigm. J Child Psychol Psychiatry. 2013;54:144–156. doi: 10.1111/j.1469-7610.2012.02601.x. [DOI] [PubMed] [Google Scholar]
- 78.de Rosnay M, et al. Transmission of social anxiety from mother to infant: an experimental study using a social referencing paradigm. Behav Res Ther. 2006;44:1165–1175. doi: 10.1016/j.brat.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 79.Blair KS, et al. Learning from other people’s fear: amygdala-based social reference learning in social anxiety disorder. Psychol Med. 2016:1–11. doi: 10.1017/S0033291716001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Remmerswaal D, Muris P, Huijding J. “Watch out for the gerbils, my child!” the role of maternal information on children’s fear in an experimental setting using real animals. Behav Ther. 2013;44:317–324. doi: 10.1016/j.beth.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 81.Remmerswaal D, Muris P, Huijding J. Transmission of Cognitive Bias and Fear From Parents to Children: An Experimental Study. J Clin Child Adolesc Psychol. 2015:1–13. doi: 10.1080/15374416.2014.987378. [DOI] [PubMed] [Google Scholar]
- 82.Askew C, et al. The effect of disgust and fear modeling on children’s disgust and fear for animals. J Abnorm Psychol. 2014;123:566–577. doi: 10.1037/a0037228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stoll K, Hall W. Vicarious birth experiences and childbirth fear: does it matter how young canadian women learn about birth? J Perinat Educ. 2013;22:226–233. doi: 10.1891/1058-1243.22.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le MT, et al. Polyvictimization Among Children and Adolescents in Low- and Lower-Middle-Income Countries: A Systematic Review and Meta-Analysis. Trauma Violence Abuse. 2016 doi: 10.1177/1524838016659489. [DOI] [PubMed] [Google Scholar]
- 85.Askew C, Field AP. The vicarious learning pathway to fear 40 years on. Clin Psychol Rev. 2008;28(7):1249–65. doi: 10.1016/j.cpr.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Bowers ME, Yehuda R. Intergenerational Transmission of Stress in Humans. Neuropsychopharmacology. 2016;41(1):232–44. doi: 10.1038/npp.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lebowitz ER, et al. Cross-generational influences on childhood anxiety disorders: pathways and mechanisms. J Neural Transm (Vienna) 2016;123(9):1053–67. doi: 10.1007/s00702-016-1565-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerull FC, Rapee RM. Mother knows best: effects of maternal modelling on the acquisition of fear and avoidance behaviour in toddlers. Behav Res Ther. 2002;40:279–287. doi: 10.1016/s0005-7967(01)00013-4. [DOI] [PubMed] [Google Scholar]
- 89.Ginsburg GS, et al. Preventing Onset of Anxiety Disorders in Offspring of Anxious Parents: A Randomized Controlled Trial of a Family-Based Intervention. Am J Psychiatry. 2015;172(12):1207–14. doi: 10.1176/appi.ajp.2015.14091178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Askew C, et al. Inhibition of vicariously learned fear in children using positive modeling and prior exposure. J Abnorm Psychol. 2016;125:279–291. doi: 10.1037/abn0000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reynolds G, Field AP, Askew C. Preventing the Development of Observationally Learnt Fears in Children by Devaluing the Model’s Negative Response. J Abnorm Child Psychol. 2015b;43:1355–1367. doi: 10.1007/s10802-015-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reynolds G, Field AP, Askew C. Reductions in Children’s Vicariously Learnt Avoidance and Heart Rate Responses Using Positive Modeling. J Clin Child Adolesc Psychol. 2016;23:1–14. doi: 10.1080/15374416.2016.1138410. [DOI] [PubMed] [Google Scholar]
- 93.Crisan LG, et al. Genetic contributions of the serotonin transporter to social learning of fear and economic decision making. Soc Cogn Affect Neurosci. 2009;4:399–408. doi: 10.1093/scan/nsp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi J, Jeong Y. Elevated emotional contagion in a mouse model of Alzheimer’s disease is associated with increased synchronization in the insula and amygdala. Sci Rep. 2017;7:46262. doi: 10.1038/srep46262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lindstrom B, et al. Co-Evolution of Social Learning and Evolutionary Preparedness in Dangerous Environments. PLoS One. 2016;11(8):e0160245. doi: 10.1371/journal.pone.0160245. [DOI] [PMC free article] [PubMed] [Google Scholar]