Abstract
Talin, a cytoskeletal protein essential in mediating integrin activation, has been previously shown to be involved in the regulation of T cell proliferation and function. Here we describe a role for talin in maintaining the homeostasis and survival of the regulatory T (Treg) cell pool. T cell-specific deletion of talin in Tln1fl/flCd4Cre mice resulted in spontaneous lymphocyte activation, primarily due to numerical and functional deficiencies of Treg cells in the periphery. Peripheral talin-deficient Treg cells were unable to maintain high expression of IL-2Rα, resulting in impaired IL-2 signaling and ultimately leading to increased apoptosis through downregulation of pro-survival proteins Bcl-2 and Mcl-1. The requirement for talin in maintaining high IL-2Rα expression by Treg cells was due, in part, to integrin LFA-1-mediated interactions between Treg cells and dendritic cells. Collectively, our data suggest a critical role for talin in Treg cell-mediated maintenance of immune homeostasis.
Introduction
Talin is a cytoskeletal regulatory protein that plays essential roles in diverse cellular processes. The protein facilitates the final stages of integrin activation and has been shown to activate β1, β2, and β3 integrins (1). Talin consists of a 190kD C-terminal flexible rod and a 47kD N-terminal globular head domain containing four sub-domains (F0, F1, F2 and F3) (1–3). The F3 sub-domain of talin, which contains a phosphotyrosine-binding (PTB) domain, binds directly to a high-affinity binding site in the β-integrin cytoplasmic tail. This interaction causes a conformational change in the integrin, shifting it from the low to the high affinity state. In addition to activating integrins, talin links integrins to the actin cytoskeleton and recruits essential signaling molecules such as phosphatidylinositol phosphate kinase (4) and TIAM1 to focal adhesions (5).
Germline deletion of talin results in embryonic lethality (5), but conditional knockout studies have revealed a role for talin in multiple immune cell types. Talin is required for fibrin clot retraction by platelets (6) and B cell homing to lymph nodes (7). In T cells, talin was one of the first proteins shown to be recruited to the immunological synapse (IS) (8), which forms between T lymphocytes and antigen-presenting cells (APCs) and facilitates T cell activation. The recruitment of talin to the IS has led to the hypothesis that talin may play a role in regulating this process. Studies with Jurkat T cells have demonstrated that talin is required for clustering and affinity regulation of the integrin LFA-1 (αLβ2) in order to facilitate contact between T cells and APCs (9, 10). Prior studies in mice with a T cell-specific deletion of talin revealed a role for the protein in the maintenance of T cell-APC contacts, contact-mediated T cell proliferation, and polarization of stable F-actin to the IS. Unexpectedly, these studies also showed that T cells isolated from the lymph nodes of these mice exhibited a phenotype consistent with prior activation, despite having reduced contact time with APCs and no defect in TCR signaling (11). Although these mice were also observed to have a reduced frequency of regulatory T (Treg) cells in the lymph nodes (11), it remains unknown whether talin is intrinsically required in naïve T cells to prevent aberrant activation or if talin plays a specific role in Treg cells in order to maintain immune homeostasis.
Treg cells, a subset of CD4+ T lymphocytes defined by expression of the transcription factor Foxp3, are indispensable for the maintenance of peripheral tolerance. Foxp3 deficiency in mice (12, 13) or humans (14, 15) leads to systemic inflammation and multi-organ autoimmunity. Deficiency in Treg cell numbers or suppressive function has been linked to various immune-mediated conditions such as inflammatory bowel disease, type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosis, and multiple sclerosis (16, 17). Treg cells are also characterized by high expression of IL-2Rα (CD25) (18). IL-2 signaling is known to reinforce the expression of Foxp3 (19, 20), and expression of Foxp3, in turn, directly upregulates the expression of IL-2Rα (21). Thus, IL-2 signaling plays a central role in the development, proliferation and homeostasis of Treg cells (22–25).
Recent evidence has revealed that Treg cells exhibit phenotypic and functional heterogeneity. Treg cells can be categorized as central (cTreg) and effector Treg (eTreg) cells based on phenotypic markers (CD62LhiCD44lo and CD62LloCD44hi), respectively, as well as localization and homeostatic requirements (26). cTreg cells express high levels of the chemokine receptor CCR7, enabling them to recirculate through lymphoid tissues. These cells are quiescent and relatively long-lived due to high expression of the anti-apoptotic proteins Bcl-2 and Mcl-1. In addition, cTreg cells are believed to be more highly dependent on IL-2 signaling and correspondingly express high levels of IL-2Rα (26). By contrast, eTreg cells are highly proliferative, localize mainly to non-lymphoid tissues, such as the liver and lung, and are short-lived and highly apoptotic (26, 27). Compared to cTreg cells, eTreg cells are also responsive to IL-2, but depend more highly on ICOS signaling for the maintenance of homeostasis and correspondingly express higher levels of ICOS (26). eTreg cells are also characterized by high expression of GITR and CD103, which facilitate trafficking to tissues (28). Most Treg cells exiting the thymus are characterized as cTreg cells, as they express high levels of CCR7 and CD62L. The subsequent differentiation of cTreg cells into eTreg cells has been shown to occur in the periphery and requires TCR signals (29, 30) and the expression of the transcription factors IRF4 and Blimp-1 (31). These subsets are functionally distinct, as cTreg cells have been proposed to prevent aberrant T cell priming in the lymphoid organs, while eTreg cells are thought to control effector T cell responses at the non-lymphoid tissue sites (26). Thus, optimal differentiation and maintenance of both subsets is required for the preservation of immune homeostasis.
To explore the role of talin in maintaining immune homeostasis, we analyzed Tln1fl/flCd4Cre mice with a T cell-specific deletion of talin. We found that talin-deficient CD4+ and CD8+ T cells exhibited spontaneous activation, which was not due to defects in T cell development or aberrant expression of co-stimulatory or inhibitory molecules by naïve peripheral T cells. Instead, Tln1fl/flCd4Cre mice exhibited a substantial deficiency in both the number and function of Treg cells. Although the numerical deficiency was primarily due to a loss of cTregs, we found that talin was required for the homeostasis of both cTreg and eTreg cells. Strikingly, talin-deficient Treg cells were unable to maintain high expression of IL-2Rα, which led to increased apoptosis of these cells by limiting the expression of pro-survival proteins Bcl-2 and Mcl-1. The requirement for talin in maintaining high IL-2Rα expression by Treg cells was due, in part, to LFA-1-mediated interactions between Treg cells and dendritic cells (DCs). Together, these findings suggest that talin plays a critical role in regulating Treg cell function, homeostasis and survival.
Materials and Methods
Mice
All animal work was approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. All mice were housed in specific pathogen-free conditions prior to use. Cd4Cre mice were purchased from Jackson Laboratories, and Tln1fl/fl and Foxp3GFP mice have been described previously (6, 32–34). For administration of IL-2/IL-2mAb complexes, 2μg of recombinant murine IL-2 (Biolegend) was combined with 10μg IL-2 monoclonal antibody JES6-1 (Bio X Cell), diluted to a volume of 200μL in PBS and incubated for 30 minutes at 37°C before intraperitoneal (i.p.) injection, as previously described (35, 36). To block LFA-1 signaling, mice were treated with 100μg anti-CD11a (M17/4) (Bio X Cell), 100μg anti-CD18 (M18/2) (Bio X Cell) and 200μg anti-ICAM-1 (YN1/1.7.4) or isotype control in PBS via i.p. injection twice weekly for three weeks.
Antibodies and flow cytometry
The following antibodies were purchased from Biolegend or eBioscience: CD4 (RM4-5), CD8 (53–6.7), Foxp3 (FJK-16s), CD44 (1M7), CD62L (MEL-14), IFNγ (XMG1.2), TNFα (MP6-XT22), IL-2 (JES6-5H4), IL-17A (TC11-18H10.1), IL-2Rα (PC61), CTLA4 (UC10-4B9), CD39 (24DMS1), CD73 (TY/11.8), GITR (DTA-1), OX40 (OX-86), PD1 (J43), CD28 (37.51), IRF4 (IRF4.3E4), Bcl-2 (BCL/10C4), ICOS (15F9), Annexin V and fixable viability dye. Anti-human Ki67 (B56) was purchased from BD Biosciences. Anti-GFP rabbit IgG was purchased from Life Technologies. For intracellular detection of cytokines, splenocytes were stimulated ex vivo with PMA (Sigma) and ionomycin (Sigma) in the presence of Brefeldin A (Sigma) for 3 hours at 37°C; cells were fixed in 4% paraformaldehyde (Electron Microscopy Services) and permeabilized with the Foxp3 Transcription Factor Fixation/Permeabilization kit (eBioscience) prior to staining. To assess pSTAT5 levels directly ex vivo, cells were fixed in Lyse/Fix buffer (BD) for 20 minutes at room temperature. Cells were then resuspended in 90% methanol and incubated for 30 minutes on ice. After washing, cells were resuspended in Perm/Wash buffer (BD) and surface and intracellular antigens were stained, including pSTAT5 (pY694; BD). To assess pSTAT5 levels in vitro, bulk splenocytes were stimulated with 0U/mL, 0.1U/mL, 1U/mL or 10U/mL of IL-2 (Peprotech) at 37°C for 30 min and the same staining protocol was used. To measure apoptosis, cells were stained with Mito Flow (Cell Technology) according to the manufacturer’s instructions. All samples were analyzed on an Accuri C6, FACS Canto, or LSR FortessaX-20 (BD Biosciences).
Treg cell suppression assays
CD4+CD25− T conventional (Tconv) cells were isolated from spleens and lymph nodes of wild-type mice by magnetic separation using the CD4+ T cell negative isolation kit (Miltenyi Biotec); a biotin-conjugated anti-CD25 (PC61, Biolegend) antibody was included to deplete Treg cells. CD4+GFP+ Treg cells were sorted with a FACS Aria 2 (BD Biosciences). Antigen-presenting cells were isolated from spleens of wild-type mice and depleted of CD3+ T cells using CD3 microbeads (Miltenyi Biotec). Tconv cells were labeled with CFSE as previously described (37). CSFE-labeled Tconv cells were co-cultured with antigen presenting cells (1:3 ratio) and Treg cells (32:1, 16:1, 8:1, 4:1, 2:1 and 1:1 ratios) in the presence of 250 ng/mL soluble anti-CD3 (2C11) for 72 hours at 37°C. Percentage suppression was calculated as: [(divided Tconv cells without Treg cells) – (divided Tconv cells with Treg cells for a given experimental condition)]/(divided Tconv cells without Treg cells) * 100.
Generation of bone marrow chimeras
Bone marrow cells were depleted of CD3+ cells using CD3 microbeads (Miltenyi Biotec). Lethally irradiated (1000 rads) RAG1-deficient mice were injected intravenously with bone marrow cells from Tln1fl/flCd4Cre mice alone or in combination with bone marrow cells from wild-type or Foxp3-deficient mice. Spleens and thymi from recipient mice were harvested for analysis 8–10 weeks after reconstitution.
Adoptive Transfer Experiments
Total CD4+ T cells were isolated from spleens of Tln1fl/flCd4Cre mice by magnetic separation using the CD4+ T cell negative isolation kit (Miltenyi Biotec) and 3×106 cells were injected into CD45.1+ wild-type recipients. Spleens were harvested from recipient mice 4 days after transfer.
Quantitative real-time PCR
Total RNA was isolated from cells with TRIzol (Life Technologies) according to manufacturer’s protocol and was converted to cDNA using an iScript Advanced cDNA synthesis Kit (Bio-Rad) according to manufacturer’s protocol. 100ng cDNA was combined with 250nM forward and reverse primers for the indicated genes and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). Samples were run on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). Samples were normalized based on expression of Rpl13a reference gene. Relative gene expression was determined based on three biological replicates and figures show one representative experiment. The following primer sequences were utilized: Rpl13a 5′GGGCAGGTTCTGGTATTGGAT, Rpl13a 3′GGCTCGGAAATGGTAGGGG, Il10 5′ATCGATTTCTCCCCTGTGAA, Il10 3′TGTCAAATTCATTCATGGCCT, Il2ra 5′CTCCCATGACAAATCGAGAAAGC, Il2ra 3′TCTCTTGGTGCATAGACTGTGT, Casp7 5′CGGAATGGGACGGACAAAGAT, Casp7 3′CTTTCCCGTAAATCAGGTCCTC, Mcl1 5′TAACAAACTGGGGCAGGATT, Mcl1 3′GTCCCGTTTCGTCCTTACAA, Bcl2 5′TCGCAGAGATGTCCAGTCAG, Bcl2 3′CCTGAAGAGTTCCTCCACCA.
Statistical analysis
An unpaired Student’s t-test (two-tailed) was used for statistical evaluation of the data between two groups, using a statistical software package (Graph Pad Prism). P values are denoted in figures as; * P<0.05, **P<0.01, *** P<0.005.
Results
Spontaneous lymphocyte activation in mice with a T cell-specific deletion of talin
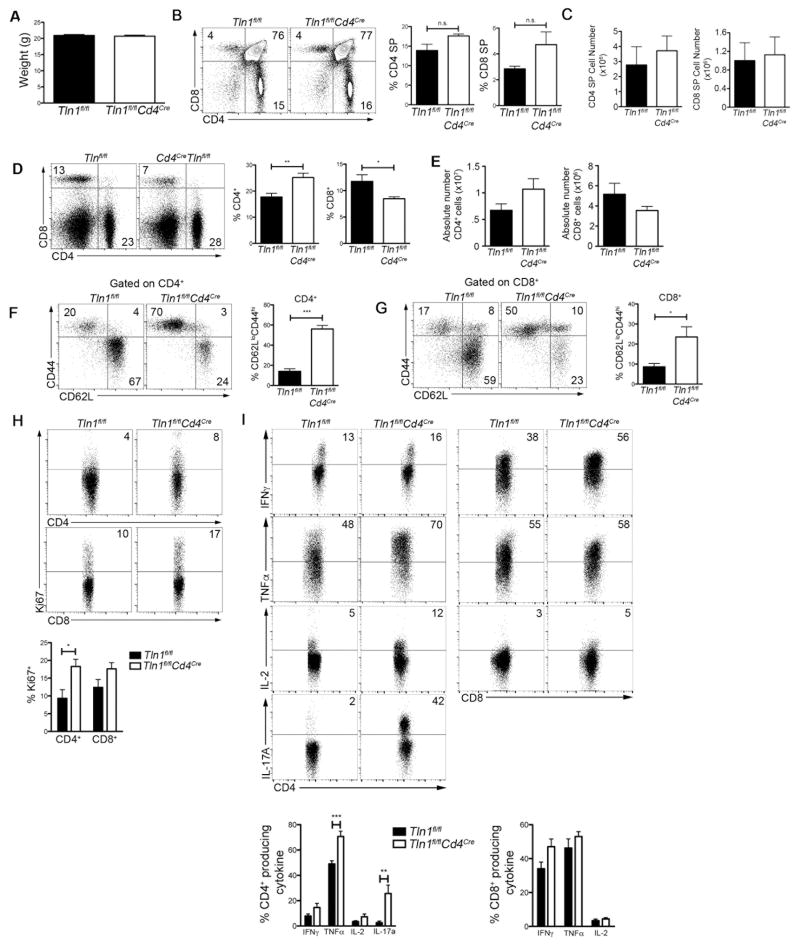
To investigate the role of talin in maintaining peripheral tolerance, we generated mice with a T cell-specific deletion of talin1 by crossing floxed talin1 mice with Cd4Cre mice (Tln1fl/flCd4Cre) in which talin was deleted at the CD4+CD8+ double-positive stage during thymic development. Compared to control Tln1fl/fl mice, Tln1fl/flCd4Cre mice were born at expected frequencies, developed normally with no overt signs of pathology and appeared healthy (Fig. 1A). Examination of thymi from control and Tln1fl/flCd4Cre mice revealed similar frequencies and numbers of CD4 and CD8 double-positive and single-positive thymocytes (Fig. 1B, 1C), suggesting that thymic T cell development after the double-positive stage was not affected in the absence of talin. Analysis of splenic T cells isolated from Tln1fl/flCd4Cre and control mice showed that although there were changes in the proportion of talin-deficient CD4+ and CD8+ T cells (Fig. 1D), no significant differences in total cell numbers were observed (Fig. 1E).
Figure 1. Spontaneous lymphocyte activation in mice with a T cell-specific deletion in talin.
(A) Weights of 8-week old Tln1fl/fl (WT) and Tln1fl/flCd4Cre (KO) mice (n=3). Percentages (B) and absolute number (C) of thymic CD4+ and CD8+ T cells isolated from WT and KO mice (n=3). Percentages (D) and absolute number (E) of CD4+ and CD8+ T cells isolated from WT or KO spleens (n=6). Expression of CD44 and CD62L in splenic CD4+ (F) and CD8+ (G) T cells from WT and KO mice (n=6). (H) Expression of Ki67 by CD4+ and CD8+ T cells (n=9). (I) IFNγ, TNFα, IL-2 and IL-17A expression by splenic CD4+ (left) and CD8+ (right) T cells from WT and KO mice after in vitro stimulation with PMA and ionomycin; displayed cells gated on CD4+CD44hi or CD8+CD44hi events (n=9). Data are representative of at least 3 independent experiments. *, P < 0.05; **, P <0.01; ***, P<0.001.
Further examination of the CD4+ and CD8+ T cell compartments revealed that talin-deficient lymphocytes in the spleen displayed an activated, antigen-experienced (CD44hiCD62Llo) phenotype (Fig. 1F, 1G). Consistent with this activated phenotype, CD4+ T cells isolated from Tln1fl/flCd4Cre mice displayed an increased proliferative capacity, as evidenced by high Ki67 expression (Fig. 1H), and were capable of producing high levels of inflammatory cytokines. In particular, talin-deficient CD4+ T cells produced high levels of TNFα and IL-17A compared to control cells (Fig. 1I). Taken together, these data show that talin is required to maintain quiescence and prevent activation of naïve T lymphocytes.
Spontaneous lymphocyte activation is controlled by factors extrinsic to naïve T cells
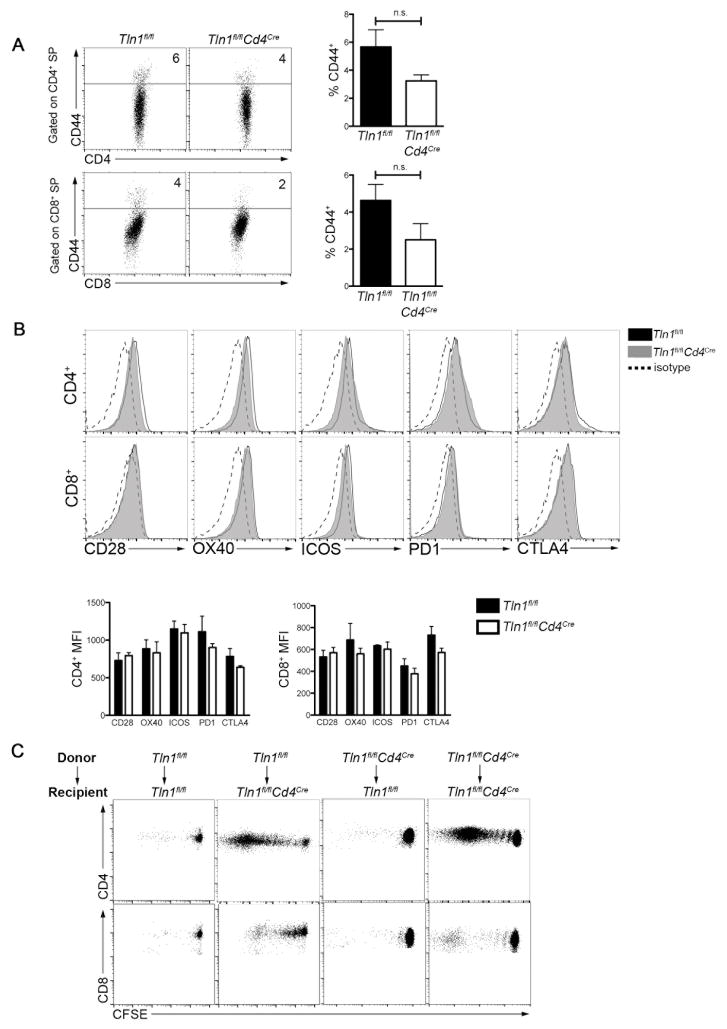
We next sought to determine the mechanisms by which deletion of talin causes aberrant T cell activation. To investigate whether T cell activation occurred during development, we examined the activation status of developing thymic T cells from Tln1fl/flCd4Cre and control mice. Thymic CD4+ or CD8+ single-positive T cells did not display an activated phenotype based on CD44 expression (Fig. 2A), suggesting that the aberrant T lymphocyte activation we observed in the periphery did not occur during T cell development.
Figure 2. Spontaneous lymphocyte activation is controlled by factors extrinsic to naïve T cells.
(A) Expression of CD44 by single-positive (SP) CD4+ and CD8+ thymocytes from Tln1fl/fl or Tln1fl/flCd4Cre mice (n=3). (B) Expression of the indicated co-stimulatory (CD28, OX40, or ICOS) or inhibitory molecules (PD1 or CTLA4) by naïve cells from Tln1fl/fl (black line) and Tln1fl/flCd4Cre (gray histogram) mice compared to isotype control (dotted line); displayed cells were gated on CD4+Foxp3−CD44lo or CD8+CD44lo cells (n=3). (C) Adoptive transfer of CFSE-labeled CD45.2 Tln1fl/fl or Tln1fl/flCd4Cre FACS-sorted naïve (CD44loCD62Lhi) T cells into CD45.1.2 Tln1fl/fl or Tln1fl/flCd4Cre recipients, followed by flow cytometric analysis of splenocytes from recipient mice 5 days later; displayed cells were gated on CD4+ or CD8+ events. Data are representative of at least 2 independent experiments.
We next hypothesized that the absence of talin might lead to increased expression of co-stimulatory receptors, or conversely, decreased expression of inhibitory receptors on naïve T cells, thereby influencing their activation threshold in the periphery. Examination of co-stimulatory (CD28, OX40, ICOS) and inhibitory receptors (PD1 and CTLA-4), however, revealed comparable expression by naïve (CD44lo) talin-deficient and control CD4+ and CD8+ T cells (Fig. 2B). Thus, factors intrinsic to naïve T cells did not appear to be responsible for the systemic T cell activation observed in Tln1fl/flCd4Cre mice.
To determine if extrinsic factors were contributing to spontaneous activation of naïve T lymphocytes in Tln1fl/flCd4Cre mice, FACS-sorted naïve (CD44loCD62Lhi) wild-type or talin-deficient T cells were labeled with the fluorescent division dye CFSE and adoptively transferred into congenic wild-type or Tln1fl/flCd4Cre recipients. Neither donor wild-type nor talin-deficient T cells proliferated when adoptively transferred into wild-type recipients. By contrast, both wild-type and talin-deficient T cells underwent proliferation when transferred into Tln1fl/flCd4Cre recipients (Fig. 2C). In summary, these results suggest that the systemic lymphocyte activation observed in Tln1fl/flCd4Cre mice resulted from factors extrinsic to naïve T cells.
Talin is required to maintain the number and function of Treg cells in the periphery
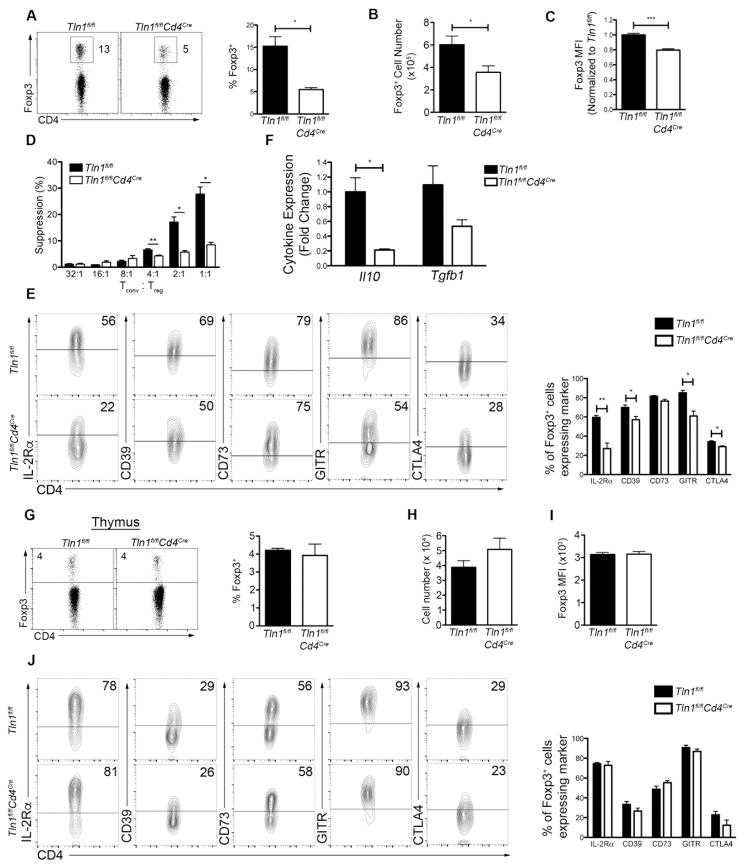
Because Treg cells are essential for controlling aberrant T cell activation, we hypothesized that a quantitative or qualitative deficiency in Treg cells might underlie the spontaneous lymphocyte activation exhibited by Tln1fl/flCd4Cre mice. To test this hypothesis, we generated Tln1fl/flCd4Cre mice expressing a GFP reporter for Foxp3 by crossing Tln1fl/flCd4Cre mice with Foxp3 GFP reporter mice (33). We observed significant reductions in the frequencies and absolute numbers of Treg cells in the spleens of Tln1fl/flCd4CreFoxp3GFP mice (Fig 3A, 3B), consistent with the previously published observation that lymph nodes from these mice harbor reduced frequencies of Foxp3+ Treg cells (11). In addition, talin-deficient Treg cells expressed significantly less Foxp3 on a per cell basis (Fig. 3C).
Figure 3. Talin deficiency leads to a reduction in Treg numbers and suppressive function.
Frequency (A) and absolute number (B) of Foxp3+ Treg cells isolated from the spleens of Tln1fl/flFoxp3GFP or Tln1fl/flCd4CreFoxp3GFP mice; displayed cells were gated on CD4+ events (n=12). (C) Foxp3 expression on a per cell basis (mean fluorescence intensity, MFI) from Foxp3+CD4+ splenic Treg cells (n=5). (D) Suppression by sorted Treg cells from Tln1fl/flFoxp3GFP or Tln1fl/flCd4CreFoxp3GFP mice at decreasing Tconv:Treg cell ratios, measured at 72 hours. (E) Expression of suppressive molecules IL-2Rα, CD39, CD73, GITR and CTLA4 on splenic Treg cells; displayed cells were gated on CD4+Foxp3+ cells (n=5). (F) Quantitative real-time PCR of Il10 and Tgfb1 transcript expression by GFP+ Treg cells isolated from Tln1fl/flFoxp3GFP or Tln1fl/fl Cd4CreFoxp3GFP mice. Cytokine mRNA expression was normalized to the abundance of Rpl13 transcript and expressed relative to transcript abundance of control Treg cells, set to one (n=3). Percentage (G) and absolute number (H) of Foxp3-expressing thymic SP CD4+ T cells from Tln1fl/flFoxp3GFP or Tln1fl/flCd4CreFoxp3GFP mice (n=3). (I) Foxp3 expression on a per cell basis (MFI) from Foxp3+CD4+ thymic Treg cells (n=3). (J) Expression of suppressive molecules IL-2Rα, CD39, CD73, GITR and CTLA4 on thymic Treg cells; displayed cells were gated on CD4+Foxp3+ cells (n=3). Data shown are mean ± SEM and are representative of at least 2 independent experiments. *, P < 0.05; **, P <0.01.
We next assessed whether expression of talin was required for Treg cell function. Using an in vitro suppression assay, we observed that Treg cells lacking talin were functionally deficient on a per cell basis (Fig. 3D). Multiple mechanisms of suppression and corresponding markers have been identified in Treg cells, including production of adenosine by CD39 and CD73; expression of the TNF family member GITR; capture of IL-2 through high expression of the high affinity IL-2 receptor chain; downregulation or blocking of co-stimulatory molecules, CD80 and CD86, on APCs through constitutive expression of CTLA-4; and production of anti-inflammatory cytokines IL-10 and TGF-β1 (23, 38). Examination of suppressive molecules revealed that talin-deficient Treg cells exhibited reduced expression of IL-2Rα, CD39, GITR and CTLA-4, but not CD73 (Fig. 3E). Analysis of anti-inflammatory cytokines at the mRNA level in talin-deficient Treg cells revealed no significant defect in the production of TGFβ-1, but a significant reduction in IL-10 production (Fig. 3F). Taken together, these data suggest that the activated phenotype of T cells in Tln1fl/flCd4Cre mice may be attributable to both the reduced suppressive capacity as well as reduced size of the Treg cell pool.
We next investigated whether the numerical deficiency of peripheral Treg cells in Tln1fl/flCd4Cre mice may be due to a defect thymic development. However, we observed similar frequencies and numbers of Treg cells in the thymi of control and Tln1fl/flCd4CreFoxp3GFP mice (Fig. 3G, 3H) as well as comparable Foxp3 expression on a per cell basis (Fig. 3I). In addition, talin-deficient Treg cells in the thymus did not display defects in the expression of Treg cell suppressive molecules, indicating that talin may be dispensable during Treg cell development (Fig. 3J). Overall, these data suggest that talin may play a specific role in maintaining peripheral Treg cell homeostasis and survival.
Spontaneous lymphocyte activation in Tln1fl/flCd4Cre mice is Treg cell-dependent
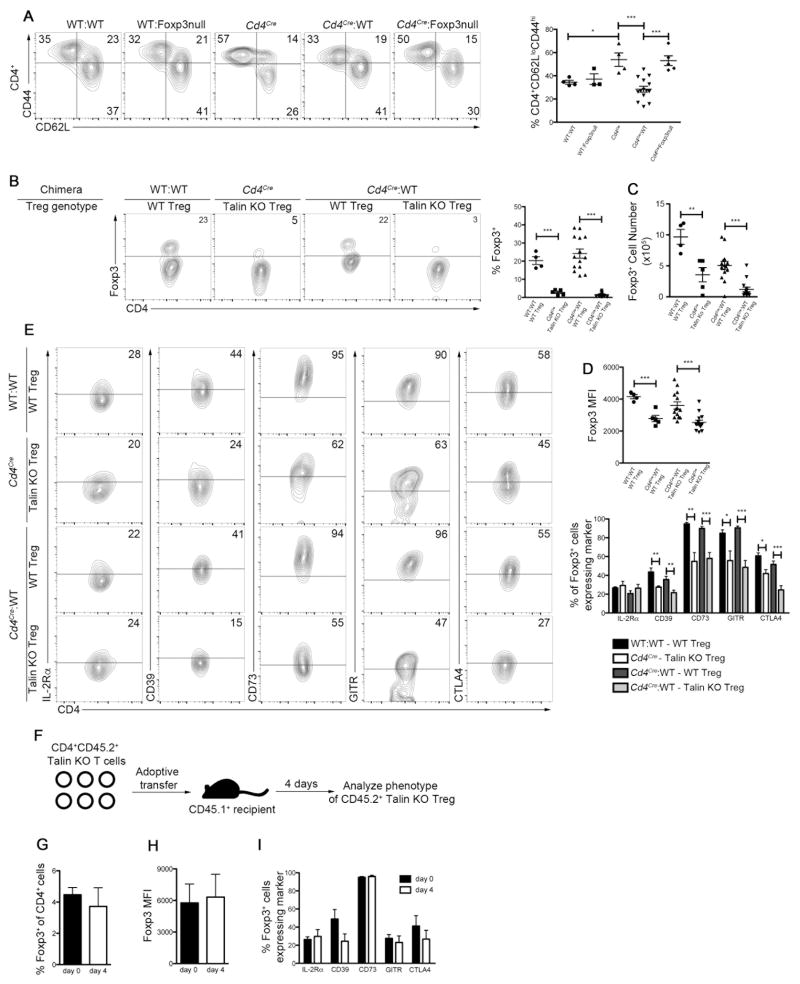
The numerical and functional deficiencies we observed in talin-deficient Treg cells led us to hypothesize that talin may be specifically required for Treg cells to prevent spontaneous lymphocyte activation. To test this possibility, we generated a series of bone marrow chimeras in which lethally irradiated RAG1-deficient mice were reconstituted with bone marrow cells from Tln1fl/flCd4Cre mice alone or in combination with bone marrow cells from wild-type or Foxp3-deficient mice (Supplemental Fig. 1). Reconstitution with Tln1fl/flCd4Cre bone marrow cells alone (Cd4Cre) or in combination with Foxp3-deficient bone marrow cells (Cd4Cre:Foxp3null) yielded mice exhibiting systemic CD4+ T cell activation that recapitulated the phenotype of Tln1fl/flCd4Cre mice (Fig. 4A). In contrast, reconstitution with Tln1fl/flCd4Cre bone marrow cells along with wild-type bone marrow cells (Cd4Cre:WT) was sufficient to prevent systemic T cell activation, as the percentage of activated CD4+CD44hiCD62Llo cells in mixed bone marrow chimeras was similar to that in bone marrow chimeras reconstituted with wild-type bone marrow (WT:WT) or wild-type bone marrow in combination with Foxp3-deficient bone marrow (WT:Foxp3null) (Fig. 4A). Further analysis revealed that talin-deficient Treg cells isolated from Cd4cre chimeras were present at significantly lower frequencies and absolute numbers and expressed significantly less Foxp3 on a per cell basis compared to wild-type Treg cells isolated from WT:WT chimeras. Moreover, talin-deficient Treg cells isolated from mixed Cd4cre:WT chimeras were also numerically deficient and exhibited a significant reduction in Foxp3 expression compared to wild-type Treg cells isolated from the same mixed chimera (Fig. 4B–D), suggesting that the spontaneous lymphocyte activation in Tln1fl/flCd4Cre mice is Treg cell-dependent.
Figure 4. Spontaneous lymphocyte activation in Tln1fl/flCd4Cre mice is Treg cell-dependent.
(A–E) Lethally irradiated RAG1-deficient mice were reconstituted with bone marrow cells from a CD45.2 wild-type donor in combination with cells from a CD45.1 wild-type donor (“WT:WT”, n=4), wild-type (CD45.2) bone marrow cells in combination with Foxp3-deficient (CD45.1) bone marrow cells (“WT:Foxp3null”, n=3), bone marrow cells from Tln1fl/flCd4Cre mice (CD45.2) alone (“Cd4Cre”, n=5), bone marrow cells from Tln1fl/flCd4Cre mice (CD45.2) in combination with wild-type (CD45.1) bone marrow cells (“Cd4Cre:WT”, n=14), and bone marrow cells from Tln1fl/flCd4Cre mice (CD45.2) in combination with Foxp3-deficient deficient (CD45.1) bone marrow cells (“Cd4Cre:Foxp3-null”, n=5). Mice were analyzed by flow cytometry 8–10 weeks after reconstitution. (A) Activation status of splenic CD4+ T cells based on CD62L and CD44 expression; displayed cells were gated on CD4+CD45.1+ events in WT:WT, WT:Foxp3null, Cd4 Cre:WT and Cd4 Cre:Foxp3null bone marrow chimeric mice and on CD4+CD45.2+ events in Cd4cre bone marrow chimeras. (B) Percentage of splenic CD4+ cells expressing Foxp3+; displayed cells were gated on CD4+CD45.2+ cells to identity talin-deficient Treg cells in Cd4cre and Cd4 Cre:WT bone marrow chimeras and on CD4+CD45.1+ cells in WT:WT and Cd4 Cre:WT chimeras to identity wild-type Treg cells. (C) Absolute numbers of talin-deficient splenic CD4+CD45.2+ Foxp3+ Treg cells in Cd4cre and Cd4cre:WT bone marrow chimeras and wild-type CD4+CD45.1+ Foxp3+ cells in WT:WT and Cd4 cre:WT chimeras. (D) Foxp3 expression on a per cell basis (MFI) from talin-deficient (CD45.2+) or wild-type (CD45.1+) CD4+Foxp3+ splenic Treg cells from WT:WT, Cd4cre and Cd4 Cre:WT bone marrow chimeras. (E) Expression of suppressive molecules IL-2Rα, CD39, CD73, GITR and CTLA4 by splenic talin-deficient (CD45.2+) or wild-type (CD45.1+) CD4+Foxp3+ splenic Treg cells from WT:WT, Cd4cre and Cd4 cre:WT bone marrow chimeras. (F) Experimental approach for the adoptive transfer of CD4+CD45.2+ T cells isolated from Tln1fl/flCd4CreFoxp3GFP mice into CD45.1+ wild-type recipients. Expression (G) and MFI (H) of Foxp3 in talin-deficient CD4+CD45.2+ Treg cells isolated from the spleens of recipient mice. (I) Expression of IL-2Rα, CD39, CD73, GITR and CTLA4 by CD4+CD45.2+GFP+ Treg cells isolated from the spleen of recipient mice. Data shown are mean ± SEM and are representative of at least 2 independent experiments. *, P < 0.05; **, P <0.01; ***, P<0.001.
Additional analysis revealed that talin-deficient Treg cells isolated from Cd4Cre:WT and Cd4Cre bone marrow chimeras exhibited decreased expression of CD39, GITR and CTLA4 compared to wild-type Treg cells, consistent with the phenotype of Treg cells isolated from Tln1fl/flCd4Cre mice (Fig. 4E). By contrast, there was no significant difference in the expression of IL-2Rα between wild-type Treg cells and talin-deficient Treg cells isolated from WT:WT, Cd4cre:WT, Cd4Cre, and Cd4Cre:Foxp3null bone marrow chimeras (Fig. 4E, Supplemental Fig. 2). However, this finding did not appear to result from a rescue of talin-deficient Treg cells through exposure to a wild-type environment; instead, it may be a peculiarity of the bone marrow chimera system, as IL-2Rα expression by wild-type Treg cells from WT:WT bone marrow chimeras was significantly reduced (Fig. 4E, Supplemental Fig. 2) relative to that by wild-type Treg cells from control Tln1fl/fl mice (Figure 3E, Supplemental Fig. 2). To test this possibility, we adoptively transferred talin-deficient Treg cells into congenically marked wild-type recipients and analyzed them four days after transfer. Analysis of talin-deficient Treg cells failed to demonstrate a significant increase in the expression of IL-2Rα, Foxp3 or any of the Treg cell suppressive molecules we examined (Fig. 4F–I), suggesting that neither the presence of wild-type Treg nor non-Treg T cells can rescue the number, phenotype or function of talin-deficient Treg cells. Taken together, these data indicate that the spontaneous lymphocyte activation observed in Tln1fl/flCd4Cre mice is primarily due to intrinsic defects in the Treg cell compartment.
Talin is required for the homeostasis of cTreg and eTreg cells
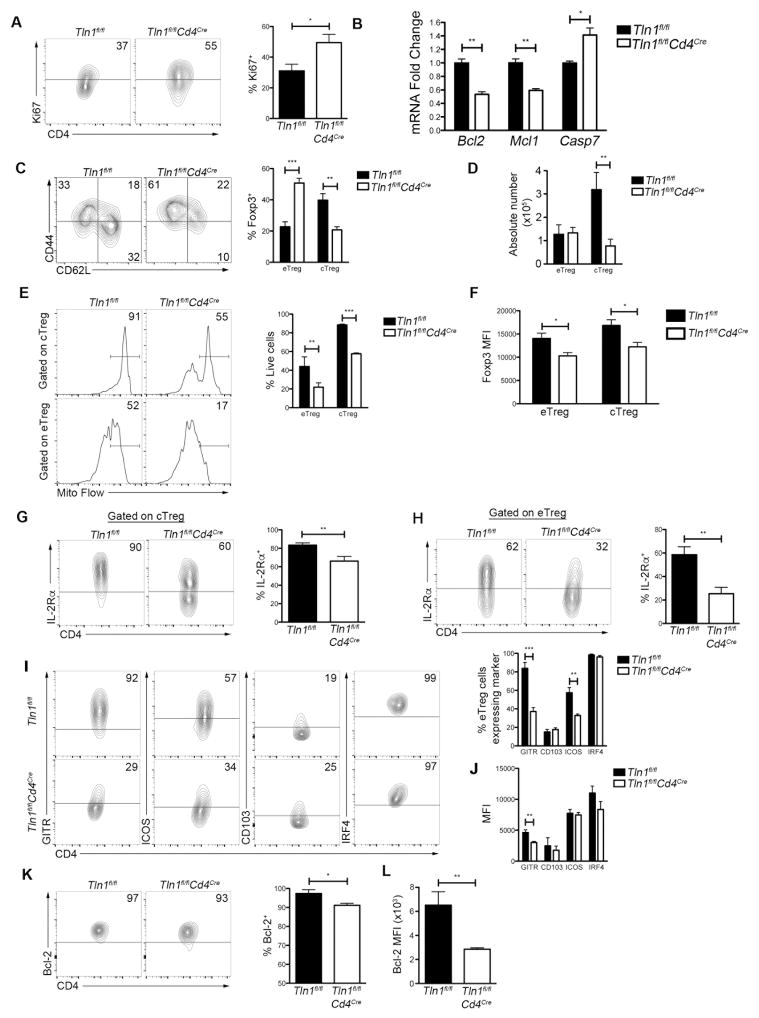
We next investigated the mechanisms accounting for the observed numerical deficiency of talin-deficient Treg cells. We initially hypothesized that talin-deficient Treg cells might have an impaired proliferative capacity. However, splenic Treg cells from Tln1fl/flCd4CreFoxp3GFP mice exhibited increased proliferation based on Ki67 expression (Fig. 5A). We next investigated whether talin-deficient Treg cells were more apoptotic than their wild-type counterparts. We assessed the expression of the pro-survival proteins Bcl-2 and Mcl-1 (39–41) and found that talin-deficient Treg cells expressed significantly lower mRNA levels of both molecules (Fig. 5B). Correspondingly, talin-deficient Treg cells expressed higher mRNA levels of the apoptosis executioner protein Caspase 7 (Casp7) (Fig. 5B), indicating that signals that promote survival may be impaired in the absence of talin.
Figure 5. Talin is required for the homeostasis of cTreg and eTreg cells.
(A) Ki67 expression in splenic Treg cells; displayed cells were gated on CD4+Foxp3+ cells (n=5). (B) Quantitative real-time PCR of Bcl2, Mcl1 and Casp7 mRNA expression by sorted GFP+ Treg cells isolated from Tln1fl/flFoxp3GFP or Tln1fl/flCd4CreFoxp3GFP mice. mRNA expression was normalized to the abundance of Rpl13 transcript and expressed relative to transcript abundance of control Treg cells, set to one (n=3). Frequency (C) and absolute number (D) of eTreg cells (CD62LloCD44hi) and cTreg cells (CD62hiCD44lo) isolated from Tln1fl/flFoxp3GFP or Tln1fl/flCd4CreFoxp3GFP mice (n=6). (E) Frequency of live CD4+Foxp3+ eTreg and cTreg cells assessed by Mito Flow staining for mitochondrial membrane potential (n=3). (F) Foxp3 expression on a per cell basis (MFI) in eTreg and cTreg cells (n=6). IL-2Rα expression by cTreg (G) and eTreg (H) cells (n=6). Frequencies of cells expressing GITR, ICOS, CD103, and IRF4 (I) and per cell expression (MFI) of these molecules (J) in eTreg cells isolated from Tln1fl/flFoxp3GFP or Tln1fl/flCd4CreFoxp3GFP mice (n=6). Frequencies of cells expressing Bcl-2 (K) and per cell expression (MFI) of Bcl-2 (L) in cTreg cells isolated from Tln1fl/flFoxp3GFP or Tln1fl/flCd4CreFoxp3GFP mice (n=6). Data shown are mean ± SEM and are representative of at least 3 independent experiments. *, P < 0.05; **, P <0.01; ***, P<0.001
We next investigated whether the dysregulation in the balance of pro- and anti-apopotic signals observed in the total Treg cell pool affected both eTreg and cTreg subsets. Although we observed an increase in the frequency of CD62LloCD44hi eTreg cells and a corresponding decrease in the frequency CD62LhiCD44lo cTreg cells (Fig. 5C), only the absolute number of cTreg cells was significantly altered (Fig. 5D), suggesting that talin may be more critical in the maintenance of the cTreg cell population. Nonetheless, both talin-deficient cTreg and eTreg cells were more highly apoptotic then their control counterparts, based on analysis using Mito Flow, a dye that measures mitochondrial membrane potential (Fig. 5E). Additionally, both talin-deficient eTreg and cTreg cells exhibited reduced expression of Foxp3 and IL-2Rα on a per cell basis (Fig. 5F–H), indicating that talin is required for the homeostasis of both Treg cell subsets. Lastly, characterization of molecules highly expressed by eTreg revealed reduced expression of GITR and ICOS, but not CD103 or IRF4, by talin-deficient eTreg cells (Fig. 5I, 5J). Further characterization of molecules highly expressed on cTreg cells revealed reduced expression of Bcl-2 by talin-deficient cTreg cells (Fig. 5K, 5L). Taken together, these findings indicate that although cTreg cells appear to be more dependent on talin for survival than eTreg cells, talin is nonetheless required for the function and homeostasis of both Treg cell subsets.
Talin mediates IL-2 responsiveness in Treg cells in a β2-integrin-dependent manner
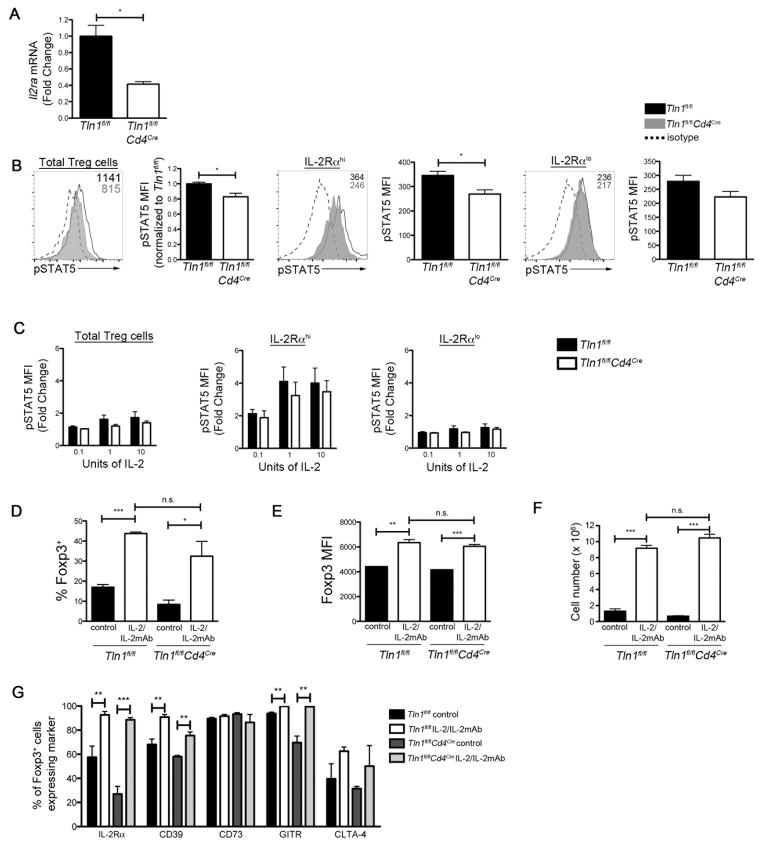
IL-2 signaling is essential for the homeostasis and survival of Treg cells (42) and has been shown to be a positive regulator of Foxp3, Mcl-1 and Bcl-2 expression in Treg cells (19, 20, 39). Moreover, IL-2 signaling directly upregulates expression of IL-2Rα (42). Having observed decreased cell surface expression of IL-2Rα (Fig. 3E, Fig. 5G, 5H) and mRNA expression of Mcl-1 and Bcl2 (Fig. 5B) in talin-deficient Treg cells, we hypothesized that IL-2 signaling in Treg cells might be impaired in the absence of talin.
Transcript levels of Il2ra were significantly lower in talin-deficient Treg cells compared to control cells (Fig. 6A), suggesting that IL-2 signaling is impaired as a consequence of talin deficiency. We therefore examined STAT5 phosphorylation, which occurs directly downstream of the IL-2R (42). When assessed directly ex vivo, talin-deficient Treg cells exhibited significantly lower levels of phosphorylated (p)STAT5 (Fig. 6B). Moreover, lower levels of pSTAT5 were observed even in IL-2Rαhi talin-deficient Treg cells compared to their control IL-2Rαhi counterparts. Both IL-2Rαlo talin-deficient and IL-2Rαlo control Treg cells (Fig. 6B) exhibited low levels of pSTAT5. We next investigated whether the inability of talin-deficient Treg cells to phosphorylate STAT5 could be rescued with high levels of IL-2 in vitro and in vivo with IL-2/IL-2 monoclonal antibody (mAb) complexes, which have been shown to target IL-2 specifically to Treg cells to induce a rapid expansion of the Treg cell pool (35, 36). Addition of exogenous IL-2 in vitro was capable of increasing STAT5 phosphorylation in talin-deficient IL-2Rαhi Treg cells to levels observed in wild-type cells (Fig. 6C). However, neither wild-type nor talin-deficient IL-2Rαlo Treg cells were capable of increasing STAT5 phosphorylation in response to exogenous IL-2 in vitro (Fig. 6C). Similarly, in vivo targeting of IL-2 to talin-deficient Treg cells with IL-2/IL-2 mAb complexes resulted in an increased frequency and absolute number of Foxp3+ talin-deficient Treg cells, along with increased Foxp3 expression on a per cell basis (Fig. 6D–F). Lastly, treatment with IL-2/IL-2 mAb complexes restored or increased the expression of suppressive molecules IL-2Rα, CD39, GITR and CTLA-4 in talin-deficient Treg cells to wild-type levels (Fig. 6G). Because only IL-2Rαhi, but not IL-2Rαlo, talin-deficient Treg cells were capable of phosphorylating STAT5 in response to exogenous IL-2 in vitro (Fig. 6C), administration of IL-2/IL-2 mAb complexes may have acted selectively on IL-2Rαhi talin-deficient Treg cells to expand the numbers of these cells. Taken together, these data show that talin plays a critical role in the maintenance of high levels of IL-2Rα by Treg cells.
Figure 6. Talin-deficient Treg cells exhibit increased apoptosis due to dysregulated IL-2 signaling.
(A) Quantitative real-time PCR of Il2ra transcript expression by GFP+ Treg cells isolated from Tln1fl/flFoxp3GFP or Tln1fl/fl Cd4CreFoxp3GFP mice. mRNA expression was normalized to the abundance of Rpl13 transcript and expressed relative to transcript abundance of control Treg cells, set to one (n=3). (B) Ex vivo pSTAT5 expression represented as histograms (left panel) and MFI (right panel) by total, IL-2Rαhi, or IL-2Rαlo Treg cells isolated from Tln1fl/flFoxp3GFP or Tln1fl/fl Cd4CreFoxp3GFP mice (n=3). (C) MFI of pSTAT5 expression after in vitro IL-2 stimulation (0.1, 1, or 10 U) in Treg cells isolated from Tln1fl/flFoxp3GFP (black line) or Tln1fl/flCd4CreFoxp3GFP mice (gray histogram) compared to isotype control (dotted line); displayed cells were gated on CD4+Foxp3+ cells (n=3). Frequency (D), Foxp3 MFI (E) and absolute number (F) of Foxp3+ Treg cells derived from Tln1fl/flFoxp3GFP and Tln1fl/flCd4CreFoxp3GFP mice given isotype or IL-2/IL-2 mAbs complexes (n=3). (G) Expression of suppressive molecules IL-2Rα, CD39, CD73, GITR and CTLA4 by Foxp3+ Treg cells derived from Tln1fl/flFoxp3GFP and Tln1fl/flCd4CreFoxp3GFP mice given isotype- or IL-2/IL-2 mAbs complexes; displayed cells were gated on CD4+Foxp3+ cells (n=3). Data shown are mean ± SEM and are representative of at least 3 independent experiments. *, P < 0.05; **, P <0.01; ***, P<.001.
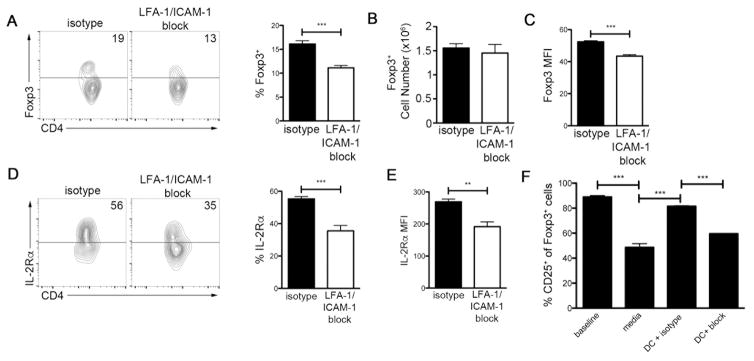
We hypothesized that talin may influence the maintenance of high levels of IL-2Rα expression by virtue of its effects on integrin activation, as talin has been shown to control activation of β1, β2 and β3 integrins (1). We focused on the integrin LFA-1 (αLβ2, CD11a/CD18) because its deficiency has been shown to result in defects in Treg cell numbers and suppressive capacity (43–45). We administered isotype or blocking anti-LFA-1 and anti-ICAM-1 mAbs to Foxp3 GFP reporter mice for three weeks. LFA-1/ICAM-1 blockade led to a significantly decreased frequency of Treg cells in the spleen (Fig. 7A) without changing the absolute number of Treg cells (Fig. 7B), presumably due to altered T cell trafficking to the lymph nodes. However, Treg cells isolated from LFA-1/ICAM-1 mAb-treated mice exhibited a reduction in Foxp3 expression on a per cell basis (Fig. 7C). Additionally, LFA-1/ICAM-1 blockade resulted in a decreased frequency of Treg cells expressing high levels of IL-2Rα (Fig 7D), as well as a reduction in the expression of IL-2Rα on a per cell basis (Fig. 7E). These findings suggested the possibility that integrin-dependent interactions between Treg cells and DCs might be critical in maintaining high expression of IL-2Rα. To test this possibility, we used a reductionist in vitro system in which sorted, wild-type Foxp3 GFP+ Treg cells were incubated with or without purified CD11c+ DCs. Incubation of Treg cells alone in serum-free media for 18 hours led to a reduction in the surface expression of IL-2Rα (Fig. 7F). By contrast, incubation of Treg cells with DCs was sufficient to maintain high IL-2Rα expression, which could be abrogated in the presence of blocking anti-LFA-1 and anti-ICAM-1 mAbs (Fig. 7F). Taken together, these data suggest that talin, by virtue of its role in facilitating integrin activation, may be required to maintain high levels of IL-2Rα that are critical for the homeostasis and survival of Treg cells.
Figure 7. Talin-mediated control of IL-2Rα expression on Treg cells is LFA-1-dependent.
Frequency (A), absolute number (B) and MFI (C) of CD4+Foxp3+ cells isolated from mice treated with isotype or LFA-1/ICAM-1 mAbs for 3 weeks (n=5). Frequency (D) and MFI (E) of CD4+Foxp3+ cells with high IL-2Rα expression isolated from mice treated with isotype or LFA-1/ICAM-1 mAbs for 3 weeks (n=5). (F) Frequency of cells with high IL-2Rα expression by sorted CD4+Foxp3+ cells directly ex vivo (‘baseline’) or cultured for 18 hours in serum-free media alone (‘media’), with purified CD11c+ DCs and isotype control mAb (‘DC + isotype’), or with purified CD11c+ DCs and blocking LFA-1 and ICAM-1 mAbs (‘DC + block’) (n=3). Data shown are mean ± SEM and are representative of at least 2 independent experiments. **, P <0.01; ***, P<.001.
Discussion
Maintenance of the Treg cell pool is essential for preventing autoimmunity. Here we show that talin deficiency in T cells resulted in spontaneous lymphocyte activation, primarily due to numerical and functional deficiencies in Treg cells. Strikingly, a reduced proportion of talin-deficient Treg cells were able to maintain high expression of IL-Rα in the periphery; moreover, talin-deficient IL-2Rαhi cells exhibited reduced STAT5 phosphorylation when assessed directly ex vivo compared to their control counterparts. High IL-2Rα expression was dependent on LFA-1-mediated Treg cell interactions with DCs and impaired IL-2Rα expression by IL-2Rαlo talin-deficient Treg cells could not be rescued, even with the addition of exogenous IL-2. Thus, our data suggest that talin, by virtue of its role in mediating integrin activation, is required for the maintenance of high IL-2Rα expression in peripheral Treg cells, thereby playing a critical role in their function, homeostasis and survival.
IL-2 signaling is a well-known mediator of Treg cell homeostasis and survival. IL-2 is produced primarily by activated CD4+ T cells in secondary lymphoid organs and can be consumed by cells expressing the high-affinity IL-2R. IL-2Rβ (CD122) and the common γ chain (CD132), which comprise the IL-2R, and IL-2Rα (46). The association of IL-2Rα with the IL-2R increases the affinity of the receptor by 10- to 100-fold and Treg cells express constitutively high levels of IL-2Rα (25). IL-2 signaling reinforces the expression of Foxp3 and IL-2Rα in Treg cells and regulates the balance between apoptosis and proliferation (24, 39). Treg cells receiving IL-2 signal upregulate the survival factors Bcl-2 and Mcl-1; conversely, in the absence of IL-2, Treg cells upregulate apoptotic factors such as Bim, Bak and Bax (24). In the thymus, talin-deficient Treg cells expressed levels of Foxp3 and IL-2Rα comparable to that of wild-type Treg cells. In the periphery, however, talin-deficient Treg cells failed to maintain high levels of IL-2Rα and consequently exhibited impaired STAT5 phosphorylation and IL-2 signaling, resulting in reduced Foxp3 expression. Impaired IL-2 signaling also led to reduced Bcl-2 and Mcl-1 expression and a two-fold reduction in total peripheral Treg cell numbers in Tln1fl/flCd4Cre mice. Taken together, our data suggest that talin is required for maintenance of Treg cell numbers owing to its role in IL-2 signaling and its downstream effects on the expression of Foxp3 and regulators that control balance between apoptosis and survival.
IL-2 signaling is known to be specifically required for the homeostasis and survival of quiescent, long-lived cTreg cells, which consequently express high levels of IL-2Rα. Conversely, highly proliferative and apoptotic eTreg cells rely on ICOS signaling to regulate their survival (25). Our data suggest that talin influences the function of both eTreg and cTreg cell subsets, although it may be more critical in the maintenance of the cTreg cell pool. Deletion of talin from T lymphocytes resulted in a loss of splenic cTreg cells, likely due to their inability to maintain high surface expression of IL-2Rα, thereby reducing the amount of IL-2 signal these cells can receive, impairing the expression of Bcl-2 and ultimately leading to increased cell death. By contrast, the absolute number of eTreg cells was not reduced in the absence of talin, perhaps because eTreg cell survival does not depend on IL-2 signaling (25). Furthermore, the observation of normal numbers of eTreg cells suggests that talin may be dispensable for the conversion of cTreg cells into eTreg cells. However, talin-deficient eTreg cells were not able to maintain wild-type expression levels of Foxp3, ICOS or GITR, which may influence the apoptotic rates and suppressive function of these cells and raises the possibility that expression of IL-2Rα may be required to maintain the expression of these molecules by eTreg cells. Thus, our data suggest that talin is required for various aspects of the homeostasis and function of the cTreg and eTreg cell pools.
Our data also suggest a requirement for integrin signaling, particularly through the integrin LFA-1, in the maintenance of high IL-2Rα expression by Treg cells. LFA-1 has been previously shown to be required for Treg cell clustering around DCs, which positions Treg cells to receive IL-2 signals by rare IL-2-expressing effector T cells that have been activated by self-antigen (47). In addition, these close intercellular interactions enable Treg cells to physically block interactions between APCs and effector T cells, facilitating the binding of CTLA-4 on Treg cells to CD80 or CD86 expressed by APCs (48). Recent evidence also suggests that the adhesion of Treg cells to DCs alters the cytoskeleton of the APCs, preventing them from activating naïve conventional T cells (49, 50) The importance of LFA-1 is further highlighted in observations from Itgb2−/− (CD18−/−) mice, which lack expression of β2 integrins. These mice develop autoimmune dermatitis, even when housed in germfree environments (49, 51). Moreover, Itgb2−/− mice harbor lower frequencies of Treg cells in the spleen and mesenteric lymph nodes and β2-deficient Treg cells display a reduced suppressive capacity both in vitro and in vivo (43). Lastly, β2 integrin-deficient Treg cells exhibit reduced expression of IL-2Rα (43), which mirrors the deficiencies we observed in talin-deficient Treg cells. Our finding that LFA-1/ICAM-1 interactions in vivo are required for high expression of IL-2Rα and Foxp3 by Treg cells provides a possible explanation for these prior observations in Itgb2−/− mice.
Because talin is known to be essential for the regulation of integrin activation, our studies suggest an essential role for integrin activation in maintaining high expression of IL-2Rα by Treg cells in the periphery and thereby influencing Treg cell homeostasis and survival. These findings are particularly intriguing in light of prior evidence linking defective Treg cell function and polymorphisms in IL-2Rα with a variety of human immune-mediated disorders, including systemic lupus erythematosis, rheumatoid arthritis, type 1 diabetes, and inflammatory bowel disease (52–56). Targeting the IL-2 pathway with low dose IL-2 or IL-2/IL-2 mAbs has been proposed as one approach to enhance Treg cell numbers and suppressive function (25). Our findings raise the possibility that therapies targeting integrins in Treg cells may help to reinforce high expression of IL-2Rα, thereby potentially enhancing their numbers and suppressive capacity.
Supplementary Material
Acknowledgments
This work was supported by NIH grants (DK093507, OD008469, and AI095277 to J.T.C.; HL078784 and HL117061 to B.G.P.; HL31950 to M.H.G.) and a Crohn’s and Colitis Foundation of America Senior Research Award (J.T.C.). J.T.C. is a Howard Hughes Medical Institute Physician-Scientist Early Career Awardee. J.E.K. and P.J.M. were supported by NIH grant T32DK007202.
We thank A. Rudensky for providing Foxp3GFP mice. We thank members of the Chang, Petrich and Ginsberg labs for helpful discussions and critical reading of the manuscript. The authors declare no conflicting financial interests.
References
- 1.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Alvarez B, de Pereda JM, Calderwood DA, Ulmer TS, Critchley D, Campbell ID, Ginsberg MH, Liddington RC. Structural determinants of integrin recognition by talin. Mol Cell. 2003;11:49–58. doi: 10.1016/s1097-2765(02)00823-7. [DOI] [PubMed] [Google Scholar]
- 3.Critchley DR, Gingras AR. Talin at a glance. Journal of cell science. 2008;121:1345–1347. doi: 10.1242/jcs.018085. [DOI] [PubMed] [Google Scholar]
- 4.Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- 5.Calderwood DA, I, Campbell D, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haling JR, Monkley SJ, Critchley DR, Petrich BG. Talin-dependent integrin activation is required for fibrin clot retraction by platelets. Blood. 2011;117:1719–1722. doi: 10.1182/blood-2010-09-305433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manevich-Mendelson E, Grabovsky V, Feigelson SW, Cinamon G, Gore Y, Goverse G, Monkley SJ, Margalit R, Melamed D, Mebius RE, Critchley DR, Shachar I, Alon R. Talin1 is required for integrin-dependent B lymphocyte homing to lymph nodes and the bone marrow but not for follicular B-cell maturation in the spleen. Blood. 2010;116:5907–5918. doi: 10.1182/blood-2010-06-293506. [DOI] [PubMed] [Google Scholar]
- 8.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 9.Simonson WT, Franco SJ, Huttenlocher A. Talin1 regulates TCR-mediated LFA-1 function. J Immunol. 2006;177:7707–7714. doi: 10.4049/jimmunol.177.11.7707. [DOI] [PubMed] [Google Scholar]
- 10.Nolz JC, Medeiros RB, Mitchell JS, Zhu P, Freedman BD, Shimizu Y, Billadeau DD. WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse. Molecular and cellular biology. 2007;27:5986–6000. doi: 10.1128/MCB.00136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wernimont SA, Wiemer AJ, Bennin DA, Monkley SJ, Ludwig T, Critchley DR, Huttenlocher A. Contact-dependent T cell activation and T cell stopping require talin1. J Immunol. 2011;187:6256–6267. doi: 10.4049/jimmunol.1102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 14.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 15.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nature genetics. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 16.Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nature reviews Immunology. 2015;15:283–294. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 19.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 20.Murawski MR, Litherland SA, Clare-Salzler MJ, Davoodi-Semiromi A. Upregulation of Foxp3 expression in mouse and human Treg is IL-2/STAT5 dependent: implications for the NOD STAT5B mutation in diabetes pathogenesis. Annals of the New York Academy of Sciences. 2006;1079:198–204. doi: 10.1196/annals.1375.031. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 22.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 25.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259:40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, Campbell DJ. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. The Journal of experimental medicine. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fohse L, Reinhardt A, Oberdorfer L, Schmitz S, Forster R, Malissen B, Prinz I. Differential postselection proliferation dynamics of alphabeta T cells, Foxp3+ regulatory T cells, and invariant NKT cells monitored by genetic pulse labeling. Journal of immunology. 2013;191:2384–2392. doi: 10.4049/jimmunol.1301359. [DOI] [PubMed] [Google Scholar]
- 28.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends in immunology. 2013;34:74–80. doi: 10.1016/j.it.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nature immunology. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, Ohkura N, Morikawa H, Poeck H, Schallenberg S, Riess D, Hein MY, Buch T, Polic B, Schonle A, Zeiser R, Schmitt-Graff A, Kretschmer K, Klein L, Korn T, Sakaguchi S, Schmidt-Supprian M. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, Kallies A. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nature immunology. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 32.Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, Tiedt R, Skoda RC, Monkley SJ, Critchley DR, Ginsberg MH. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. The Journal of experimental medicine. 2007;204:3103–3111. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 36.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of experimental medicine. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arsenio J, Kakaradov B, Metz PJ, Kim SH, Yeo GW, Chang JT. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nature immunology. 2014;15:365–372. doi: 10.1038/ni.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, Humblet-Baron S, Schonefeldt S, Herold MJ, Hildeman D, Strasser A, Bouillet P, Lu LF, Matthys P, Freitas AA, Luther RJ, Weaver CT, Dooley J, Gray DH, Liston A. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nature immunology. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama K, Nakayama K, Negishi I, Kuida K, Shinkai Y, Louie MC, Fields LE, Lucas PJ, Stewart V, Alt FW, et al. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science. 1993;261:1584–1588. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- 41.Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- 42.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 43.Marski M, Kandula S, Turner JR, Abraham C. CD18 is required for optimal development and function of CD4+CD25+ T regulatory cells. J Immunol. 2005;175:7889–7897. doi: 10.4049/jimmunol.175.12.7889. [DOI] [PubMed] [Google Scholar]
- 44.Gultner S, Kuhlmann T, Hesse A, Weber JP, Riemer C, Baier M, Hutloff A. Reduced Treg frequency in LFA-1-deficient mice allows enhanced T effector differentiation and pathology in EAE. European journal of immunology. 2010;40:3403–3412. doi: 10.1002/eji.201040576. [DOI] [PubMed] [Google Scholar]
- 45.Wohler J, Bullard D, Schoeb T, Barnum S. LFA-1 is critical for regulatory T cell homeostasis and function. Molecular immunology. 2009;46:2424–2428. doi: 10.1016/j.molimm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malek TR. The biology of interleukin-2. Annual review of immunology. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z, Gerner MY, Van Panhuys N, Levine AG, Rudensky AY, Germain RN. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. 2015;528:225–230. doi: 10.1038/nature16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Ganguly A, Mucsi AD, Meng J, Yan J, Detampel P, Munro F, Zhang Z, Wu M, Hari A, Stenner MD, Zheng W, Kubes P, Xia T, Amrein MW, Qi H, Shi Y. Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy. The Journal of experimental medicine. 2017;214:327–338. doi: 10.1084/jem.20160620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan J, Liu B, Shi Y, Qi H. Class II MHC-independent suppressive adhesion of dendritic cells by regulatory T cells in vivo. The Journal of experimental medicine. 2017;214:319–326. doi: 10.1084/jem.20160629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, Montgomery CA, Rich S, Beaudet AL. Spontaneous skin ulceration and defective T cell function in CD18 null mice. The Journal of experimental medicine. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouzid D, Amouri A, Fourati H, Marques I, Abida O, Tahri N, Goncalves CP, Masmoudi H. Polymorphisms in the IL2RA and IL2RB genes in inflammatory bowel disease risk. Genet Test Mol Biomarkers. 2013;17:833–839. doi: 10.1089/gtmb.2013.0291. [DOI] [PubMed] [Google Scholar]
- 53.Orru V, Steri M, Sole G, Sidore C, Virdis F, Dei M, Lai S, Zoledziewska M, Busonero F, Mulas A, Floris M, Mentzen WI, Urru SA, Olla S, Marongiu M, Piras MG, Lobina M, Maschio A, Pitzalis M, Urru MF, Marcelli M, Cusano R, Deidda F, Serra V, Oppo M, Pilu R, Reinier F, Berutti R, Pireddu L, Zara I, Porcu E, Kwong A, Brennan C, Tarrier B, Lyons R, Kang HM, Uzzau S, Atzeni R, Valentini M, Firinu D, Leoni L, Rotta G, Naitza S, Angius A, Congia M, Whalen MB, Jones CM, Schlessinger D, Abecasis GR, Fiorillo E, Sanna S, Cucca F. Genetic variants regulating immune cell levels in health and disease. Cell. 2013;155:242–256. doi: 10.1016/j.cell.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vella A, Cooper JD, Lowe CE, Walker N, Nutland S, Widmer B, Jones R, Ring SM, McArdle W, Pembrey ME, Strachan DP, Dunger DB, Twells RC, Clayton DG, Todd JA. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet. 2005;76:773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lowe CE, Cooper JD, Brusko T, Walker NM, Smyth DJ, Bailey R, Bourget K, Plagnol V, Field S, Atkinson M, Clayton DG, Wicker LS, Todd JA. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nature genetics. 2007;39:1074–1082. doi: 10.1038/ng2102. [DOI] [PubMed] [Google Scholar]
- 56.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, Chen SL, Rosa R, Cumiskey AM, Serreze DV, Gregory S, Rogers J, Lyons PA, Healy B, Smink LJ, Todd JA, Peterson LB, Wicker LS, Santamaria P. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nature genetics. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.