Abstract
This study explored the relationships among multimodal imaging, clinical features, and language impairment in patients with left temporal lobe epilepsy (LTLE). Fourteen patients with LTLE and 26 controls underwent structural MRI, functional MRI, diffusion tensor imaging, and neuropsychological language tasks. Laterality indices were calculated for each imaging modality and a principal component (PC) was derived from language measures. Correlations were performed among imaging measures, as well as to the language PC. In controls, better language performance was associated with stronger left-lateralized temporo-parietal and temporo-occipital activations. In LTLE, better language performance was associated with stronger right-lateralized inferior frontal, temporo-parietal, and temporo-occipital activations. These right-lateralized activations in LTLE were associated with right-lateralized arcuate fasciculus fractional anisotropy. These data suggest that interhemispheric language reorganization in LTLE is associated with alterations to perisylvian white matter. These concurrent structural and functional shifts from left to right may help to mitigate language impairment in LTLE.
Keywords: Temporal Lobe Epilepsy, Cortical Thickness, DTI, Functional MRI, Language
1. Introduction
Temporal lobe epilepsy (TLE) is the most common localization-related epilepsy in adults and it is highly refractory to pharmacological treatment (Zimprich et al., 2004). Due to the involvement of the temporal lobes in language and semantic processing (Pujol, Deus, Losilla, & Capdevila, 1999; Springer et al., 1999), patients with chronic TLE often have language impairment, including poor auditory naming, visual naming, and verbal fluency (Bell et al., 2001; Hamberger & Tamny, 1999; N’Kaoua, Lespinet, Barsse, Rougier, & Claverie, 2001; Oyegbile et al., 2004). This is particularly characteristic of patients with left TLE (LTLE) who may have damage to the left hippocampus, lateral temporal neocortex, and perisylvian white matter (Ahmadi et al., 2009; Lin, Riley, Juranek, & Cramer, 2008; McDonald et al., 2008).
Standard of care for patients with well-localized pharmacoresistant TLE is anterior temporal lobectomy (ATL), which typically consists of the removal of anterior portion of the hippocampus, parahippocampal gyrus, and amygdala, with variable resection of the temporal lobe neocortex and underlying white matter (Jette, Sander, & Kezzer, 2016; Wiebe, Blume, Girvin, & Eliasziw, 2001). Thus, removal of the left (i.e., typically language dominant) temporal lobe may exacerbate language deficits in patients with LTLE if language networks have not relocated in response to injury (Hermann et al., 1999; Bonelli et al., 2012). Fortunately, in many patients with LTLE, language networks may “shift” to homologous regions in the right hemisphere in an adaptive process called reorganization. This is most frequently observed in patients with an early age of seizure onset, left-handedness and the presence of mesial temporal sclerosis (MTS) on magnetic resonance imaging (MRI) (Pataraia et al., 2004; Springer et al., 1999). However, because the likelihood and degree of reorganization differs significantly across patients, a major goal of the presurgical evaluation is to localize language networks in TLE in efforts to quantify the risk for language decline following ATL.
In recent years, functional MRI (fMRI) has emerged as a popular method for lateralizing language networks in patients with LTLE, and there is some evidence that it can successfully predict language decline following ATL (Bonelli et al., 2012; Sabsevitz et al., 2003). Specifically, studies have shown that patients with LTLE who have a more bilateral or right-sided language activation pattern in perisylvian regions, including inferior prefrontal and posterior superior temporal lobe regions, experience less decline on neuropsychological measures of language than those with a left-lateralized pattern (Sabsevitz et al., 2003). However, only a portion of the patients with LTLE with right-sided language on fMRI have preserved language functions, suggesting that the blood-oxygen-level dependent (BOLD) response may not reflect all the factors involved in successful language reorganization (Labudda, Mertens, Janszky, Bien, & Woermann, 2012).
Recent studies have suggested that language reorganization may depend on the degree of structural or microstructural alterations within perisylvian networks, but the evidence in patients with TLE is mixed. In healthy controls, some studies have shown a relationship between structural asymmetry of arcuate fasciculus (ARC) and functional asymmetry (e.g., Propper et al., 2010), whereas others have not (e.g., Vernooij et al., 2007). In patients with LTLE, Powell et al. (2007) found that rightward asymmetry in fractional anisotropy (FA) of frontotemporal fiber tracts in LTLE was associated with rightward asymmetry in fMRI language activations, suggesting that language reorganization in LTLE may be explained by alterations to the left hemisphere white matter. Conversely, Rodrigo et al. (2008) found that an association between reduced asymmetry in FA of the ARC and reduced asymmetry in fMRI activations was only observed in patients with right TLE, but not in patients with LTLE, suggesting a de-coupling of white matter microstructure and the BOLD response. This inconsistency highlights the complexity of language reorganization in patients with LTLE, warranting additional research into the underlying architecture of language networks in TLE.
There is also emerging evidence that reorganization within neocortical structures may accompany and/or facilitate a shift in the BOLD response. Labudda et al., (2012) studied 20 LTLE patients with typical and 20 LTLE patients with atypical (i.e., right-sided) language dominance on fMRI. They found that LTLE patients with atypical language dominance had increased gray matter volumes within right-sided temporo-lateral and frontal regions relative to those with typical language dominance. Furthermore, the degree of atypical fronto-temporal language activation correlated with temporal and frontal lobe gray matter volumes. However, patients with typical and atypical language dominance did not differ in terms of language performance. These findings complement those from multimodal imaging studies of fMRI- diffusion tensor imaging (DTI), suggesting that functional reorganization within language networks may depend, in part, on underlying structural changes. However, these structural and functional “shifts” do not always co-occur, nor do they appear to necessarily facilitate language performance.
Although existing studies have examined associations between fMRI-DTI or fMRI-volumetric MRI to understand language reorganization in TLE, no studies have combined all three imaging modalities to our knowledge. Therefore, the goals of this study were (1) to characterize the relationship between fMRI language lateralization and asymmetries in both DTI and volumetric MRI measures, and (2) to determine the contribution of each brain imaging derived measure to language performance in LTLE. In addition, we examined the contribution of important clinical/demographic variables (i.e., age of seizure onset, handedness, presence of MTS) to both our imaging and neuropsychological measures. Such information could yield critical insight into the neurobiological underpinnings of language reorganization in LTLE, which may help to explain variability in pre-operative language functioning as well as postoperative language decline following left ATL.
2. Methods
2.1. Participants
This study was approved by the Institutional Review Board at the University of California, San Diego (UCSD) and all participants provided informed consent according to the Declaration of Helsinki. Fourteen patients with a diagnosis of LTLE and 26 healthy controls were included in this study. All patients were medically-refractory and under evaluation for surgical treatment at the UCSD Epilepsy Center and were diagnosed by board-certified neurologists with expertise in epileptology, according to the criteria defined by the International League Against Epilepsy (Kwan et al., 2010). Patients were classified as LTLE based on seizure onsets recorded by video-EEG telemetry, supported by seizure semiology and neuroimaging results. Clinical MRI scans were available on all patients (i.e., T1-weighted, T2-weighted, and coronal FLAIR sequences with 1mm slices through the mesial temporal lobe). MRIs were visually inspected by a board-certified neuroradiologist for detection of MTS and the exclusion of contralateral temporal lobe structural abnormalities. MRI findings revealed that nine patients had ipsilateral MTS and five patients had normal MRIs. No patients showed evidence of contralateral MTS or extra-hippocampal pathology on clinical MRI. The clinical characteristics and medication information for all patients are presented in Table 1. Control participants were screened for neurological or psychiatric conditions.
Table 1.
Clinical information for the LTLE patients with atypical and typical language dominance
| ID | Sex | Handedness | Age | Age of Onset | MTS | First Language* | Medications | Seizure Frequency (#/month) |
|---|---|---|---|---|---|---|---|---|
| Atypical (A) | ||||||||
| A1 | F | Right | 27 | 13 | Yes | Vietnamese | levetiracetam, lamotrigine | 4 |
| A2 | F | Left | 43 | 6 | Yes | English | carbamazepine, phenobarbital, divalproex sodium | 5 |
| A3 | F | Right | 51 | 5 | Yes | English | levetiracetam, valproid acid, clonazepam | 3 |
| A4 | F | Left | 50 | 40 | Yes | Spanish | carbamezapine | 8 |
| A5 | F | Right | 53 | 5 | No | English | phenytoin, methylphenylbarbital | 8 |
| A6 | F | Right | 26 | 24 | No | English | topiramate, oxcarbezapine | 5 |
|
| ||||||||
| Typical (T) | ||||||||
| T1 | M | Right | 53 | 35 | Yes | English | carbamezapine, lamotrigine, levetiracetam | 6 |
| T2 | M | Right | 48 | 38 | No | English | phenytoin, zonisamide, lacosimide | 2 |
| T3 | M | Right | 61 | 33 | Yes | English | lamotrigine, lacosimide, divalproex sodium | 10 |
| T4 | F | Right | 35 | 1 | Yes | English | carbamezapine | 10 |
| T5 | F | Left | 33 | 1.5 | No | English | lamotrigine, lorazepman, phenytoin | 5 |
| T6 | M | Right | 33 | 12 | Yes | English | levetiracetam, lamotrigine | 60 |
| T7 | F | Right | 36 | 18 | Yes | English and Japanese | carbamezapine | 30 |
MTS indicates mesial temporal sclerosis
All patients were fluent in English based on a language screening measure
The mean age of the LTLE group (M = 40.1, SD = 12) was not statistically different from the control group (M = 36.3, SD = 14.2), t(38) = 0.85, p = .4. However, the healthy controls attained more years of education (M = 15.9, SD = 2.4) than patients with LTLE (M = 14.1, SD = 1.8), t(38) = −2.46, p = .019. The distribution of the sex and handedness were comparable between groups (sex: χ2 = 1.71, p = .191; handedness: χ2 = 3.13, p = .077), and the distribution of the language status (i.e., English as first language versus second language) was also comparable between groups (χ2 = 1.91, p = .386). One patient and three controls’ fMRI data was removed due to excessive head motion.
2.2. Materials and Procedures
fMRI, volumetric MRI, DTI, and neuropsychological testing of language were performed on all participants according to the procedures described below.
2.2.1. Neuropsychological tasks
Participants were administered the Boston Naming Test, a visual confrontation naming measure (BNT; Kaplan, Goodglass, & Weintraub, 1983); Auditory Naming Test, an auditory naming test in which participants are provided with verbal cues (ANT; Hamberger & Seidel, 2003), and Category Fluency (CF) and Letter Fluency (LF) subtests from the Delis-Kaplan Executive Function System (Delis, Kaplan, & Kramer, 2001), as part of a larger neuropsychological test battery. Category fluency and letter fluency are measures of sematic and phonemic fluency, respectively. Age-corrected scaled scores were calculated for the BNT, CF, and LF based on normative data provided in the test manuals; and z-scores were calculated for the ANT based on normative data published in Hamberger and Seidel (2003).
2.2.2. FMRI language task
During fMRI scanning, the participants performed an event-related semantic judgment task that has previously shown strong perisylvian activations in both healthy controls and patients with TLE (McDonald et al., 2010; Thesen et al., 2012). Two runs of the task were presented. In each run, participants were visually presented with one stimulus at a time as light gray letters on a black background in Arial font. For each run, the stimuli consisted of 80 novel words (NW) that were presented only once, 80 “old” words that were repeated 10 times thus 800 words altogether, 80 false font stimuli (FF), and 24 target words (animals). The NW stimuli were nouns with 4 to 8 letters, with a written lexical frequency of 3–80 per 10 million (Francis & Kucera, 1982). The FF stimuli were comprised of alphabet-like characters that were matched in size and number of characters to each NW stimulus in order to control for visual features of the stimuli (McDonald et al., 2009). The target words consisted of moderate to low frequency animal names. Participants were instructed to respond to the presence of target words by pressing a button. The stimuli were presented in pseudo-random order with a rapid 900-ms stimulus onset asynchrony followed by a 600-ms crosshair. Temporal jittering with 198 500-ms null baseline trials (presenting only a visual crosshair) was optimally inserted throughout the runs, using the program Optseq2 (Dale, 1999). Presentation software (Neurobehavioral Systems, Inc, Albany, CA, U.S.A.) was used to present stimuli and collect participants’ responses. In this present study, the contrast between NW and FF stimuli was used as the primary contrast to model lexical-semantic processing. This contrast has been shown to be robust for language mapping in patients with TLE and healthy controls (McDonald et al., 2010; Thesen et al., 2012).
2.3. Data Acquisition
All patients were self-reported to have remained seizure-free for a minimum of 24 hours prior to the MRI scan. All imaging was performed on a General Electric Discovery MR750 3T scanner with an 8-channel phased-array head coil at the Keck Center for Functional MRI at UCSD. The sequence of image acquisition was a conventional three-plane localizer, GE calibration scan, a T1-weighted 3D customized FSPGR structural sequence (TR = 8.08 ms, TE = 3.16 ms, TI = 600 ms, flip angle = 8°, FOV = 256 mm, matrix = 256 × 192, slice thickness = 1.2 mm), two functional T2*-sensitive echo-planar imaging (EPI) scans (TR = 3000 ms, TE = 30 ms, flip angle = 90°, FOV = 220 mm, matrix = 64 × 64, slice thickness = 2.5 mm), and a 30-directional diffusion weighted sequence using a b-value of 1000 mm2/s with an additional b=0 volume (TR = 8000 ms, TE = 82.9 ms, flip angle = 90°, FOV = 240 mm, matrix = 96 × 96m, slice thickness = 2.5 mm, echo-spacing = 588 ms). The diffusion-weighted and fMRI scans were acquired for each individual using two different phase encoding directions (forward and reverse) to correct for geometric distortions in the EPI images (Holland, Kuperman, & Dale, 2010). For fMRI, the order and combination of the two phase encoding directions and two word lists were counterbalanced across the participants to control for order effects.
2.4. Pre-Processing of Imaging Data
2.4.1. Volumetric and cortical thickness
Individual T1-weighted images were used to construct models of each participant’s cortical surfaces using FreeSurfer software 5.1.0 (http://surfer.nmr.mgh.harvard.edu) (Dale, Fischl, & Sereno, 1999; Dale & Sereno, 1993). The reconstructed surfaces were visually inspected for any defects and manually edited according to established software guidelines. The cortical surface was then parcellated into regions of interest (ROIs) according to the Destrieux atlas (Destrieux, Fischl, Dale, & Halgren, 2010).
2.4.2. Functional MRI
The fMRI data analysis was carried out using Analysis of Functional NeuroImages (AFNI; Cox, 1996), SUMA (Saad & Reynolds, 2012), and Matlab programs (MathWorks, Natick, MA). Each participant’s data was preprocessed with the following steps. In order to accurately co-register functional and structural MRI datasets, any distortions caused by gradient nonlinearities and B0 magnetic field inhomogeneities in both functional and structural MRI were minimized according to Holland, Kuperman, and Dale (2010). Head motion between scans was removed by rigid-body registration to the first functional run and head motion within scan was removed using AFNI’s 3dvolreg. Each time series was shifted so that each slice was aligned to the first acquired slice using AFNI’s 3dTshift. EPI datasets were aligned to one another and to the T1-weighted images, and then were resampled to 2.5 mm3 isotropic voxels. Both cortical parcellations and subcortical volume segmentations were imported, aligned, and applied to the EPI using SUMA’s @SUMA_Make_Spec_FS. The time series data were also scaled by computing the mean of each voxel time series in order to calculate the percent signal change using AFNI’s 3dTstat and 3dcalc. Then, the preprocessed time series data for each individual were analyzed based on the general linear model using AFNI’s 3dDeconvolve with tent function. Six separate regressors spaced at a TR of 3 seconds for each stimulus type were used to cover a 15-sec period after stimulus onset. Thus, for each voxel, six amplitudes for each stimulus class were generated. Additional regressors were used to model motion residuals. Baseline drifts were also modeled using quadratic polynomials. The regression coefficients that formed the response function for the first through second post-stimulus time points (corresponding to 3–6 sec post-stimulus onset) were then used in general linear tests, for the contrast of NW and FF (NW-FF).
2.4.3. DTI
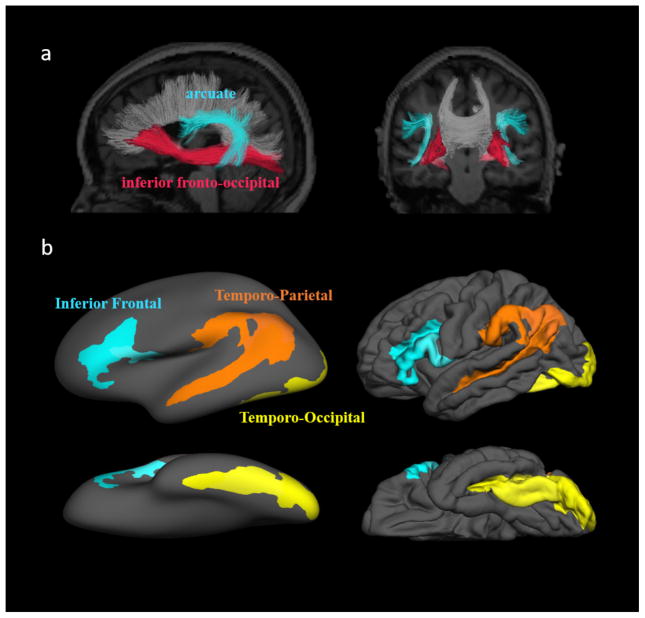
FA and mean diffusivity (MD) values were derived using an automated probabilistic diffusion tensor atlas of fiber track locations and orientation that was developed using in-house software written in Matlab and C++. This fiber track atlas has been validated in both healthy controls and patients with TLE (Hagler et al., 2009). A full description of the atlas and the steps used to create this atlas is provided in Hagler et al. (2009). For each participant, T1-weighted images were used to nonlinearly register the brain to a common space, and diffusion tensor orientation estimates were compared to the atlas to obtain a map of the relative probability that a voxel belongs to a particular fiber tract given the location and similarity of diffusion orientations. Voxels identified with FreeSurfer as cerebrospinal fluid (CSF) or gray matter were excluded from the fibers of interest (see the description in Hagler et al., 2009). Guided by the atlas, fiber tracts were segmented for each individual and mean FA and MD values were calculated based on that participant’s diffusion data. For this study, FA and MD were calculated for both the left and right ARC and inferior fronto-occipital fasciculus (IFOF) (Figure 1a). The ARC was considered the primary tract of interest due to its well-established role in the language network and putative connectivity between our cortical regions of interest in left inferior prefrontal (Broca’s area) and posterior superior temporal lobe regions (Wernicke’s area) (Catani & Mesulam, 2008; Friederici & Gierhan, 2013). The IFOF was also included due to evidence that this fiber tract is involved in semantic processing and object naming (Mandonnet, Nouet, Gatignol, Capelle, & Duffau, 2007), and is believed to subserve the direct ventral pathway for language (Almairac, Herbet, Moritz-Gasser, de Champfleur, & Duffau, 2015).
Figure 1.
a. The two white matter tracts of interest shown for a single individual. b. The three language regions of interest selected for the functional and volumetric MRI analyses. The inferior frontal region consists of the opercular, orbital, and triangular part of the inferior frontal gyrus (index 12, 13, and 14) and the inferior frontal sulcus (index 52) in the Destrieux altas. The temporo-parietal region consists of the angular gyrus (index 25), supramarginal gyrus (index 26), and superior temporal sulcus (index 73). The temporo-occipital region consists of the inferior occipital gyrus and sulcus (index 2), lateral occipito-temporal gyrus (index 21), occipital pole (index 42), anterior and posterior transverse collateral sulcus (index 50 and 51), and lateral occipito-temporal sulcus (index 60).
2.5. Post-Processing of Imaging Data
2.5.1. Laterality indices in MRI datasets
Multiple-parcelled Destrieux regions were combined to create three ROIs: temporo-occipital (TO), temporo-parietal (TP), and inferior frontal (IF) regions. Selection of these ROIs was guided by previous fMRI and magnetoencephalography (MEG) studies that have identified these three regions as critical to different aspects of language processing, including visual word form processing, lexical access, and phonological processing, respectively (e.g., Dehaene & Cohen, 2011; Poldrack et al., 1999; Thesen et al., 2012). These regions are also frequently used to determine language laterality in patients with TLE (e.g., Adcock, Wise, Oxbury, Oxbury, Matthews, 2003; Binder et al., 1996). The exact placement of each ROI was determined by the statistical activation maps of the NW-FF contrast from the healthy control group in this present study. The three ROIs are displayed in Figure 1b. For cortical thickness data, average thickness was calculated for the IF, TP, and TO regions separately for each hemisphere. For fMRI data, the number of activated voxels above the threshold of p = .01 with cluster size > 2.6 were counted within each of the ROIs for each hemisphere. For DTI data, average FA and MD values of the ARC and IFOF were calculated separately for the two hemispheres. Laterality indices (LI: [L-R]/[L+R]) between the two hemispheres then were calculated for all the brain imaging measures. A more positive LI indicates a more leftward asymmetry in the measures.
2.6. Statistical Analyses
2.6.1. Between and within group comparisons
Group differences were tested for each of the four neuropsychological measures and for each of the LIs across the ROIs in the three imaging modalities with either a Welch U test or Wilcoxon-Mann-Whitney (WMW) test, according to recommendations in Skovlund and Fenstad (2001). The equality of variances between the two groups was assessed by Levene’s test. Due to the unequal sample sizes between groups, a WMW test was performed and the Z value was reported if the variances were equal, whereas a Welch U test was performed and the F value was reported if the variances were unequal. Post-hoc paired t-tests were performed to test for significant differences between the right and left hemisphere imaging measures (i.e., to determine whether there was a laterality effect within each group) when the between group difference was not significant.
2.6.2. Development of the language principal component (PC)
Principal component analysis was used to reduce the number of neuropsychological variables (BNT, ANT, CF, and LF), and therefore, the likelihood of Type I errors, and to determine if the four language tasks could be reduced to a single meaningful “language” score based on the rule of eigenvalue greater than 1. This newly generated PC variable was named “Language PC,” and it was used to represent language performance in this study.
2.6.3. Correlation and regression analyses
Spearman rho correlations were performed to examine the relationships between the structural (DTI and volumetric MRI) and functional MRI imaging laterality indices, as well as test the relationship of all brain imaging laterality indices to the Language PC, co-varying for years of education and language status. The associations between brain imaging measures and clinical variables (i.e., age of seizure onset, MTS, and handedness) were also evaluated. Due to multiple comparisons made in this study and the possibility of Type I errors, a randomization test with 10000 repetitions was performed to determine the probability of getting the observed correlations. The randomization test was only performed on correlations with p < .05.
Hierarchical multiple regression was then used to build a language prediction model in LTLE. In addition, commonality analysis was used to determine the distinct contributions of variance from potential confounding variables, and ANOVA was used to test the significance of the incremental variances. To build this model, we first selected brain imaging variables based on whether they were directly correlated with the Language PC (“direct” variables) or indirectly related to the Language PC (correlated with the “direct” variables). These indirect variables were included in the model in order to test whether they mediate the relationship to the Language PC. The sequence of entering these variables was listed as follows: The first group of variables to be entered consisted of the cortical thickness and white matter laterality measures (Block 1). The second group of variables to be entered consisted of the group of BOLD laterality measures (Block 2). We entered the structural variables before functional variables due to preclinical evidence that structural changes appear to precede functional ones (O’Reilly et al., 2013). This allowed us to test whether functional laterality contributes additional variance beyond the structural information. Due to the high covariance among these measures, the commonality and ANOVA analyses were performed during each step to determine whether each modality contributed additional variance to the Language PC.
3. Results
Descriptive and inferential statistics for the neuropsychological and the brain imaging measures for each group are presented in Table 2.
Table 2.
Descriptive and inferential statistics for the language and imaging measures
| Variables | Control | LTLE | Statistical Result | p |
|---|---|---|---|---|
| Neuropsychological Task | ||||
| BNT ss | 10.68 (2.56) | 6.08 (1.55) | Z = −4.65 | < .001*** |
| ANT z | −0.48 (1.54) | −8.65 (11.76) | F(1, 12.2) = 6.23 | .028* |
| Letter Fluency ss | 13.08 (3.55) | 9 (3.62) | Z = −2.99 | .003** |
| Category Fluency ss | 13.23 (3.08) | 7.14 (2.88) | Z = −4.46 | <.001*** |
| Language PC | 0.87 (0.97) | −1.66 (1.51) | Z = −4.46 | <.001*** |
|
| ||||
| fMRI LI | ||||
| IF | 0.57 (0.27) | 0.31 (0.45) | F(1, 17.1) = 3.48 | .080+ |
| TP | 0.39 (0.33) | 0.34 (0.42) | Z = −0.16 | .869 |
| TO | 0.49 (0.29) | 0.13 (0.44) | F(1, 17.9) = 6.78 | .018* |
|
| ||||
| Cortical Thickness LI | ||||
| IF | 0 (0.02) | 0 (0.03) | F(1, 19.2) = 0.02 | .898 |
| TP | −0.02 (0.02) | −0.02 (0.02) | Z = 0.58 | .561 |
| TO | −0.01 (0.02) | −0.03 (0.02) | Z = −1.21 | .228 |
|
| ||||
| DTI LI FA | ||||
| IFOF | 0.01 (0.02) | 0 (0.02) | Z = −1.32 | .186 |
| ARC | 0.02 (0.02) | 0.01 (0.01) | Z = −1.48 | .140 |
|
| ||||
| DTI LI MD | ||||
| IFOF | 0.01 (0.02) | 0.03 (0.03) | F(1, 15.7) = 3.59 | .077+ |
| ARC | 0.01 (0.01) | 0.02 (0.02) | F(1, 14.6) = 3.96 | .066+ |
Note: In the column of statistical result, Z value is the result of Wilcoxon-Mann-Whitney test, and F value is the result of Welch U test.
< .001;
< .01;
< .05;
Not statistical significant, but shown a possible trend.
Patients with LTLE performed significantly worse than the controls on all neuropsychological measures of language. The four language tasks were highly correlated with each other across all participants (all Pearson r’s ranged from .49 to .67). The eigenvalues of principal component analysis were 2.74, 0.51, 0.45, and 0.3, and the first principal component accounted for 69% of the total variance using the four language measures. The loading values of the four language measures on the first principal component were: 0.81 in BNT, 0.78 in ANT, 0.83 in LF, and 0.88 in CF.
The fMRI laterality index was significantly lower in the LTLE group compared to the controls in the TO region, with a trend toward a lower laterality in the IF region. Post-hoc paired t-tests revealed that controls showed significantly greater activation in the left than in the right in IF and TP regions (IF: t(22) = −6.14, p < .001, TP: t(22) = −3.56, p = .002), whereas patients with LTLE only showed greater activation in the left TP region (IF: t(12) = −1.64, p = .126, TP: t(12) = −3.08, p = .01).
There were no significant differences in FA laterality between patients and controls in the two tracts of interest. In controls, FA values were greater in the left compared to the right for the ARC (t(24) = −4.52, p < .001), whereas there was a trend for greater left than right FA in the IFOF (t(24) = −1.85, p = .077). In patients with LTLE, there was only a trend for left greater than right FA was observed in the ARC (t(12) = −2.13, p = .055), but not in the IFOF (t(12) = 0.84, p = .418). In addition, patients with LTLE showed a trend for higher MD laterality in the ARC and IFOF compared with controls. In controls, MD values on the left were significantly greater than the right in the ARC (t(24) = −2.73, p = .012), but a trend in the IFOF (t(24) = −1.73, p = .096). In the patients with LTLE, the MD values on the left were significantly greater than the right in these two tracts (ARC: t(12) = −3.1, p = .009; IFOF: t(12) = −2.80, p = .016).
There were no differences between the patients with LTLE and controls in cortical thickness laterality for the three ROIs (IF, TP and TO). The paired t-tests revealed that both controls and the patients with LTLE had greater right than left cortical thickness in the TP (controls: t(25) = 4.37, p < .001; LTLE: t(13) = 2.39, p = .03) and TO regions (controls: t(25) = 3.81, p < .001; LTLE: t(13) = 4.39, p < .001), but not in the IF region (controls: t(25) = 0.64, p = .53; LTLE: t(13) = 0.31, p = .76).
3.1. Correlations between Clinical Variables and Brain Laterality Measures
In patients with LTLE, earlier age of seizure onset was associated with greater right-lateralized FA in the IFOF (ρ = 0.75, p = .003, randomization p = .001) and greater left-lateralized MD in IFOF and ARC (IFOF: ρ = −0.64, p = .018, randomization p = .01; ARC: ρ = −0.56, p = .049, randomization p = .027). Earlier age of seizure onset was also associated with greater right-lateralized cortical thickness in the TP region (ρ= 0.58, p = .031, randomization p = .018). Age of seizure onset was not associated with BOLD laterality in any of the ROIs. The presence of MTS was associated with greater right-lateralized cortical thickness in the IF region (b = 0.02, t = 2.51, p = .034) with a trend in the TP region (b = 0.01, t = 2.02, p = .074), but it was not associated with DTI or BOLD laterality. Left-handedness was associated with right-lateralized BOLD responses in IF region (b = −0.33, t = −2.42, p = .042), but not with any of other imaging laterality indices.
3.2. Correlations between fMRI, Cortical Thickness, and DTI Laterality
All of the correlations among imaging measures are displayed in Table 3. In controls, there were no significant associations observed between laterality of fMRI and laterality of cortical thickness or DTI measures. Conversely, in the patients with LTLE, greater right-lateralized BOLD responses in both the IF and TP regions were associated with greater right-lateralized FA in the ARC. In addition, greater right-lateralized BOLD responses in the TO region were associated with greater right-lateralized cortical thickness in the same region.
Table 3.
Correlations between different modalities in the controls and the patients with LTLE groups. Rp indicates the p value for randomization test. LI indicates the laterality index.
| Variable | Variable | Control | LTLE | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ρ | p | Rp | ρ | P | Rp | ||
| fMRI LI | Cortical Thickness LI | ||||||
| IF | IF | 0.27 | .206 | 0.07 | .817 | ||
| TP | TP | −0.13 | .565 | 0.21 | .482 | ||
| TO | TO | −0.09 | .675 | 0.70 | .008* | .005* | |
|
| |||||||
| fMRI LI | DTI LI FA | ||||||
| IF | IFOF | −0.03 | .904 | 0.48 | .112 | ||
| TO | IFOF | 0.09 | .687 | 0.14 | .665 | ||
| IF | ARC | 0.08 | .727 | 0.67 | .017* | .011* | |
| TP | ARC | −0.06 | .785 | 0.59 | .045* | .024* | |
|
| |||||||
| fMRI LI | DTI LI MD | ||||||
| IF | IFOF | 0.08 | .73 | −0.24 | .443 | ||
| TO | IFOF | −0.08 | .715 | −0.06 | .846 | ||
| IF | ARC | 0.04 | .872 | −0.22 | .499 | ||
| TP | ARC | −0.09 | .677 | 0.03 | .931 | ||
|
| |||||||
| Neuropsychology | fMRI LI | ||||||
| Language PC | IF | 0.33 | .139 | −0.66 | .026* | .014* | |
| Language PC | TP | 0.52 | .012* | .007* | −0.69 | .019* | .012* |
| Language PC | TO | 0.58 | .005* | .002* | −0.74 | .010* | .006* |
|
| |||||||
| Neuropsychology | Cortical Thickness LI | ||||||
| Language PC | IF | 0.10 | .648 | −0.16 | .618 | ||
| Language PC | TP | −0.13 | .536 | −0.16 | .618 | ||
| Language PC | TO | −0.10 | .619 | −0.68 | .015* | .008* | |
|
| |||||||
| Neuropsychology | DTI LI FA | ||||||
| Language PC | IFOF | 0.01 | .961 | −0.29 | .386 | ||
| Language PC | ARC | −0.32 | .126 | −0.46 | .151 | ||
|
| |||||||
| Neuropsychology | DTI LI MD | ||||||
| Language PC | IFOF | −0.13 | .560 | 0.22 | .519 | ||
| Language PC | ARC | −0.07 | .753 | 0.18 | .593 | ||
3.3. Correlations between Brain Imaging Measures and the Language PC
In controls, greater left-lateralized BOLD responses in the TP and TO regions were associated with better performance on the Language PC (Table 3). In the patients with LTLE, greater right-lateralized BOLD responses in all three ROIs and cortical thickness in the TO region were associated with better performance on the Language PC.
3.4. Proposed Language Model in the Patients with LTLE
Since all three BOLD responses (IF, TP, and TO) and cortical thickness in TO region were directly correlated with the Language PC, they were all included in the hierarchical model. FA of the ARC was also included in the model because it was associated with BOLD responses. The first step was to enter the structural variables (i.e., cortical thickness in the TO region and FA in the ARC) into the regression model. These two structural variables accounted for a total of 60% (R2adj = 0.49) of the variance. A follow-up commonality analysis revealed that the unique variance accounted for by FA in the ARC was 39% (total variance = 0.51), whereas cortical thickness laterality in the TO region accounted for 9% of the unique variance (total variance = 0.21). FA in the ARC (p = .02) accounted for significant variance in Language PC, but cortical thickness in the TO region did not (p = .185). Thus, only FA in ARC was retained in the model. After controlling for ARC FA, the three BOLD responses were entered. These three BOLD variables accounted for an additional 27% of the variance. A follow-up commonality analysis revealed that these three BOLD variables were highly co-linear (common variance: IF = 0.11, TP = 0.14, TO = 0.13), but BOLD in the TP had highest unique variance (IF = 0.01; TP = 0.11, TO = 0.01). To reduce the number of predictors in the model, only FA in the ARC and BOLD in the TP were retained. In total, these two variables explained up to 76% of the variance (R2adj = 0.70, p = .006) with the effect size of 0.70 (recommended by Yin & Fan, 2001) in Language PC.
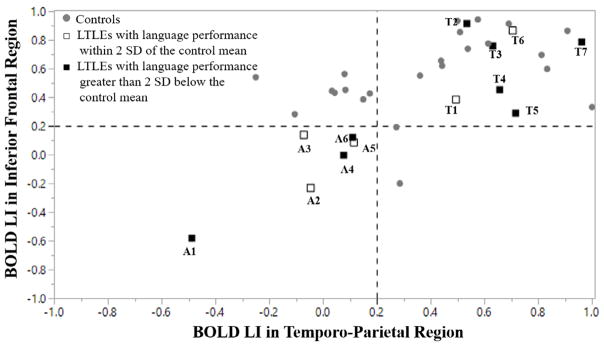
3.5. Typical versus Atypical Language Dominance
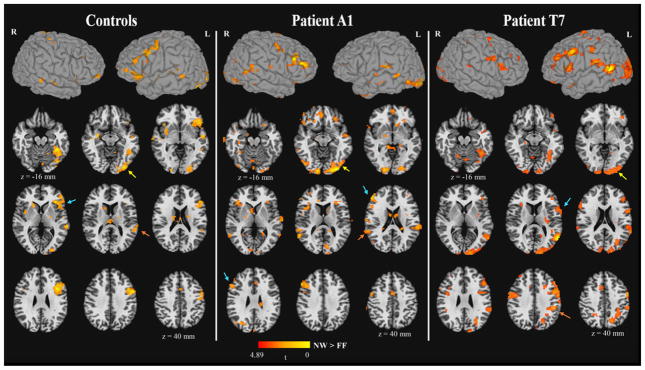
Commensurate with much of the clinical fMRI literature (e.g., Spring et al., 1999; Yuan et al., 2006; Gaillard et al., 2002), the patients were divided into those with “typical” and “atypical” language dominance based on their BOLD laterality in the IF and TP regions. If the individual’s BOLD LI was below 0.2 for both IF and TP regions, then that individual was categorized as “atypical.” The value of 0.2 for laterality index was chosen based on previous cut-offs used to characterize atypical language dominance (e.g., Janszky, Mertens, Janszky, Ebner, & Woermann, 2006; Labudda et al., 2012; Springer et al., 1999). Figure 2 plots the laterality index of BOLD responses in the IF and TP regions for all the patients with LTLE and controls. Six patients with LTLE were categorized as atypical based on this criterion, whereas 7 patients with LTLE were categorized as typical. Descriptive results showed that 50% (3 of 6) of the patients with LTLE with atypical language dominance scored less than 2 standard deviations below the mean of controls on the Language PC, whereas 71% (5 of 7)1 of the patients with LTLE with typical language dominance scored greater than 2 standard deviations below the mean of controls. To illustrate this at the individual patient level, Patient A1 had the most right-lateralized language activations (i.e., most atypical patient), and Patient T7 had the most left-lateralized language activations (i.e., most typical patient). Patient T7 obtained the lowest score on the Language PC. Both Patient A1 and Patient T7’s functional activation maps were presented in the middle and the right panel in Figure 3, respectively. Patient A1’s FA in the ARC (LI = 0.01) showed a lower leftward asymmetry relative to Patient T7 (LI = 0.035), who showed a pattern for left lateralized asymmetry.
Figure 2.
The plot for atypical (A) and typical (T) language dominance in both controls and patients with LTLE. The lower left quadrant indicates atypical language dominance. The empty square indicates that patient’s language performance was greater than 2 standard deviations below of the mean in the control group, whereas the solid square indicates that patient’ language performance was less than 2 standard deviations below the mean of the control.
Figure 3.
The fMRI activation maps2 for the contrast of NW > FF in the control group, Patient A1, and Patient T7. The activation in the inferior frontal, temporo-parietal, and temporo-occipital regions were indicated by the cyan, orange, yellow arrows, respectively. The z coordinate was presented in Talairach space. p < .01, cluster size > 25 voxels, corrected α < .05.
Clinical characteristics of the atypical and typical patients with LTLE were represented in Table 1. The results of the descriptive statistics showed that the patients with atypical language dominance tended to have an earlier age of seizure onset (M = 15.5, SD = 14.1) and longer seizure history (M = 26.2, SD = 19.9) than typical language dominance patients (onset: M = 19.8, SD = 15.8; history: M = 22.9, SD = 8.6). In addition, atypical patients with LTLE (ANT: M = −5.37, SD = 2.15; BNT: M = 6.17, SD = 1.32; LF: M = 9.5, SD = 4.04; CF: M = 8.17; SD = 1.33) tended to have better performances than typical patients with LTLE across all four tasks (ANT: M = −12.45, SD = 17.17; BNT: M = 6, SD = 2; LF: M = 8.43, SD = 3.74; CF: M = 6.14; SD = 3.76). Thirty-three percent of patients with atypical language dominance (2 of 6) were left-handed whereas 14% of typical patients with LTLE (1 of 7) were left-handed. Sixty-seven percent of patients with atypical language dominance (4 of 6) had MTS whereas 71% of typical patients with LTLE (5 of 7) had MTS. However, the sample sizes for both groups were too small to perform inferential statistics.
4. Discussion
With increasing use of fMRI for language localization in patients with TLE and other neurosurgical populations, there is great interest in understanding (1) the relationship between BOLD responses and underlying structural pathology, and (2) the association among different imaging measures and language performance in TLE. This is particularly relevant for the patients with LTLE, whose language networks may reorganize in response to an early epileptic insult. In this study, we showed that healthy controls had a left-lateralized pattern of language activation on fMRI, and also had a higher FA of the left relative to the right ARC at the group level. These findings are similar to those revealed by Powell et al. (2006) and Rodrigo et al. (2008) and are thought to reflect natural asymmetries intrinsic to a healthy perisylvian network. In addition, our results revealed that higher left-lateralized activations in our controls were associated with stronger performances on neuropsychological measures of language. Conversely, patients with LTLE showed the opposite pattern. That is, greater activations in the right hemisphere language homologues were associated with stronger language performances. Furthermore, this left to right “shift” in the BOLD response in the patients with LTLE was associated with a reduced asymmetry in FA of the ARC. This suggests that the degree of disruption within perisylvian white matter in the left hemisphere may result in an adaptive shift in language networks in LTLE. It is of interest that DTI laterality was directly associated with fMRI laterality in patients with LTLE, whereas this correlation did not hold in controls. This may be due to the restricted range of fMRI laterality values in healthy controls relative to the much broader range of values observed in patients with LTLE. This finding is in line with some existing literature that has failed to observe direct associations between DTI-fMRI in controls (e.g., Vernooij et al., 2007).
At the individual subject level, descriptive analysis further revealed that 71% of the patients with typical language dominance, whereas only 50% of the patients with atypical language dominance were impaired in their language performance compared with controls. These data add to the existing literature by not only demonstrating an association between functional and microstructural measures of reorganization in LTLE, but also by revealing a direct association of imaging measures to language performance at the group and individual subject levels.
Despite fMRI-DTI correlations, we did not find group differences in cortical thickness for either the IF or TP regions, nor did we find cortical thickness laterality within these regions to be associated with shifts in the BOLD response or language performances. This is not too surprising since there are data to suggest that cortical thickness does not necessarily correlate with the magnitude of the local BOLD response (Barnett, 2012; Hegarty et al., 2012; Squeglia et al., 2013). In addition, although white matter along late myelinating fiber tracts has been shown to be highly affected and this effect was greater in the side of seizure focus in TLE (Ahmadi et al. 2009; Kemmotsu et al., 2011; Lee et al., 2013), cortical thinning appears to follow a more bilateral pattern, perhaps making the laterality index less remarkable (Lin et al., 2007; McDonald et al., 2008). Furthermore, an earlier age of seizure onset has been associated with greater white matter alterations along frontotemporal white matter in TLE, whereas cortical thinning is more frequently associated with a longer disease duration (Bernhardt et al., 2009; Kemmotsu et. al, 2011; McDonald et al, 2008). These data suggest that white matter versus neocortical alterations in TLE may be due to different underlying disease characteristics (i.e., neurodevelopmental versus neurodegenerative, respectively), thus differentially affecting language abilities in LTLE. However, it is of interest that in the patients with LTLE with atypical language dominance, 67% showed right > left cortical thickness in the IF region and 83% showed right > left cortical thickness in the TP region, and this pattern was further influenced by the presence of MTS. Conversely, in patients with LTLE with typical language dominance only 29% and 43% showed this pattern, respectively. These trends in the data are consistent with those found in Labudda et al. (2012) and suggest that functional reorganization within language networks may also depend on damage to the underlying cortex, but perhaps to a lesser degree. Unlike Labudda et al. (2012), our data revealed a strong association between inter-hemispheric shifts in the BOLD response in all three regions and language performances in patients with LTLE. The reason for these discrepant findings is unclear, but they could be due to a number of methodological factors, including differences in task design (i.e., semantic judgment versus verbal fluency), different approaches for estimating cortical structure (i.e., cortical thickness versus cortical volumes from voxel-based morphometry), and/or differences in our outcome measures (i.e., a composite measure of language versus a single measure of verbal fluency). In particular, we propose that because our Language PC was based on four language measures, it might provide a more comprehensive estimate of language functioning than using a single measure.
It is worth noting that a rightward asymmetry in the BOLD response in the TO region was associated with rightward asymmetry in TO thickness and stronger language performance. Although the TO region was of secondary interest in our analysis due to its location outside the perisylvian network, this region is reliably activated during functional tasks of word reading and is known to play a critical role in letter and visual word-form processing (McDonald et al., 2010, Thesen et al., 2012; Petersen, Fox, Snyder, & Raichle, 1990). Thus, these results suggest that structural and functional reorganization in some patients with LTLE may occur in the stage of perceiving visual word-forms.
Proposed Model of Language Network Reorganization in LTLE
The three cortical ROIs selected in our study have previously been shown to be engaged in three different stages of lexical-semantic processing using this same visual semantic judgment task measured by fMRI, MEG, and intracranial recordings (see McDonald et al., 2009; McDonald et al., 2010; Thesen et al., 2012). In addition, the two fiber tracts selected for this study, the ARC and IFOF, are thought to subserve the dorsal (phonological) and ventral (semantic) language streams, respectively, and both have been associated with language impairment in TLE (Kucukboyaci et al., 2014; McDonald et al., 2008; Osipowicz, Sperling, Sharan, & Tracy, 2016; Pustina et al., 2014). Thus, we considered all of these cortical regions and white matter tracts in our analysis. Based on our results, we propose the following. An early age of seizure onset in LTLE likely disrupts the development of left hemisphere fiber tracts, including the ARC and IFOF, reflected by the observed correlation between earlier age of seizure onset and rightward laterality of both language pathways. However, only the rightward shift in the ARC is associated with rightward shifts in both TP and IF BOLD responses, indicating a propensity for perisylvian structures to reorganize together. This functional shift in the IF region may co-localize with a shift in adjacent motor regions, increasing the likelihood of left-handedness (Berl et al., 2014). Together, they explained a total of 76% of the variance in language performances. Thus, early alterations to the left ARC may increase the likelihood of an inter-hemispheric shift in the BOLD response to right-hemisphere language homologues, but the combination of microstructural and functional reorganization allows for the most successful language outcomes in LTLE. It is of note that our model assumes a temporal relationship that cannot be confirmed with the current dataset. Nevertheless, these data support a growing literature suggesting that structural reorganization of the ARC underpins functional plasticity within cerebral networks (Bernhardt, Bernasconi, Hong, Dery, & Bernasconi, 2015; Bernhardt, Chen, He, Evans, & Bernasconi, 2011; Liao et al., 2010; Vaessen et al., 2012).
Our study has several limitations. First, the sample size of our LTLE group is rather small, limiting our ability to perform inferential statistics between patients with atypical and typical language dominance. However, even with our limited sample size, we observed many robust correlations. In addition, our multimodal imaging approach necessitated that we perform a sizable number of comparisons. Instead of using an approach that may have been too conservative (i.e., Bonferroni) to correct for multiple comparisons, we adopted both non-parametric and randomization tests. Even though randomization tests are less conservative than other methods, they are typically robust (Sham & Purcell, 2014). Furthermore, we limited our total number of comparisons by creating a single language composite. Second, average cortical thickness in this study was derived within the regions of functional activation. However, fiber tracts were derived using probabilistic tractography. An alternative approach would have been to use the functional data to guide the fiber tracking (i.e., a deterministic approach). This may have led to increased certainty that our fibers of interest innervated our cortical ROIs. However, deterministic tractography also has limitations in that it very user-dependent and subject to both intra- and inter-operator bias. Third, in white matter, FA and MD were used to interpret the integrity of fiber tracts. However, their validity remains limited due to the problem of crossing fibers (even using 30 DTI directions), partial voluming, and noise (Nucifora, Verma, Lee, & Melhem, 2007). Fourth, language laterality in this study was based on a single fMRI semantic judgment task that did not measure all aspects of language functions. We appreciate that language is not a monolithic construct and a panel of fMRI language paradigms that tap both expressive and receptive language abilities may be optimal for probing language network reorganization in TLE (Gaillard et al., 2004). However, practical limitations of patient fatigue and scanner time can make performing multiple fMRI tasks in a clinical setting a challenge. Fifth, the selected ROIs in fMRI and cortical thickness were based on the average activation map in the controls. However, the Language PC was derived from language production tasks that all shared a semantic component. Thus, these selected ROIs may not directly reflect the specific components included in the Language PC. Sixth, even though the observed relationship among DTI-fMRI-Language PC led us to conclude that alterations in ARC microstructure may lead to functional reorganization that mitigates language impairment in the patients with LTLE, this causal relationship cannot be established in our study. In a rhesus monkeys model, O’Reilly et al. (2013) demonstrate that disruption of white matter connections causes near-total abolition of functional connectivity, providing some support for this directional relationship. However, the temporal nature of this structure-function relationship and the true contributions of the right hemisphere to language cannot be determined in our study. Lesion or disruptive neuro-stimulation studies would provide more direct support for these hypotheses. Seventh, there are many different patterns of language reorganization that may occur in patients (Berl et al., 2014). Although inter-hemispheric reorganization is believed to be the most frequent pattern observed in patients with LTLE, intra-hemispheric reorganization (i.e., language regions shifting within the same hemisphere, most often to regions adjacent to perisylvian cortex) can also occur (Bell et al., 2002; Brázdil, Zákopčan, Kuba, Fanfrdlová, & Rektor, 2003; Mbwana et al., 2009). Because we selected three “language-specific” ROIs and their homologues, our analysis would not have captured intra-hemispheric shifts. However, intra-hemispheric reorganization is most likely to occur in patients with space-occupying lesions (Hamberger, McClelland, McKhann, Williams, & Goodman, 2007) and these patients were excluded from our study. Finally, this present study does not address the important question as to whether our imaging approach can successfully predict post-operative language outcomes. Answering this question often requires multi-center efforts with large, well-characterized patient cohorts, and this study is currently underway.
5. Conclusions
This study combined DTI, fMRI, structural MRI and neuropsychological measures to better characterize language network reorganization in the patients with LTLE. We demonstrated that interhemispheric shifts in language in LTLE are associated with damage to perisylvian white matter. Together, structural and functional shifts reflect an adaptive process that appears to mitigate language impairment in LTLE.
Highlights.
Multimodal imaging can help to localize language networks in LTLE
Interhemispheric language reorganization is associated with alternations to the ARC
Structural and functional shifts mitigate language impairment in LTLE
Acknowledgments
Funding: This work was supported by the National Institutes of Health [R01 NS065838 to C.R.M.].
Footnotes
Two patients with LTLE with typical language dominance did not have a Language PC score because they were missing data for one of the four neuropsychological measures. One patient’s language performances were less than 2 standard deviation below the mean of controls on two of the three available measures (ANT and CF) whereas the other patient’s performances were greater than 2 standard deviation below the mean of controls on three available measures. Therefore, one patient was included in the impaired group and another patient was included in the unimpaired group in the individual subject analyses.
Each participant’s anatomical and functional data were transformed into N27 atlas space (Mazziotta et al., 2001) for creating individual and group statistical maps. The functional data for each participant were then smoothed using a 4-mm full-width half maximum (FWHM) Gaussian kernel in AFNI’s 3dmerge program. The statistical maps were corrected for multiple comparisons by using a combined significance level of p < .01 and a cluster size > 25 voxels, for a corrected α of .05 as determined by 3dClustSim.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18(2):423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Ahmadi ME, Hagler DJ, McDonald CR, Tecoma ES, Iragui VJ, Dale AM, Halgren E. Side matters: Diffusion tensor imaging tractography in left and right temporal lobe epilepsy. American Journal of Neuroradiology. 2009;30(9):1740–1747. doi: 10.3174/ajnr.a1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almairac F, Herbet G, Moritz-Gasser S, de Champfleur NM, Duffau H. The left inferior fronto-occipital fasciculus subserves language semantics: A multilevel lesion study. Brain Structure and Function. 2015;220(4):1983–1995. doi: 10.1007/s00429-014-0773-1. [DOI] [PubMed] [Google Scholar]
- Barnett A. Unpublished master’s thesis. University of Toronto; Toronto, Ontario, Canada: 2012. Memory functioning in patients with unilateral temporal lobe epilepsy: Neuroimaging indicators of functional integrity in the hippocampus and beyond. [Google Scholar]
- Bell B, Hermann B, Seidenberg M, Davies K, Cariski D, Rosenbek J, Woodard A, Rutecki P, Bishop M. Ipsilateral reorganization of language in early-onset left temporal lobe epilepsy. Epilepsy & Behavior. 2002;3(2):158–164. doi: 10.1006/ebeh.2002.0322. [DOI] [PubMed] [Google Scholar]
- Bell BD, Hermann BP, Woodard AR, Jones JE, Rutecki PA, Sheth R, Dow CC, Seidenberg M. Object naming and semantic knowledge in temporal lobe epilepsy. Neuropsychology. 2001;15(4):434–43. doi: 10.1037/0894-4105.15.4.434. [DOI] [PubMed] [Google Scholar]
- Berl MM, Zimmaro LA, Khan OI, Dustin I, Ritzl E, Duke ES, Sepeta LN, Sato S, Theodore WH, Gaillard WD. Characterization of atypical language activation patterns in focal epilepsy. Annals of Neurology. 2014;75(1):33–42. doi: 10.1002/ana.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi N, Hong SJ, Dery S, Bernasconi A. Subregional mesiotemporal network topology is altered in temporal lobe epilepsy. Cerebral Cortex. 2015;26(7):3237–3248. doi: 10.1093/cercor/bhv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cerebral Cortex. 2011;21(9):2147–2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology. 2009;72:1747–1754. doi: 10.1212/01.wnl.0000345969.57574.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, Benbadis S, Frost JA, Rao SM, Haughton VM. Determination of language dominance using functional MRI A comparison with the Wada test. Neurology. 1996;46(4):978–984. doi: 10.1212/01.wnl.0000397065.05305.5d. [DOI] [PubMed] [Google Scholar]
- Bonelli SB, Thompson PJ, Yogarajah M, Vollmar C, Powell RHW, Symms MR, McEvoy AW, Micallef C, Koepp MJ, Duncan JS. Imaging language networks before and after anterior temporal lobe resection: results of a longitudinal fMRI study. Epilepsia. 2012;53(4):639–650. doi: 10.1111/j.1528-1167.2012.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brázdil M, Zákopčan J, Kuba R, Fanfrdlová Z, Rektor I. Atypical hemispheric language dominance in left temporal lobe epilepsy as a result of the reorganization of language functions. Epilepsy & Behavior. 2003;4(4):414–419. doi: 10.1016/s1525-5050(03)00119-7. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. Journal of Cognitive Neuroscience. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends in Cognitive Sciences. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Destrieux C, Fischl B, Dale AM, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage: Lexicon and grammar. Boston, MA: Houghton Mifflin; 1982. [Google Scholar]
- Friederici AD, Gierhan SM. The language network. Current Opinion in Neurobiology. 2013;23(2):250–254. doi: 10.1016/j.conb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Jabbari B, Vezina LG, Frattali C, Theodore WH. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59(2):256–265. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Vezina LG, Frattali C, Theodore WH. fMRI language task panel improves determination of language dominance. Neurology. 2004;63(8):1403–1408. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Ahmadi ME, Kuperman J, Holland D, McDonald CR, Halgren E, Dale AM. Automated white matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Human Brain Mapping. 2009;30(5):1535–1547. doi: 10.1002/hbm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, McClelland S, McKhann GM, Williams AC, Goodman RR. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space occupying temporal lobe lesions. Epilepsia. 2007;48(3):531–538. doi: 10.1111/j.1528-1167.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT. Auditory and visual naming tests: Normative and patient data for accuracy, response time, and tip-of-the-tongue. Journal of the International Neuropsychological Society. 2003;9(3):479–489. doi: 10.1017/s135561770393013x. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Tamny TR. Auditory naming and temporal lobe epilepsy. Epilepsy Research. 1999;35(3):229–243. doi: 10.1016/s0920-1211(99)00023-6. [DOI] [PubMed] [Google Scholar]
- Hegarty CE, Foland-Ross LC, Narr KL, Townsend JD, Bookheimer SY, Thompson PM, Altshuler LL. Anterior cingulate activation relates to local cortical thickness. Neuroreport. 2012;23(7):420–424. doi: 10.1097/WNR.0b013e3283525a95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Perrine K, Chelune GJ, Barr W, Loring DW, Strauss E, Trenerry MR, Westerveld M. Visual confrontation naming following left anterior temporal lobectomy: A comparison of surgical approaches. Neuropsychology. 1999;13(1):3–9. doi: 10.1037/0894-4105.13.1.3. [DOI] [PubMed] [Google Scholar]
- Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in echo planar imaging. Neuroimage. 2010;50(1):175–183. doi: 10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janszky J, Mertens M, Janszky I, Ebner A, Woermann FG. Left sided interictal epileptic activity induces shift of language lateralization in temporal lobe epilepsy: An fMRI study. Epilepsia. 2006;47(5):921–927. doi: 10.1111/j.1528-1167.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Jetté N, Sander JW, Keezer MR. Surgical treatment for epilepsy: The potential gap between evidence and practice. The Lancet Neurology. 2016;15(9):982–994. doi: 10.1016/s1474-4422(16)30127-2. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston naming test. 2. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- Kemmotsu N, Girard HM, Bernhardt BC, Bonilha L, Lin JJ, Tecoma ES, Tecoma ES, Iragui VJ, Hagler DJ, Halgren E, McDonald CR. MRI analysis in temporal lobe epilepsy: Cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia. 2011;52(12):2257–2266. doi: 10.1111/j.1528-1167.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukboyaci NE, Kemmotsu N, Leyden KM, Girard HM, Tecoma ES, Iragui VJ, McDonald CR. Integration of multimodal MRI data via PCA to explain language performance. NeuroImage: Clinical. 2014;5:197–207. doi: 10.1016/j.nicl.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- Labudda K, Mertens M, Janszky J, Bien CG, Woermann FG. Atypical language lateralisation associated with right fronto-temporal grey matter increases – A combined fMRI and VBM study in left-sided mesial temporal lobe epilepsy patients. Neuroimage. 2012;59(1):728–737. doi: 10.1016/j.neuroimage.2011.07.053. [DOI] [PubMed] [Google Scholar]
- Lee CY, Tabesh A, Benitez A, Helpern JA, Jensen JH, Bonilha L. Microstructural integrity of early versus late myelinating white matter tracts in medial temporal lobe epilepsy. Epilepsia. 2013;54(10):1801–1809. doi: 10.1111/epi.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, Luo C, Lu G, Chen H. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PloS one. 2010;5(1):e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Riley JD, Juranek J, Cramer SC. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Research. 2008;82(2):162–170. doi: 10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Luders E, Toga AW, Engel J, Thompson PM. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cerebral cortex. 2007;17(9):2007–2018. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130(3):623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philosophical Transactions of the Royal Society B: Biological Sciences. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbwana J, Berl MM, Ritzl EK, Rosenberger L, Mayo J, Weinstein S, Conry JA, Pearl PL, Shamim S, Moore EN, Sato S, Vezina LG, Theodore WH, Gaillard WD. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain. 2009;132(2):347–356. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71(23):1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Hagler DJ, Jr, Carlson C, Devinksy O, Kuzniecky R, Barr W, Gharapetian L, Trongnetrpunya A, Dale AM, Halgren E. Distributed source modeling of language with magnetoencephalography: Application to patients with intractable epilepsy. Epilepsia. 2009;50(10):2256–2266. doi: 10.1111/j.1528-1167.2009.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Carlson C, Blumberg M, Girard HM, Trongnetrpunya A, Sherfey JS, Devinsky O, Kuzniecky R, Dolye WK, Cash SS, Leonard MK, Hagler DJ, Dale AM, Halgren E. Multimodal imaging of repetition priming: Using fMRI, MEG, and intracranial EEG to reveal spatiotemporal profiles of word processing. Neuroimage. 2010;53(2):707–717. doi: 10.1016/j.neuroimage.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Kaoua B, Lespinet V, Barsse A, Rougier A, Claverie B. Exploration of hemispheric specialization and lexico-semantic processing in unilateral temporal lobe epilepsy with fluency tasks. Neuropsychologia. 2001;39(6):635–642. doi: 10.1016/s0028-3932(00)00139-1. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: Exploring brain microstructure and connectivity 1. Radiology. 2007;245(2):367–384. doi: 10.1148/radiol.2452060445. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Croxson PL, Jbabdi S, Sallet J, Noonan MP, Mars RB, Browning PGF, Wilson CRE, Mitchell AS, Miller KL, Rushworth MFS, Baxter MG. Causal effect of disconnection lesions on interhemispheric functional connectivity in rhesus monkeys. Proceedings of the National Academy of Sciences. 2013;110(34):13982–13987. doi: 10.1073/pnas.1305062110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipowicz K, Sperling MR, Sharan AD, Tracy JI. Functional MRI, resting state fMRI, and DTI for predicting verbal fluency outcome following resective surgery for temporal lobe epilepsy. Journal of Neurosurgery. 2016;124(4):929–937. doi: 10.3171/2014.9.jns131422. [DOI] [PubMed] [Google Scholar]
- Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, Seidenberg M, Hermann BP. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62(10):1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- Pataraia E, Simos PG, Castillo EM, Billingsley-Marshall RL, McGregor AL, Breier JI, Sarkari S, Papanicolaou AC. Reorganization of language-specific cortex in patients with lesions or mesial temporal epilepsy. Neurology. 2004;63(10):1825–1832. doi: 10.1212/01.wnl.0000144180.85779.9a. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Snyder AZ, Raichle ME. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990;249(4972):1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Powell HWR, Parker GJM, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CAM, Barker GJ, Koepp MJ, Duncan JS. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007;36(1):209–221. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Powell HWR, Parker GJM, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CAM, Barker GJ, Noppeney U, Koepp MJ, Duncan JS. Hemispheric asymmetries in language-related pathways: A combined functional MRI and tractography study. Neuroimage. 2006;32(1):388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Propper RE, O’Donnell LJ, Whalen S, Tie Y, Norton IH, Suarez RO, Zollei L, Radmanesh A, Golby AJ. A combined fMRI and DTI examination of functional language lateralization and arcuate fasciculus structure: Effects of degree versus direction of hand preference. Brain and Cognition. 2010;73(2):85–92. doi: 10.1016/j.bandc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52(5):1038–1038. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Pustina D, Doucet G, Evans J, Sharan A, Sperling M, Skidmore C, Tracy J. Distinct types of white matter changes are observed after anterior temporal lobectomy in epilepsy. PloS One. 2014;9(8):1–13. doi: 10.1371/journal.pone.0104211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo S, Oppenheim C, Chassoux F, Hodel J, De Vanssay A, Baudoin Chial S, Devaux B, Meder JF. Language lateralization in temporal lobe epilepsy using functional MRI and probabilistic tractography. Epilepsia. 2008;49(8):1367–1376. doi: 10.1111/j.1528-1167.2008.01607.x. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC. Suma. Neuroimage. 2012;62(2):768–773. doi: 10.1016/j.neuroimage.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL, Mueller WM, Binder JR. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60(11):1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- Sham PC, Purcell SM. Statistical power and significance testing in large-scale genetic studies. Nature Reviews Genetics. 2014;15(5):335–346. doi: 10.1038/nrg3706. [DOI] [PubMed] [Google Scholar]
- Skovlund E, Fenstad GU. Should we always choose a nonparametric test when comparing two apparently nonnormal distributions? Journal of Clinical Epidemiology. 2001;54(1):86–92. doi: 10.1016/s0895-4356(00)00264-x. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PSF, Brewer CC, Perry HM, Morris GL, Mueller WM. Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain. 1999;122:2033–2045. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, McKenna BS, Jacobus J, Castro N, Sorg SF, Tapert SF. BOLD response to working memory not related to cortical thickness during early adolescence. Brain Research. 2013;1537:59–68. doi: 10.1016/j.brainres.2013.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen T, McDonald CR, Carlson C, Doyle W, Cash S, Sherfey J, Felsovalyi O, Girard H, Barr W, Devinsky O, Kuzniecky R, Halgren E. Sequential then interactive processing of letters and words in the left fusiform gyrus. Nature Communications. 2012;3:1284. doi: 10.1038/ncomms2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen MJ, Jansen JFA, Vlooswijk MCG, Hofman PAM, Majoie HJM, Aldenkamp AP, Backes WH. White matter network abnormalities are associated with cognitive decline in chronic epilepsy. Cerebral Cortex. 2012;22(9):2139–2147. doi: 10.1093/cercor/bhr298. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right-and left-handed healthy subjects: A combined fMRI and DTI study. Neuroimage. 2007;35(3):1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. New England Journal of Medicine. 2001;345(5):311–318. doi: 10.1056/nejm200108023450501. [DOI] [PubMed] [Google Scholar]
- Yin P, Fan X. Estimating R2 shrinkage in multiple regression: A comparison of different analytical methods. The Journal of Experimental Education. 2001;69(2):203–224. doi: 10.1080/00220970109600656. [DOI] [Google Scholar]
- Yuan W, Szaflarski JP, Schmithorst VJ, Schapiro M, Byars AW, Strawsburg RH, Holland SK. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47(3):593–600. doi: 10.1111/j.1528-1167.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich F, Sunder-Plassmann R, Stogmann E, Gleiss A, Dal-Bianco A, Zimprich A, Plumer S, Baumgartner C, Mannhalter C. Association of an ABCB1 gene haplotype with pharmacoresistance in temporal lobe epilepsy. Neurology. 2004;63(6):1087–1089. doi: 10.1212/01.wnl.0000141021.42763.f6. [DOI] [PubMed] [Google Scholar]