Abstract
Sarcomeres are the basic contractile units of muscle, and their lengths influence muscle force-generating capacity. Despite their importance, in vivo sarcomere lengths remain unknown for many human muscles. Second harmonic generation (SHG) microendoscopy is a minimally invasive technique for imaging sarcomeres in vivo and measuring their lengths. In this study, we used SHG microendoscopy to visualize sarcomeres of the human vastus lateralis, a large knee extensor muscle important for mobility, to examine how sarcomere lengths change with knee flexion and thus affect the muscle’s force-generating capacity. We acquired in vivo sarcomere images of several muscle fibers of the resting vastus lateralis in six healthy individuals. Mean sarcomere lengths increased (p = 0.031) from 2.84±0.16 μm at 50° of knee flexion to 3.17±0.13 μm at 110° of knee flexion. The standard deviation of sarcomere lengths among different fibers within a muscle was 0.21±0.09 μm. Our results suggest that the sarcomeres of the resting vastus lateralis at 50° of knee flexion are near optimal length. At a knee flexion angle of 110° the resting sarcomeres of vastus lateralis are longer than optimal length. These results show a smaller sarcomere length change and greater conservation of force-generating capacity with knee flexion than estimated in previous studies.
Keywords: muscle, sarcomere, strength, knee, microendoscopy
Introduction
The force generated by a skeletal muscle is influenced by the length of its sarcomeres according to the sliding filament theory (Gordon et al., 1966). A muscle spanning a joint assumes different lengths as the joint is flexed and extended. As the muscle changes length its sarcomeres also change length, altering the muscle’s force-generating capacity. Thus, muscle force-generating capacity (i.e., the maximum isometric force a muscle can generate) can be greatly affected by joint angle.
The forces generated by the vastus lateralis muscle, the largest knee extensor (Ward et al., 2009a), play an important role in supporting body weight during walking (Liu et al., 2006) and running (Hamner et al., 2010), and enabling activities such as climbing stairs (Andriacchi et al., 1980), rising from a chair (Hughes et al., 1996), and jumping (Pandy and Zajac, 1991). Several studies have estimated how the force-generating capacity of the vastus lateralis changes with knee flexion using dynamometry experiments (Ichinose et al., 1997) and musculoskeletal models (Arnold et al., 2010; Cutts, 1988; Herzog et al., 1990). By measuring knee extension moment and estimating parameters including the knee extensor moment arm and relative muscle cross sectional areas, Ichinose et al., (1997) estimated the force of the vastus lateralis at different knee flexion angles. Other studies estimated the force-generating capacity using musculoskeletal models based on muscle architecture and sarcomere lengths measured in cadavers (Arnold et al., 2010; Cutts, 1988; Herzog et al., 1990). Sarcomere lengths are generally measured in only one fixed body position in cadavers, and they may differ from in vivo sarcomere lengths due to rigor mortis and fixation effects (Huang et al., 2011).
Direct measurement of sarcomere lengths at different joint angles provides insight into how sarcomere lengths vary with body posture and influence muscle force-generating capacity. Laser diffraction is a useful technique (Lieber et al., 1994) for in vivo measurement of sarcomere lengths, but it requires surgical access to the muscle and only provides an average across multiple fibers. Boakes et al. (2007) used intraoperative laser diffraction to measure vastus lateralis sarcomere lengths in one subject undergoing a femoral lengthening. This study provided valuable data, but the sarcomere lengths of the vastus lateralis at multiple knee angles in healthy individuals remain unknown. Furthermore, variation of sarcomere lengths of different fibers within the same muscle is unstudied, leading researchers to assume all sarcomeres are the same length in computational musculoskeletal models. Previous investigations have revealed inhomogeneity in serial sarcomere lengths within human muscle fibers (Cromie et al., 2013; Gollapudi and Lin, 2009), but studying the variance in mean sarcomere lengths among neighboring fibers in live humans has not previously been possible.
Second harmonic generation (SHG) microendoscopy is a technique that can directly image sarcomeres in vivo (Llewellyn et al., 2008). Sarcomere visualization from muscle fibers is possible due to an intrinsic second harmonic signal that arises from the interaction of pulsed laser light with myosin filaments (Plotnikov et al., 2006). The technique has been used to measure sarcomere lengths in the forearm muscles of humans with a large, tabletop system (Cromie et al., 2013; Llewellyn et al., 2008). With the recent advent of miniaturized SHG microendoscopy (Sanchez et al., 2015), it is now possible to image sarcomeres in previously inaccessible lower extremity muscles.
Our long-term goal is to understand how changes in sarcomere length with knee flexion affect the force-generating capacity of the vastus lateralis. In this study, we used SHG microendoscopy to measure: (1) the lengths of the sarcomeres in the vastus lateralis at different knee flexion angles, and (2) the variation in sarcomere lengths among fibers within the vastus lateralis. These direct measurements reveal the joint angle at which sarcomeres reach their optimal length and demonstrate the changes in sarcomere lengths with knee flexion.
Methods
Sarcomere Imaging System
We acquired images in humans with a custom-built fiber-coupled microendoscopy system recently introduced by Sanchez et al. (2015) and illustrated in Figure 1. A high power femtosecond fiber laser provided ultrashort pulses (200 fs) of excitation light at a 1030 nm wavelength. A second harmonic generation signal arises from laser interaction with myosin containing bands (Plotnikov et al., 2006). A dual axis MEMS mirror (Mirrorcle Technologies; Richmond, CA) directed the laser scan pattern for an acquisition time of 0.47 seconds per 512 pixel by 512 pixel image. The SHG signal collected from the muscle was returned through the probe to a collection fiber connected to a photomultiplier tube (Hamamatsu; Hamamatsu City, Japan). The microendoscope had an optical resolution of 1.47 μm within the plane of the image and a field of view of 78 μm by 78 μm.
Figure 1.
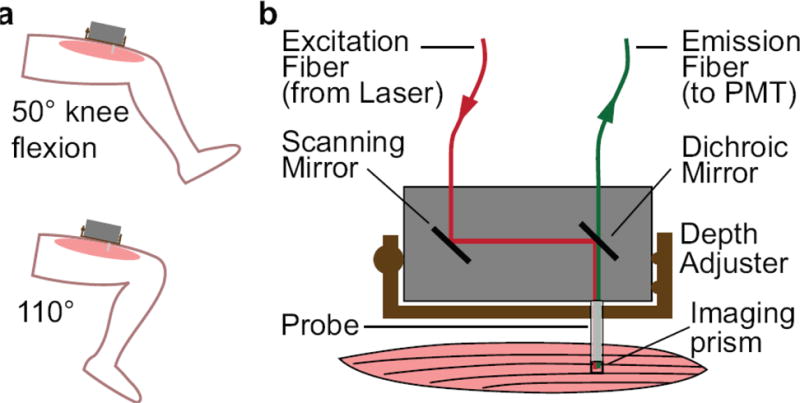
Experimental setup for imaging sarcomeres in the vastus lateralis muscle. a) A second harmonic generation (SHG) microendoscope was affixed to the thigh to image sarcomeres with the knee at 50° and 110° of flexion. b) Excitation laser light was delivered via an optical fiber to a scanning mirror and a dichroic mirror, into a microendoscope probe, and out the side of the tip of the probe into the muscle with an imaging prism. The emission signal was collected back through the probe and dichroic mirror into a fiber connected to a photomultiplier tube (PMT). A depth adjuster allowed for alteration of the probe insertion depth to image different muscle fibers.
The microendoscope probe consisted of a 500 μm diameter Gradient Refractive Index (GRIN) objective and relay in series with a prism to direct the laser beam out the side of the probe (Grintech; Jena, Germany). The GRIN assembly was secured in a 15 mm long 20-gauge stainless steel tube with a tri-faceted tip that enabled delivery of the probe into the muscle.
Human subject image collection
Six unimpaired adults (2 men, 4 women) with no history of injury or neuromuscular disease participated in this study. Subjects were aged 30±6 years, with average height of 168±11 cm and average mass of 61±12 kg. The Stanford University Institutional Review Board approved the experimental protocol, and all subjects gave informed consent.
Participants rested comfortably with the microendoscope probe inserted to the left vastus lateralis. We defined the knee angle, measured using a handheld goniometer, as the angle between the line extending from the greater trochanter of the femur to the lateral epicondyle of the femur and the line from the lateral epicondyle of the femur to lateral malleolus of the tibia. Full knee extension was defined as 0° (Figure 1a). We used custom-made braces to secure the subjects’ limbs at 50° and 110° of knee flexion. Using ultrasound guidance, we determined the muscle location and fiber orientation. We inserted a sterilized microendoscope probe approximately one third of the distance from the greater trochanter to the lateral epicondyle, lateral to the rectus femoris, such that the imaging window of the probe prism ran parallel to the muscle fibers. The microendoscope was attached to a translating depth adjuster that allows controlled extraction of the probe from the muscle in 1 mm increments over a range of 1 cm; this allowed us to image different fibers within the muscle with a single probe insertion.
In each subject, we inserted the microendoscope probe at 50° of knee flexion, acquired images at different depths, and then removed the probe. We then reinserted the probe within 1 cm of the original insertion with the knee at 110° of flexion. In 3 subjects, we collected images on two different days. In each subject at each joint angle, at least 3 different fibers were imaged and their sarcomere lengths were analyzed.
Sarcomere length analysis
We determined the mean sarcomere lengths from our images using Matlab (MathWorks; Natick, MA). We averaged 4 successive images to reduce noise. We rotated the averaged images to align the fibers vertically. For the rotated image, we multiplied each pixel column with a Hamming window and performed a one-dimensional discrete Fourier transform. A bandpass filter was applied to only preserve feature periods between 2 and 5 μm. We window-averaged power spectra of adjacent columns to further reduce noise. We then selected a threshold for the peak power spectrum magnitude in each column normalized by the maximum power spectrum magnitude in the image to only measure columns that corresponded to sarcomeres by visual inspection. For the remaining columns, we used a least squares fit of a Gaussian curve to the frequency spectrum to determine the sarcomere length in each column. The precision of our measurement of mean sarcomere lengths is in the tens of nanometers because we measure more than 20 serial sarcomeres within our images (Sanchez et al., 2015). We inspected the image to determine the columns that belonged to the same fiber. The mean sarcomere length of each fiber was then determined by averaging the measured lengths from columns belonging to the same fiber, averaging at least 20 columns with a standard deviation of less than 0.1 μm. We further adjusted the mean sarcomere length to account for stretching due to the probe as described below.
We compared sarcomere lengths between knee flexion angles by Fisher’s sign test. We determined sarcomere variability within the muscle from the mean sarcomere length of different fibers collected from the same subject at the same knee flexion angle. We used a Fisher’s sign test to compare variances across the two knee flexion angles.
We used the theoretical model of the force–length relationship described by Gollapudi and Lin (2009) to calculate the force-generating capacity based on sarcomere lengths, and to estimate sarcomere lengths based on force-generating capacity reported in previous studies. The theoretical model is based on the sliding filament model (Gordon et al., 1966) and electron microscopy measurements of human filaments (Walker and Schrodt, 1974). The plateau extends from 2.64 μm to 2.81 μm, and the ascending limb has a slope of 0.57 units of normalized force per micrometer starting at 1.7 μm, whereas the descending limb has a slope of −0.70 units of normalized force per micrometer (see Fig. 7 of Gollapudi and Lin (2009) and Fig. 4a).
Figure 4.
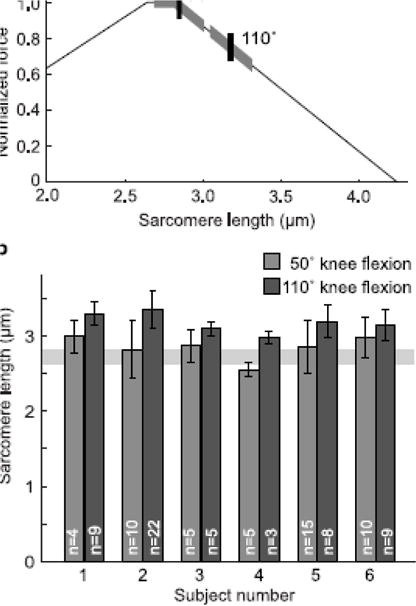
Sarcomere lengths in the vastus lateralis at 50° and 110° of knee flexion. a) Mean (vertical tick) and standard deviation (thick highlighting on curve) of sarcomere lengths measured in n = 6 subjects in the vastus laterals at 50° and 110° of knee flexion, plotted with a theoretical force-length curve as described by Gollapudi and Lin (2009). b) Mean sarcomere lengths of the vastus lateralis in each of 6 subjects at 50° and 110° of knee flexion. Standard deviation error bars show the variation among multiple (n = 3–22) fibers within each subject. The shaded horizontal region represents the plateau of the force-length curve.
Probe influence determination
To ensure accurate measurements with the microendoscope probe, we characterized the amount of stretch induced in muscle fibers as they curved around the 20-gauge probe following insertion (Figure 2a). We inserted probes into rat hamstring muscles and analyzed sarcomere lengths close to and far from the insertion site. All surgical procedures were approved by the Stanford University Administrative Panel on Lab Animal Care.
Figure 2.
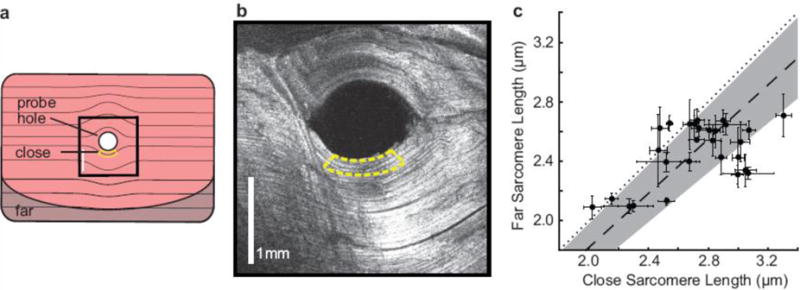
Probe insertion into muscle increases sarcomere lengths. a) Schematic of a rat muscle fixed after insertion of a microendoscope probe and sectioned orthogonal to the probe axis. The yellow border represents a close region within the 250 μm imaging distance of the probe. The darker shaded region represents a far region, which is more than 3 mm away from the probe hole edge. The black square marks the location of b) SHG image of muscle fibers curved around the probe insertion hole taken with a commercial laser scanning microscopy system. The yellow border is the close region. c) Mean and standard error of the mean of sarcomere lengths measured close to and far from the probe insertion site in n = 28 muscle samples. Far measurements are 91±8% the length of close measurements (mean ± standard deviation, dashed line ± shaded region), generally falling below the 1:1 dotted line.
Male Sprague Dawley rats (300–325 grams, Charles River Laboratories) were deeply anesthetized with 5% isoflurane. Under anesthesia, we pinned the legs of the rats at various joint angles to induce a wide range of stretch in the hind limb muscles. We then inserted a microendoscope probe into the hamstring muscles in each leg and transcardially perfused the rat with 4% paraformaldehyde for fixation. After fixation, we removed the probe. The probe left a visible hole in the muscle, and we removed sections of muscles around the probe insertion hole.
We used a commercial laser scanning microscope (Prairie Technologies; Middleton, WI) and a Titanium:Sapphire laser (Chameleon, Coherent; Santa Clara, CA) providing pulses at 960 nm to image 28 muscle samples. For each muscle section, we compared sarcomere images from two regions. The first region, called “close,” was within 250 μm of the probe hole edge and represented sarcomeres within the imaging distance of the microendoscope probe (within yellow bordered regions in Figure 2a and 2b). The second region, called “far,” was more than 3 mm away from the insertion hole, where we expect there to be no effect of the probe. For each sample, we took images from at least four different locations in the “close” region and at least four locations in the “far” region. We determined the sarcomere lengths in the images using the same algorithm as described above for human sarcomere images and averaged together lengths from images from the same region.
We found that sarcomeres close to the insertion site are longer (p < 0.001, paired ratio t-test) than sarcomeres far from the insertion site (Figure 2c). The far measurements were on average 91 ± 8% of the close measurements (mean ± standard deviation, n=28 muscle samples). The average coefficient of variation of the unperturbed far measurements in the 28 samples was 8.7%. The coefficient of variation of the correction ratio is 9.2%. Considering these similarities, we believe that the high variability in the correction ratio is due to the inherent differences in sarcomere lengths (O’Connor et al., 2016). Assuming the probe similarly stretches human muscle fibers, we adjusted our human sarcomere lengths by 91%.
Results
We successfully collected in vivo sarcomere images (Figure 3) and measured sarcomere lengths and fiber-to-fiber sarcomere length variability in the vastus lateralis of healthy individuals. Sarcomeres in the vastus lateralis were longer at 110° of knee flexion than at 50° of knee flexion (p = .031). At 50° of knee flexion, the average resting sarcomere length was 2.84±0.16 μm; at 110° flexion, the average sarcomere length was 3.17±0.13 μm (mean ± standard deviation, n=6 subjects) (Figure 4a). At 50° of knee flexion, sarcomere lengths were near the plateau region of the force–length curve, with the average sarcomere length corresponding to 98% of the maximum force-generating capacity. At 110° of knee flexion, sarcomere lengths were on the descending limb of the force–length curve, with the average sarcomere length corresponding to 75% of the maximum force-generating capacity (Figure 4a).
Figure 3.
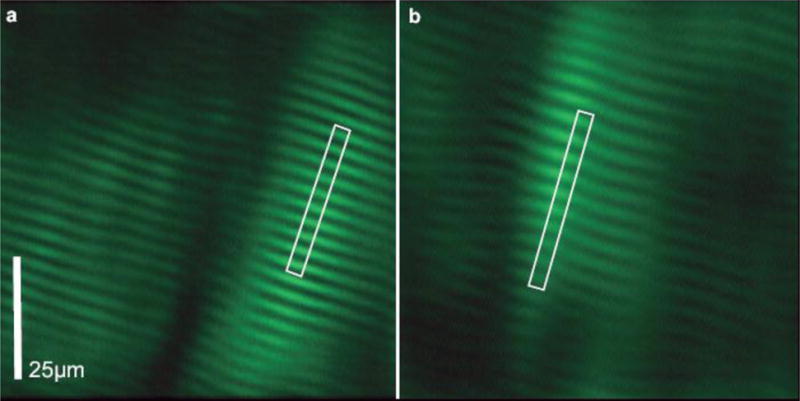
SHG microendoscopy images of sarcomeres in the vastus lateralis of one subject with the knee at a) 50° of flexion (average sarcomere length 2.76 μm), showing 2 fibers, and b) 110° of flexion (average sarcomere length 3.26 μm). Bright regions of the image are myosin-containing bands. Images are low pass filtered and window-and-level adjusted for presentation. White rectangles outline the length of 10 sarcomeres.
Sarcomere lengths varied substantially between fibers within a subject’s vastus lateralis (see standard deviation error bars in Fig. 4b). Average standard deviations of sarcomere lengths from different fibers within a muscle at one joint angle were 0.21±0.09 μm, and coefficients of variation were 7.1±3.4% (mean ± standard deviation of standard deviation or coefficient of variation, n=12 muscle positions). Standard deviations of sarcomere measurements within muscles were larger (p = 0.031) at 50° of knee flexion (0.25±0.10 μm) than at 110° knee flexion (0.17±0.07 μm, mean ± standard deviation of standard deviation, n=6 muscles at each knee angle).
Discussion
This paper reports the first direct observations of human sarcomeres at multiple joint angles in a lower extremity muscle. We found that the resting sarcomeres of the vastus lateralis are near the plateau of the sarcomere force–length curve at 50° of knee flexion. Sarcomeres increase in length with knee flexion greater than 50°, presumably decreasing their active force-generating capacity as the sarcomeres traverse the descending limb of the force–length curve.
Sarcomere lengths of resting muscle, as reported here, are likely longer than those in active muscle due to stretching of the tendon and shortening of muscle fibers with activation (Fukunaga et al., 1997; Ichinose et al., 1997). Ultrasound measurements of the vastus lateralis tendon estimate the tendon strain to be approximately 8% during maximum voluntary activation (Stafilidis et al., 2005), which results in fiber shortening of approximately 10%. Assuming 10% sarcomere shortening in the vastus lateralis, our measurements result in maximally active sarcomere lengths of 2.56 μm at 50° and 2.85 μm at 110° of knee flexion. This suggests that maximally active sarcomeres traverse the plateau of the force–length curve between these two joint angles. Future microendoscopy studies in active muscle should investigate the effects of activation on sarcomere shortening in vivo.
We compared our measurements to sarcomere lengths estimated from previous studies that report normalized force versus joint angle relationships for the vastus lateralis (Figure 5a). At 50° of knee flexion, we measured resting sarcomere lengths longer than active sarcomere lengths estimated by Herzog et al. (1990) and Ichinose et al. (1997), but shorter than resting sarcomere lengths estimated by Arnold et al. (2010). In contrast, at 110° of knee flexion, we measured sarcomere lengths approximately equal to those estimated from Herzog et al. (1990) and Ichinose et al. (1997). The change in sarcomere length with knee flexion measured by microendoscopy is smaller than predicted by previous studies (Figure 5b). We believe this discrepancy to result mainly from modeling assumptions in previous studies, namely the assumption that the vastus lateralis has a single moment arm such that all fibers change in length by the same amount with knee flexion. Arnold et al. (2010) and Herzog et al. (1990) made this assumption in developing their musculoskeletal models, and Ichinose et al. (1997) estimated muscle force from measured joint moments and assumed a single moment arm for all the knee extensors. Our results suggest that the force-generating capacity is more constant across that range of motion than has been previously estimated.
Figure 5.
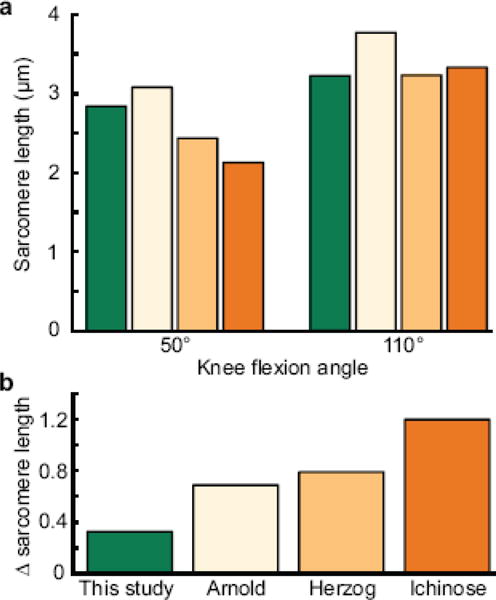
Comparison of sarcomere lengths in the vastus lateralis reported here (green bars) to lengths estimated from Arnold et al. (2010) (tan bars), Herzog et al. (1990) (light orange bars), and Ichinose et al. (1997) (dark orange bars). a) Average sarcomere lengths at 50° and 110° of knee flexion from the different studies. b) Differences in sarcomere lengths between the two knee flexion angles measured in this study and previous modeling and dynamometry studies show microendoscopy measures a smaller change in sarcomere length between the two knee angles.
Previous studies support the theory that fibers within a muscle change length by different amounts with joint flexion. Blemker and Delp (2006) reported that simple muscle models of the rectus femoris and vastus intermedius, which assume that a muscle has the same moment arm for all fibers, overestimate changes in muscle fiber lengths with knee flexion compared to three-dimensional models of muscle architecture and mechanics. They theorize that the overestimation is due to the omission of complex architectural features in simple models. The vastus lateralis originates from a wide region of the femur and inserts to long tendon on the lateral side of the patella (Bardeen, 1921). These complex architectural features likely cause different fibers to change length by different amounts with knee flexion. Indeed, Visser and Hoogkamer (1990) found that the medial fibers of the vastus lateralis have a larger moment arm than the lateral fibers, suggesting that simple models may not accurately represent changes in fiber lengths and thus produce errors in estimating sarcomere lengths.
The measured change in sarcomere lengths in the vastus lateralis between 50° and 110° of knee flexion is comparable with the change in fascicle length observed via ultrasound. Fukunaga et al. (1997) measured a 14% increase in fascicle length in resting muscle between these two joint angles. We measured an increase in mean sarcomere length of 12%. A 14% increase in mean sarcomere length measured in this study at 50° would result in a length within one standard deviation of our mean measured at 110°.
We observed variation of sarcomere lengths among fibers in the vastus lateralis. Prior studies hypothesized that sarcomere length variation among fibers may broaden the force–length relationship of the whole muscle as different sarcomeres reach optimal length at different muscle lengths (Herzog and ter Keurs, 1988). We found that sarcomere variability decreased with greater knee flexion, perhaps because as the vastus lateralis is stretched, the increased passive tension in the muscle increases sarcomere length uniformity. The variability we observed among fibers of the vastus lateralis (standard deviation of 0.21 μm) is of similar magnitude to the variability within a muscle fiber of the extensor carpi radialis brevis (0.20 μm) reported by Cromie et al. (2013).
Resting sarcomeres cannot shorten below the length at which passive tension starts. While the slack length of the whole vastus lateralis muscle is unknown, previous studies in various human muscle fibers have found slack sarcomere lengths from 2.06 to 2.39 μm (Boakes et al., 2007; Ward et al., 2009b). In comparison, we measured the average sarcomere lengths of 2.84 μm at 50° flexion, suggesting that the sarcomeres studied here were likely under passive tension.
The theoretical model for the force–length relationship used to predict the force generating capacity of sarcomeres (see Fig. 4b) differs from experimental results, which show a wider plateau and less steep slopes for the ascending and descending limbs of the of the force– length relationship (Gollapudi and Lin, 2009). Such an effect further diminishes the change in force-generating capacity of sarcomeres with joint angle, indicating that a better understanding of the force–length relationship is required for more accurate estimation of force-generating capacity.
In this study, we directly measured sarcomere lengths and sarcomere length variability among fibers in the human vastus lateralis at two knee angles, demonstrating the use of SHG microendoscopy in understanding a muscle’s force-generating capacity range. As this technique is capable of imaging individual fibers, future studies may investigate how complex muscle features and regional differences affect sarcomere length and variability, leading to more accurate biomechanical models. Intraoperative studies have provided valuable insight into sarcomere lengths in muscle contracture (Mathewson et al., 2015; Pontén et al., 2007; Smith et al., 2011), and SHG imaging of muscle biopsies has been used to assess muscle diseases (Liu et al., 2013; Plotnikov et al., 2008). Our microendoscopy technique provides a less invasive method for measuring sarcomere lengths in a variety of muscles and patients without surgery, and for collecting valuable SHG images in real time in the sarcomeres’ native environment. This technique will expand our understanding of muscle force-generating capacity in both healthy and diseased subjects, enabling new diagnoses and interventions for disease.
Acknowledgments
We thank Melinda Cromie and Kate Montgomery for helpful feedback on the manuscript. This work was supported by NIH R24 HD065690 and a NSF Graduate Research Fellowship to Xuefeng Chen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
To disseminate the microendoscopy technology described here, G.N.S., M.J.S. and S.L.D. have co-founded Zebra Medical Technologies Inc. and have a financial interest in the company.
References
- Andriacchi TP, Andersson GB, Fermier RW, Stern D, Galante JO. A study of lower-limb mechanics during stair-climbing. J Bone Jt Surg. 1980;62:749–757. [PubMed] [Google Scholar]
- Arnold EM, Ward SR, Lieber RL, Delp SL. A model of the lower limb for analysis of human movement. Ann Biomed Eng. 2010;38:269–279. doi: 10.1007/s10439-009-9852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeen C. In: Morris’s Human Anatomy. Jackson C, editor. Morris’s Human Anatomy P Blakistons’s Son & Co; Philadelphia: 1921. p. 502. [Google Scholar]
- Blemker SS, Delp SL. Rectus femoris and vastus intermedius fiber excursions predicted by three-dimensional muscle models. J Biomech. 2006;39:1383–1391. doi: 10.1016/j.jbiomech.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Boakes JL, Foran J, Ward SR, Lieber RL. Muscle adaptation by serial sarcomere addition 1 year after femoral lengthening. Clin Orthop Relat Res. 2007;456:250–253. doi: 10.1097/01.blo.0000246563.58091.af. [DOI] [PubMed] [Google Scholar]
- Cromie MJ, Sanchez GN, Schnitzer MJ, Delp SL. Sarcomere lengths in human extensor carpi radialis brevis measured by microendoscopy. Muscle Nerve. 2013;48:286–292. doi: 10.1002/mus.23760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutts A. The range of sarcomere lengths in the muscles of the human lower limb. J Anat. 1988;160:79–88. [PMC free article] [PubMed] [Google Scholar]
- Fukunaga T, Ichinose Y, Ito M. Determination of fascicle length and pennation in a contracting human muscle in vivo. J Appl Physiol. 1997;82:354–358. doi: 10.1152/jappl.1997.82.1.354. [DOI] [PubMed] [Google Scholar]
- Gollapudi SK, Lin DC. Experimental determination of sarcomere force–length relationship in type-I human skeletal muscle fibers. J Biomech. 2009;42:2011–2016. doi: 10.1016/j.jbiomech.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner SR, Seth A, Delp SL. Muscle contributions to propulsion and support during running. J Biomech. 2010;43:2709–2716. doi: 10.1016/j.jbiomech.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W, Abrahamse SK, ter Keurs H. Theoretical determination of force–length relations of intact human skeletal muscles using the cross-bridge model. Pflügers Arch. 1990:113–119. doi: 10.1007/BF00370231. [DOI] [PubMed] [Google Scholar]
- Herzog W, ter Keurs H. Force–length relation of in-vivo human rectus femoris muscles. Pflügers Arch. 1988;411:642–647. doi: 10.1007/BF00580860. [DOI] [PubMed] [Google Scholar]
- Huang SH, Hsiao CD, Lin DS, Chow CY, Chang CJ, Liau I. Imaging of zebrafish in vivo with second-harmonic generation reveals shortened sarcomeres associated with myopathy induced by statin. PLoS One. 2011;6:e24764. doi: 10.1371/journal.pone.0024764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MA, Myers BS, Schenkman ML. The role of strength in rising from a chair in the functionally impaired elderly. J Biomech. 1996;29:1509–1513. [PubMed] [Google Scholar]
- Ichinose Y, Kawakami Y, Ito M, Fukunaga T. Estimation of active force–length characteristics of human vastus lateralis muscle. Acta Anat (Basel) 1997;159:78–83. doi: 10.1159/000147969. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Loren GJ, Fridén J. In vivo measurement of human wrist extensor muscle sarcomere length changes. J Neurophysiol. 1994;71:874–881. doi: 10.1152/jn.1994.71.3.874. [DOI] [PubMed] [Google Scholar]
- Liu MQ, Anderson FC, Pandy MG, Delp SL. Muscles that support the body also modulate forward progression during walking. J Biomech. 2006;39:2623–2630. doi: 10.1016/j.jbiomech.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Liu W, Raben N, Ralston E. Quantitative evaluation of skeletal muscle defects in second harmonic generation images. J Biomed Opt. 2013;18:26005. doi: 10.1117/1.JBO.18.2.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn ME, Barretto RPJ, Delp SL, Schnitzer MJ. Minimally invasive high-speed imaging of sarcomere contractile dynamics in mice and humans. Nature. 2008;454:784–788. doi: 10.1038/nature07104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson MA, Ward SR, Chambers HG, Lieber RL. High resolution muscle measurements provide insights into equinus contractures in patients with cerebral palsy. J Orthop Res. 2015;33:33–39. doi: 10.1002/jor.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor SM, Cheng EJ, Young KW, Ward SR, Lieber RL. Quantification of sarcomere length distribution in whole muscle frozen sections. J Exp Biol. 2016;219:1432–1436. doi: 10.1242/jeb.132084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandy MG, Zajac FE. Optimal muscular coordination strategies for jumping. J Biomech. 1991;24:1–10. doi: 10.1016/0021-9290(91)90321-d. [DOI] [PubMed] [Google Scholar]
- Plotnikov SV, Kenny AM, Walsh SJ, Zubrowski B, Joseph C, Scranton VL, Kuchel GA, Dauser D, Xu M, Pilbeam CC, Adams DJ, Dougherty RP, Campagnola PJ, Mohler WA. Measurement of muscle disease by quantitative second-harmonic generation imaging. J Biomed Opt. 2008;13:044018. doi: 10.1117/1.2967536. [DOI] [PubMed] [Google Scholar]
- Plotnikov SV, Millard AC, Campagnola PJ, Mohler WA. Characterization of the myosin-based source for second-harmonic generation from muscle sarcomeres. Biophys J. 2006;90:693–703. doi: 10.1529/biophysj.105.071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén E, Gantelius S, Lieber RL. Intraoperative muscle measurements reveal a relationship between contracture formation and muscle remodeling. Muscle Nerve. 2007;36:47–54. doi: 10.1002/mus.20780. [DOI] [PubMed] [Google Scholar]
- Sanchez GN, Sinha S, Liske H, Chen X, Nguyen V, Delp SL, Schnitzer MJ. In vivo imaging of human sarcomere twitch dynamics in individual motor units. Neuron. 2015;88:1109–1120. doi: 10.1016/j.neuron.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 2011;589:2625–2639. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafilidis S, Karamanidis K, Morey-Klapsing G, DeMonte G, Brüggemann GP, Arampatzis A. Strain and elongation of the vastus lateralis aponeurosis and tendon in vivo during maximal isometric contraction. Eur J Appl Physiol. 2005;94:317–322. doi: 10.1007/s00421-004-1301-4. [DOI] [PubMed] [Google Scholar]
- Visser JJ, Hoogkamer JE. Length and moment arm of human leg muscles as a function of knee and hip-joint angles. Eur J Appl Physiol. 1990;61:453–460. doi: 10.1007/BF00236067. [DOI] [PubMed] [Google Scholar]
- Walker SM, Schrodt GR. I segment lengths and thin filament periods in skeletal muscle fibers of the rhesus monkey and the human. Anat Rec. 1974;178:63–82. doi: 10.1002/ar.1091780107. [DOI] [PubMed] [Google Scholar]
- Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture accurate? Clin Orthop Relat Res. 2009a;467:1074–1082. doi: 10.1007/s11999-008-0594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SR, Tomiya A, Regev GJ, Thacker BE, Benzl RC, Kim CW, Lieber RL. Passive mechanical properties of the lumbar multifidus muscle support its role as a stabilizer. J Biomech. 2009b;42:1384–1389. doi: 10.1016/j.jbiomech.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]


