Abstract
Objectives:
Methicillin-resistant Staphylococcus aureus (MRSA) is an important health care-associated and community-associated pathogen and causes a large number of infections worldwide. For the purpose of application to topical treatment of MRSA infection, we examined the antimicrobial effects of lysophosphatidylcholine (LPC) on MRSA strains. We also investigated the combination effect of LPC and gentamicin on MRSA growth.
Methods:
The LPC minimum inhibitory concentrations (MIC) for Gram-positive (S. aureus, Staphylococcus epidermidis, and Streptococcus pneumoniae) and Gram-negative (Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, and Pseudomonas aeruginosa) bacteria were measured by the broth microdilution method. The mechanism of LPC-mediated MRSA killing was investigated by membrane permeability analysis with DiSC3(5) fluorescence and growth curve analysis. Lastly, the effects of LPC on gentamicin-induced bactericidal activity were determined in combination treatment studies with 15 gentamicin-resistant MRSA isolates from the skin, nose, or ears.
Results:
The LPC MIC for Gram-positive bacteria varied between 32 µg/ml and >2048 µg/ml, whereas that for all Gram-negative bacteria was >2048 µg/ml. Consistently, membrane permeability analysis showed that LPC was substantially more effective in inducing membrane permeability in Gram-positive bacteria than in Gram-negative counterparts. Growth curve analysis in cotreatment studies demonstrated that LPC has intrinsic bactericidal effects and can also potentiate gentamicin sensitivity in resistant MRSA strains.
Conclusions:
Our study demonstrates that LPC exhibits intrinsic antimicrobial effects and can enhance the antimicrobial effects of gentamicin for resistant MRSA strains, suggesting that LPC may be a beneficial additive in topical antibiotics for superficial skin infections.
Keywords: cell membrane permeability, combination treatment study, lysophosphatidylcholine, methicillin-resistant Staphylococcus aureus
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is an important cause of both health care-associated and community-associated (CA) infections and has also developed resistance to various antimicrobial agents (β-lactams, quinolones, and aminoglycosides).1 CA-MRSA has become increasingly prevalent in Japan,2 and almost all healthy individuals are colonized with CA-MRSA. However, some strains can cause skin and soft-tissue infections and life-threatening necrotizing pneumonia even in the absence of predisposing conditions.3 The rising number of antimicrobial-resistant strains has limited the treatment options in infections and is associated with clinical treatment failure.4 ‘Anti-infectious drugs’ include not only compounds that inhibit the growth of pathogenic microorganisms statically or kill them but also compounds that control microbial survival or pathogenicity, potentiate the activities of known antimicrobials, or enhance the activity of the host immune system against microbial infection.
Lysophosphatidylcholine (LPC; also called lysolecithin) is a component of phospholipids in eukaryotic cells and is synthesized by the liver in mammals from phosphatidylcholine through phospholipase A2-mediated hydrolysis. LPC has been reported to reduce mortality in animal models of sepsis induced from both Gram-positive and Gram-negative bacteria, and modulation of inflammation accounted for the survival effect.5 A recent clinical study suggested that the serum level of LPC might be a mortality predictor in septic shock syndrome.6
Although LPC has been considered an immune modulator and shown to stimulate certain leukocyte activities critical for the initiation and maintenance of inflammation,7 information about the direct antimicrobial effects of LPC is limited. Here, we studied the antimicrobial efficacy of LPC on MRSA to evaluate LPC as a topical ointment.
Materials and methods
Strains
Thirty-two strains of clinically isolated MRSA from Tokyo Medical University Hospital and two reference strains (ATCC 33591 and ATCC 43300) were used in the present study. In addition, various other strains were included in a comparative analysis, including methicillin-sensitive S. aureus (MSSA; 20 clinical isolates, ATCC 25923, and ATCC 29213), Staphylococcus epidermidis (16 clinical isolates and ATCC 29887), Streptococcus pneumoniae (18 clinical isolates), Escherichia coli (19 clinical isolates and ATCC 25922), Enterobacter cloacae (20 clinical isolates), Klebsiella pneumoniae (20 clinical isolates), and Pseudomonas aeruginosa (19 clinical isolates and ATCC 27853).
Antimicrobial susceptibility testing
The minimum inhibitory concentration (MIC) of LPC (Egg Yolk Lysolecithin LPC-1, Kewpie) for each isolate was determined following a broth microdilution method according to the Clinical and Laboratory Standards Institute protocol with some modifications. The inoculum was adjusted to a cell density of 5 × 105 CFU/ml in Mueller-Hinton broth (Eiken Chemical) supplemented with 25 mg/l Ca2+ and 12.5 mg/l Mg2+ (CA-MHB). Bacteria were plated in triplicate, incubated for 20 h at 35°C, and then judged visually.
Membrane permeability assay
The effects of LPC on bacterial membrane permeability were assessed with the membrane potential-sensitive fluorescent dye DiSC3(5) (3,3′-dipropylthiadicarbocyanine iodide; Invitrogen), as described previously.8 Bacteria were grown at 35°C in CA-MHB, with shaking, to mid-logarithmic phase (0.5–0.6 OD600). The cells were harvested, washed once with buffer (5 mM HEPES pH 7.2, 5 mM glucose), and resuspended in the same buffer, and then supplemented with 100 mM KCl and 2 µM DiSC3(5) to 0.05 OD600. The mixture was then incubated for 15 min at room temperature to enable dye uptake and fluorescence quenching. The change in fluorescence was measured immediately after the addition of serial concentrations of LPC (16–256 µg/ml), using a fluorescence microplate reader (excitation: 622 nm; emission: 670 nm; Mithras LB940, Berthold Technologies). Measurements were recorded every minute for 10 min to analyse treatment kinetics. Saline-treated bacteria served as a negative control.
Colony enumeration
The effect of LPC treatment on MRSA ATCC 33591 growth was examined by colony counting. Bacteria were cultured to log-phase growth in CA-MHB and then diluted to a final concentration of 106 cells/ml. The cultures were subsequently treated with LPC at 128 or 256 µg/ml; incubated at 35°C for 0, 3, 6, and 24 h; serially diluted; and then plated on heart infusion agar (Eiken Chemical). The plates were incubated at 35°C for 24 h, and the surviving MRSA cells were counted.
LPC and gentamicin combination treatment
To determine the bactericidal efficacy of LPC in combination with gentamicin (Gentamicin Sulfate, Wako) in a topical ointment, we analysed the combined effects in vitro on CA-MRSA isolated from skin, nose, or ears. Among the 24 CA-MRSA isolates tested (Table 1), 15 were gentamicin-resistant (MIC ⩾ 16 µg/ml). MIC of gentamicin combined with 32 µg/ml LPC was measured for 15 gentamicin-resistant CA-MRSA isolates, following a broth microdilution method.
Table 1.
CA-MRSA isolates from skin, nose, or ear.
| Strain no. | Age | Sex | Sample | SCC mec | MIC of LPC (A) | MIC of GM (B) | MIC of GM combined with 32 µg/ml of LPC (C) | C/B |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | F | Skin | Type II | 128 | 0.25 | – | |
| 2 | 7 | M | Pus | Type II | >2048 | 8 | – | |
| 3 | 0 | M | Nose | Type II | >2048 | >256 | 64 | <1/4 |
| 8 | 27 | M | Ear | Type V | 64 | 256 | 64 | 1/4 |
| 12 | 7 | F | Pus | Type IV | 64 | 64 | 32 | 1/2 |
| 13 | 64 | M | Ulcer | Type IV | 32 | 0.25 | – | |
| 18 | 6 | M | Nose | Type II | 64 | 128 | 32 | 1/4 |
| 19 | 63 | F | Ear | Type II | 64 | >256 | 256 | 1/2 |
| 22 | 4 | M | Nose | Type II | 64 | 256 | 128 | 1/2 |
| 24 | 7 | F | Ear | Type V | 128 | 256 | 32 | 1/8 |
| 26 | 68 | M | Nose | Type IV | 32 | 4 | – | |
| 27 | 68 | F | Ear | Type IV | 64 | 128 | 32 | 1/4 |
| 29 | 2 | M | Skin | Type V | 2048 | 0.25 | – | |
| 31 | 0 | M | Nose | Type IV | 32 | 4 | – | |
| 35 | 5 | M | Wound | Type IV | 256 | 8 | – | |
| 36 | 45 | M | Skin | Type IV | 64 | 0.25 | – | |
| 40 | 34 | M | Nose | Type V | 2048 | 0.25 | – | |
| 41 | 0 | F | Nasal discharge | Type V | 128 | 128 | 4 | 1/32 |
| 43 | 0 | F | Nose | Type II | 2048 | 256 | 64 | 1/4 |
| 46 | 37 | F | Pus | Type V | 128 | 128 | 16 | 1/8 |
| 47 | 3 | F | Pus | Type V | >2048 | 256 | 16 | <1/16 |
| 48 | 1 | F | Skin | Type V | >2048 | 256 | 16 | <1/16 |
| 49 | 53 | M | Pus | Type IV | 2048 | 128 | 32 | 1/4 |
| 50 | 66 | M | Skin | Type IV | >2048 | 256 | 32 | <1/8 |
GM, gentamicin; LPC, lysophosphatidylcholine.
Results
LPC MIC
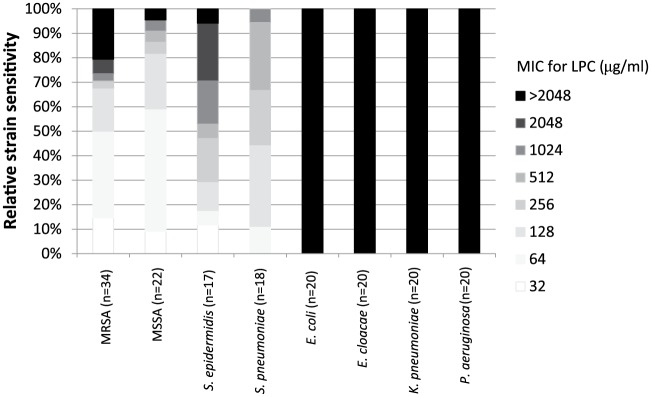
As shown in Figure 1, the LPC MIC for Gram-positive bacteria varied between 32 µg/ml and >2048 µg/ml. The MIC50 of MRSA and MSSA was 64 µg/ml, which was lower than that of S. epidermidis (512 µg/ml) and S. pneumoniae (256 µg/ml). All strains of Gram-negative bacteria showed an LPC MIC >2048 µg/ml.
Figure 1.
Minimum inhibitory concentrations (MICs) for lysophosphatidylcholine (LPC) for various Gram-positive and Gram-negative bacteria. The LPC MIC for each isolate was determined according to a broth microdilution method.
LPC-induced bacterial membrane permeability
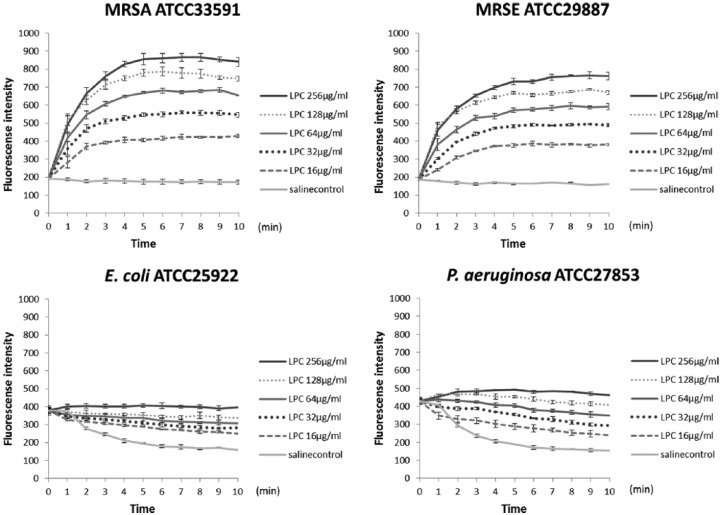
Figure 2 shows the results from membrane permeability assays for four species of bacteria. MRSA ATCC33591 (LPC MIC, 128 µg/ml) exhibited membrane depolarization with LPC in a concentration-dependent manner. Furthermore, Gram-positive methicillin-resistant S. epidermidis (MRSE) ATCC 29887 also showed membrane depolarization, which was not observed in the Gram-negative bacteria E. coli ATCC 25922 and P. aeruginosa ATCC 27853.
Figure 2.
LPC-induced bacterial membrane permeability. Membrane permeability was determined by DiSC3(5) fluorescence analysis after treatment with serial concentration of LPC or saline vehicle. Data represent the mean ± standard deviation from three experiments.
Effect of LPC on bacterial growth
Figure 3 shows the growth curve of MRSA ATCC 33591. Notably, cell growth markedly decreased 6 h after exposure to the MIC and 2× MIC of LPC, demonstrating the bactericidal effect of LPC on MRSA.
Figure 3.
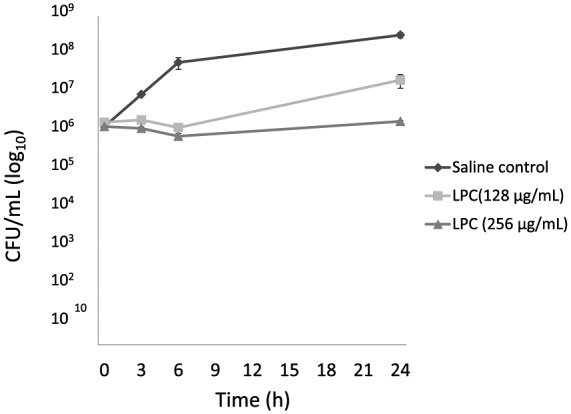
Effect of LPC on MRSA ATCC 33591 growth. Cells (106 cells/ml) were treated with LPC and enumerated by serial dilution colony counting. Gray square, 128 µg/ml LPC; gray triangle, 256 µg/ml LPC; black diamond, untreated control. Data represent the mean ± standard deviation from three experiments.
Efficacy of LPC and gentamicin combination treatment
As shown in Table 1, the addition of 32 µg/ml LPC was sufficient to decrease the gentamicin MIC for all 15 gentamicin-resistant CA-MRSA by ⩽ 1/2 compared to that observed with gentamicin alone, indicating that LPC showed a beneficial effect on gentamicin efficacy.
Discussion
LPC is an important lipid mediator involved in the pathogenesis of various diseases, including diabetes, atherosclerosis, rheumatoid arthritis, bronchial asthma, psoriatic skin, and biliary tract cancer.9–13 The anti-infective effect of LPC is mainly attributed to modulation of inflammation such as upregulation of adhesion molecules, monocyte chemotactic protein-1, and growth factors.14–16
It has been proposed that LPC interacts primarily with the membrane interface and promotes bilayer damage. As shown in our study, Gram-negative bacteria had uniformly high MICs for LPC in vitro, which may have resulted from the outer membrane preventing the interaction between LPC and bacterial cytoplasmic membrane. The beneficial effects of LPC alone and the antibiotic combination treatment against Gram-negative species such as Acinetobacter baumannii and E. coli have been previously reported in murine models of sepsis and pneumonia.17–19 These reports suggested that LPC can effectively treat sepsis and microbial infections by stimulating immune cells that regulate cytokine homeostasis in some Gram-negative infections.
Our in vitro study showed that 2 mg/ml LPC suppressed the growth of most MRSA. Because MRSA is a Gram-positive bacterium, LPC can directly induce MRSA killing by interacting with the cytoplasmic membrane and inducing membrane depolarization and increased membrane permeability, as shown in our permeability assays. However, the MIC of LPC for MRSA strains ranged from 32 µg/ml to >2048 µg/ml and the bactericidal effect differed depending on strains. We did observe some discrepancy between membrane depolarization and growth inhibition. For instance, a few MRSA isolates exhibited high LPC resistance (MIC > 2048 µg/ml), but membrane depolarization was observed with LPC. These results suggested that MRSA strains may have LPC resistance mechanisms other than membrane interaction. Furthermore, we also showed that LPC acts in synergy with gentamicin for inhibition of CA-MRSA growth. All gentamicin-resistant strains showed decrease of the MIC by ⩽ 1/2 for the combination with LPC compared with that for gentamicin alone. It is believed that the membrane perturbation induced by LPC facilitates the entry of the antimicrobial agent into the cells and makes it more effective.20
Because LPC is known to have low toxicity to the skin and is used as a component in cosmetics, it is also appropriate as a component of ointments. Although the mechanism underlying this bactericidal effect remains poorly understood, our results suggests that topical use of LPC may be potentially beneficial in the treatment of superficial skin MRSA infections. Further studies including in vivo evaluation are urgently required.
Acknowledgments
The authors thank Kewpie Corp. for the donation of lysophosphatidylcholine. We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work supported by the Kewpie Corp.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Matsumoto has been granted research support to the institution from Kewpie Corp. The other authors declare that there is no conflict of interest.
Contributor Information
Haruko Miyazaki, Department of Microbiology, Tokyo Medical University, 6-1-1 Shinjuku, Shinjuku-ku, Tokyo 160-8402, Japan.
Naoko Midorikawa, Department of Microbiology, Tokyo Medical University, Tokyo, Japan.
Saki Fujimoto, Department of Microbiology, Tokyo Medical University, Tokyo, Japan.
Natsumi Miyoshi, Department of Microbiology, Tokyo Medical University, Tokyo, Japan.
Hideto Yoshida, Department of Fine Chemicals, Institute of Product Development, Research & Development Division, Kewpie Corporation, Tokyo, Japan.
Tetsuya Matsumoto, Department of Microbiology, Tokyo Medical University, Tokyo, Japan.
References
- 1. Tomasz A. Multiple-antibiotic resistant pathogenic bacteria. N Engl J Med 1994; 330: 1247–1251. [DOI] [PubMed] [Google Scholar]
- 2. Yamaguchi T, Okamura S, Miura Y, et al. Molecular characterization of community-associated methicillin-resistant Staphylococcus aureus isolated from skin and pus samples of outpatients in Japan. Microb Drug Resist 2015; 21: 441–447. [DOI] [PubMed] [Google Scholar]
- 3. From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus–Minnesota and North Dakota, 1997–1999. JAMA 1999; 282: 1123–1125. [PubMed] [Google Scholar]
- 4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52: 285–292. [DOI] [PubMed] [Google Scholar]
- 5. Murch O, Collin M, Sepodes B, et al. Lysophosphatidylcholine reduces the organ injury and dysfunction in rodent models of gram-negative and gram-positive shock. Br J Pharmacol 2006; 148: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrario M, Cambiaghi A, Brunelli L, et al. Mortality prediction in patients with severe septic shock; a pilot study using a target metabolomics approach. Sci Rep 2016; 6: 20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Rensburg CE, Joone GK, O’Sullivan JF, et al. Antimicrobial activities of clofazimine and B669 are mediated by lysophospholipids. Antimicrob Agents Chemother 1992; 36: 2729–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu M, Maier E, Benz R, et al. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 1999; 38: 7235–7242. [DOI] [PubMed] [Google Scholar]
- 9. Iwase M, Sonoki K, Sasaki N, et al. Lysophosphatidylcholine contents in plasma LDL in patients with type 2 diabetes mellitus: relation with lipoprotein-associated phospholipase A2 and effects of simvastatin treatment. Atherosclerosis 2008; 196: 931–936. [DOI] [PubMed] [Google Scholar]
- 10. Fuchs B, Schiller J, Wagner U, et al. The phosphatidylcholine/lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: investigations by 31P NMR and MALDI-TOF MS. Clin Biochem 2005; 38: 925–933. [DOI] [PubMed] [Google Scholar]
- 11. Mehta D, Gupta S, Gaur SN, et al. Increased leukocyte phospholipase A2 activity and plasma lysophosphatidylcholine levels in asthma and rhinitis and their relationship to airway sensitivity to histamine. Am Rev Respir Dis 1990; 142: 157–161. [DOI] [PubMed] [Google Scholar]
- 12. Ryborg AK, Gron B, Kragballe K. Increased lysophosphatidylcholine content in lesional psoriatic skin. Br J Dermatol 1995; 133: 398–402. [DOI] [PubMed] [Google Scholar]
- 13. Shimizu R, Kanno K, Sugiyama A, et al. Cholangiocyte senescence caused by lysophosphatidylcholine as a potential implication in carcinogenesis. J Hepatobiliary Pancreat Sci 2015; 22: 675–682. [DOI] [PubMed] [Google Scholar]
- 14. Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A 1988; 85: 2805–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kume N, Gimbrone MA., Jr. Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J Clin Invest 1994; 93: 907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol 2005; 25: 923–931. [DOI] [PubMed] [Google Scholar]
- 17. Smani Y, Domínguez-Herrera J, Ibáñez-Martínez J, et al. Therapeutic efficacy of lysophosphatidylcholine in severe infections caused by Acinetobacter baumannii. Antimicrob Agents Chemother 2015; 59: 3920–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parra Millán R, Jiménez Mejías ME, Sánchez Encinales V, et al. Efficacy of lysophosphatidylcholine in combination with antimicrobial agents against Acinetobacter baumannii in experimental murine peritoneal sepsis and pneumonia models. Antimicrob Agents Chemother 2016; 60: 4464–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan JJ, Jung JS, Lee JE, et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med 2004; 10: 161–167. [DOI] [PubMed] [Google Scholar]
- 20. Vaara M, Porro M. Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria. Antimicrob Agents Chemother 1996; 40: 1801–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]