Abstract
Vertical growth of plants is a dynamic process that is influenced by genetic and environmental factors and has a pronounced effect on overall plant architecture and biomass composition. We have performed six controlled growth trials of an interspecific Setaria italica x Setaria viridis recombinant inbred line population to assess how the genetic architecture of plant height is influenced by developmental queues, water availability and planting density. The non-destructive nature of plant height measurements has enabled us to monitor height throughout the plant life cycle in both field and controlled environments. We find that plant height is reduced under water limitation and high density planting and affected by growth environment (field vs. growth chamber). The results support a model where plant height is a heritable, polygenic trait and that the major genetic loci that influence plant height function independent of growth environment. The identity and contribution of loci that influence height changes dynamically throughout development and the reduction of growth observed in water limited environments is a consequence of delayed progression through the genetic program which establishes plant height in Setaria. In this population, alleles inherited from the weedy S. viridis parent act to increase plant height early, whereas a larger number of small effect alleles inherited from the domesticated S. italica parent collectively act to increase plant height later in development.
Author summary
Growth is a dynamic process that responds to a changing environment. Most of the methods that we have for measuring are static and collecting information throughout an organisms lifecycle is labor and cost prohibitive. Advances in imaging and robotics technology have enabled novel approaches to understanding how plants adapt to the environment. Using the model grass Setaria and new methods for measuring parameters from images, we investigate the genetic architecture of plant height in response to water availability and planting density. Height is one of the most influential components of plant architecture, determining tradeoffs between competition and resource allocation and is an important trait for boosting yields. The non-destructive nature of plant height measurements has enabled us to monitor growth throughout the plant life cycle in both field and controlled environments. We identified several loci controlling height in a population derived from a wild strain of Setaria viridis and its domesticated relative Setaria italica, as well as the developmental time in which these loci act. In this population, alleles inherited from the wild parent act to increase plant height early, whereas a larger number of small effect alleles inherited from the domesticated parent collectively act to increase plant height later in development.
Introduction
Organismal growth is a complex process that is influenced by genetic and environmental factors in a time dependent manner. Understanding the dynamics of these interactions is a critical step in understanding how traits are determined. Height is one of the most influential components of plant architecture. Due to ease of measurement, high heritability and agronomic importance, plant height has been an attractive topic for scientific inquiry for over a century [1]. Plant investment in vertical growth has adaptive value because it helps a plant to out-compete neighbors for access to solar radiation. However, vertical growth requires a concomitant energetic allocation for construction of stem tissue at the expense of allocation to lateral growth and other processes. Greater height growth also amplifies hydraulic cost [2], and increases the risk of lodging [3]. The benefit to cost ratio of such adaptation is likely directly influenced by growth environment [4]. Adaptations introgressed into wheat and rice varieties during the Green Revolution that led to reduced height, increased yield, harvest uniformity, improved carbon partitioning and nutrient and water use efficiency. In biomass crops, height has been shown to be positively correlated with above ground biomass [5,6]. Thus, depending on the crop and breeding objective either increased or decreased height may be targeted.
Plant height in the Poaceae, a family of grasses that includes the cereal crops and bioenergy grasses, is a function of internode length and number, the increase of which is terminated at reproductive maturity. Plant height within the grasses is a highly heritable, polygenic trait [7–14]. Genetic studies of height in maize, sorghum, sugarcane, wheat, barley, setaria and rice have identified well over 100 QTL [7–16], and enabled the cloning of multiple loci that contribute large effects [9,15–36]. Forward genetic screens of mutant populations has also been an effective approach to identify causative genes associated with height [24,37]. Although plant height exhibits a strong degree of genetic determinism, it is influenced by environmental factors such as water availability and planting density and exhibits dynamic behavior throughout the plant life cycle [10,38–42].
The strong influence of environment on plant height suggests that multiple signals impinge on the regulation of plant height throughout development. Surprisingly, relatively few studies have assessed how the temporal genetic architecture of this dynamic trait changes through developmental time [14,43]. A major challenge of performing such experiments is the difficulties associated with growing large populations in conditions with contrasting environmental variables while capturing high precision measurements of phenotypes at appropriate intervals.
Recent use of modern high-throughput phenotyping (phenomics) technology is beginning to alleviate these technical obstacles [44,45]. Utilizing genetic model systems that possess desirable growth attributes and tractable genetics [46–48] in combination with phenomics technology improves our ability to obtain a more holistic systems view of growth through developmental time and in the face of environmental challenge. As a model system, plants in the genus Setaria, possesses many favorable experimental and life history attributes that make temporal genetic analysis of complex traits possible and the genetic dissection of these traits more feasible than in larger evolutionarily related crop plants like maize and sorghum [46,47]. In this study we conduct a large scale and multi-environment analysis of plant height. We identify thirty seven QTL associated with plant height throughout development within a Setaria recombinant inbred population (RIL) population [49–51] across twelve different experiments varying water availability and planting density. Analysis of ortholog positions suggests that known height affecting genes from the Poaceae account for only a fraction of the loci identified. The results of this study are discussed in light of development and environmental variation and suggest a strategy for the fine scale manipulation of plant height in the Poaceae.
Materials and methods
Plant material
A Setaria F7 RIL population comprised of 217 individuals was used for genetic mapping. The RIL population was generated through an interspecific cross between the wild-type S. viridis accession, A10, and the domesticated S. italica accession, B100 [49–51]. Plant height measurements of individuals within this population were collected in six different trials. Four of the six trials were conducted at field sites located at University of Illinois-Champaign. Two field trials designed to assess plant phenotypic response to planting density and water availability were each conducted in both 2013 and 2014. The controlled environment trials were performed in a growth chamber at Carnegie Institute for Science, Stanford, CA and using the Bellweather Phenotyping Facility at Donald Danforth Plant Science Center [52].
Illinois
Field experiments were conducted on the South Farms at the University of Illinois Urbana-Champaign in summers 2013 and 2014. The field site is rain-fed, tile-drained, has a deep and organically rich Flanagan/Drummer series type soil [53].
For each field experiment, seeds were first germinated in a greenhouse in plug trays (128sq, T.O. Plastics) containing a media mix composed of sphagnum, vermiculite, fine bark, bark ash, gypsum, slow-release nitrogen, dolomitic limestone, and a wetting agent (Metro-Mix 360, Sun Gro Horticulture). Plugs were provided with ample water daily by clear water or by fertigation every other day (EXCEL CAL MAG 15-5-5). Seedlings were hand transplanted into genotype specific subplots (dimensions 7 x 6) within a mechanically tilled field approximately 1 week after sewing. Control over water availability was achieved by growing plants under retractable awning rainout shelters at 5 cm spacing to block precipitation from experimental plots and re-applying differential drip irrigation regimes to wet and dry replicates. Each awning is referred to as a plot and the genotype specific blocks within each awning are called subplots. Planting density experiments were performed by growing each genotype specific subplot at 5 cm (dense) and 25 cm (sparse) spacing.
Carnegie Institute for Science
Plants (F7 RIL population) were grown in deep pots (Stuewe & Sons, Oregon, D25L) with one plant per pot (one plant per RIL was phenotyped). Seeds were directly germinated in 14 inch Deepots filled with a soil mixture containing 75% Pro-Mix (PRO-MIX(r) PGX soil, Premier Tech, Canada) and 25% river sand, imbibed in water to pot capacity. Pot capacity, defined as the amount of water that soil in a pot can hold against the pull of gravity, was estimated to be 230 ml. Plants were grown in a growth chamber (12 hours light at 31°C and 12 hours dark at 23°C with constant relative humidity at 54–55%). Well-watered plants were watered back to field capacity once every third day whereas for water deficit experiments, seeds were sown in soil imbibed to pot capacity and no further water was added. To prevent water loss, the bottom of each pot was covered with a plastic bag.
Donald Danforth Plant Science Center
After a six week stratification in moist long fiber sphagnum moss (Luster Leaf Products Inc., USA) at 4 C, Setaria seeds were planted in four inch diameter (10 cm) white pots pre-filled with ~470 cm3 of Metro-Mix 360 soil (Hummert, USA) and 0.5 g of Osmocote Classic 14-14-14 fertilizer (Everris, USA). After planting, seeds were given 7 days to germinate in a Conviron growth chamber with long day photoperiod (16 h day/8 h night; light intensity 230 μmol/m2/s) at 31°C day/21°C night before being loaded onto the Bellweather Phenotyping System using a random block design. Plants were grown on the system for 25 days under long day photoperiod (16 h day/8 h night; light intensity 500 μmol/m2/s) cycling the temperature to match the photoperiod (31°C day/21°C night) and maintaining the relative humidity between 40–80%. Weighing and watering of plants was performed between 2–3 times per day to maintain soil volumetric water content at either 40% full-capacity (FC) (drought) or 100% FC (well-watered) as determined by [52]. Water limitation began 15 days after planting. Individual plants were imaged at 4 different angular rotations (0°, 90° 180°, 270°) every other day.
The effect of pot size was evaluated by growing parental lines of Setaria viridis (A10) and Setaria italica (B100) in four different size pots: 160 cm3 (T.O. Plastics, 715348C), 473 cm3 (Pöppelmann, VCC 10 F US), 950 cm3 (Stuewe & Sons, MT38) and 6033 cm3 (Nursery Supplies, C600) filled with Metro-Mix 360 soil (Sun Gro Horticulture). Seeds were given 7 days to germinate in a greenhouse and watered daily by the Donald Danforth Plant Science Center greenhouse staff. Plants were grown for 50 days under long day photoperiod (14 h day/ 10 h night) cycling the temperature to match photoperiod (27°C day/ 23°C night) and maintaining the relative humidity between 40–100%.
Phenotyping plant height and panicle emergence
In the field, plant height was measured as the distance from the plant base to the uppermost leaf collar on the culm. In 2013 this was measured by judging the average height of the canopy in a subplot relative to a yard stick and recorded the measurement using a Janam XP20 hand held scanner [Janam Technologies; Woodbury, NY]. In 2014 height was directly measured repeatedly on three tagged plants in each subplot. In each field experiment, a direct culm height measurement was taken at the final destructive biomass harvest. In 2013 this was done on three representative plants from each subplot; in 2014 this was done on the same-tagged plants as the in-field repeated height measurements.
In the 2013 Density experiment, height was measured at 19, 25, 31, 38, 45, 52, 59, and 67 days after seed sowing. Final biomass harvest began 87 days after seed sowing. In the 2013 Drought experiment, height was measured at 21, 29, 36, 43, 50, 59, and 67 days after seed sowing. Final biomass harvest began 92 days after seed sowing. In the 2014 Drought experiment, height was measured at 25, 33, 40, and 47 days after seed sowing. Final biomass harvest began 59 days after seed sowing. In the 2014 Density experiment, height was measured at 25 and 46 days after seed sowing. Final biomass harvest began 67 days after seed sowing.
Panicle emergence was measured as the number of days after sowing at which the panicle head was seen past the collar of the culm flag leaf in at least half of the individuals in a genotype specific subplot.
At Carnegie, plant height was measured from the base of the plant to the tip of the panicle (at 48 days after seed sowing).
Five different measurement functions encoded within the PlantCV software package were used to estimate plant height in the images collected at the Bellweather Phenotyping Facility [52] (S1 Fig). These include length of the plant object along the y-axis (extent_y), length of the plant object from the top of the pot to the maximal y-axis point identified as the plant object (height_above_bound), the distance from the top of the pot to the y-coordinate reported as the center of mass of the plant object (centroid_y), the distance from the top of the pot to the y-coordinate identified as the center of the ellipse that encompasses the plant object (ellipse_y) and an additional function designed to estimate the distance between the top of the pot and y-coordinate where average plant width is maximized (canopy_height). Canopy height is calculated by dividing the height_above_bound measure into 20 different equally sized scoring windows (divide the object into 5% intervals along the y-axis, scoring is based upon median width within each interval) and measuring the median plant width within each window. The function reports the average of y-coordinates that comprises the scoring window and median width within the scoring window where the object (plant) is the widest (canopy_width).
Centroid_y and ellipse_center_y were the most and least heritable measurements of plant height respectively (S2a Fig and S2 Table). Height_above_bound and extent_y exhibited the largest proportion of variance attributed to water limitation (S2b Fig and S3 Table). Summarization of height measurements derived from four rotational side view images was performed by calculating the mean, median, maximum or minimum of each distribution. Summarization method does not appear to influence the heritability of this these traits (S3 Fig). All height metrics are reported as the average value estimated from all individual rotational images at each plant time point. Lengths of objects measured in pixels were converted to centimeters (cm) using the calibration reported by [52].
Plant height of parental lines grown in the greenhouse at the Donald Danforth Plant Science Center were measured as the distance from the base of the plant to most distant leaf collar on the culm using a tape measure. Measurements were recorded every other day during the workweek for a span of 50 days.
Genotyping RILs
A de novo genetic map of the A10 x B100 Setaria RIL population was constructed through genotyping by sequencing (GBS) using the methods described in [54,55]. Briefly, leaf tissue was collected from plants in the 2014 planting density experiment in addition to greenhouse trials. High molecular weight DNA was extracted using the CTAB method [56] and quantified using a Nanodrop 1000 Spectrophotometer [Thermo Scientific; Waltham, MA]. DNA was then double-digested using the restriction enzymes Pst1 and MspI and a unique barcode and common adaptors were ligated onto their respective fragment ends [57]. After size selection and cleanup using Agencourt AMPure XP beads [Beckman Coulter; Brea, Ca], barcoded samples were pooled and submitted for sequencing (100-bp single end reads) using two Illumina Hi-Seq lanes [Illumina; San Diego, CA].
The resulting sequence reads were processed using the Tassel 3.0 GBS pipeline [58]. Raw sequence reads were filtered to remove any tags that were not present 20 times at minimum within in each lane of sequencing and anchored to the S. viridis genome version 1.1 (Phytozome) using bowtie2 [59] with default parameters. Anchored SNP polymorphisms exhibiting an inbreeding coefficient less than 0.9 were removed and identical taxa within and between library preps (2 sequencing lanes) were called as heterozygous if the ratio between alleles in and between all identical taxa and/or library preps was less than 0.8. All polymorphisms categorized as heterozygous were called as missing values for the remainder of the analysis, yielding 17,612 SNPs.
The genetic map was generated using the R/qtl software [60]. Genotype calls within the combined A10 taxa were compared to the S. viridis reference genome version 1.1 (Phytozome) and all missing and non-congruent polymorphisms were removed. The remaining sites were filtered to remove SNPs not present in > 90% of the unified taxa yielding 217 RILs and 3,295 markers. All polymorphisms were then recoded to reflect their parent of origin. Dubious genotype calls were identified by examining the recombination frequency between adjacent markers. Based upon manual inspection of histograms generated of recombination fraction between adjacent markers, all markers exhibiting a recombination frequency between adjacent markers greater than 36% (80 recombinations / 217 individuals) were considered to be erroneous and removed from the map. Next imputation of missing genotypes was performed at all sites that did not exhibit recombination between missing makers (using the R/qtl fill.geno() function and specifying the ‘no_dbl_XO’ method) and duplicate markers were removed from the genetic map. Finally, the genetic distance between markers was calculated using the Kosambi mapping function assuming a genotype error rate of 0.005. Prior to QTL mapping, individuals missing more than 10% of their genotype calls are removed yielding a genetic map containing 208 individuals genotyped at 1,595 sites with 99.6% coverage. Based on pairwise similarity between identical individuals, the GBS map constructed here is highly consistent with other genetic maps of this population described in the literature [49,61].
Partitioning trait variance, calculating heritability and estimating line values
Variance components corresponding to broad sense heritability and total variance explained were estimated using a mixed linear model using the R package lme4 [62]. Broad sense heritability was calculated using two methods. Within an individual experiment, broad sense heritability on a line-estimate basis was calculated using the following formula:
in which ntreatment is the harmonic mean of the number of treatment blocks in which each line was observed and nreplicates is the harmonic mean of number of replicates of each genotype in the experiment. Heritability within treatment blocks was calculated by fitting a linear model with genotype as the only explanatory factor within each treatment block.
The proportion of variance attributed to genotype divided by total variance within each treatment block is reported as broad sense heritability within treatment.
Total variance explained was calculated by fitting a linear model including factors genotype, treatment, plot and genotype x treatment effects across all phenotypic values in all treatments. The proportion of variance that is incorporated into these factors divided by the total variance in the experiment is reported as total variance explained.
Best linear unbiased predictors (BLUPs) of plant height for each genotype were predicted from a linear mixed effect model independently within all individual time points of field drought and density experiments in 2013 and 2014 using lme4 [62]. The following terms were included in the model:
In which Yijk is the individual observation of plant height, μ is the overall mean, genotypei is the effect of the ith genotype, treatmentj is the effect of the jth treatment, plot(treatment)k(j) is the effect of the kth plot nested within the jth treatment, and (genotype x treatment)ij is a term describing the interaction between the ith genotype and jth treatment and εijk is the random error term. As fixed effects we entered treatment and plot nested within treatment into the model. The terms genotype and genotype by treatment interactions were modeled as random effects.
Logistic regression was used to model the line values of plant height in experiments performed at the Bellweather Phenotyping Facility. Three types of logistic regression models considered (three-parameter logistic, four-parameter logistic and Gompertz) and estimates from the model with the lowest Akaike information criterion (AIC) score were used. Modeling was performed using R functions described in [63].
QTL analysis
QTL mapping was performed at each time point within treatment blocks and on the numerical difference, relative difference and trait ratio calculated between treatment blocks using functions encoded within the R/qtl and funqtl package [60,64]. The functions were called by a set of custom python and R scripts (https://github.com/maxjfeldman/foxy_qtl_pipeline). Two complimentary analysis methods were utilized. First, a single QTL model genome scan using Haley-Knott regression was performed to identify QTL exhibiting LOD score peaks greater than a permutation based significance threshold (α = 0.05, n = 1000). Next, a stepwise forward/backward selection procedure was used to identify an additive, multiple QTL model based upon maximization of penalized LOD score. Both procedures were performed at each time point, within treatment blocks and on the numerical difference relative difference and trait ratio calculated between phenotypic values measured in treatment blocks at each time point. QTL associated with difference or ratio composite traits may identify loci associated with genotype by environment interaction [65]. QTLs across different experiments were considered to be the same if they were in a 20cM window around the most commonly identified marker in that window. Tests for epistatic interaction between QTL were performed using the scantwo function in the R/qtl package [60]. Epistasis between QTL was evaluated by comparing the log10 likelihood ratio of a model describing the additive and interaction between two QTL with a model only containing the additive interaction between two QTL. The significance threshold was determined through permutation (α = 0.05, n = 100).
The function-valued approach described by Kwak et al., 2016 [64] was used to identify QTL associated with the average (SLOD) and maximum (MLOD) score at each locus throughout the experiment [64]. The genotypic mean height within treatments was estimated using a logistic function and the QTL significance threshold was determined based upon permutation-based likelihood of observing the empirical SLOD or MLOD test statistic. Across all grow outs, separate; independent linkage mapping analysis performed at each time point identified a larger number of QTL locations relative to similar function valued analysis based on the SLOD and MLOD statistics calculated at each individual marker throughout the experimental time course.
After refinement of QTL position estimates, the significance of fit for the full multiple QTL model was assessed using type III analysis of variance (ANOVA). The contribution of individual loci was assessed using drop-one-term, type III ANOVA. The proportion of variance explained by in addition to the allelic effect size of each locus were determined by comparing the fit of the full model to a sub-model with one of the terms removed. All putative protein coding genes (Setaria viridis genome version 1.1) found within a 1.5-logarithm of the odds (LOD) confidence interval were reported for each QTL.
Candidate gene analysis
A search for orthologous genes associated with plant height (putative causal genes) was performed within the 37 unique QTL intervals identified throughout the genome. A curated list of plant height genes cloned from maize, sorghum and rice was generated through literature review and searching Maize GDB with search terms including “height” and “dwarf”. The coding sequence name and genomic coordinates of each of these genes was downloaded from their species-specific repository (Maize GDB, Rice Genome Annotation Project, Phytozome). Regions of genomic synteny between Setaria viridis and maize, sorghum or rice were identified using the synmap function encoded by the CoGe web tool (default parameters) [66]. The genomic coordinates of coding sequences of interest were extracted from the CoGe text output using a custom python script (https://github.com/maxjfeldman/Feldman_Setaria_Height_2016). Reciprocal BLAST searches were used to identify the location of orthologous genes not found in syntenic blocks. The location of these sequences was plotted across the genome to examine their location relative to the coordinates of the QTL and median confidence interval at each location.
Results
Evaluating different measurements of plant height
Height in the field was measured using two different methods of approximation. An analysis of variance indicates that on average, measuring the exact height to the collar of the uppermost leaf on the culm of a subset of three individuals within a plot (as performed in 2014) was approximately 5.8% more heritable than attempting to estimate the average height to the collar of the uppermost leaf on the culm of all plants within the plot (as performed in 2013) (S1 Table). An increase in heritability between years was not observed for flowering time between the 2013 and 2014 field seasons, suggesting that the increase in heritability was not due to differences in environment or management (S1 Table).
Five different automated methods of measuring plant height (height_above_bound, extent_y, centroid_y, ellipse_center_y, canopy_height—see definitions in methods) from 2-D images captured at the Bellweather Phenotyping Facility were evaluated (S1 Fig). Among these measurements, height_above_bound, extent_y and centroid_y were highly correlated (R2 > 0.97) (S2 Table) and all three were highly heritable on average (h2 > 0.85) and showed clear effects of water limitation (S2 Fig, S3 and S4 Tables). Summary statistic does not greatly influence the heritability of height type measurements (S3 Fig). The mean value of height_above_bound based upon four rotational images was the primary metric used for the remainder of this study as it is intuitively simple to understand, highly heritable, strongly correlated with other height metrics, sensitive to treatment effects and previously validated against manual measurements [52].
Variation in plant height
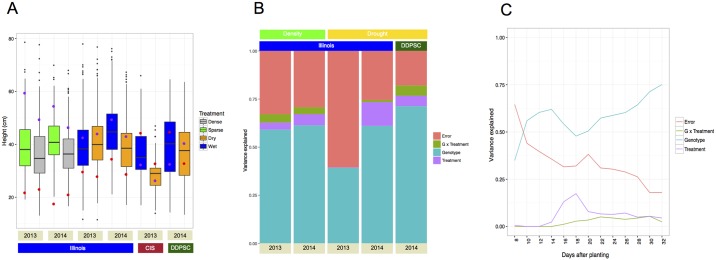
Height of individuals within the S. viridis (A10) X S. italica (B100) RIL population was evaluated under very diverse growing conditions. Variation resulted from different water availability and planting density treatments. In addition, day length and temperature varied between plant-outs at three locations (Illinois field site, DDPSC growth chamber, Carnegie growth chamber) and two different planting dates each year at the Illinois field site. Although the mean and variance of final height differed significantly between grow outs (Fig 1a), the coefficient of variation remained relatively constant across all replicated experiments and treatments (Table 1 and S5 Table). The proportion of variance attributed to experimental factors (genotype versus treatment versus error) also varied between grow outs (Fig 1b) and throughout time in a given experiment (Fig 1c and S4 Table). Across all experiments the mapping population size averaged ~160 individuals with a minimum count of 146 (Table 1). Final height estimated near the end of each these experiments averaged 38 cm but every experiment exhibited at least a 20 cm difference between the 5th and 95th percentile (Table 1). Modest transgressive segregation was apparent across all experimental grow outs (Fig 1a). At the end of all eight field grow outs, the B100 parental line was taller than the A10 parent, whereas in three out of four grow outs conducted in artificial environments at Carnegie and Bellweather, A10 was taller (Fig 1a).
Fig 1. A large proportion of variance can be attributed to genotype and treatment factors, the effect of which changes throughout time and across trial locations.
Field grow outs were performed at the University of Illinois Champaign-Urbana (Illinois), whereas green house experiments were performed at the Carnegie Institute for Science (CIS) and the Donald Danforth Plant Science Center (DDPSC). Both water availability and planting density experiments were conducted at Illinois in 2013 and 2014. A) Boxplot summaries of plant heights at the end of each experiment. Red points indicate the mean height of the A10 parental line and purple points denote the mean height of the B100 parental line. B) The proportion of variance that can be attributed to experimental factors at the end of each study that included replication. C) The proportion of variance that can be attributed to experimental factors throughout the Bellweather 2014 grow out.
Table 1. Summary of experimental metrics from field and controlled environments.
| Location | Environment | Year | Timepoint | Treatment | Summarization method | Genotypes planted | Mapping pop. size | Replication | Plant Height 5% | Plant Height 50% | Plant Height 95% | Mean | Variance | CV | Correlation with panicle emergence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Illinois | Field | 2013 | 92 | Dry | BLUP | 183 | 159 | 2.90 | 25.47 | 39.95 | 56.80 | 40.50 | 98.93 | 0.33 | 0.30 |
| Illinois | Field | 2013 | 92 | Wet | BLUP | 170 | 159 | 2.99 | 24.20 | 38.29 | 56.20 | 39.16 | 98.19 | 0.37 | 0.31 |
| Illinois | Field | 2013 | 87 | Dense | BLUP | 183 | 169 | 2.40 | 21.04 | 34.68 | 51.67 | 36.03 | 96.63 | 0.34 | 0.69 |
| Illinois | Field | 2013 | 87 | Sparse | BLUP | 182 | 168 | 2.96 | 22.15 | 38.06 | 59.70 | 39.19 | 118.10 | 0.34 | 0.75 |
| Illinois | Field | 2014 | 59 | Dry | BLUP | 157 | 146 | 3.00 | 25.42 | 38.53 | 57.28 | 38.90 | 88.61 | 0.28 | 0.37 |
| Illinois | Field | 2014 | 59 | Wet | BLUP | 157 | 146 | 3.00 | 30.41 | 44.70 | 64.79 | 45.28 | 102.31 | 0.28 | 0.37 |
| Illinois | Field | 2014 | 67 | Dense | BLUP | 188 | 174 | 1.97 | 21.87 | 36.34 | 53.36 | 36.76 | 81.59 | 0.31 | 0.49 |
| Illinois | Field | 2014 | 67 | Sparse | BLUP | 187 | 172 | 1.93 | 27.13 | 40.76 | 56.44 | 41.59 | 82.06 | 0.29 | 0.42 |
| Stanford | Growth chamber | 2013 | NA | Dry | None | 160 | 149 | 1.00 | 18.00 | 29.00 | 38.45 | 28.31 | 40.05 | 0.22 | NA |
| Stanford | Growth chamber | 2013 | NA | Wet | None | 166 | 155 | 1.00 | 23.43 | 35.00 | 51.08 | 36.65 | 82.21 | 0.25 | NA |
| Danforth | Growth chamber | 2014 | 30 | Dry | Logistic regression | 187 | 157 | 2.91 | 18.85 | 37.64 | 58.36 | 37.44 | 134.69 | 0.31 | NA |
| Danforth | Growth chamber | 2014 | 30 | Wet | Logistic regression | 185 | 167 | 2.89 | 20.60 | 40.21 | 59.72 | 39.40 | 146.29 | 0.31 | NA |
| AVERAGE | AVERAGE | NA | NA | NA | NA | 175.42 | 160.08 | 2.41 | 23.22 | 37.76 | 55.32 | 38.27 | 97.47 | 0.30 | 0.46 |
Within each trial and treatment, the heritability of final plant height exceeded 0.84 (S1 Table). The mean heritability of plant height in this population across all time points was 0.82 and tended to be higher in controlled environments (average H2 = 0.86 at Bellweather and Carnegie, average H2 = 0.83 in field at Illinois). Generally, the proportion of height variance attributed to genotype increased as the plant approached maturity (Fig 1c and S6 Table). The effects of water limitation and planting density treatments did not dramatically influence the heritability of plant height in this study (S1 Table).
The block structure of the field experimental design and the frequent repeated measurements on the Bellweather phenotyping system enabled modeling of the underlying trait phenotypes. Best linear unbiased estimated line values of height within individual experiment and time points were used to account for variation attributed to plot and treatment effects in field studies. Logistic regression was used estimate the expected value of repeated temporal measurements of height collected at the Bellweather Phenotyping Facility.
Variation of plant height in response to growth environment and experimental treatment
Water limitation experiments were conducted in all three locations; each of which differed by genotypes planted, statistical power and the severity of treatment administered in addition to other latent variables inherent to growth location and experimental design. The effect of these factors was assessed at a single time point representative of final plant size using type III analysis of variance [67]. Within drought trials, genotype had the strongest effect on height, followed by trial location and treatment effect nested within location (S7 Table). Similar to the correlation of height and days to anthesis in maize [13], a significant positive correlation between final height and day of panicle emergence was observed in each field grow out (Table 1), suggesting that different planting dates, with their different day lengths and temperatures could explain some of the trial location variation.
The water limitation treatment varied widely between experiments, simulating several possible scenarios plants could experience. Field trials at Illinois and the controlled growth trial at Carnegie were designed as progressive drought experiments, where after an initial growth period, water is withheld. In the Bellweather phenotyping experiment, a reduced water availability set point was maintained after an initial dry down. The controlled growth experiments all showed a significant effect of drought, as did the 2014 field experiment (despite an end of season flood). In retrospect, water was not withheld early enough in the 2013 field experiment to detect a significant drought effect on height although soil probes showed a substantial difference in water availability within 3 weeks (S8 Table). The effect of withholding water completely soon after germination in the Carnegie experiment made the observed height effect the most pronounced of any grow out observed (S5 Fig). Height appears less responsive to planting density than water limitation, with most of the variance attributed to genotypic and plot nested within treatment followed by the variance attributed to year and lastly planting density (S7 Table). Nonetheless, in both 2013 and 2014, planting density had a significant effect on height by the end of the experiment.
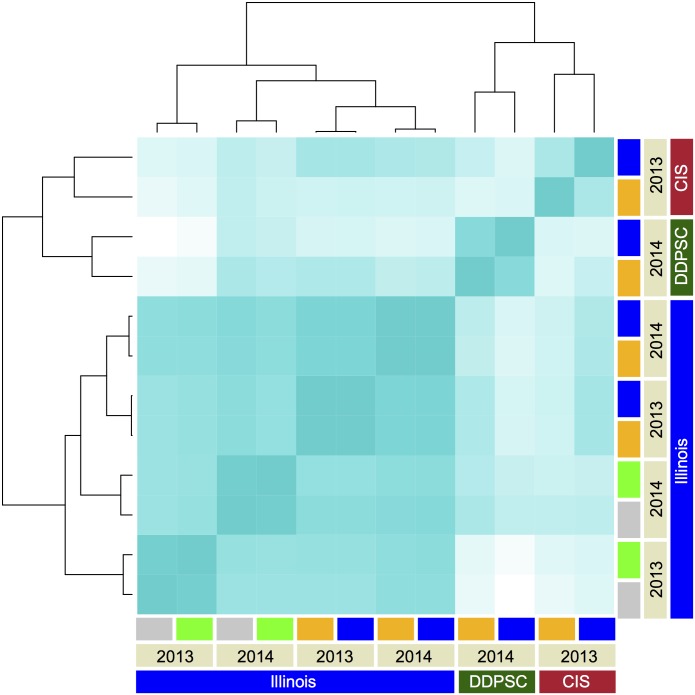
Using the rank order of lines by phenotype as a proxy for how similar the experiments are to each other, the effect of experimental location is apparent, as well as the difference between controlled environment and the field. (Fig 2 and S4 Fig). The well-watered treatment at Bellweather, the drought treatment at Carnegie and the 2013 field density experiment are the most divergent environments encountered within this study (Fig 2).
Fig 2. Rank order correlation of plant height measured at the end of each experiment.
Grow outs of the A10 X B100 RIL population were performed in three different locations including the University of Illinois Champaign-Urbana, Carnegie Institute for Science (CIS) and Donald Danforth Plant Science Center (DDPSC). Treatment block is indicated by color bar at the edge of the dendrogram (blue corresponds to wet, orange represents to dry, green indicates sparse and grey denotes dense planting). The intensity of color shading is proportional to the magnitude of Pearson’s correlation coefficient (0.0 is white whereas 1.0 is blue).
Genetic map construction for the Setaria A10 X B100 RIL population
Two previous genetic maps have been constructed for this population [49,61] but neither included all of the lines used in this study. We constructed a de novo genetic map and genotyped all phenotyped RILs to verify the identity of genetic stocks [68]. DNA samples were prepared from 183 field grown and 34 greenhouse grown RIL lines (in addition to the two parental genotypes) and GBS libraries were prepared and sequenced [54,55]. After alignment to the recently completed S. viridis genome (Phytozome v1.1) using bowtie2 [59] and filtering to select SNPs that match the sequenced parent samples and removal of duplicates, the final genetic map consists of 217 individuals, spanning 1,088 cM, calculated using 1,595 markers (S9 Table). After imputation, the average spacing between markers is 0.7 cM with the greatest distance between markers being 14.4 cM (S9 Table). The map size, coverage and identity of individuals in this RIL population are largely identical between map builds [49,61].
Architecture of height QTL in different environments
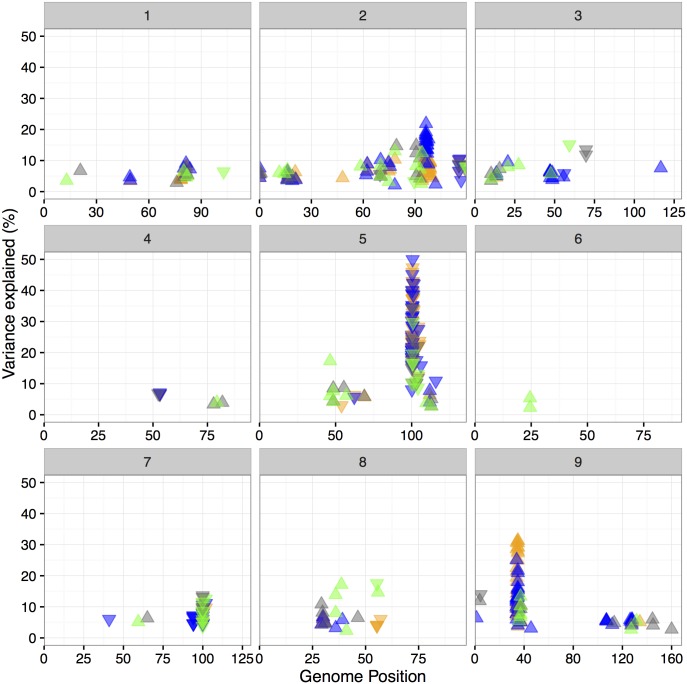
We implemented a standard stepwise forward/backward selection procedure to identify the optimal, additive, multiple QTL model based upon penalized LOD score [60]. Mapping was performed on all traits, at each time point within treatment blocks and on the numerical difference, relative difference and trait ratio between phenotypic values by treatment blocks. This procedure identified a total of 153 individual SNPs associated with height across all time points and treatments within the 12 experimental grow outs (Fig 3 and S10 Table). No significant epistatic interaction between QTL was detected. Many of the individually identified SNP positions group into clusters of linked loci that are likely representative of a single QTL location. Groups of closely linked QTL markers (10 cM radius) were collapsed into the most frequently observed SNP marker in each cluster, resulting in 37 unique QTLs (S11 Table and S6 Fig). A substantial proportion of these QTL were observed at least once in three (17/37) or four (12/37) treatment groups, suggesting that the genetic architecture of height is similar across all experimental treatments contrasts considered in this study (S11 Table). Confirming this observation, Mauro-Herrera et al, with the same population grown in the greenhouse and field and height measured at three different timepoints, identified 7 of the 12 QTLs [16].
Fig 3. One hundred and fifty three unique QTL positions were identified across all experiments.
Each box corresponds to an individual chromosome, where the values along the x-axis are chromosome position and values along the y-axis denote the proportion of genetic variance explained by the QTL. Each triangle represents a single QTL detected, where the color indicates the treatment condition the QTL was identified in (blue represents wet, orange corresponds to dry, green indicates sparse planting density whereas grey denotes dense planting.) and direction of the arrow corresponds the directional effect of the B100 parental allele.
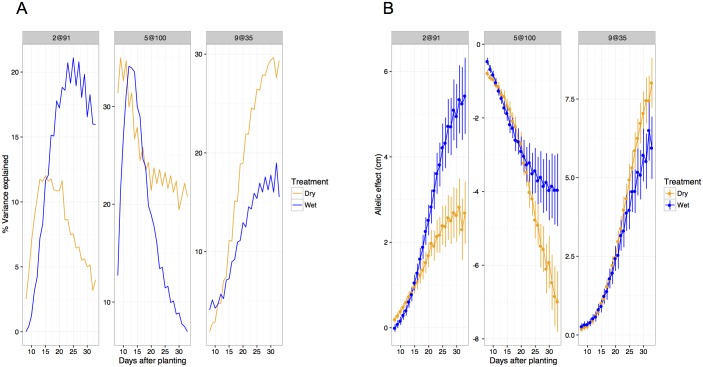
A static QTL model including only the 12 QTL locations identified at least once in all treatment groups was fit at a time point representative of final plant size in each experiment. Across all experimental grow outs these 12 QTL explained between 25–64% of the additive genetic variance and 53% on average (Fig 4). Across all experimental time points in this study the three loci that explain the greatest proportion of variance in any single time point are included within this set of 12 QTL. The most frequently observed QTL (marker: S5_41999990) was identified in each of the 12 experiments and explained the largest percentage of additive genetic variance (mean of 19%). The B100 allele at this QTL position is associated with reduced plant height. The individual contribution of the other 11 QTL at this representative time point on average, did not explain more than 7% of the additive genetic variance alone, but cumulatively these 11 QTL account for greater than 33%. The B100 allele at nine of these twelve positions are associated with increased plant height.
Fig 4. Twelve QTL were identified across all experimental treatment groups.
Cells shaded red indicate a positive effect from inheriting the B100 parental allele whereas cells shaded in blue indicate a positive effect of inheriting the allele derived from the A10 parent. Values within the cells and intensity of coloration report the percentage of additive genetic variance explained. Loci are identified by either their approximate location in genetic map units (5@100 is a QTL on chromosome 5 at 100 cM) or by the location of their strongest associated marker (S5_41999990 is the best scoring marker on chromosome 5 at nucleotide position 41999990).
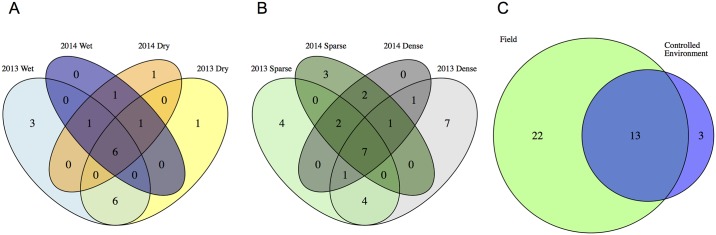
Across all field trials performed, the number of QTL shared between treatment blocks and across years is greater than the number of QTL unique to any treatment block across growth years or both treatment blocks within a single growth year (Fig 5 and S7 Fig). No overlap between QTL detected exclusively in well-watered or water-limited conditions was observed between any of the experiments (Fig 5a and S7 Fig). Within planting density experiments a single QTL (marker: S9_52254840) was identified uniquely within the high planting density treatment block in both years (Fig 5b and S7 Fig). A majority (13/16) of the QTL detected in controlled environments were also detected in field environments (Fig 5c).
Fig 5. QTL associated with plant height are shared across experimental contrasts.
A) No QTL unique to either treatment block were observed across both water limitation field trials in 2013 and 2014. B) In planting density experiments, one QTL was identified uniquely within the high density treatment block in field trials in both years. C) Almost all QTL identified in controlled environments are also identified in field environments.
The results from QTL scans performed on the numerical difference, relative difference and the ratio of plant heights measured between treatment blocks at all time points largely overlapped with the results from the QTL analysis performed on measured value of plant height within treatments (S8 Fig and S12 Table). In total, 18 of the 29 unique QTL associated with these numerical metrics of difference in plant height between treatment blocks were also identified during our QTL analysis of plant height (S8 Fig and S12 Table). In contrast to the QTL locations identified for plant height, most of which were found in multiple experiments, of the 11 difference QTLs that do not overlap 10 were only found in one experiment (S8 Fig and S12 Table). While there are genetic loci that appear to be responsive to different environments, the majority of the environmentally responsive loci are due to relative differences in the timing of the loci growth affect between treatments.
Temporal architecture of plant height QTL
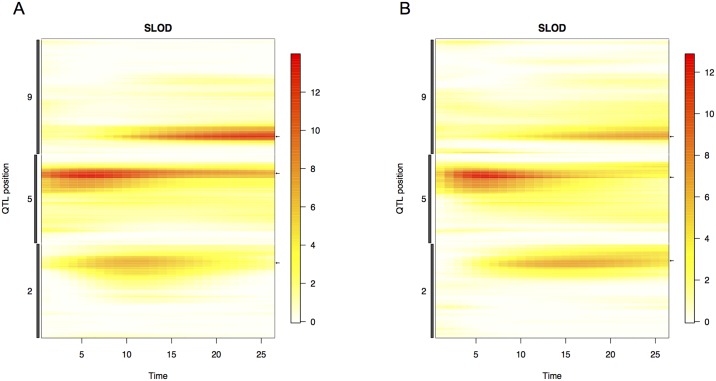
Height is a dynamic trait that changes at different rates throughout the plant life cycle. To determine which growth stage the identified loci influence, we examined the best fit multiple-QTL model produced at each separate independent time point and performed an analysis using the average LOD (SLOD) and maximum LOD (MLOD) function-valued approaches as described by [64,69]. Across all grow outs, linkage mapping analysis performed independently at each time point identified a larger number of QTL locations relative to the function-valued analysis. The positional locations of major QTL detected using each of these methods varied slightly, but in all cases it is likely that the same QTL are identified. To simplify discussion, we will refer to these QTL regions using notation that describes the approximate location of the QTL on each chromosome (QTL detected on chromosome 5 at position 99.1 cM and at position 105.2 will be reported as 5@100 for example).
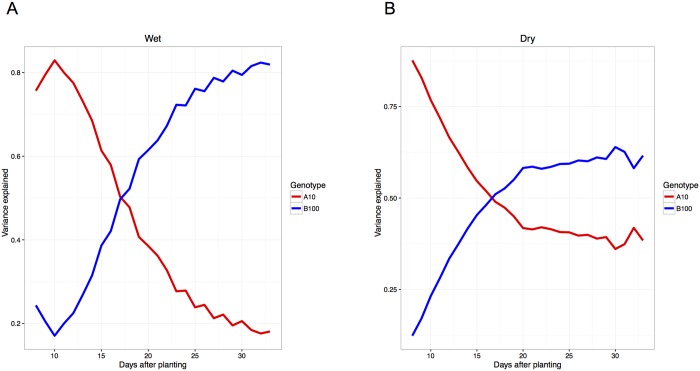
The relative significance of each QTL location changes substantially throughout the life cycle of plant (Fig 6 and S9 Fig). In all experimental grow outs, plant height at early stages of development is strongly associated with a single major QTL located at 5@100. In the dataset with the highest temporal resolution (Bellweather), the proportional contribution of this QTL diminishes over time while the contribution of other QTL located at 2@91 and 9@35 increase (Fig 7a). The proportion of variance explained by 5@100 diminished late in plant development in all field grow outs (S10 Fig). However, height measurements started later and continued longer through plant development under field conditions. This revealed that the proportion of variance explained by 5@100 peaked between 30–40 days after sowing, before starting to decline. This is in contrast to its declining role throughout the growth period (8–32 days) under controlled environment conditions. Three out of four field studies also detected 9@35, and again the proportion of variance explained by this QTL diminished at late developmental stages only assessed in the field (S10 Fig). The magnitude of allelic effects contributed by individual loci generally increase through time in all growth environments (Fig 7b and S11 Fig) highlighting the cumulative nature of developmental traits.
Fig 6. Three major QTLs account for the most significant proportion of variance across all time points during the Bellweather experiment.
The significance of association at each QTL location is plotted over the course of the experiment. Coloring of the heat map is reflective of LOD score at genome location (cM) at a given time point. Increased red coloring is indicative of higher LOD score. A) Plot of LOD score at loci that significantly influence plant height in the water-limited treatment block of the Bellweather experiments as calculated using the SLOD test statistic. B) Plot of LOD score at loci that significantly influence plant height in the well-watered treatment block of the Bellweather experiments as calculated using the SLOD test statistic.
Fig 7. The temporal influence of individual loci influences effect sizes throughout development.
QTL location is indicated with the following notation (chromosome@position). A) The proportion of variance attributed to the QTL at position at 2@91 and 9@35 is increases throughout the plant life cycle whereas the influence of the major QTL at position 5@100 decreases as the plant approaches maturity. B) The effect sizes of each locus are cumulative through out development. Although by the end of the experiment the proportion of variance explained by the locus at 5@100 is very low, the effect size continues to grow in magnitude.
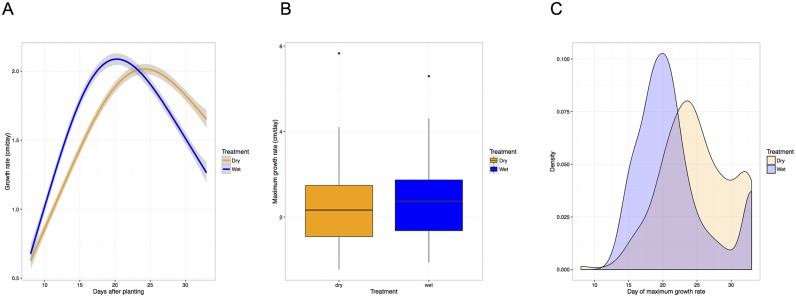
Although the identity of the major loci that influence plant height in this population is relatively unresponsive to treatment, the proportional contribution of each locus throughout time appears responsive to water treatment (Fig 7). Water-limited plants exhibit slightly decreased average and maximal rates of growth (Fig 8). In addition, the day at which maximal growth rate is achieved is significantly delayed in water-limited plants (Fig 8). QTLs associated with maximal and daily growth rate are essentially identical to those detected for height, although the time at which these QTL exhibit the greatest influence appears shifted toward earlier time points in the experiment (S12 Fig). No significant QTL were identified for either the day at which maximal growth rate occurs or the difference of this trait between treatments.
Fig 8. A delay in the developmental program that controls vertical growth is likely more influential on final plant height observed rather than maximal growth rate.
A) Vertical growth rate plotted through time indicate rates of vertical growth vary dramatically throughout development. B) No significant differences (α = 0.05) in the rate of maximal vertical growth was not observed between water level treatment blocks. C) Plants within the well-watered treatment block achieve their maximal vertical growth rate significantly earlier than their water limited counterparts.
A temporal multi locus model of Setaria plant height
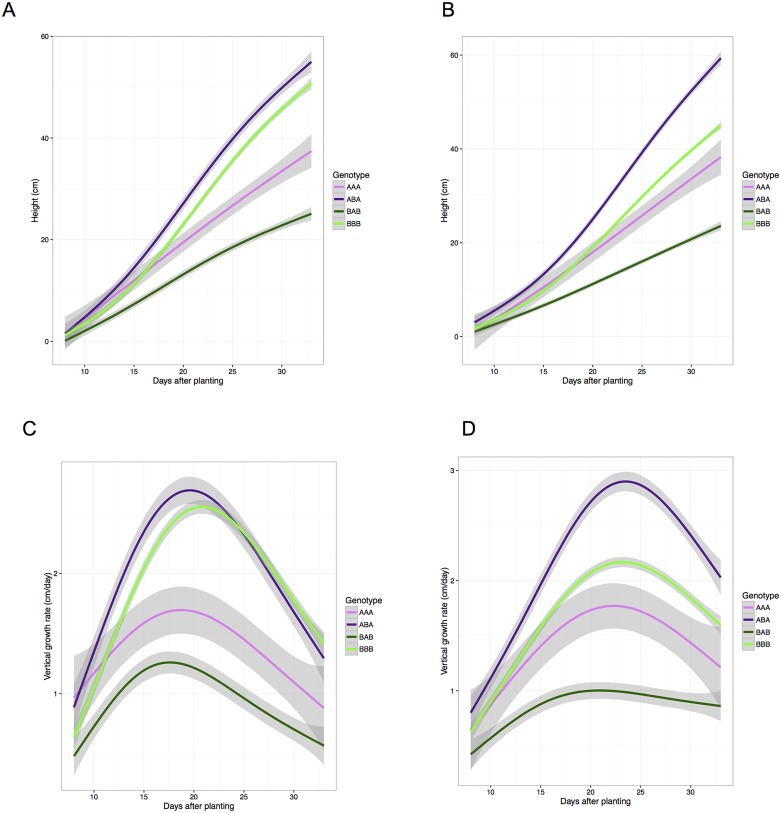
The SLOD based function-valued QTL analysis of our highest resolution dataset identified a simple, additive, QTL model comprised of loci on chromosomes 2, 5 and 9 (2@91, 5@100 and 9@35) which provides a framework to link many of the experiments. The same causative QTL loci were identified in the wet and dry treatment blocks of the grow outs at Bellweather. Early in plant development, individuals that inherit the B100 allele at position 5@100 will be shorter than those that inherit the A10 allele (Fig 9). The effects of this locus are then counterbalanced by the influence of the QTL at position 2@91 and later by 9@35 where the B100 allele increases plant height at maturity. If the RIL genotypes are divided into four genotypic classes based on these three loci: all A10 (AAA), all B100 (BBB) and combinations where the 5@100 loci is discordant (ABA and BAB), the effect of these loci is clear. The BAB class is the tallest and the ABA class is the shortest, throughout the growth cycle. The AAA and BBB classes, which include the parent genotypes, however, have an inflection point between the early growth, where AAA is initially taller and the later stages where the BBB class becomes and remains taller for the remainder of the experiment (Fig 9). In the well-watered treatment block of the Bellwether grow out, individuals that have inherited the AAA loci exhibit the largest initial growth rate of all genotypes but are over taken by both the BBB and BAB within 13 days after planting (Fig 9c). Individuals that inherit the BBB allele combination maintain the highest rate of vertical growth at the end of this experiment in the well-watered treatment block (Fig 9c).
Fig 9. Plants that inherit A10 alleles at 5@100 exhibit greater plant height initially, whereas those that inherit alleles from the B100 parent at 2@91 and 9@35 generally achieve greater final heights. Genotypes are reported as three character string where individual characters represent the parental origin of the allele at locus 2@91, 5@100 and 9@35 respectively.
A) The plant height of individuals that that exhibit parental genotypes (AAA and BBB) and those that are segregating at the locus at chromosome 5 (BAB and ABA) in well-watered conditions. B) The plant height of individuals that that exhibit parental genotypes (AAA and BBB) and those that are segregating at the locus at chromosome 5 (BAB and ABA) in water-limited conditions. C) The vertical growth rate of individuals that that exhibit parental genotypes (AAA and BBB) and those that are segregating at the locus at chromosome 5 (BAB and ABA) in well-watered conditions. D) The vertical growth rate of individuals that that exhibit parental genotypes (AAA and BBB) and those that are segregating at the locus at chromosome 5 (BAB and ABA) in water-limited conditions.
The 5@100 QTL is found in all of the experiments with the A10 contributing the tall allele. The 2@91 and 9@35 QTLs are present in many other experiments, but there are also several additional QTLs with large effect and B100 contributing the taller allele. In most of the experiments, a fairly simple model with the 5@100 QTL driving early growth and several B100 tall QTLs taking over later explains the observed variation (Fig 10). The exceptions are three field grow outs (2013 Dry, 2014 Dry/Wet) where A10 high QTLs drive early growth, but the B100 QTLs do not appear to be the major loci influencing growth at later developmental time points. Closer inspection of the variance explained by the QTLs in these experiments reveals that at later time points, the percent variance explained by the QTLs is low, despite the trait heritability remaining high (S13 Fig). Running the QTL analysis with a lower permutation threshold cutoff (α = 0.25) reveals the presence of several additional small effect QTLs with B100 tall alleles. Given the limitations of the population size, it is reasonable to predict that there may be more small effect QTLs that could be contributing to make B100 taller in this experiment. This chronological genetic model of plant height may in part explain the differences in plant height observed when parental lines are grown in controlled environments versus field settings.
Fig 10. The proportional contribution of parental alleles that increase plant height changes throughout development during the 2014 experiment at Bellweather.
Alleles derived from the A10 parent increase plant height early in development whereas the alleles that increase plant height later in development are inherited from the B100 parent. A) The proportion of additive genetic variance contributed by parental alleles plotted throughout all time points in the well-watered (wet) treatment block of the 2014 Bellweather experiment. B) The proportion of additive genetic variance contributed by parental alleles plotted throughout all time points in the water-limited (dry) treatment block of the 2014 Bellweather experiment.
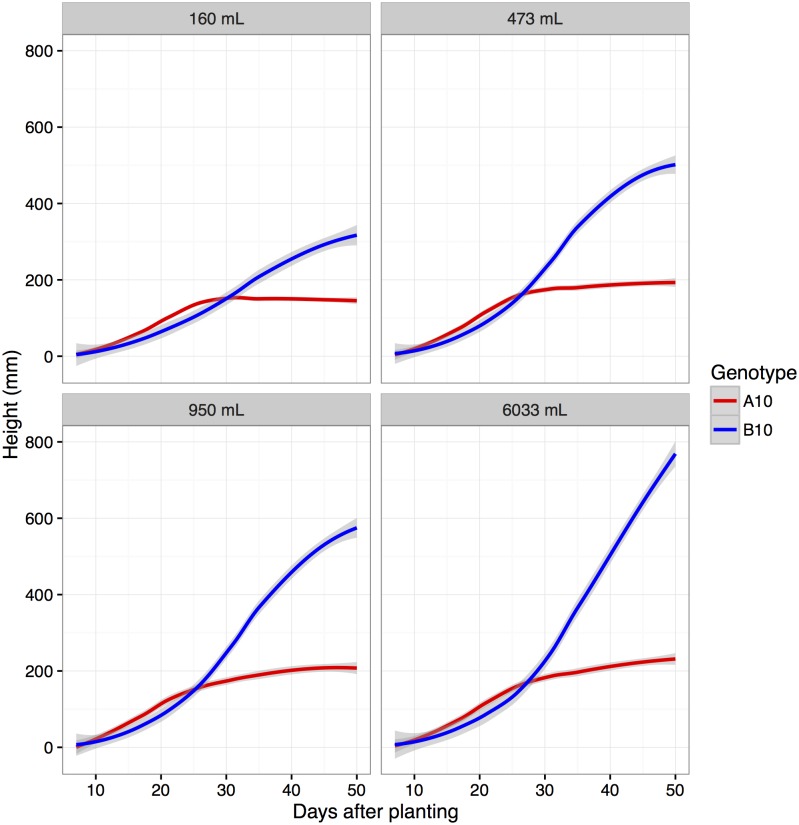
Experiments performed in controlled environmental settings potentially end before the effects of the B100 alleles at positions 2@91 and 9@35 can fully materialize or may reflect limitations in growth due to pot size constraints. The results of a glass house experiment designed to test these factors, suggests that the pot size used during in the Bellweather trial does not dramatically limit vertical growth given the duration of this experiment (experiment ends prior to 35 days after planting; Fig 11). Clearly, pot size is a significantly influential factor for larger plants, later in development (S14 Fig).
Fig 11. In replicated greenhouse experiments, the height of the A10 parent is greater than the B100 parent for the first 25 days after planting, after which the B100 parent becomes taller.
Plants were grown in pots of four different sizes. Pots with volume greater than or equal 473 mL did not exhibit limitations in height at time points before 30 days after planting.
Candidate genes
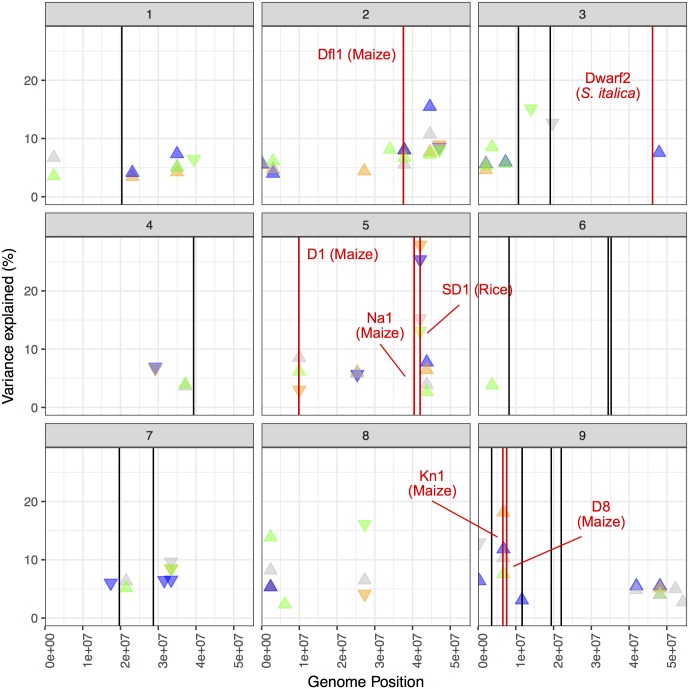
The 37 unique QTL locations and their corresponding median 1.5 LOD confidence interval were compared to the genomic location of the Setaria orthologs of plant height genes previously cloned from monocot grasses (S13 Table). Analysis using CoGe [66] identified the genomic location of syntenic orthologs in S. viridis for 16 of the 20 previously cloned height genes examined. BLAST searches were utilized to identify the location of the remaining 4 reference genes. Comparison of these two lists identified several cases of genomic colocalization (Fig 12, S14 Table).
Fig 12. The genomic coordinates of orthologous genes known to effect plant height colocalize with QTL.
Colocalization of orthologous candidate genes is illustrated by plotting a vertical line in red (with gene name and species). The location of all other orthologs are plotted as black vertical lines. Each box corresponds to an individual chromosome, where the values along the x-axis are chromosome position and values along the y-axis denote the proportion of genetic variance explained by the QTL. Each triangle represents a single QTL detected, where the color indicates the treatment condition the QTL was identified in (blue represents wet, orange corresponds to dry, green indicates sparse planting density whereas grey denotes dense planting.) and direction of the arrow corresponds the directional effect of the B100 parental allele.
Several promising candidate genes were identified for two of the three major QTL identified during this study. The QTL of greatest influence on plant height (5@100) colocalizes with the S. viridis ortholog of rice SD-1 (semi-dwarfing), a putative gibberellin 20-oxidase enzyme [36]. This gene lies within 3.2 kB of the peak LOD score and exhibits non-synonymous sequence polymorphisms in parental species coding sequence [16]. An alternative candidate gene associated with this QTL was located 156 kB away from the peak LOD score (outside the 1.5 LOD confidence interval) is the S. viridis orthologue of the maize Na1, a 3-oxo-5-alpha-steroid 4-dehydrogenase enzyme required for brassinosteroid biosynthesis [19]. Two potential candidate genes appear to colocalize with QTL on 9@35. The S. viridis orthologs of maize Kn1 (15 kB from peak LOD position) and maize D8 (836 kB from peak LOD position) are located within the 1.5 LOD confidence interval of this QTL [23,24]. These candidate genes were also identified in another study using the same interspecific RIL population [16]. None of the S. viridis orthologs in our table of previously cloned height genes overlap with the QTL confidence interval on 2@91.
This colocalization approach also identified reasonable candidates for two smaller effect QTL located on 2@70 and 5@48. The S. viridis orthologue of maize Dfl1, a basic leucine zipper protein involved in modulating flowering time and plant stature [29] is located on chromosome 2 approximately 252 kB from the position of maximal LOD score for the QTL on 2@70. The QTL on 5@48 colocalizes (103 kB from position of maximal LOD score) with the orthologue of maize D1, a gibberellic acid (GA) 3-oxidase enzyme, which is the first committed step of GA biosynthesis [20]. The candidate gene for the S. italic D2 dwarf locus (15) is located just outside the confidence interval for a single environment QTL on the distal arm of Chr. 3, suggesting that this locus has only a minor, if any, role in controlling height in this population. Although several other candidate genes were found within the confidence intervals of QTL on 1@49, 3@69, 7@59, 9@1 and 9@54, the candidates were not in particularly close proximity to the position of maximal LOD score or the QTL were only found in a single environment (Fig 12).
Discussion
Although several end point analyses of plant height have been performed in grasses [7–14], we lack an understanding of the temporal interplay of genetics and environment over development. A major impediment is the challenge of performing replicated field trials that impose treatment effects and the difficulty of repeatedly measuring any trait manually. The use of model systems, robust field design strategies and high-throughput phenotyping platforms alleviate many of these obstacles.
The proportion of energy that grasses invest into vertical growth influences vegetative biomass and grain yield, which are important feed and biofuel traits [2,70,71]. As demonstrated here, using platforms with high frequency and non-destructive measurements of plant height provides temporal resolution of QTL effects. The results of 12 coordinated grow outs of a Setaria RIL population in three different experimental locations suggest that plant height is specified as a functionally continuous developmental process that is under strong and robust genetic control but can be influenced by both water availability and planting density.
As expected [40–42], more aggressive water limitation treatments led to a greater reduction in plant height. However, no significant difference in the maximal plant growth rate was observed, rather it was delayed by approximately 3 days in water limited plants. Another surprising finding was that RILs planted at lower planting densities were taller than their densely planted counterpart, counter to the expectation that the shade avoidance response would result in increased plant height in densely grown stands [42,72] and may reflect resource limitations that inhibit growth of densely planted stands. Trial location was the most influential variable affecting plant height after genotype which may be the result of differences in day length and temperature influencing flowering time. In all field trials, flowering time was positively correlated with plant height at maturity.
Our results indicate that the identity of the genetic components that most strongly influence plant height are not dependent upon growth environment. Rather, delayed progression through this “core” genetic program that establishes mature plant height is a key element of G x E responses. Twelve genetic loci are found in each of the four different treatment regimens and are responsible for the strongest effects in every experiment, although many smaller effect QTL were identified across individual time points in multiple independent replicated trials. Overall, the identity of genetic loci identified in this study are highly congruent with an independent study of height performed on the same population [16].
The alleles that increase plant height are found in both parental lines, with the allele of largest positive effect (5@100) inherited from the shorter A10 parental line. The effects of this locus are realized early, as growth rate approaches its maximum value. Later in development, the number of loci that contribute to plant height increases. A vast majority of the loci that increase plant height late in development are inherited from the B100 parent. The dynamics of the late-appearing genetic loci are clearly dependent upon environmental factors as they are more variable between experiments. The balance of early alleles favoring height coming from the wild parent and later alleles coming from the domesticated parent may reflect the results of domestication on growth habit, with the wild parent favoring rapid growth and flowering while the domesticated favors longer growth cycles and larger plants.
Given the number of genes that have been cloned for plant height in the Poaceae, it is not surprising to find several candidate orthologs located near our identified loci. The close linkage of the 5@100 peak LOD score marker with the ortholog of SD-1 strongly suggests that this gene is responsible for the phenotype, but confirmation will require further experiments. Several other promising candidates were found for other QTLs, but several have multiple possible candidates, while others are within linkage disequilibrium, but farther away from the peak LOD SNP. Several QTLs have no known candidate orthologs within their confidence intervals, suggesting that they result from novel height altering loci.
Despite stark differences in rank order among genotypes and the relative height of the parents observed within field and controlled environments, the QTL detected largely overlap. The temporal QTL model provides the explanation for these conflicting observations across experiments conducted at different time windows. Plants grown at the Bellweather Phenotyping Facility are limited to the first 30–40 days of growth due to size constraints, whereas in the field data can be collected from more mature plants but cannot be reliably collected before 20 days after planting due to the need to establish seedlings after transplanting. These results demonstrate that adding temporal analysis to phenotyping can greatly improve our ability to detect and interpret the factors underlying complex traits.
The dense marker map created for this study can be combined with diverse germplasm studies to fine map causal variants and identify tightly linked markers for use in breeding programs. These tools can be used to modulate plant height and vertical growth rate throughout plant development given a predicted precipitation regimen or planting density. These approaches should be applicable across diverse species and traits for improvement of both food and biofuel crops.
Conclusions
By combining field and controlled environmental analysis with quantitative genetics we were able to dissect the genetic and environmental components of plant height in Setaria. The identity of genetic components that determine plant height within this RIL population exhibits a strong degree of genetic canalization, but the contribution of these loci is influenced by temporal and environmental factors. Temporal analysis revealed that alleles from the wild parent act early in development, while alleles from the domesticated parent act later. This approach can be used to dissect all of the traits that can be measured in a high throughput manner by emerging phenotyping systems to better understand plant adaptation.
Supporting information
Extent_y metric is in blue, height_above_bound is in green, centroid_y is in red, elipse_center_y is in orange and canopy_height is illustrated in purple.
(PNG)
A) The broadsense heritability of ‘height_above_bound’, ‘Centroid_Y’, ‘Extent_Y’, ‘Ellipse_Center_Y’, and canopy height plotted across all time points in the Bellweather experiment. B) The proportion of variance attributed to treatment as measured using ‘height_above_bound’, ‘Centroid_Y’, ‘Extent_Y’, ‘Ellipse_Center_Y’, and canopy height metrics throughout all time points in the Bellweather experiment. C) The proportion of variance attributed to genotype x treatment partition as measured using ‘height_above_bound’, ‘Centroid_Y’, ‘Extent_Y’, ‘Ellipse_Center_Y’, and canopy height metrics throughout all time points in the Bellweather experiment.
(PDF)
The broad sense heritability of ‘height_above_bound’ is roughly equivalent given the 4 types of summarization functions is used (mean, median, max, min).
(PDF)
(PDF)
(PDF)
Each box corresponds to an individual chromosome, where the values along the x-axis are chromosome position (centimorgan) and values along the y-axis denote the proportion of genetic variance explained by the QTL. Each triangle represents a single QTL detected, where the color indicates the treatment condition the QTL was identified in (blue represents wet, orange corresponds to dry, green indicates sparse planting density whereas grey denotes dense planting.) and direction of the arrow corresponds the directional effect of the B100 parental allele.
(PDF)
A) Overlap between QTL detected in treatment block and experimental locations within controlled environmental studies (Bellweather at Donald Danforth Plant Science Center compared to the growth chamber experiment at Carnegie Institute for Science). B) Among QTL found exclusively in wet treatment blocks there is no overlap between all experiments. C) Among QTL found exclusively in dry treatment blocks there is no overlap between all experiments. D) Among QTL found exclusively in sparse treatment blocks there is no overlap between all experiments. E) Among QTL found exclusively in dense treatment blocks there is one QTL found in both 2013 and 2014.
(PDF)
The location of trait difference QTLs is plotted as circles colored by their difference type. QTLs detected by mapping on the numerical difference between treatment blocks are plotted in cyan. QTLs detected by mapping on the relative difference between treatment blocks are plotted in purple. QTLs detected by mapping on the ratio between treatment blocks are plotted in red.
(PDF)
(PDF)
(PDF)
(PDF)
Loci at which the A10 allele contributes larger plant height are plotted in blue, whereas loci at which the B100 allele contributes to larger plant height are plotted in red. A) Water limited environment (Dry). B) Well-watered environment (Wet).
(PDF)
The discrepancy is particularly evident during the drought experiment performed in 2014 and in the dry treatment block in the 2013 drought experiment. A) The results of the drought experiment in 2014 B) The results of the drought experiment in 2013.
(PDF)
(PDF)
(XLSX)
(CSV)
(CSV)
(CSV)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(CSV)
(CSV)
(CSV)
(CSV)
(XLSX)
(CSV)
Acknowledgments
This project would not have been possible without the dedicated efforts of the entire Setaria team (Baxter, Leakey, Dinneny, Brutnell, Rhee, Cousins and Voytas labs) and field crews (UIUC undergraduates and local high school students) who planted, nurtured and harvested over 100,000 plants over two years. Particularly, Mark Holmes, Hannah Schlake, Amanda Youssef, Sarah Keeley, Kara Barto, Jonny Yockey, Finey Ruan, Zack Reynado, Mitch Dickey, George Gunter, Marshall Alston-Yeagle, Audrey Rouse, and Andrew Chancellor for their collection of in-field measurements. We'd also like to thank Bradley Dalsing, Christopher Montes, Alex Hathcock, Kannan Puthuval and Dyson McMillan Singer the SoyFACE site technicians, for helping to maintain the experimental infrastructure. The authors are extremely grateful for their hard work. The authors would additionally like to thank Melinda Darnell, Malia Gehan and Noah Fahlgren for their assistance conducting the experiment at the Bellwether Phenotyping Facility and helpful discussions regarding analysis of the data.
Data Availability
Sequence data is deposited at the SRA (SRP108884). All Image data is deposited on CyVerse (plantcv.danforthcenter.org/pages/data-sets/setaria_height.html). All scripts and derived data can be found on github (https://github.com/maxjfeldman/Feldman_Setaria_Height_2016).
Funding Statement
Funding for this project was provided by award number DE-SC0008769 from the U.S. Department of Energy to TB, IB, ADBL and JD. IB is supported by the US Department of Agriculture—Agricultural Research Service. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mendel G. Versuche über Pflanzenhybriden. Verhandlungen Naturforschenden Vereines Brunn 4 3. 1866;44. [Google Scholar]
- 2.Mencuccini M. The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ. 2003;26(1):163–82. [Google Scholar]
- 3.Falster DS, Westoby M. Plant height and evolutionary games. Trends Ecol Evol. 2003. July;18(7):337–43. [Google Scholar]
- 4.Givnish TJ. On the Adaptive Significance of Leaf Height in Forest Herbs. Am Nat. 1982. September;120(3):353–81. [Google Scholar]
- 5.Slavov G, Allison G, Bosch M. Advances in the genetic dissection of plant cell walls: tools and resources available in Miscanthus. Front Plant Sci. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnoult S, Brancourt-Hulmel M. A Review on Miscanthus Biomass Production and Composition for Bioenergy Use: Genotypic and Environmental Variability and Implications for Breeding. BioEnergy Res. 2015. June;8(2):502–26. [Google Scholar]
- 7.Austin DF, Lee M. Genetic resolution and verification of quantitative trait loci for flowering and plant height with recombinant inbred lines of maize. Genome. 1996. October 1;39(5):957–68. [DOI] [PubMed] [Google Scholar]
- 8.Beavis WD, Grant D, Albertsen M, Fincher R. Quantitative trait loci for plant height in four maize populations and their associations with qualitative genetic loci. Theor Appl Genet 1991. December;83(2). [DOI] [PubMed] [Google Scholar]
- 9.Huang N, Courtois B, Khush GS, Lin H, Wang G, Wu P, et al. Heredity—Abstract of article: Association of quantitative trait loci for plant height with major dwarfing genes in rice. Heredity. 1996. August;77(2):130–7. [Google Scholar]
- 10.Li Z, Pinson SRM, Stansel JW, Park WD. Identification of quantitative trait loci (QTLs) for heading date and plant height in cultivated rice (Oryza sativa L.). Theor Appl Genet. 1995. July;91(2). [DOI] [PubMed] [Google Scholar]
- 11.Lin YR, Schertz KF, Paterson AH. Comparative analysis of QTLs affecting plant height and maturity across the Poaceae, in reference to an interspecific sorghum population. Genetics. 1995. September 1;141(1):391–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ming R, Del Monte TA, Hernandez E, Moore PH, Irvine JE, Paterson AH. Comparative analysis of QTLs affecting plant height and flowering among closely-related diploid and polyploid genomes. Genome. 2002. October 1;45(5):794–803. [DOI] [PubMed] [Google Scholar]
- 13.Peiffer JA, Romay MC, Gore MA, Flint-Garcia SA, Zhang Z, Millard MJ, et al. The Genetic Architecture Of Maize Height. Genetics. 2014. April 1;196(4):1337–56. doi: 10.1534/genetics.113.159152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan J, Zhu J, He C, Benmoussa M, Wu P. Molecular Dissection of Developmental Behavior of Plant Height in Rice (Oryza sativa L.). Genetics. 1998. November 1;150(3):1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue C, Zhi H, Fang X, Liu X, Tang S, Chai Y, et al. Characterization and Fine Mapping of SiDwarf2 (D2) in Foxtail Millet. Crop Sci. 2016;56(1):95. [Google Scholar]
- 16.Mauro-Herrera M, Doust AN. Development and Genetic Control of Plant Architecture and Biomass in the Panicoid Grass, Setaria. PLoS ONE. 2016. March 17;11(3):e0151346 doi: 10.1371/journal.pone.0151346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez MGS, Becraft PW, Yin Y, Lübberstedt T. From dwarves to giants? Plant height manipulation for biomass yield. Trends Plant Sci. 2009;14(8):454–61. doi: 10.1016/j.tplants.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 18.Winkler RG. The Maize Dwarf3 Gene Encodes a Cytochrome P450-Mediated Early Step in Gibberellin Biosynthesis. Plant Cell. 1995. August 1;7(8):1307–17. doi: 10.1105/tpc.7.8.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartwig T, Chuck GS, Fujioka S, Klempien A, Weizbauer R, Potluri DPV, et al. Brassinosteroid control of sex determination in maize. Proc Natl Acad Sci. 2011. December 6;108(49):19814–9. doi: 10.1073/pnas.1108359108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Hou M, Liu L, Wu S, Shen Y, Ishiyama K, et al. The Maize DWARF1 Encodes a Gibberellin 3-Oxidase and Is Dual Localized to the Nucleus and Cytosol. Plant Physiol. 2014. December 1;166(4):2028–39. doi: 10.1104/pp.114.247486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleary AL, Smith LG. The Tangled1 gene is required for spatial control of cytoskeletal arrays associated with cell division during maize leaf development. Plant Cell. 1998. November;10(11):1875–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki M, Latshaw S, Sato Y, Settles AM, Koch KE, Hannah LC, et al. The Maize Viviparous8 Locus, Encoding a Putative ALTERED MERISTEM PROGRAM1-Like Peptidase, Regulates Abscisic Acid Accumulation and Coordinates Embryo and Endosperm Development. Plant Physiol. 2008. March 1;146(3):1193–206. doi: 10.1104/pp.107.114108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hake S, Vollbrecht E, Freeling M. Cloning Knotted, the dominant morphological mutant in maize using Ds2 as a transposon tag. EMBO J. 1989. January;8(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, et al. “Green revolution” genes encode mutant gibberellin response modulators. Nature. 1999. July 15;400(6741):256–61. doi: 10.1038/22307 [DOI] [PubMed] [Google Scholar]
- 25.Makarevitch I, Thompson A, Muehlbauer GJ, Springer NM. Brd1 Gene in Maize Encodes a Brassinosteroid C-6 Oxidase. Wu S-B, editor. PLoS ONE. 2012. January 26;7(1):e30798 doi: 10.1371/journal.pone.0030798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bensen RJ. Cloning and Characterization of the Maize An1 Gene. Plant Cell. 1995. January 1;7(1):75–84. doi: 10.1105/tpc.7.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colasanti J, Yuan Z, Sundaresan V. The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell. 1998. May 15;93(4):593–603. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin M, Walbot V. Cloning of a mutable bz2 allele of maize by transposon tagging and differential hybridization. Genetics. 1987. December;117(4):771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muszynski MG, Dam T, Li B, Shirbroun DM, Hou Z, Bruggemann E, et al. delayed flowering1 Encodes a Basic Leucine Zipper Protein That Mediates Floral Inductive Signals at the Shoot Apex in Maize. Plant Physiol. 2006. December 1;142(4):1523–36. doi: 10.1104/pp.106.088815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Best NB, Hartwig T, Budka J, Fujioka S, Johal G (Guri) S, Schulz B, et al. nana plant2 encodes a maize ortholog of the Arabidopsis brassinosteroid biosynthesis protein Dwarf1, identifying developmental interactions between brassinosteroids and gibberellins. Plant Physiol. 2016. June 10;pp.00399.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawit SJ, Wych HM, Xu D, Kundu S, Tomes DT. Maize DELLA Proteins dwarf plant8 and dwarf plant9 as Modulators of Plant Development. Plant Cell Physiol. 2010. November 1;51(11):1854–68. doi: 10.1093/pcp/pcq153 [DOI] [PubMed] [Google Scholar]
- 32.Fu J, Ren F, Lu X, Mao H, Xu M, Degenhardt J, et al. A Tandem Array of ent-Kaurene Synthases in Maize with Roles in Gibberellin and More Specialized Metabolism. Plant Physiol. 2016. February;170(2):742–51. doi: 10.1104/pp.15.01727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilley J, Truong S, Olson S, Morishige D, Mullet J. Identification of Dw1, a Regulator of Sorghum Stem Internode Length. Subudhi PK, editor. PLoS ONE. 2016. March 10;11(3):e0151271 doi: 10.1371/journal.pone.0151271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Multani DS. Loss of an MDR Transporter in Compact Stalks of Maize br2 and Sorghum dw3 Mutants. Science. 2003. October 3;302(5642):81–4. doi: 10.1126/science.1086072 [DOI] [PubMed] [Google Scholar]
- 35.Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A. Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the alpha-subunit of GTP-binding protein. Proc Natl Acad Sci U S A. 1999. August 31;96(18):10284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spielmeyer W, Ellis MH, Chandler PM. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc Natl Acad Sci. 2002. June 25;99(13):9043–8. doi: 10.1073/pnas.132266399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldmann KA, Marks MD, Christianson ML, Quatrano RS. A Dwarf Mutant of Arabidopsis Generated by T-DNA Insertion Mutagenesis. Science. 1989. March 10;243(4896):1351–4. doi: 10.1126/science.243.4896.1351 [DOI] [PubMed] [Google Scholar]
- 38.Edmeades GO, Daynard TB. The Development of Plant-to-Plant Variability in Maize at Different Planting Densities. Can J Plant Sci. 1979. July 1;59(3):561–76. [Google Scholar]
- 39.Sari-Gorla M, Krajewski P, Di Fonzo N, Villa M, Frova C. Genetic analysis of drought tolerance in maize by molecular markers. II. Plant height and flowering. Theor Appl Genet. 1999. July 23;99(1–2):289–95. [Google Scholar]
- 40.Wu X, Wang Z, Chang X, Jing R. Genetic dissection of the developmental behaviours of plant height in wheat under diverse water regimes. J Exp Bot. 2010. May 24;erq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu X, Chang X, Jing R. Genetic Insight into Yield-Associated Traits of Wheat Grown in Multiple Rain-Fed Environments. PLoS ONE. 2012. February 17;7(2):e31249 doi: 10.1371/journal.pone.0031249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglas BJ, Morrison IN, Thomas AG, Maw MG. THE BIOLOGY OF CANADIAN WEEDS.: 70. Setaria viridis (L.) Beauv. Can J Plant Sci. 1985. July 1;65(3):669–90. [Google Scholar]
- 43.Würschum T, Liu W, Busemeyer L, Tucker MR, Reif JC, Weissmann EA, et al. Mapping dynamic QTL for plant height in triticale. BMC Genet. 2014;15:59 doi: 10.1186/1471-2156-15-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furbank RT, Tester M. Phenomics—technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 2011. December 1;16(12):635–44. doi: 10.1016/j.tplants.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 45.Honsdorf N, March TJ, Berger B, Tester M, Pillen K. High-Throughput Phenotyping to Detect Drought Tolerance QTL in Wild Barley Introgression Lines. PLoS ONE. 2014. May 13;9(5):e97047 doi: 10.1371/journal.pone.0097047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brutnell TP, Bennetzen JL, Vogel JP. Brachypodium distachyon and Setaria viridis: Model Genetic Systems for the Grasses. Annu Rev Plant Biol. 2015;66(1):465–85. [DOI] [PubMed] [Google Scholar]
- 47.Brutnell TP, Wang L, Swartwood K, Goldschmidt A, Jackson D, Zhu X-G, et al. Setaria viridis: A Model for C4 Photosynthesis. Plant Cell. 2010. August 1;22(8):2537–44. doi: 10.1105/tpc.110.075309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Somerville C, Koornneef M. A fortunate choice: the history of Arabidopsis as a model plant. Nat Rev Genet. 2002. November;3(11):883–9. doi: 10.1038/nrg927 [DOI] [PubMed] [Google Scholar]
- 49.Bennetzen JL, Schmutz J, Wang H, Percifield R, Hawkins J, Pontaroli AC, et al. Reference genome sequence of the model plant Setaria. Nat Biotechnol. 2012. June;30(6):555–61. doi: 10.1038/nbt.2196 [DOI] [PubMed] [Google Scholar]
- 50.Devos KM, Wang ZM, Beales J, Sasaki T, Gale MD. Comparative genetic maps of foxtail millet (Setaria italica) and rice (Oryza sativa). Theor Appl Genet. 1998. January 23;96(1):63–8. [Google Scholar]
- 51.Wang ZM, Devos KM, Liu CJ, Wang RQ, Gale MD. Construction of RFLP-based maps of foxtail millet, Setaria italica (L.) P. Beauv. Theor Appl Genet. 1998. January 23;96(1):31–6. [Google Scholar]
- 52.Fahlgren N, Feldman M, Gehan MA, Wilson MS, Shyu C, Bryant DW, et al. A Versatile Phenotyping System and Analytics Platform Reveals Diverse Temporal Responses to Water Availability in Setaria. Mol Plant. 2015. October 5;8(10):1520–35. doi: 10.1016/j.molp.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 53.Long SP, Ainsworth EA, Rogers A, Ort DR. RISING ATMOSPHERIC CARBON DIOXIDE: Plants FACE the Future. Annu Rev Plant Biol. 2004;55(1):591–628. [DOI] [PubMed] [Google Scholar]
- 54.Huang P, Feldman M, Schroder S, Bahri BA, Diao X, Zhi H, et al. Population genetics of Setaria viridis, a new model system. Mol Ecol. 2014. October 1;23(20):4912–25. doi: 10.1111/mec.12907 [DOI] [PubMed] [Google Scholar]
- 55.Schröder S, Bahri BA, Eudy DM, Layton DJ, Kellogg EA, Devos KM. Genetic diversity and origin of North American green foxtail [Setaria viridis (L.) Beauv.] accessions. Genet Resour Crop Evol. 2016. January 25 [Google Scholar]
- 56.Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980. October 10;8(19):4321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poland JA, Brown PJ, Sorrells ME, Jannink J-L. Development of High-Density Genetic Maps for Barley and Wheat Using a Novel Two-Enzyme Genotyping-by-Sequencing Approach. PLoS ONE. 2012. February 28;7(2):e32253 doi: 10.1371/journal.pone.0032253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glaubitz JC, Casstevens TM, Lu F, Harriman J, Elshire RJ, Sun Q, et al. TASSEL-GBS: A High Capacity Genotyping by Sequencing Analysis Pipeline. PLoS ONE. 2014. February 28;9(2):e90346 doi: 10.1371/journal.pone.0090346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012. March 4;9(4):357–9. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Broman KW, Wu H, Sen Ś, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003. May 1;19(7):889–90. [DOI] [PubMed] [Google Scholar]
- 61.Mauro-Herrera M, Wang X, Barbier H, Brutnell TP, Devos KM, Doust AN. Genetic Control and Comparative Genomic Analysis of Flowering Time in Setaria (Poaceae). G3 Genes Genomes Genetics. 2013. February 1;3(2):283–95. doi: 10.1534/g3.112.005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015. October 7;67. [Google Scholar]
- 63.Paine CET, Marthews TR, Vogt DR, Purves D, Rees M, Hector A, et al. How to fit nonlinear plant growth models and calculate growth rates: an update for ecologists. Methods Ecol Evol. 2012. April 1;3(2):245–56. [Google Scholar]
- 64.Kwak I-Y, Moore CR, Spalding EP, Broman KW. Mapping Quantitative Trait Loci Underlying Function-Valued Traits Using Functional Principal Component Analysis and Multi-Trait Mapping. G3 Genes Genomes Genetics. 2016. January 1;6(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Des Marais DL, Hernandez KM, Juenger TE. Genotype-by-Environment Interaction and Plasticity: Exploring Genomic Responses of Plants to the Abiotic Environment. Annu Rev Ecol Evol Syst. 2013. November 23;44(1):5–29. [Google Scholar]
- 66.Lyons E, Pedersen B, Kane J, Alam M, Ming R, Tang H, et al. Finding and Comparing Syntenic Regions among Arabidopsis and the Outgroups Papaya, Poplar, and Grape: CoGe with Rosids. Plant Physiol. 2008. December 1;148(4):1772–81. doi: 10.1104/pp.108.124867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fox J, Weisberg S. An R Companion to Applied Regression Second. Thousand Oaks, CA: Sage; [Google Scholar]
- 68.Bergelson J, Buckler ES, Ecker JR, Nordborg M, Weigel D. A Proposal Regarding Best Practices for Validating the Identity of Genetic Stocks and the Effects of Genetic Variants: Table 1. Plant Cell. 2016. March;28(3):606–9. doi: 10.1105/tpc.15.00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore CR, Johnson LS, Kwak I-Y, Livny M, Broman KW, Spalding EP. High-Throughput Computer Vision Introduces the Time Axis to a Quantitative Trait Map of a Plant Growth Response. Genetics. 2013. November 1;195(3):1077–86. doi: 10.1534/genetics.113.153346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boe A, Beck DL. Yield Components of Biomass in Switchgrass. Crop Sci. 2008;48(4):1306. [Google Scholar]
- 71.Das MK, Fuentes RG, Taliaferro CM. Genetic Variability and Trait Relationships in Switchgrass. Crop Sci. 2004;44(2):443. [Google Scholar]
- 72.Aphalo PJ, Ballaré CL, Scopel AL. Plant-plant signalling, the shade-avoidance response and competition. J Exp Bot. 1999. November 1;50(340):1629–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extent_y metric is in blue, height_above_bound is in green, centroid_y is in red, elipse_center_y is in orange and canopy_height is illustrated in purple.
(PNG)
A) The broadsense heritability of ‘height_above_bound’, ‘Centroid_Y’, ‘Extent_Y’, ‘Ellipse_Center_Y’, and canopy height plotted across all time points in the Bellweather experiment. B) The proportion of variance attributed to treatment as measured using ‘height_above_bound’, ‘Centroid_Y’, ‘Extent_Y’, ‘Ellipse_Center_Y’, and canopy height metrics throughout all time points in the Bellweather experiment. C) The proportion of variance attributed to genotype x treatment partition as measured using ‘height_above_bound’, ‘Centroid_Y’, ‘Extent_Y’, ‘Ellipse_Center_Y’, and canopy height metrics throughout all time points in the Bellweather experiment.
(PDF)
The broad sense heritability of ‘height_above_bound’ is roughly equivalent given the 4 types of summarization functions is used (mean, median, max, min).
(PDF)
(PDF)
(PDF)
Each box corresponds to an individual chromosome, where the values along the x-axis are chromosome position (centimorgan) and values along the y-axis denote the proportion of genetic variance explained by the QTL. Each triangle represents a single QTL detected, where the color indicates the treatment condition the QTL was identified in (blue represents wet, orange corresponds to dry, green indicates sparse planting density whereas grey denotes dense planting.) and direction of the arrow corresponds the directional effect of the B100 parental allele.
(PDF)
A) Overlap between QTL detected in treatment block and experimental locations within controlled environmental studies (Bellweather at Donald Danforth Plant Science Center compared to the growth chamber experiment at Carnegie Institute for Science). B) Among QTL found exclusively in wet treatment blocks there is no overlap between all experiments. C) Among QTL found exclusively in dry treatment blocks there is no overlap between all experiments. D) Among QTL found exclusively in sparse treatment blocks there is no overlap between all experiments. E) Among QTL found exclusively in dense treatment blocks there is one QTL found in both 2013 and 2014.
(PDF)
The location of trait difference QTLs is plotted as circles colored by their difference type. QTLs detected by mapping on the numerical difference between treatment blocks are plotted in cyan. QTLs detected by mapping on the relative difference between treatment blocks are plotted in purple. QTLs detected by mapping on the ratio between treatment blocks are plotted in red.
(PDF)
(PDF)
(PDF)
(PDF)
Loci at which the A10 allele contributes larger plant height are plotted in blue, whereas loci at which the B100 allele contributes to larger plant height are plotted in red. A) Water limited environment (Dry). B) Well-watered environment (Wet).
(PDF)
The discrepancy is particularly evident during the drought experiment performed in 2014 and in the dry treatment block in the 2013 drought experiment. A) The results of the drought experiment in 2014 B) The results of the drought experiment in 2013.
(PDF)
(PDF)
(XLSX)
(CSV)
(CSV)
(CSV)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(CSV)
(CSV)
(CSV)
(CSV)
(XLSX)
(CSV)
Data Availability Statement
Sequence data is deposited at the SRA (SRP108884). All Image data is deposited on CyVerse (plantcv.danforthcenter.org/pages/data-sets/setaria_height.html). All scripts and derived data can be found on github (https://github.com/maxjfeldman/Feldman_Setaria_Height_2016).