Abstract
Lobiopa insularis is a newly reported pest of strawberry in Argentina. We investigated characteristics of its biology in the laboratory, including survivorship and reproduction. We also estimated population growth for L. insularis fed ripe strawberry fruits. Lobiopa insularis was not observed ovipositing on strawberry fruits. A higher proportion of egg masses were recorded from a depth of 1 cm within the soil than on either the soil surface or deeper than 1cm (i.e. between 1and 2 cm) within the soil. The duration of preimaginal developmental stages represented ~18.5% of the total life cycle, while the adult stage represented 81.5%. Survival from egg to adult was 64.20% and mean longevity of females and males adults was 121.84, (SE = 8.86) and 118.58 (SE = 5.90) days, respectively. Females laid eggs only when they were with a male, so reproductive period was dependent on male presence. The number of eggs/female/day was 18.01 (SE = 1.71); and total fecundity was 1655 (ES = 249.53) eggs/female. The long life span of adults and high reproductive output, i.e high fecundity and long reproductive period, indicate that availability and concentration of suitable developmental resources are important factors in the population dynamics of Lobiopa insularis associated with strawberry crops.
Introduction
The life history of an organism refers to the pattern of growth, resource accumulation, differentiation and reproduction exhibited on average by a given species during the sequence of events that occur during its lifetime: e.g. birth, growth during pre-reproductive and reproductive periods [1]. Characteristics of the life cycle and life history of a species greatly influence its population dynamics [2, 3] and are also influenced by the availability of resources which vary significantly over time [4–7]. These factors, coupled with the abilities of polyphagous insects to disperse and use richer habitat patches can lead to pest outbreaks [8–10]. The schedules of survival and age specific reproduction, i.e. life table data, provide insights into the process of population growth [11, 12] and pest population dynamics.
Lobiopa insularis (Castelnau) (Coleoptera: Nitidulidae) is a polyphagous species, whose life cycle comprises the egg, three larval instars, pupal and adult stages. When full grown, the larvae fall to the ground and bury themselves to pupate [13]. The adults can disperse long distances and overwinter [14].
Adults can be strongly attracted to ripe fruits in agricultural settings such as peaches, blueberries, raspberries, strawberries, pineapples, apples, melons, tomatoes, corn, stored corn, and dried fruit products, settings where they feed and reproduce. The host range may include small fruits [14, 15]. In natural ecosystems, species of Lobiopa are often encountered in subcortical spaces of dead or decaying trees, sap flows, fermenting fruit and flower falls, and occasionally in decomposing leaf litter and debris ([16–21], Cline pers. obs.). This species has also been reported from honeybee hives in North America [22]. The genus Lobiopa was recently reviewed [18], which included an overview of the inclusive species and their respective biologies and distributions.
Lobiopa insularis has been known as a strawberry pest in USA [14, 23], Brazil [24] and Argentina [13]. In addition to direct feeding damage to the fruit by adults and larvae, they also serve as fungal dispersal agents, increasing yield losses [25–28]. In these countries, current control of L. insularis in strawberry crops is mainly based on cultural practices. Specifically, techniques include the harvest of strawberries and other fruits in the field immediately upon ripening, and removing damaged, diseased and overripe fruits [14].
Herein, we characterize oviposition traits, analyze survivorship and reproduction, and estimate population growth of L. insularis fed ripe strawberry fruits.
Materials and methods
Lobiopa insularis colonies were established in the laboratory starting with 16 males and 16 females placed in a plastic container (250 ml) with moistened filter paper in the bottom for oviposition, and fed ripe strawberries. All individuals were collected during October 2007 from commercial strawberry plots in Buenos Aires, Argentina. Several specimens were vouchered at the California State Collection of Arthropods and at the Museum of Natural Sciences of La Plata (La Plata National University, Argentina). The material was labelled as voucher material for this project.
Colonies and experiments were performed under controlled conditions (25 ±1°C, 60–70% RH and 14:10 h L-D).
A trial was performed to assess oviposition preference sites. The experimental unit was a glass container (35 cm length, 30 cm height, 3 cm width) (4 replicates). The containers were filled with sterilized moist soil with two intact ripe strawberries added to the soil surface. Five pairs of adults were introduced to each unit. The number of egg masses and number of eggs per mass were recorded by site for the following: a) on strawberry fruit, b) on soil surface, c) at a depth of 1 cm in soil (just below the soil surface down to a depth of 1 cm), and d) in soil deeper than 1 cm. Observations were made after three days. The proportion of egg masses and the number of eggs per egg mass for each site were analyzed by ANOVA.
To assess survivorship, reproduction, and population growth, three cohorts of 142, 81 and 94 eggs (less than one day old) were individually positioned in plastic containers with ripe strawberries and sterilized soil. The development of the larvae and pupae as well as the adult emergence were recorded.
The duration of developmental stages was estimated and female and male longevity among cohorts was compared by one way ANOVA. If no differences were determined, one way ANOVA was performed to compare longevity between both sexes using individuals of all cohorts.
For each cohort, at the beginning of interval x (each interval lasted one day) we recorded the number of individuals alive, N(x), and their developmental stage (S1 Data). Once in the adult stage the sex was determined according to [29]. This procedure involved observation of the VIII abdominal segment (the so-called “anal sclerite”), which is heavily sclerotized and externally visible in males. The Kaplan-Meier product-limit survival curve analysis [30] was used to examine survival curves. Survival curves of females and males of the three cohorts were compared using a multiple comparison Chi-square test. Comparisons between female and male curves were then tested by the Gehan-Wilcoxon test [31].
Fecundity and fertility were obtained from 25 pairs of newly hatched adults that were kept in individual containers. The number of eggs was counted daily and the daily rate of oviposition calculated as the number of eggs/female/day. Fertility was calculated as the total number of emerged larvae/the total number of eggs and expressed as a percentage.
When the male of a pair died before the female, the female was sustained until death without a male replacement (S1 Data). For each pair, we recorded the following: duration of pre-oviposition period, number of eggs oviposited daily by each female, total number of eggs deposited during a female’s lifetime, day of first oviposition (α), day of last oviposition (ω), duration of reproductive interval (ω – α), and in cases when the male died before the female we also recorded the number of eggs oviposited daily by the female after the death of the male.
We estimated lx and mx distributions calculated for age (x) expressed in days following standard procedures and considering that reproduction typically occurs in the middle of the interval (x, x+1). The corresponding survival to the midpoint of an age interval, the pivotal age, was calculated as: Lx = (Lx+ Lx+1)/2 [2, 12]. We estimated lx and mx (S1 Table) distributions calculated for pivotal age (x) expressed in days following standard procedures [2]. The following statistics were also calculated based on standard procedure [12]: reproductive rate ; mean generation time ; intrinsic rate of natural increase and reproductive value .
Results and discussion
Lobiopa insularis was not observed ovipositing on strawberry fruits. The proportion of egg masses was higher at a depth of one cm in soil (0.55, SE: 0.05) than on soil surface (0.32, SE: 0.04) and deeper than one cm (0.19, SE: 0.02) (F = 19.549; df = 2, 9; p<0.001). The number of eggs per egg mass was similar among the soil depths (on soil surface: 13.53, SE: 0.90; at a depth of one cm in soil: 13.60, SE: 0.61 and deeper than one cm: 13.31, SE: 0.02) (F = 0.025; df = 2, 76; p = 0.976). No egg masses were recorded more than 2 cm deep. This may reflect the non-specialized ovipositor found in members of this genus. Some nitidulids, such as Pocadius [32] and Neohebascus [33] within Nitidulinae, have specialized egg laying apparati due to the specialized hosts that they utilize. However, Lobiopa, much like other related nitiduline genera (i.e. Lasiodactylus and Stelidota) [16, 34] and non-related genera in disparate subfamilies possess a more generalized ovipositor for laying eggs directly on a substrate.
The duration of preimaginal developmental stages represented 18.5% of the duration of the entire life cycle, whereas the adult stage represented 81.5% (Table 1).
Table 1. Mean and standard deviation of duration (days) of developmental stages of Lobiopa insularis fed ripe strawberry fruits.
| Egg | Instar 1 | Instar 2 | Instar 3 | Pupa | Adult | |
|---|---|---|---|---|---|---|
| Cohort 1 | 4.56(0.92) n = 123 | 1.19(0.52) n = 123 | 1.28(0.45) n = 123 | 13.27(1.65) n = 123 | 7.58(1.53) n = 120 | 117.31(62.97) n = 91 |
| Cohort 2 | 4.36(0.79) n = 68 | 1.14(0.42) n = 65 | 1.24(0.43) n = 65 | 12.73(1.86) n = 65 | 7.76(1.43) n = 55 | 119.12(64.82) n = 55 |
| Cohort 3 | 4.87(1.11) n = 80 | 1.25(0.59) n = 78 | 1.20(0.40) n = 78 | 13.30(1.68) n = 78 | 7.61(1.50) n = 58 | 1129.81(64.12) n = 57 |
| Mean | 4.60(0.94) n = 271 | 1.19(0.51) n = 266 | 1.24(0.43) n = 266 | 13.10(1.73) n = 266 | 7.65(1.49) n = 233 | 122.08(63.97) n = 203 |
Development was shorter than reported by [35], when the larvae were fed an artificial diet.
Mean longevity of adult females was 121.84, SE = 8.86 and was similar in all cohorts (F = 0.44; df = 2, 121; P = 0.64) while mean longevity of adult males was 118.58, SE = 5.90 and as above was similar in all cohorts (F = 0.32; df = 2, 107; P = 0.72). Mean longevity did not differ between both sexes (F = 0.15; df = 1, 132; P = 0.69). Maximum adult longevity was 219 days for females and 211 for males. These values are lower than those observed by [35] for L. insularis fed on an artifitial diet: 306 days. Although lower than in this species, high longevity values were also observed in other nitidulids, i.e. Carpophilus lugubris: 101.3 and 115.2 days for females and males respectively [36], Stelidota ferruginea: 123 days for females [37] and Stelidota octomaculata: 125 days for females [38].
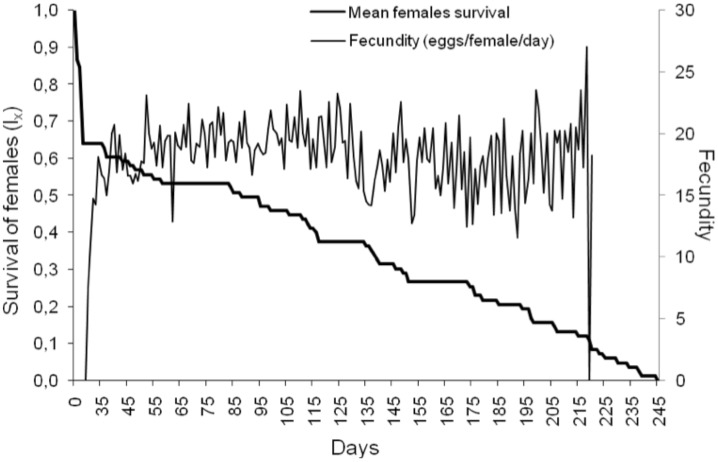
Female and male survival curves for all cohorts were similar (Chi2 = 0.10, df = 3, p = 0.950 and Chi2 = 0.88, df = 3, p = 0.644, respectivelly). Survival of females (Fig 1) and males were similar (Gehan-Wilcoxon test statistic = 0.17, p = 0.865). Survival from egg to adult (64.20%) was higher than that obtained by [35] (43.5%).
Fig 1. Survival and fecundity of Lobiopa insularis fed strawberry.
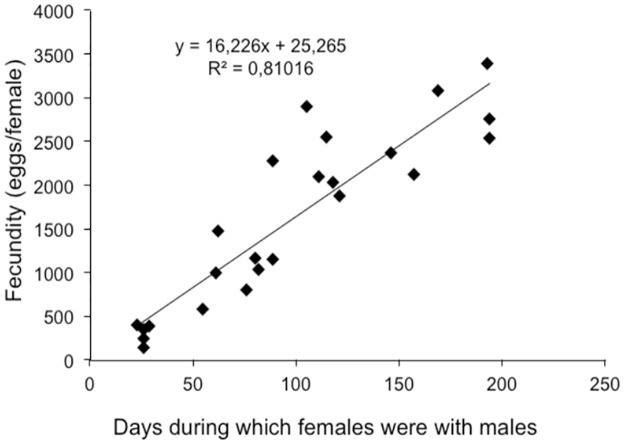
The number of eggs/female/day (18.1±3.74) was also higher in our study than [35], who reported a daily fecundity of 13.9±5.8 eggs/female/day. The pre-reproductive period was shorter than the reproductive and post-reproductive periods, which were significantly longer and more variable than the former (Table 2). The reproductive period and the fecundity (number of eggs per female) were dependent on the presence of males (Fig 2). The isolation of pairs in this study demonstrated that L. insularis females laid their eggs only when they were in the presence of a male. Therefore, mating would be essential for oviposition.
Table 2. Reproductive attributes of Lobiopa insularis fed ripe strawberry fruits.
| Attribute | Mean | SE |
|---|---|---|
| Pre-reproductive period (days) | 6.20 | 0.26 |
| Age of first oviposition, α (day) | 7.20 | 0.33 |
| Age of last oviposition, ω (day) | 97.76 | 11.26 |
| Reproductive period (days) | 91.44 | 11.17 |
| Post-reproductive period (days) | 21.88 | 5.87 |
| Number of oviposition days (days) | 87.44 | 10.97 |
| Proportion of reproductive period with oviposition | 0.82 | 0.03 |
| Intervals without oviposition (number) | 3.20 | 0.54 |
| Duration of intervals without oviposition (days) | 1.27 | 0.10 |
| Number of eggs/day/female | 18.01 | 1.71 |
| Number of oviposition days after male death | 1.90 | 0.28 |
| Eggs/female | 1655.35 | 249.53 |
Fig 2. Relationship between fertility and the time lapse during which females were with males.
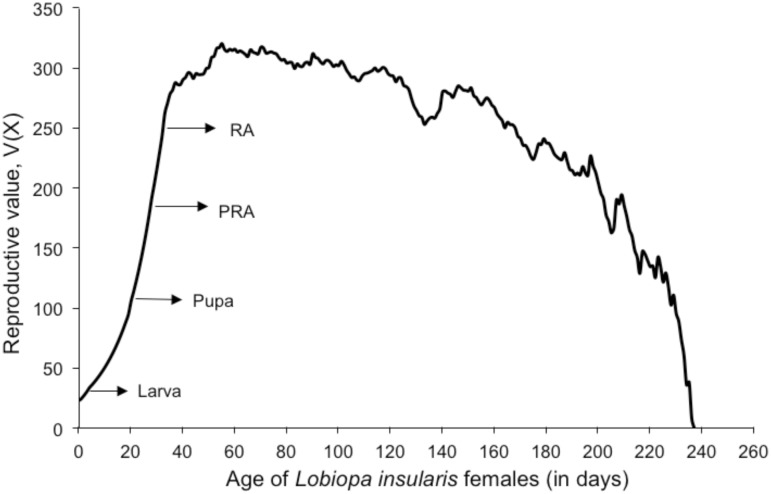
Natural selection favors individuals that make the highest proportionate contribution to the population to which they belong, and one measure of an individual’s contribution is it’s reproductive value, V(x) [1]. This measure provides a basis for estimating the present and future contribution of a female age (x) to the population growth rate, r [12]. As is common in other insects, L. insularis exhibited the greatest V(x) value in the adult stage, however not at the onset of reproduction (age x = α) but rather between ages x = 53 (corresponding to day 26 in the adult stage) to x = 60 (corresponding to day 33 in the adult stage). This time is effectively 20 days after the onset of the reproductive period (Fig 3).
Fig 3. Reproductive value for different ages of Lobiopa insularis females (in days) fed ripe strawberry fruits.
Arrows indicate the onset of the larval, pupal and adult developmental stages. The pre-reproductive stage (PRA) was differentiated from the onset of the reproductive stage (RA).
The long life span of adults and high reproductive attributes, including high fecundity and lengthy reproductive period (Table 2), are features of other nitidulid life cycles [14, 36, 37]. The longevity exhibited by adults together in conjunction with a long oviposition period indicates a broad overlap of successive generations during the year [14, 37]. Species that are subject to uncertain environments frequently exhibit extended longevity. Long life span as an adaptation for increasing fitness in uncertain environments is supported by empirical evidence in other groups [39] as well as by mathematical models [40, 41]. At least some individuals must be capable of surviving long, unpredictable periods of unfavorable conditions to ensure population replacement. Species that must find food sources that are scarce and/or widely dispersed tend to be long lived [42]. An overlap of successive generations may be advantageous in subcortical habitats where these beetles typically occur. These microhabitats provide a type of “refugia” wherein larval and pupal stages may have adequate protection from predators and parasitoids as well as non-ephemeral fungal food sources when conditions are optimal. Therefore, overlapping generations may be possible and advantageous. Other subcortical mycophagous cucujoid beetles have expressed this type of reproductive strategy.
Females laid eggs only in the presence of a male, which may indicate that females would have little sperm accumulation capacity within the spermatheca during mating. This characteristic could negatively affect the rate of population increase (Table 3) in natural settings. Insect natality usually is higher at intermediate population densities than at low or high densities. At low densities, difficulties in attracting mates may limit mating [43]. Clearly, if unmated females must find a mate to reproduce after finding a habitable patch, their value as founders is negligible.
Table 3. Population parameters of Lobiopa insularis fed ripe strawberry fruits.
| Cohort 1 (n = 142) | Cohort 2 (n = 81) | Cohort 3 (n = 94) | Mean | SD | |
|---|---|---|---|---|---|
| T | 102.399 | 110.123 | 114.007 | 108.843 | 5.909 |
| r | 0.062 | 0.059 | 0.058 | 0.060 | 0.002 |
| R0 | 570.127 | 663.985 | 780.644 | 671.585 | 105.464 |
Different mechanisms ensure breeding at a site of colonization such as long-distance attraction via pheromones, or through males accompanying females via phoretic or mating swarms [43]. The concentration of a food resource when the strawberry crop harvest is not done with proper cultural practices attracts L. insularis adults and thereby increases the rate of meetings between sexes and consequently affects female fecundity. The high fecundity coupled with a long period of resource availability (i.e.the harvest period lasts approximately four months) would lead to high local population increase of this species in strawberry fields. The scarcity of parasitoids, possibly due to recent colonization of strawberry crops or parasitoid satiation, could enhance the pest population increase. The encyrtid Zeteticontus insularis (Howard) was cited by [44] as parasitizing L. insularis, however it has never been registered for strawberry crops in Argentina. In the USA and Brasil, the only parasitoid registered on L. insularis is the larval parasitoid Brachyserphus abruptus (Hymenoptera: Proctotrupidae) [14, 45, 46].
Availability and concentration of resources appear to be the most important factors affecting the population dynamics of Lobiopa insularis associated with strawberry crops. However, soil predators (e.g. staphylinids and carabids) [47] may deserve more attention as potential control agents for eggs and pupae that are buried in the soil.
Supporting information
(XLSX)
Acknowledgments
This research was supported by grant from BID FONCyT, PICT 2012–1624, and Programa de Incentivos UNLP, N712.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was partially supported by grants from BID FONCyT, PICT 2012-1624, and Programa de Incentivos UNLP, N712. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Begon M, Harper JL, Townsend CR. Ecology: From individuals to ecosystem. Fourth edition Blackwell Scientific Publications Ltd; Oxford, U.K; 2006. [Google Scholar]
- 2.Carey JR. Applied demography for biologists with special emphasis on insects. Oxford University Press; 1993. [Google Scholar]
- 3.Price PW, Denno RF, Eubanks MD, Finke DL, Kaplan I. Insect Ecology Behaviour, populations and communities. Cambridge University Press; 2011. [Google Scholar]
- 4.Pimm SL. Food Webs. Chapman and Hall, London; 1982. [Google Scholar]
- 5.Denno RF, McClure MS. Variable plants and herbivores in natural and managed systems. Academic Press, New York, New York, USA; 1983. [Google Scholar]
- 6.Hunter MD, Ohgushi T, Price PW. 1992. Effects of resource distribution on animal-plant interactions. Academic Press, San Diego, California, 505 pp. [Google Scholar]
- 7.Berryman AA, Kindlmann P. Population Systems: A General Introduction. Springer; 2008. [Google Scholar]
- 8.Rabb RL. A sharp focus on insect populations and pest management from a widearea view. Bull Entomol Soc Am. 1978;24: 55–61. [Google Scholar]
- 9.Stinner RE. Biological monitoring essentials in studying wide-area moth movement In: Rabb RL, Kennedy GG, editors. Movement of Highly Mobile Insects: Concepts and Methodology in Research. Raleigh: NC State Univ; 1979. p. 199–211. [Google Scholar]
- 10.Kennedy GG, Storer NP. Life Systems of Polyphagous Arthropod Pests in Temporally Unstable Cropping Systems. Annu Rev Entomol. 2000;45: 467–493. doi: 10.1146/annurev.ento.45.1.467 [DOI] [PubMed] [Google Scholar]
- 11.Stearns SC. The evolution of life histories. New York: Oxford Univ. Press; 1992. [Google Scholar]
- 12.Ebert TA. Plant and animal populations: methods in demography. San Diego: Academic Press; 1999. [Google Scholar]
- 13.Cluigt N, Liljesthröm G, Greco N. Análisis espacio-temporal de Lobiopa insularis (Coleoptera: Nitidulidae) en el cultivo de frutilla". 30° Congreso Argentino de Horticultura y 1° Simposio Internacional sobre Cultivos Protegidos, organizado por la ASAHO, 25 a 28 de septiembre de 2007 en la ciudad de La Plata, Buenos Aires, Argentina.
- 14.Myers L. Sap beetle (of Florida), Nitidulidae (Insecta: Coleoptera: Nitidulidae). Entomology and Nematology departament, Florida cooperative extension service, institute of food and agricultural sciences, University of Florida. EENY256, 2004;7 pp.
- 15.Loughner RL, Loeb GM, Demchak K, Schloemann S. Evaluation of strawberry sap beetle (Coleoptera: Nitidulidae) use of habitats surrounding strawberry plantings as food resources and overwintering sites. Environ Entomol. 2007;36: 1059–1065. [DOI] [PubMed] [Google Scholar]
- 16.Parsons CT. A revision of the Nearctic Nitidulidae (Coleoptera). Bulletin of the Museum of Comparative Zoology, Harvard: 1943;92: 121–278. [Google Scholar]
- 17.Cline AR. 2005. Revision of Pocadius Erichson (Coleoptera: Nitidulidae). Ph.D. dissertation, Louisiana State University, 384pp. Baton Rouge, LA. USA.
- 18.Cline AR, Kinnee SA. A new species of sap beetle (Coleoptera: Nitidulidae) from Baja California Sur, Mexico, with a review of the genus Lobiopa Erichson. Pan-Pac Entomol. 2012;88: 202–211. [Google Scholar]
- 19.Majka CG, Cline AR. Nitidulidae and Kateretidae (Coleoptera: Cucujoidea) of the Maritime provinces of Canada. I. New records from Nova Scotia and Prince Edward Island. Can Entomol. 2006;138: 314–332. [Google Scholar]
- 20.Majka CG, Webster R, Cline AR. New records of Nitidulidae and Kateretidae (Coleoptera) from New Brunswick, Canada. Zookeys 2008;2: 337–356. [Google Scholar]
- 21.Price MB, Young DK. An annotated checklist of Wisconsin sap and short-winged flower beetles (Coleoptera: Nitidulidae, Kateretidae). Insecta Mundi 2006;20: 69–84. [Google Scholar]
- 22.Ellis JD, Delaplane KS, Cline AR, McHugh JV. The association of multiple sap beetle species (Coleoptera: Nitidulidae) with western honeybee (Apis mellifera) colonies in North America. J Apicult Res. 2008; 47: 188–189. [Google Scholar]
- 23.Price JF. Sap beetle and fruit damage final quality in March. Berry Times; 2001. Volume 1. [Google Scholar]
- 24.Bortolozzo AR,Valdebenito Sanhueza RM, de Melo GWB, Dekovaleski A, Bernardi J, Hoffmann A et al. Produçao de morangos no sistema semi-hidroponico. Bentos Gonçalves, RS: Embrapa, Circular Técnica; 62 Outubro 2007. [Google Scholar]
- 25.Dowd PF, Nelson TC. Seasonal variation of sap beetle (Coleoptera: Nitidulidae) populations in central Illinois. J Econ Entomol. 1994;93: 1714–1720. [DOI] [PubMed] [Google Scholar]
- 26.Williams RN, Salles LAB. Nitidulidae associated with fruit crops in Rio Grande do Sul, Brazil. Fla Entomol. 1986;69: 298–302. [Google Scholar]
- 27.Salles LAB. 2005. Pragas do morangueiro In: Sistema de produção do morango. Bento Gonçalves: Embrapa Uva e Vinho (Sistemas de Produção 5) 2016, 26 August http://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Morango/SistemaProducaoMorango/cap07.htm#broca [Google Scholar]
- 28.Rondon SI, Price JF, Cantliffe DJ. Sap Beetle (Coleoptera: Nitidulidae) Management in Strawberries. University of Florida; 2011;p. 1–4. [Google Scholar]
- 29.Guimarães JA, Michereff filho M, Ribeiro, MGP de M; Liz RS de, Guedes ÍMR. Ocorrência e manejo da broca-do-morangueiro no Distrito Federal. Brasília: Embrapa Hortaliças (Embrapa Hortaliças 2009;Comunicado técnico, 74).
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53: 457–481. [Google Scholar]
- 31.Gehan EA, Thomas DG. The performance of some two sample tests in small samples with and without censoring. Biometrika. 1969;56: 127–132. [Google Scholar]
- 32.Cline AR. Revision of the sap beetle genus Pocadius Erichson, 1843 (Coleoptera: Nitidulidae: Nitidulinae). Zootaxa. 2008;1799: 1–120. [Google Scholar]
- 33.Cline AR. A new sap beetle (Nitidulidae: Nitidulinae) genus from the Neotropics, with commentary on the Pocadius complex of genera. Zootaxa. 2009;2237: 34–42. [Google Scholar]
- 34.Cline AR. Review of Lasiodactylus Perety, with descriptions of three new species (Coleoptera: Nitidulidae: Nitidulinae). Coleopts Bull. 2004;58: 355–368. [Google Scholar]
- 35.Bortoli LC, Machota R, Botton M. Biologia e tabela de vida de fértilidade da broca-do-morangueiro criada em dieta artificial. Pesq agropec bras Brasilia. 2014;49: 144–147. [Google Scholar]
- 36.Sanford JW, Luckman WH. Observations on the biology and control of the dusky sap beetle in Illinois. Proceedings North Central Branch Entomological Society of America 1963;18: 39–43. [Google Scholar]
- 37.Galford JR, Williams RN, Beacom M. Notes on the Biology and Hosts of Stelidota ferruginea (Coleoptera:Nitidulidae) Res. Pap. NE-654. Radnor, PA: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station; 1991a; 4 pp. [Google Scholar]
- 38.Galford JR, Williams RN, Daugherty A. Life History and Notes on the Biology of Stelidota octomaculata (Coleoptera:NitiduIidae) Res. Pap. NE-644. Radnor, PA: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station; 1991b; 7 pp. [Google Scholar]
- 39.Tinkle DW. The concept of reproductive effort and its relation the evolution of life histories of lizards. Am Nat. 1969;103: 501–516. [Google Scholar]
- 40.Tuljapurkar S. An uncertain life: Demography in random environments. Theor Popul Biol. 1989;35: 227–294. [DOI] [PubMed] [Google Scholar]
- 41.Tuljapurkar S. Delayed reproduction and fitness in variable environments. P Natl Acad Sci-Biol. 1990;87: 1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carey JR. Insect biodemography. Annu Rev Entomol. 2001;46: 79–110. doi: 10.1146/annurev.ento.46.1.79 [DOI] [PubMed] [Google Scholar]
- 43.Schowalter TD. Insect ecology An ecosystem approach, 2nd edition Elsevier, Burlington, USA; 2006. [Google Scholar]
- 44.De Santis L. Encírtidos de la República Argentina (Hymenoptera: Chalcidoidea). Anales de la Comisión de Investigaciones Científicas de la provincia de Buenos Aires. 1964;4: 9–422. [Google Scholar]
- 45.Hayashi C. A new statistical approach to estimating the size of an animal population: the case of a hare population. Math Sci. 1978;3: 117–130. [Google Scholar]
- 46.Williams RN, Weiss MJ, Miller KV, Werner JJ. A summary of experiments for control of sap beetles which attack fruit crops. Research circular- Ohio Agricultural Research and Development Center 1984;283: 66–68. [Google Scholar]
- 47.Sunderland KD . Invertebrate pest control by carabids In: Holland JM,editors. The Agroecology of carabid beetles. Intercept, Andover, UK; 2002. p. 165–214. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.