Abstract
Sudden cardiac death (SCD) is a devastating event afflicting 350,000 Americans annually, despite the availability of life-saving preventive therapy, the implantable cardioverter defibrillator (ICD). SCD prevention strategies are hampered by overreliance on global left ventricular ejection fraction (LVEF) below 35% as the most important criterion to determine ICD candidacy. Annually in the U.S. alone, this results in approximately 130,000 ICD placements at a cost of over $3 billion but only a 5% incidence per year of appropriate firings. This approach further fails to identify individuals who experience the majority, as many as 80%, of SCD events, which occur in the setting of more preserved LVEF. Better risk stratification is needed to improve care and should be guided by direct pathophysiologic markers of arrhythmic substrate, such as specific LV structural abnormalities. There is an increasing body of literature to support the prognostic value of cardiac magnetic resonance imaging with late gadolinium enhancement (CMR-LGE) in phenotyping the LV to identify those at highest risk for SCD. CMR has unparalleled tissue characterization ability and provides exquisite detail about myocardial structure and composition, abnormalities of which form the direct, pathophysiologic substrate for SCD. Here we review the evolution and current state of CMR for imaging the arrhythmic substrate, both as a research tool and for clinical applications.
Keywords: sudden cardiac death, arrhythmia, cardiac magnetic resonance imaging, cardiomyopathy, infarct size, fibrosis, late gadolinium enhancement, ventricular tachycardia arrhythmia, risk prediction, left ventricular dysfunction
Sudden cardiac death (SCD) remains a significant public health care burden.1 Estimated potential years of life lost due to SCD approach 2 million in men and 1.3 million in women, and often strikes in the prime of life.2 Significant progress in medical and device therapy has improved treatment of the precursors of SCD, coronary heart disease and heart failure (HF). Clinical guideline recommendations emphasize identification of candidates with cardiomyopathy for appropriateness of primary prevention implantable cardioverter defibrillators (ICDs). Nonetheless, despite declines in overall cardiovascular deaths, there remains a disproportionately high contribution from SCD, rates of which exceed all non-cardiac deaths except overall cancer and accidents.2 SCD prevention is limited by the lack of robust approaches to identify patients at the highest risk and distinguish them from those who will not benefit from the costly therapy. Current practice guidelines for selecting candidates for ICD therapy for the primary prevention of SCD rely on left ventricular ejection fraction (LVEF) below 30 to 35% as the sole LV structural abnormality.3 LVEF, however, is an inadequate surrogate of the underlying myocardial phenotype predisposing to SCD and thus is insensitive and nonspecific.4 The same LVEF can represent multiple cardiomyopathic derangements. Those with LVEF below 35% account for <20% of all SCD events.4 No LVEF cutoff discriminates well between sudden and non-sudden modes of cardiac death.4 In fact, LVEF is indirectly related to mechanisms of arrhythmia and subject to considerable spontaneous variability.4–6 A 2010 workshop highlighted this predicament and recommended that “research should assess new approaches that may provide incremental information on SCD risk beyond LVEF,” including “new imaging methods of cardiac structure and physiology.”1 It is increasingly apparent that cardiac magnetic resonance imaging (CMR) is particularly well-suited as the imaging modality of choice for SCD risk stratification, particularly compared to other modalities (Table 1).
Table 1.
CMR vs. Other Imaging Modalities for SCD Risk Stratification
|
Echocardiography Uses
|
|
Cardiac blood pool imaging (multigated acquisition scan, MUGA) Uses
|
|
Single photo emission tomography (SPECT) Uses
|
|
Positron emission tomography Uses
|
|
Computed tomography Uses
|
|
CMR Uses
|
LVEF quantification by CMR and impact on decision-making
Although an LVEF below 30 to 35% determines eligibility for primary prevention ICD placement, current clinical guidelines do not specify by which imaging modality to measure LVEF. Echocardiography is the most widely used technique, both clinically and in the randomized ICD trials that inform current practice guidelines. No prior ICD trials included CMR. However, CMR is accepted as the modality of choice for LVEF assessment because of its high accuracy and reproducibility, particularly when compared to echocardiography. Multiple studies report modest agreement and variable biases between LVEFs quantified by echocardiography and CMR.7 At lower LVEF, echocardiography generally overestimates CMR LVEF by at least 3–5%,7, 8 which may impact ICD eligibility, most notably in the intermediate LVEF range, if threshold cutoffs are used interchangeably. At higher LVEFs, echo may underestimate CMR as highlighted by the differential normal LVEF ranges for the two modalities. For echo, normal LVEF ranges are 52–74%9 compared to 57–77% for CMR (excluding papillary muscles from the LV cavity).10 How these LVEF differences affect clinical outcomes remain undetermined but highlight the lack of interchangeability among imaging modalities and the need for further investigation of modality-specific thresholds.
CMR assessment of myocardial scar and scar heterogeneity
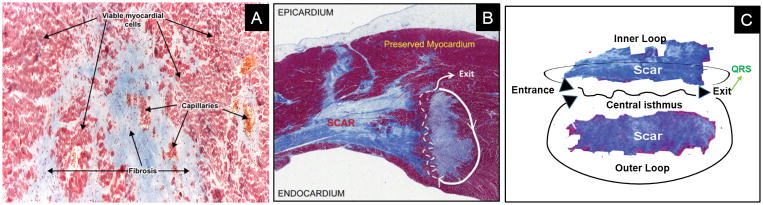
Myocardial fibrosis is a major pathophysiologic determinant of arrhythmic propensity in both ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM). Myocardial injury causes extensive structural and functional cardiac remodeling with resultant myocardial loss, with or without compensatory myocyte hypertrophy, and replacement of the extracellular matrix with fibrosis.11, 12 The extent and architecture of fibrosis, even in the absence of contractile dysfunction, lead to electrophysiologic derangements that increase propensity for ventricular arrhythmias and SCD due to scar-related re-entry.13, 14 It is increasingly recognized that scar heterogeneity within the myocardium is especially arrhythmogenic.15–17 The intermingling of viable myocytes and collagen produces spatial heterogeneity and anisotropy leading to slow conduction, fixed and functional conduction block, enhanced excitability, and dispersion of refractoriness, all of which promote the development and propagation of re-entrant ventricular tachyarrhythmias (Figure 1).13, 18–22 Hence, identifying and characterizing the underlying arrhythmogenic substrate of myocardial scar have great potential to improve SCD risk stratification.
Figure 1. Mechanisms of scar re-entry.
Heterogeneously distributed scar forms electrical conduction barriers but also facilitate the formation of critical isthmuses of viable myocytes that support re-entrant circuits (Panels A and B, collagen bundles shown in blue on Masson’s trichrome staining). Panel C: Wavefronts can enter the proximal end of the isthmus (entrance), exiting from the distal end (exit) and then propagating throughout the ventricle to form the QRS complex. The wavefront can re-enter the isthmus from channels within the infarct (inner loop) or via an outer loop at the border of the infarct zone with the normal myocardium. Reprinted with permission from: Danciu M.21 and Ajijola A. et al.22
CMR with late gadolinium enhancement (CMR-LGE) using segmented inversion-recovery acquisition techniques has unparalleled ability to characterize myocardial tissue composition. It is increasingly used to advance our understanding of the pathophysiology, diagnosis, and treatment of ventricular arrhythmias. A growing body of literature demonstrates the strong prognostic significance of CMR scar indices, including presence of scar, scar transmurality, total scar extent and extent of myocardial tissue heterogeneity (gray zone).
CMR in chronic ICM: pathophysiologic correlates
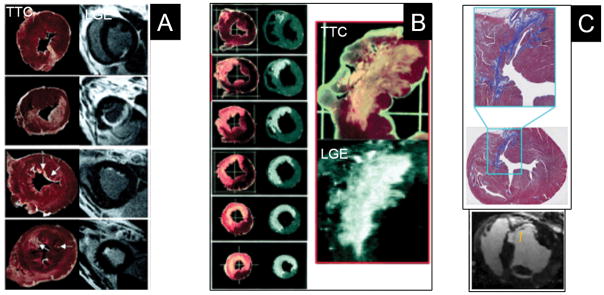
CMR-LGE was first utilized to quantify acute myocardial infarct (MI) size. It is based on differential T1 shortening properties of gadolinium contrast and the increased contrast volume of distribution (extracellular volume, ECV of gadolinium) from loss of cell membrane integrity that result in demarcation of bright, hyperenhanced necrotic tissue with elevated signal intensity (SI) and differentiation from unenhanced, dark-appearing normal myocardium. Similarly, in chronic infarction, myocyte loss and replacement by collagenous scar result in increased gadolinium ECV and delayed contrast washout kinetics leading to persist hyperenhancement when imaging is performed 15–20 minutes after contrast injection, hence the moniker, LGE (Figure 2). Total scar extent by CMR-LGE correlates closely with pathologic quantification of irreversible myocardial injury at all stages of infarct evolution beyond the acute MI period (Figure 3).23, 24 Scar size predicts major adverse cardiovascular outcomes post-MI independently of LVEF and LV volumes.25
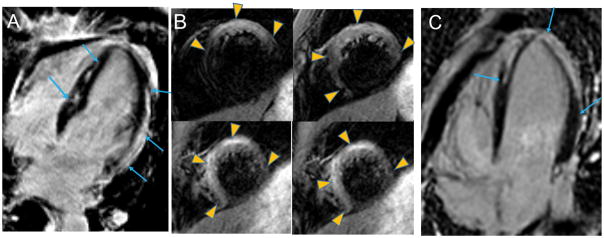
Figure 2. Ischemic scar (between arrows).
Panel A: non-transmural scar of the inferior and inferoseptal walls. Panel B: thinned transmural scar in the territory of the left anterior descending coronary artery. Panel C: two subendocardial infarcts of the anterolateral and inferolateral walls. Panel D: chronic inferior infarct with wall thinning (left image) and quantification (right image) of core (red) and peri-infarct, gray regions (yellow) using the FWHM method.
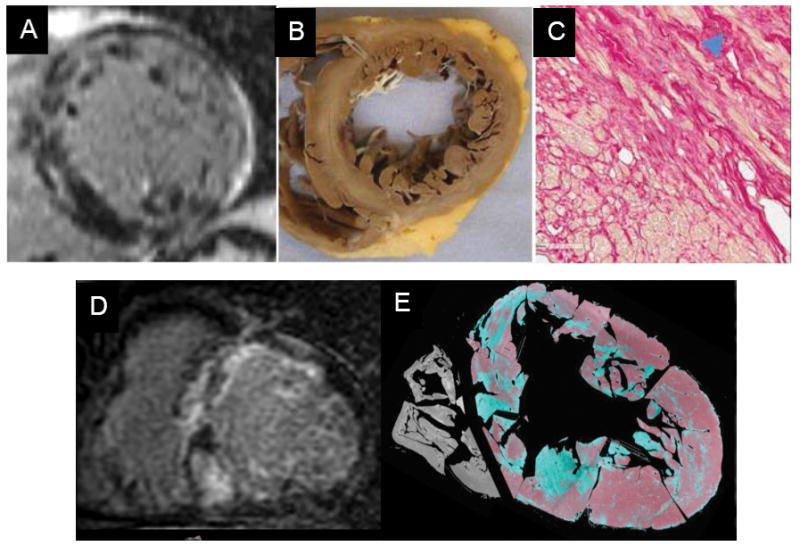
Figure 3. Pathologic correlates of LGE post-MI.
The region of LGE closely matches the extent of infarction determined by pathology at all stages of infarct healing. Panels A (1 day post-MI) and B (3 days post-MI) show pathologic cross-sections stained with tetrazolium chloride (TTC) in which the pale regions represent regions of infarction and corresponding CMR-LGE images. Panel C (6 weeks post-MI) shows Masson’s trichrome staining in which collagenous scar appears blue and corresponding CMR-LGE images. Reprinted from Kim et al.23 and Zhang et al.24 with permission.
In addition to total scar extent, the complex architecture of MI contributes to re-entrant forms of ventricular arrhythmogenesis. The interspersion of fibrotic areas with viable myocyte myocytes and heterogeneous spatial geometry of scar underlie potentially arrhythmogenic substrate.26 Regions of densely fibrotic tissue can be distinguished from infarct border zones with intermingled collagen bundles and viable myocytes by quantifying relative differences in CMR SI reflective of differential contrast kinetics within the scar.27–30 Within the hyperenhanced (bright) area seen on CMR, regions of very elevated SI reflecting homogeneously dense fibrosis (infarct “core”) can be partitioned from peripheral regions with intermediately elevated SI (“peri-infarct” or “gray zone”) reflecting a mixture of collagen bands and viable myocytes.27 Different SI threshold definitions have been used in the literature to define infarct core and gray zone.27, 29, 30 These include using an SI cutoff of above 50% of peak SI (full-width half-maximum, FWHM) within the total hyperenhanced region to define core; SI thresholds between peak normal myocardial SI and FWHM to define gray; between 35% and 50% of peak hyperenhanced SI to define gray; and SI between 2 to 3 standard deviations of mean SI in normal myocardium to define gray zone.
There are limited direct head-to-head comparisons of histopathology and CMR-LGE quantification of infarct heterogeneity. An ex-vivo experimental swine infarct model showed that the gray, border zones, as defined by a moderate severity, histopathologic (Picrosirius Red staining) fibrosis grade of 20–70%, had intermediate SI by CMR-LGE. In contrast, dense core regions had distinctly elevated SI corresponding to a severe histopathologic grade of ≥70% fibrosis.31 A patient study provided additional support for the differential contrast characteristics within infarcts.32 Core regions (defined using FWHM) corresponded to regions with an elevated gadolinium ECV fraction of 42% compared to 25% in normal myocardium. In contrast, border regions with SI above 2 standard deviations above mean normal myocardium corresponded to areas with intermediately elevated ECV fraction of 32%, reinforcing the concept of differential tissue composition based on CMR-LGE thresholds.
Correlations with electroanatomic voltage mapping (EAVM) and results of electrophysiologic ablation procedures also corroborate the pathophysiologic importance of detailed infarct phenotyping by CMR-LGE. High-resolution experimental models of ventricular tachycardia (VT) show that critical re-entrant isthmuses occur in infarct border zones.33 Substrate mapping and targeted ablation focusing on the heterogeneous border zones result in more successful therapy of inducible VTs.34 Subsequent patient studies also suggest the close proximity of critical VT isthmus sites to the core-gray transition zone and CMR-derived tissue characterization not only correlates with but improves upon the EAVM identification of VT ablation targets.35, 36 Other recent studies have used segmented myocardial shells to delineate layers of core as distinct from border zones and thereby locate border zone channels that correlate with VT isthmuses identified by electroanatomic pacemapping.37, 38
CMR in chronic NICM: pathologic correlates
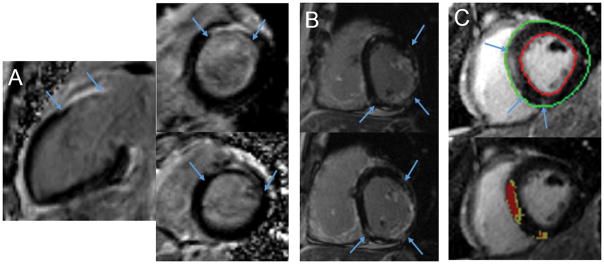
Replacement fibrosis is common in dilated NICM of unknown etiology. Several patterns of focal fibrosis by CMR-LGE have been described (Figure 4). These include most commonly, midwall enhancement, sparing the endocardium; subepicardial enhancement; and patchy foci not following a coronary artery territory.39 In some patients, an infarct pattern, i.e. coronary distribution with endocardial to epicardial involvement, is seen in the absence of epicardial coronary disease. While this may be a feature of the cardiomyopathy, it may also reflect epicardial coronary artery recanalization, spasm or an embolic episode. Comparison between in-vivo CMR-LGE and ex vivo histopathology of explanted hearts shows excellent agreement among the locations and patterns of fibrosis (Figure 5).40–42 In patients with no LGE, histopathology confirms the absence of fibrosis.
Figure 4. Nonischemic scar (between arrows).
Panel A: CMR images showing basal septal scar in a 45 year-old woman with strong family history of ventricular arrhythmia and SCD. EPS showed inducible monomorphic VT with right bundle, inferior axis morphology. An ICD was placed and subsequently discharged for monomorphic VT. Panel B: patchy inferior, inferoseptal and inferolateral LGE in a 65 year-old with NICM and multiple episodes of VT. Panel C: septal and inferior RV insertion LGE sparing the endocardium (top image) with quantification (lower image) of core (red) and gray regions (yellow) using the FWHM method.
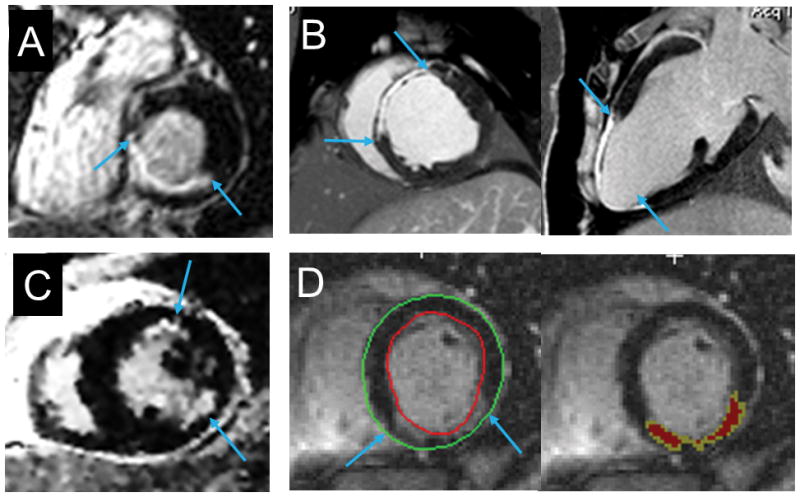
Figure 5. Pathologic correlates of nonischemic scar.
Panels A (pre-transplant CMR-LGE), B (post-transplant gross macroscopic cross-section) and C (post-transplant microscopic cross-section with fibrotic bundles, blue arrow) showing that the LGE with a midwall, near-circumferential pattern mirrors the distribution of pathologic replacement fibrosis. Panel D (pre-transplant CMR-LGE) shows diffuse LGE corresponding to regions of fibrosis confirmed by Masson’s trichrome staining (in green) on post-transplant histopathology (Panel E). E Reprinted from Iles et al.41 and Halliday et al.42 with permission of the publishers.
Electrophysiologic substrate mapping studies in NICM also corroborate the pathophysiologic relationship between CMR-LGE scar characteristics and arrhythmogenesis. CMR-LGE scar mapping in NICM improves the identification of critical VT components during EAVM of potential VT ablation sites.43, 44 The pattern of LGE (subendocardial, transmural, mid-myocardial vs. subepicardial) may be of particular utility for guiding the ablation approach (endocardial vs. epicardial) in NICM.45
Results of meta-analyses in chronic ICM and NICM
A growing number of publications and meta-analyses demonstrate the prognostic value of CMR-LGE scar presence and extent for SCD risk stratification.46–49 A recent meta-analysis identified 19 studies comprising 2850 ICM and NICM patients who experienced 423 combined arrhythmic events (sudden death, aborted sudden death, VT/VF, and appropriate ICD therapy) with an annualized event rate of 5.3%.47, 50 The pooled OR for arrhythmic events in patients with LGE above the designated study-defined thresholds was 5.62 for all patients. The OR was 5.05 in ICM and 6.27 in NICM with no significant difference in risk between ICM and NICM, suggesting similar predictive power of abnormal LGE (presence and greater extent) among ischemic and non-ischemic etiologies.
A number of subgroup analyses have been performed within the meta-analyses. Four studies specifically assessed gray zone extent (459 patients and 86 events) with a similar number of studies assessing core scar extent as a predictor.46 Gray zone extent more strongly predicted ventricular arrhythmic events than core (RR 5.94 vs. 3.82).46 Studies were also grouped by mean LVEF below (n=11, 1178 patients) or above 30% (n=8, 1672 patients).47 The OR for an arrhythmic outcome for abnormal LGE findings was double for LVEF below 30% compared to LVEF above 30% (9.56 vs. 4.48, p=0.02). However, those with LVEF above 30% in existing studies tend to be secondary prevention ICD recipients which may result in survivor biases and inadequately reflect risk stratification for primary prevention.
Acute MI
Assessing arrhythmic risk in the acute MI period poses a particular dilemma. Infarct characteristics and LV remodeling evolve considerably in the initial days to weeks post-MI. Not only can LVEF recover but infarcted regions tend to shrink as the acute edema resolves and tissue healing occurs. Current guidelines exclude routine ICD placement in acute MI patients with low LVEF within 40 days because of negative results from two clinical trials which showed no mortality reduction with ICDs.51 However, SCD risk remains highest within the first 30 days post-MI across all LVEF categories, though highest in those with LVEF below 30%.52 In the Valsartan in Acute Myocardial Infarction Trial (VALIANT), among those with LV dysfunction or HF complicating acute MI, 19% of all SCDs or aborted SCDs occurred in the first 30 days post-MI with an absolute rate of 1.4% per month, decreasing to 0.14% per month after 2 years.53 Thus, there remains considerable interest in better risk-stratifying these patients.
Limited studies have focused on CMR-LGE for arrhythmic risk assessment early post-MI. One multi-center study enrolled 162 patients with large reperfused ST-elevation MI (STEMI).54 CMR-LGE was performed at 3–4 days post-MI with 24 Holter monitoring at 1 month. Size of the infarct penumbra (i.e. peri-infarct, gray zone) relative to total infarct size most strongly and independently predicted Holter VT burden. Another single-center study of 440 acute STEMI patients incorporated CMR-LGE at 1 week post-MI with 2 year median follow-up for SCD, sustained VT or VF documented by electrocardiography or ICD, which occurred in 2.5% of patients (n=11).55 Mean LVEF was 52±13% with total scar size of 21±15%. On multivariate analysis, the strongest predictors of the combined arrhythmic endpoint were LVEF below 37% combined with infarct size above 30% (area under the curve, AUC, 0.87) and accounted for all but one of the arrhythmic events (n=10 of 11).
A recent clinical study highlighted changes in the temporal course of infarct tissue heterogeneity and influencing factors.56 Twenty-one patients with reperfused STEMI underwent CMR-LGE at 2 days, 3 weeks and 6 months post-MI. Core infarct sizes declined significantly over time in both patients with and without microvascular obstruction (no-reflow). In contrast, size of the peri-infarct (gray) zone declined only in those without microvascular obstruction, remaining unchanged in those with microvascular obstruction.
CMR-LGE in other arrhythmogenic LV cardiomyopathies
Hypertrophic cardiomyopathy (HCM)
Replacement fibrosis is common in HCM and detectable in 42–73% of patients by CMR.57 LGE pattern in HCM (Figure 6) is heterogeneously distributed but most commonly occurs in regions of hypertrophy and tends not to follow a coronary artery territory.58 Involvement of both the ventricular septum and free wall occurs in over 30% of patients.58 Other patterns include confinement of LGE to the free wall, septum, apex, RV-LV insertion points, RV free wall or papillary muscles or combinations, thereof.58 CMR-LGE has been included in clinical practice guidelines as an American College of Cardiology/American Heart Association (ACC/AHA) class IIb consensus recommendation since 2011: “In selected patients with known HCM, when SCD risk stratification is inconclusive, CMR-LGE may be considered in resolving clinical decision-making.”59 The initial HCM-CMR data supporting this recommendation comprised 4 published studies, 1063 patients, 3.1 year follow-up with 30 SCD/aborted SCDs.60 A more recent meta-analysis57 confirmed these results in 5 studies of 2993 patients with median follow-up of 36.8 months and included 81 SCD or aborted SCD events. LGE and SCD were strongly associated (OR 3.41, p<0.001). Larger amounts of LGE conferred a higher risk with each 10% increase corresponding to a 36% rise in SCD risk.61 Based on these cumulative data, it has been proposed that both the presence and amount of LGE be integrated into a personalized approach to SCD risk assessment.61
Figure 6. HCM.
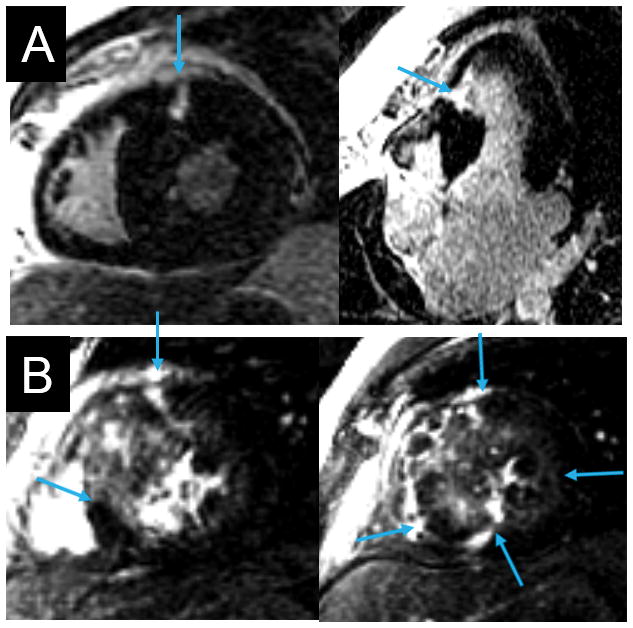
Two patients (Panels A and B) with HCM. Panel A: focal fibrosis (arrows) in non-coronary territories. Panel B: extensive, diffusely distributed LGE (arrows).
Cardiac sarcoidosis
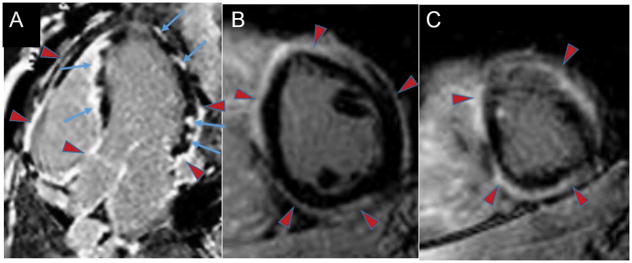
Sarcoidosis is a multi-system, granulomatous inflammatory disease with variable cardiac involvement that is difficult to diagnose and may be subclinical. Ventricular arrhythmias are a common disease manifestation and arrhythmic SCD may be the first presentation. The acute phase is characterized by myocardial inflammation of varying degree and reversibility and is modestly responsive to steroid therapy.62 Irreversible injury with granulomatous scar formation is common and forms the basis of chronic myocardial scarring which promotes macroreentrant ventricular arrhythmias. CMR-LGE detects chronic myocardial involvement with higher prevalence compared to non-imaging clinical diagnostic criteria such as the modified Japanese Ministry of Health guidelines.63 The presence of CMR-LGE was added to the recent 2014 Heart Rhythm Society (HRS) consensus guidelines as a criterion contributing to a probable clinical diagnosis of cardiac sarcoid.62 Typical CMR-LGE features (Figure 7) include non-vascular territory involvement with mid-myocardial or sub-epicardial LGE but infarct patterns may also be seen (i.e. distribution along a coronary territory with transmural or subendocardial enhancement). Basal septal aneurysm formation is a rare but specific CMR feature of cardiac sarcoid.
Figure 7. Cardiac sarcoidosis.
33-year-old who presented with intermittent third degree heart block and CMR-LGE demonstrating extensive cardiac involvement and sarcoidosis on lymph node biopsy. Panel A (4 chamber apical view): patchy LV lateral wall LGE and LGE of the right ventricular side of the ventricular septum with endocardial sparing (blue arrows). There is also LGE (red arrowheads) of the epicardium and pericardium of the basal to mid RV free wall; atria; and LV pericardium. Panels B (mid-ventricular short axis slice) and C (apical short axis slice): extensive epicardial and pericardial (red arrowheads) LGE. Despite steroid therapy, he subsequently developed VT storm.
Two recent meta-analyses investigated the prognostic role of CMR-LGE with similar results.64, 65 One included ten studies with 760 sarcoid patients and mean follow-up of 3.0±1.1 years.65 The other identified seven studies with 694 patients.64 A composite endpoint was evaluated as either a primary or secondary endpoint: all-cause mortality, ventricular arrhythmia, ICD shock and SCD. The prevalence of LGE ranged from 13–89%. Compared to those who were LGE negative, patients with LGE had a higher risk of the composite outcome (OR 10.74, p<0.00001) with an increased annualized event rate of 11.9% vs. 1.1% (p<0.0001). Data from several studies suggest that the prognostic value of LGE was independent of and stronger than LVEF and persisted among those with LVEF above 50%.64, 65 The 2014 HRS consensus statement62 had previously indicated that CMR-LGE for the purpose of SCD risk stratification may be considered (ACC/AHA class IIb recommendation). Furthermore, if LVEF is in the intermediate range (36–49%) despite optimal medical therapy and a period of immunosuppression, CMR with or without an electrophysiologic study may be considered to help risk stratify these patients. The meta-analysis results support this recommendation.
Acute and chronic myocarditis (Figure 8)
Figure 8. Acute and chronic myocarditis.
Panels A and B: 29-year-old who presented with acute myocarditis with peak troponin-I 72 ng/mL; peak creatine phosphokinase 2742 U/L, CK-MB 331μg/L. LVEF was mildly reduced (Video 1) but he was asymptomatic from the arrhythmia and HF standpoint during a 6 day hospitalization. Panel A: mid-septal and lateral epicardial wall LGE (arrows) and pericardial enhancement. Panel B: T2 weighted edema imaging with extensive edema (arrowheads). He died suddenly at home 2 days post-discharge.
Panel C: 22 year-old with documented acute myocarditis 15 months previously. CMR-LGE showed LGE of the distal segments of the LV with endocardial sparing (arrows) and pericardium. LV function was normal with no regional wall motion abnormalities (Video 2). Two years later, the patient developed palpitations and syncope with large burden of multifocal PVCs on Holter (>5%). EPS showed easily inducible monomorphic and polymorphic VT. An ICD was implanted and subsequently fired multiple times for MVT at 250 beats per minute.
The natural history of acute myocarditis varies considerably. However, acute viral myocarditis may account for a large proportion of SCD in young people.66 In the initial disease phase, active viral replication leads to direct injury and lysis accompanied by innate immune activation. The resultant myocardial damage is generally asymptomatic and most patients recover. In some, however, disease activity persists and triggers an adaptive autoimmune response with profound myocardial inflammation, causing HF and arrhythmias. Recovery occurs in resistant individuals (>90%) while progression to a dilated cardiomyopathy is seen in other susceptible people. Acutely, active myocardial inflammation is arrhythmogenic due to triggered activity and abnormal automaticity. In chronic myocarditis, myocardial replacement fibrosis promotes re-entrant arrhythmic mechanisms.
An early series of 32 patients with clinically-suspected acute myocarditis showed the ability of CMR to diagnose myocarditis and guide endomyocardial biopsy.67 LGE was present in 88% with typical features consisting of patchy involvement with epicardial predominance and frequent localization to the lateral free wall. At 3 month follow-up, the amount of enhanced tissue had declined in all patients who initially had LGE and completely resolved in 15%. Histopathologic analysis of the biopsy specimens obtained from LGE-positive regions showed active myocarditis in 90%. To address sampling issues with endomyocardial biopsy, an experimental model of acute myocarditis was subsequently studied. It showed close correlation between CMR-LGE and both histologic severity of myocarditis (r=0.96, p<0.05) and topographic distribution of histologic inflammation.
A follow-up CMR study evaluated 128 myocarditis patients with documented parvovirus B19 (PVB19) and/or herpesvirus 6 (HHV6) myocardial infections.68 Among the 87 patients with biopsy-proven active myocarditis, 95% had LGE and the LGE pattern appeared to be related to viral type: lateral wall involvement correlated with PVB19 infection and anteroseptal mid-wall involvement with HHV6. At average follow-up of 138 days, repeat CMR showed persistent LGE in 73%, most of whom were infected with PVB19. At initial CMR, 15 patients had evidence of healing myocarditis on biopsy with LGE in 40%. Follow-up CMR was performed in 4 healing myocarditis patients, none of whom had LGE on the second scan.
Another CMR study of 405 suspected myocarditis patients showed that LGE absence was associated with no major adverse cardiac events (cardiac death, SCD, ICD discharge, aborted SCD) after 1591 days compared to a 5.6% event rate in those with abnormal CMR findings (reduced LVEF, abnormal LV volume, or LGE presence).69 LGE prevalence was 28% and typically localized to the subepicardium or midwall regions. Subsequently, a study of chronic dilated NICM demonstrated that midwall fibrosis correlates with increased frequency of a secondary composite arrhythmic endpoint consisting of SCD or aborted SCD.40 These patients may represent those in whom chronic myocarditis with midwall involvement progressed to a dilated NICM and forms the basis for increased arrhythmic propensity.
Future directions
There remains a paucity of randomized control trial (RCT) data using CMR-LGE to guide decision-making for SCD prevention. However, there is ever-increasing need to improve our SCD risk stratification approach, as evidenced by the recently reported Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Filure on Mortality (DANISH trial).70 DANISH randomized 556 symptomatic HF patients with LVEF below 36% to usual care or ICD with primary outcome of all-cause death. After 67.6 months, there was no statistical difference in mortality among those with and without an ICD. SCD rates were relatively low overall consistent with improved outcome with comprehensive HF medical therapy and cardiac resynchronization therapy (CRT). These results further underscore the need for a more targeted approach to identifying the arrhythmogenic substrate and arrhythmically vulnerable patient. Consideration should be given to incorporating CMR-LGE into SCD prevention clinical guidelines, as has been done in HCM, based on the copious existing literature.
Ongoing studies continue to evaluate the utility of CMR-LGE for SCD risk prediction and recognize the need to incorporate multiple risk factors including CMR-LGE in order to significantly impact SCD prognostication. It has been suggested that improved diagnostic accuracy for any new SCD risk algorithm requires a clinically significant AUC level approaching 0.90.71 No single risk factor to date has that discriminant power. For LVEF, AUC is 0.62. However, combining parameters, particularly those that are complementary (such as CMR-determined substrate and assessment of electrophysiologic triggers) may achieve the requisite high levels of diagnostic accuracy. Because many pathologies are associated with a high prevalence of scar involvement, it is likely that increased scar extent above a certain threshold (rather than binary presence/absence) will be a better-performing risk factor. Identification of clinically meaningful cut-offs, particularly those that are disease-specific, will require RCTs. Several RCT and prospective observational studies are underway that will add to the current evidence base (Table 2).72–74
Table 2.
In-progress Studies of CMR for SCD Risk Stratification
| Study title | Number of patients and sites | Patient cohort | Study Design | CMR metrics | Follow-up |
|---|---|---|---|---|---|
| HCMR72 |
|
|
|
|
5 years for composite endpoint:
|
| PROTECT-ICD Trial73 |
|
|
|
|
2 years for combined endpoint of
|
| CMR-GUIDE74 |
|
|
|
|
3 years for composite of
|
| PROSe-ICD Extension www.clinicaltrials.gov, NCT00733590 |
|
|
|
|
5 years for appropriate ICD firing or SCD |
HCMR = Hypertrophic Cardiomyopathy Registry
PROTECT-ICD = Programmed Ventricular Stimulation to Risk Stratify for Early Cardioverter-Defibrillator Implantation to Prevent Tachyarrhythmias Following Acute Myocardial Infarction
LV = left ventricle
RV = right ventricle
RCT = randomized control trial
ECV = extracellular volume
CMR-GUIDE = Cardiovascular Magnetic Resonance GUIDEd management of mild-moderate LV systolic dysfunction
ILR = implantable loop recorder
PROSe-ICD = Prospective Observational Study of the ICD in Sudden Cardiac Death Prevention
Early acute MI
Observational studies suggest that the demonstration of inducible, sustained VT on invasive electrophysiology study (EPS) beyond 4 days after acute MI in patients with reduced LVEF (below 40%) predicts arrhythmic risk.52 As such, current ICD guidelines suggest prophylactic ICD implantation may be appropriate under these conditions.3 However, a lack of proven efficacy of such a strategy and concern with the negative predictive value of EPS have contributed to a decline in such an approach. A multi-center trial is underway to determine the efficacy of a combined EPS and CMR-guided strategy for early acute MI SCD risk stratification, the Programmed Ventricular Stimulation to Risk Stratify for Early Cardioverter-Defibrillator Implantation to Prevent Tachyarrhythmias Following Acute Myocardial Infarction (PROTECT-ICD) trial.73 Enrollment targets patients with ST-elevation or non-ST-elevation MI and LVEF below 41% at a minimum of day 3 post-MI. Patients are randomized to EPS-directed ICD implantation versus usual care and followed for 2 years. A cohort of 1058 patients (529 per arm) is planned with 400 patients undergoing CMR-LGE. Endpoints include whether tissue heterogeneity or scar size by CMR predict inducible VT at invasive EPS as well as SCD or ventricular tachyarrhythmia at follow-up.
ICM
In addition to substrate assessment in chronic ICM, evidence supports the predictive value of determining electrical irritability to identify candidates for primary prevention ICDs. The randomized controlled Multicenter Unsustained Tachycardia Trial (MUSTT)75 showed a significant reduction in the number needed to treat when results of programmed stimulation during invasive EPS were incorporated into decision-making. Inducible ventricular arrhythmia during invasive EPS remains a class I indication for ICD placement in patients with prior MI, LVEF below 41% and non-sustained VT.3 A novel proof-of-concept study recently demonstrated the potential for risk prediction based on patient-specific, CMR-derived 3-dimensional virtual heart and computational electrophysiologic modeling.76 The heart models can be noninvasively subjected to a rigorous electrophysiologic stimulation protocol to determine VT inducibility. This virtual arrhythmic risk predictor strongly predicted SCD outcomes in a small retrospective cohort of 41 patients (HR 4.05, p=0.03, which exceeded other single risk predictors). Further validation is needed as well as application to NICM patients in whom inducibility is also a potentially strong risk predictor.77
ICM and NICM with mild-moderate LV dysfunction
A multi-centre RCT is underway in Australia and Europe targeting ICM and NICM patients with LVEF between 36% and 50% who are not currently targeted for prophylactic ICDs but comprise the majority of SCDs. Cardiovascular Magnetic Resonance GUIDEd management of mild-moderate LV systolic dysfunction (CMR GUIDE)74 will test the hypothesis that a strategy of CMR-guided ICD placement reduces SCD or ventricular arrhythmia during 3 year follow-up. Patients with scar/fibrosis (n=428) will be randomized to receive ICD or an implantable loop recorder. Those without scar/fibrosis (n=521) will be followed in a registry. Results are expected in December 2020.
HCM
A multinational prospective observational study is underway in 2750 HCM patients to assess prognostic predictors of 5 year cardiovascular outcomes.72 All patients undergo CMR imaging for analysis of LV volumes, mass, hypertrophy distribution, LGE and T1 mapping pre and post-contrast. In addition to clinical data, other measured biomarkers include genomic DNA analysis and serum markers of collagen metabolism, myocardial injury, and hemodynamic stress. The primary endpoint is the composite of cardiac death (SCD and HF death), aborted SCD (with or without an ICD) and need for heart transplantation.
Assessment of LV remodeling
Although CMR accurately and reproducibly quantifies LV dimensions, CMR volumes and masses are not strong independent discriminants of SCD outcomes, particularly when compared to LGE scar. However, the ability to quantify LV shape indices by CMR that may better identify phenotypically high risk individuals, particularly in ICM, is promising.78 A recent proof-of-concept study reported differences in regional LV curvature by CMR that were associated with increased SCD risk despite similar global LV volumes and masses.79
CMR further has the potential to predict and track the temporal course of cardiomyopathy. Both positive and negative LV remodeling, due either to the natural history of the myopathic process or resulting from therapeutic interventions, may significantly impact SCD risk. Baseline CMR scar extent prior to ICD insertion was recently shown to predict subsequent LVEF trajectory with a trend toward fewer arrhythmic outcomes in those with improved LVEF.5 The advent of protocols to safely image patients with indwelling ICDs and CRT devices and reduce device-related artifacts80 provides additional opportunities. Scar imaging appears to predict less effective cardiac resynchronization and increased SCD risk when the LV lead is positioned over regions of scar.81 CMR can potentially be used to further monitor response to CRT as defined by improvement in dyssynchrony and LVEF and reduction in chamber sizes and mitral regurgitation, all of which may reduce subsequent SCD risk. CMR can also be potentially used to investigate suboptimal lead positioning post-implantation in non-responders and explore mechanisms to better understand the phenomenon of CRT-induced proarrhythmia.82 An observational study is underway to examine the association between temporal changes in scar extent and characteristics as well as LV chamber remodeling and subsequent arrhythmic risk in ICD and CRT-D recipients undergoing their first ICD generator change. Repeat CMR-LGE will be performed in patients originally enrolled in the Prospective Observational Study of the ICD in Sudden Cardiac Death Prevention who have not yet had an appropriate ICD shock (PROSe-ICD, NCT00733590).
New CMR techniques for SCD risk assessment
T1 mapping
The CMR-LGE technique relies on detecting relative differences in SI and requires a region of normal remote myocardium. Hence, while highly accurate and reproducible for identifying focal regions of scar or fibrosis, it is insensitive to diffuse fibrosis, which is common in NICM. The T1 mapping technique, both native, non-contrast and after contrast administration, measures extracellular matrix expansion and thus better detects diffuse fibrosis.83, 84 Small studies have reported correlation coefficients of 0.49–0.98 between histologic assessment of ECV fraction and that of T1 mapping for quantifying fibrosis associated with valvular heart disease and cardiomyopathy.84 A recent study of 131 ICM and NICM patients performed T1 mapping and CMR-LGE prior to ICD implantation with average follow-up of 425 days for appropriate ICD discharges or sustained ventricular arrhythmias.85 Both gray zone assessment and native T1 mapping independently predicted the outcome with respective net reclassification improvements of 62.5% and 43.9% in the primary prevention ICD cohort. Native T1 mapping has the advantage of lack of contrast requirement. Further studies are needed to establish standardized, reproducible protocols and normative values as well as compare the diagnostic accuracy of T1 mapping over and above existing LGE indices.
Edema imaging
Myocardial edema imaging combined with CMR-LGE may help differentiate myocardial necrosis from reversible inflammation in inflammatory cardiomyopathies. Existing techniques improve the diagnosis of myocarditis and sarcoidosis. Whether a combined imaging approach improves identification of SCD risk remains uncertain.
Conclusions and comments
Progress in SCD risk stratification remains impeded by a one-size-fits all approach with over-reliance on LVEF, lack of patient-specific personalization, and failure to incorporate pathophysiologically-driven risk factors.7 CMR is a powerful and ideal technique to address these limitations with the potential to transform the field. Current constraints to widespread CMR implementation include the lack of consensus regarding quantitative infarct and tissue heterogeneity standards as well as definitive outcome studies. It is thus perhaps timely to consider a prospective randomized controlled trial of purely CMR-guided decision-making for primary prevention ICD insertions across a wide range of LVEF values, including both ischemic and non-ischemic etiologies, to challenge the current concept of a low LVEF threshold.7 Such a trial would require long-term, adjudicated arrhythmic outcome assessment. In the interim, there would appear to be sufficient published data to support the incorporation of CMR metrics in SCD risk stratification in clinically indeterminate situations, similar to that done with HCM. This will require expert consensus for inclusion in clinical practice guidelines.
Supplementary Material
Footnotes
Disclosures
KCW was principal investigator on NIH/NHLBI grant R01HL103812 which studied CMR-LGE predictors of SCD. She is a Johns Hopkins in Health funded investigator.
References
- 1.Fishman GI, Chugh SS, DiMarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen P-S, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O’Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng Z-J. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stecker EC, Reinier K, Marijon E, Narayanan K, Teodorescu C, Uy-Evanado A, Gunson K, Jui J, Chugh SS. Public health burden of sudden cardiac death in the United States. Circ Arrhythm Electrophysiol. 2014;7:212–217. doi: 10.1161/CIRCEP.113.001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, Kapa S, Kremers MS, Lindsay BD, Stevenson LW. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 Appropriate Use Criteria for Implantable Cardioverter-Defibrillators and Cardiac Resynchronization Therapy: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013;61:1318–1368. doi: 10.1016/j.jacc.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Wellens HJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, Huikuri HV, Kaab S, La Rovere MT, Malik M, Myerburg RJ, Simoons ML, Swedberg K, Tijssen J, Voors AA, Wilde AA. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J. 2014;35:1642–1651. doi: 10.1093/eurheartj/ehu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Guallar E, Weiss RG, Stillabower M, Gerstenblith G, Tomaselli GF, Wu KC. Associations between scar characteristics by cardiac magnetic resonance and changes in left ventricular ejection fraction in primary prevention defibrillator recipients. Heart Rhythm. 2016;13:1661–1666. doi: 10.1016/j.hrthm.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcox JE, Fonarow GC, Ardehali H, Bonow RO, Butler J, Sauer AJ, Epstein SE, Khan SS, Kim RJ, Sabbah HN, Diez J, Gheorghiade M. “Targeting the Heart” in Heart Failure: Myocardial Recovery in Heart Failure With Reduced Ejection Fraction. JACC. Heart failure. 2015;3:661–669. doi: 10.1016/j.jchf.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Wu KC, Calkins H. Powerlessness of a Number: Why Left Ventricular Ejection Fraction Matters Less for Sudden Cardiac Death Risk Assessment. Circ Cardiovasc Imaging. 2016;9:e005519. doi: 10.1161/CIRCIMAGING.116.005519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rijnierse MT, van der Lingen AL, Weiland MT, de Haan S, Nijveldt R, Beek AM, van Rossum AC, Allaart CP. Clinical Impact of Cardiac Magnetic Resonance Imaging Versus Echocardiography-Guided Patient Selection for Primary Prevention Implantable Cardioverter Defibrillator Therapy. Am J Cardiol. 2015;116:406–412. doi: 10.1016/j.amjcard.2015.04.059. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European heart journal cardiovascular Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 10.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unverferth DV, Baker PB, Swift SE, Chaffee R, Fetters JK, Uretsky BF, Thompson ME, Leier CV. Extent of myocardial fibrosis and cellular hypertrophy in dilated cardiomyopathy. Am J Cardiol. 1986;57:816–820. doi: 10.1016/0002-9149(86)90620-x. [DOI] [PubMed] [Google Scholar]
- 12.Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol. 1987;60:1340–1355. doi: 10.1016/0002-9149(87)90618-7. [DOI] [PubMed] [Google Scholar]
- 13.Aliot EM, Stevenson WG, Almendral-Garrote JM, Bogun F, Calkins CH, Delacretaz E, Della Bella P, Hindricks G, Jaïs P, Josephson ME, Kautzner J, Kay GN, Kuck K-H, Lerman BB, Marchlinski F, Reddy V, Schalij M-J, Schilling R, Soejima K, Wilber D. EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart Rhythm. 2009;6:886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Anter E, Josephson ME. Ischemic heart disease. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: from cell to bedside. Philadelphia, PA: Saunders; 2014. pp. 849–857. [Google Scholar]
- 15.de Bakker JM, Stein M, van Rijen HV. Three-dimensional anatomic structure as substrate for ventricular tachycardia/ventricular fibrillation. Heart Rhythm. 2005;2:777–779. doi: 10.1016/j.hrthm.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Dickfeld T. Pursuing the “Holy Grail”. Circ Cardiovasc Imaging. 2012;5:167–170. doi: 10.1161/CIRCIMAGING.112.972935. [DOI] [PubMed] [Google Scholar]
- 17.Peters NS, Wit AL. Myocardial architecture and ventricular arrhythmogenesis. Circulation. 1998;97:1746–1754. doi: 10.1161/01.cir.97.17.1746. [DOI] [PubMed] [Google Scholar]
- 18.Dillon SM, Allessie MA, Ursell PC, Wit AL. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ Res. 1988;63:182–206. doi: 10.1161/01.res.63.1.182. [DOI] [PubMed] [Google Scholar]
- 19.Wit AL, Dillon SM, Coromilas J, Saltman AE, Waldecker B. Anisotropic reentry in the epicardial border zone of myocardial infarcts. Ann N Y Acad Sci. 1990;591:86–108. doi: 10.1111/j.1749-6632.1990.tb15083.x. [DOI] [PubMed] [Google Scholar]
- 20.Betensky BP, Dixit S. Sudden cardiac death in patients with nonischemic cardiomyopathy. Indian heart journal. 2014;66(Suppl 1):S35–45. doi: 10.1016/j.ihj.2013.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danciu M. Atlas of Pathology. Gr. T. Popa University of Medicine and Pharmacy; Iasi, Romania: 2010. Ischemic fibrosis of myocardium. Retrieved 02/11/2017 from http://www.pathologyatlas.ro/cardiovascular-pathology.php. [Google Scholar]
- 22.Ajijola OA, Tung R, Shivkumar K. Ventricular tachycardia in ischemic heart disease substrates. Indian heart journal. 2014;66(Suppl 1):S24–34. doi: 10.1016/j.ihj.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Athavale P, Pop M, Wright GA. Multicontrast reconstruction using compressed sensing with low rank and spatially varying edge-preserving constraints for high-resolution MR characterization of myocardial infarction. Magn Reson Med. 2016 doi: 10.1002/mrm.26402. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, Nichols M, Ben-Yehuda O. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol. 2016;67:1674–1683. doi: 10.1016/j.jacc.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 26.Raymond JM, Sacher F, Winslow R, Tedrow U, Stevenson WG. Catheter ablation for scar-related ventricular tachycardias. Current problems in cardiology. 2009;34:225–270. doi: 10.1016/j.cpcardiol.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marban E, Tomaselli GF, Lima JA, Wu KC. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 30.Roes SD, Borleffs CJW, van der Geest RJ, Westenberg JJM, Marsan NA, Kaandorp TAM, Reiber JHC, Zeppenfeld K, Lamb HJ, de Roos A, Schalij MJ, Bax JJ. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circulation-Cardiovascular Imaging. 2009;2:183–190. doi: 10.1161/CIRCIMAGING.108.826529. [DOI] [PubMed] [Google Scholar]
- 31.Pop M, Ghugre NR, Ramanan V, Morikawa L, Stanisz G, Dick AJ, Wright GA. Quantification of fibrosis in infarcted swine hearts by ex vivo late gadolinium-enhancement and diffusion-weighted MRI methods. Physics in medicine and biology. 2013;58:5009–5028. doi: 10.1088/0031-9155/58/15/5009. [DOI] [PubMed] [Google Scholar]
- 32.Hwang SH, Choi EY, Park CH, Paek MY, Greiser A, Kim TH, Choi BW. Evaluation of extracellular volume fraction thresholds corresponding to myocardial late-gadolinium enhancement using cardiac magnetic resonance. Int J Cardiovasc Imaging. 2014;30(Suppl 2):137–144. doi: 10.1007/s10554-014-0489-6. [DOI] [PubMed] [Google Scholar]
- 33.Ashikaga H, Sasano T, Dong J, Zviman MM, Evers R, Hopenfeld B, Castro V, Helm RH, Dickfeld T, Nazarian S, Donahue JK, Berger RD, Calkins H, Abraham MR, Marban E, Lardo AC, McVeigh ER, Halperin HR. Magnetic resonance-based anatomical analysis of scar-related ventricular tachycardia: implications for catheter ablation. Circ Res. 2007;101:939–947. doi: 10.1161/CIRCRESAHA.107.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Estner HL, Zviman MM, Herzka D, Miller F, Castro V, Nazarian S, Ashikaga H, Dori Y, Berger RD, Calkins H, Lardo AC, Halperin HR. The critical isthmus sites of ischemic ventricular tachycardia are in zones of tissue heterogeneity, visualized by magnetic resonance imaging. Heart Rhythm. 2011;8:1942–1949. doi: 10.1016/j.hrthm.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Piers SR, Tao Q, de Riva Silva M, Siebelink HM, Schalij MJ, van der Geest RJ, Zeppenfeld K. CMR-based identification of critical isthmus sites of ischemic and nonischemic ventricular tachycardia. JACC Cardiovasc Imaging. 2014;7:774–784. doi: 10.1016/j.jcmg.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Piers SR, Zeppenfeld K. Imaging-guided Ventricular Tachycardia Ablation. Arrhythmia & electrophysiology review. 2013;2:128–134. doi: 10.15420/aer.2013.2.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andreu D, Ortiz-Perez JT, Fernandez-Armenta J, Guiu E, Acosta J, Prat-Gonzalez S, De Caralt TM, Perea RJ, Garrido C, Mont L, Brugada J, Berruezo A. 3D delayed-enhanced magnetic resonance sequences improve conducting channel delineation prior to ventricular tachycardia ablation. Europace. 2015;17:938–945. doi: 10.1093/europace/euu310. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Armenta J, Berruezo A, Andreu D, Camara O, Silva E, Serra L, Barbarito V, Carotenutto L, Evertz R, Ortiz-Perez JT, De Caralt TM, Perea RJ, Sitges M, Mont L, Frangi A, Brugada J. Three-dimensional architecture of scar and conducting channels based on high resolution ce-CMR: insights for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2013;6:528–537. doi: 10.1161/CIRCEP.113.000264. [DOI] [PubMed] [Google Scholar]
- 39.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marban E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O’Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 41.Iles LM, Ellims AH, Llewellyn H, Hare JL, Kaye DM, McLean CA, Taylor AJ. Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. European heart journal cardiovascular Imaging. 2015;16:14–22. doi: 10.1093/ehjci/jeu182. [DOI] [PubMed] [Google Scholar]
- 42.Halliday B, Gulati A, Ali A, Guha K, Newsome SJ, Arzanauskaite M, Vassiliou VS, Lota AS, Izgi C, Tayal U, Khalique Z, Stirrat C, Auger D, Pareek N, Ismail TF, Rosen SD, Vazir A, Alpendurada F, Gregson J, Frenneaux MP, Cowie MR, Cleland JG, Cook SA, Pennell DJ, Prasad SK. Association Between Mid-Wall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients with Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.026910. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogun FM, Desjardins B, Good E, Gupta S, Crawford T, Oral H, Ebinger M, Pelosi F, Chugh A, Jongnarangsin K, Morady F. Delayed-Enhanced Magnetic Resonance Imaging in Nonischemic Cardiomyopathy: Utility for Identifying the Ventricular Arrhythmia Substrate. Journal of the American College of Cardiology. 2009;53:1138–1145. doi: 10.1016/j.jacc.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreu D, Ortiz-Perez JT, Boussy T, Fernandez-Armenta J, de Caralt TM, Perea RJ, Prat-Gonzalez S, Mont L, Brugada J, Berruezo A. Usefulness of contrast-enhanced cardiac magnetic resonance in identifying the ventricular arrhythmia substrate and the approach needed for ablation. Eur Heart J. 2014;35:1316–1326. doi: 10.1093/eurheartj/eht510. [DOI] [PubMed] [Google Scholar]
- 45.Bisbal F, Fernandez-Armenta J, Berruezo A, Mont L, Brugada J. Use of MRI to guide electrophysiology procedures. Heart. 2014;100:1975–1984. doi: 10.1136/heartjnl-2013-304692. [DOI] [PubMed] [Google Scholar]
- 46.Scott PA, Rosengarten JA, Curzen NP, Morgan JM. Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: a meta-analysis. Eur J Heart Fail. 2013;15:1019–1027. doi: 10.1093/eurjhf/hft053. [DOI] [PubMed] [Google Scholar]
- 47.Disertori M, Rigoni M, Pace N, Casolo G, Mase M, Gonzini L, Lucci D, Nollo G, Ravelli F. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction: A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9:1046–1055. doi: 10.1016/j.jcmg.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 48.Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014;7:250–258. doi: 10.1161/CIRCIMAGING.113.001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Marco A, Anguera I, Schmitt M, Klem I, Neilan T, White JA, Sramko M, Masci PG, Barison A, McKenna P, Mordi I, Haugaa KH, Leyva F, Rodriguez Capitan J, Satoh H, Nabeta T, Dallaglio PD, Campbell NG, Sabate X, Cequier A. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC. Heart failure. 2017;5:28–38. doi: 10.1016/j.jchf.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Bilchick KC. The Fault Is in Our Scars: LGE and Ventricular Arrhythmia Risk in LV Dysfunction. JACC Cardiovasc Imaging. 2016;9:1056–1058. doi: 10.1016/j.jcmg.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 51.Kusumoto FM, Calkins H, Boehmer J, Buxton AE, Chung MK, Gold MR, Hohnloser SH, Indik J, Lee R, Mehra MR, Menon V, Page RL, Shen WK, Slotwiner DJ, Stevenson LW, Varosy PD, Welikovitch L. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94–125. doi: 10.1161/CIR.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 52.Zaman S, Kovoor P. Sudden cardiac death early after myocardial infarction: pathogenesis, risk stratification, and primary prevention. Circulation. 2014;129:2426–2435. doi: 10.1161/CIRCULATIONAHA.113.007497. [DOI] [PubMed] [Google Scholar]
- 53.Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L, White H, Van de Werf F, Pieper K, Califf RM, Pfeffer MA Valsartan in Acute Myocardial Infarction Trial I. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 54.Robbers LF, Delewi R, Nijveldt R, Hirsch A, Beek AM, Kemme MJ, van Beurden Y, van der Laan AM, van der Vleuten PA, Tio RA, Zijlstra F, Piek JJ, van Rossum AC. Myocardial infarct heterogeneity assessment by late gadolinium enhancement cardiovascular magnetic resonance imaging shows predictive value for ventricular arrhythmia development after acute myocardial infarction. European heart journal cardiovascular Imaging. 2013;14:1150–1158. doi: 10.1093/ehjci/jet111. [DOI] [PubMed] [Google Scholar]
- 55.Izquierdo M, Ruiz-Granell R, Bonanad C, Chaustre F, Gomez C, Ferrero A, Lopez-Lereu P, Monmeneu JV, Nunez J, Chorro FJ, Bodi V. Value of early cardiovascular magnetic resonance for the prediction of adverse arrhythmic cardiac events after a first noncomplicated ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. 2013;6:755–761. doi: 10.1161/CIRCIMAGING.113.000702. [DOI] [PubMed] [Google Scholar]
- 56.Roifman I, Ghugre NR, Vira T, Zia MI, Zavodni A, Pop M, Connelly KA, Wright GA. Assessment of the longitudinal changes in infarct heterogeneity post myocardial infarction. BMC cardiovascular disorders. 2016;16:198. doi: 10.1186/s12872-016-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, He Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9:1392–1402. doi: 10.1016/j.jcmg.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 58.Maron MS. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2012;14:13. doi: 10.1186/1532-429X-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW American College of Cardiology Foundation/American Heart Association Task Force on Practice G, American Association for Thoracic S, American Society of E, American Society of Nuclear C, Heart Failure Society of A, Heart Rhythm S, Society for Cardiovascular A Interventions Society of Thoracic S. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–831. doi: 10.1161/CIR.0b013e318223e2bd. [DOI] [PubMed] [Google Scholar]
- 60.Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370–377. doi: 10.1016/j.jcmg.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 61.Maron BJ, Maron MS. LGE Means Better Selection of HCM Patients for Primary Prevention Implantable Defibrillators. JACC Cardiovasc Imaging. 2016;9:1403–1406. doi: 10.1016/j.jcmg.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 62.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 63.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hulten E, Agarwal V, Cahill M, Cole G, Vita T, Parrish S, Bittencourt MS, Murthy VL, Kwong R, Di Carli MF, Blankstein R. Presence of Late Gadolinium Enhancement by Cardiac Magnetic Resonance Among Patients With Suspected Cardiac Sarcoidosis Is Associated With Adverse Cardiovascular Prognosis: A Systematic Review and Meta-Analysis. Circ Cardiovasc Imaging. 2016;9:e005001. doi: 10.1161/CIRCIMAGING.116.005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coleman GC, Shaw PW, Balfour PC, Jr, Gonzalez JA, Kramer CM, Patel AR, Salerno M. Prognostic Value of Myocardial Scarring on CMR in Patients With Cardiac Sarcoidosis. JACC Cardiovasc Imaging. 2017;10:411–420. doi: 10.1016/j.jcmg.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baksi AJ, Kanaganayagam GS, Prasad SK. Arrhythmias in viral myocarditis and pericarditis. Cardiac electrophysiology clinics. 2015;7:269–281. doi: 10.1016/j.ccep.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 67.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 68.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 69.Schumm J, Greulich S, Wagner A, Grun S, Ong P, Bentz K, Klingel K, Kandolf R, Bruder O, Schneider S, Sechtem U, Mahrholdt H. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson. 2014;16:14. doi: 10.1186/1532-429X-16-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp-Pedersen C, Pehrson S, Investigators D. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 71.Goldberger JJ, Buxton AE, Cain M, Costantini O, Exner DV, Knight BP, Lloyd-Jones D, Kadish AH, Lee B, Moss A, Myerburg R, Olgin J, Passman R, Rosenbaum D, Stevenson W, Zareba W, Zipes DP. Risk stratification for arrhythmic sudden cardiac death: identifying the roadblocks. Circulation. 2011;123:2423–2430. doi: 10.1161/CIRCULATIONAHA.110.959734. [DOI] [PubMed] [Google Scholar]
- 72.Kramer CM, Appelbaum E, Desai MY, Desvigne-Nickens P, DiMarco JP, Friedrich MG, Geller N, Heckler S, Ho CY, Jerosch-Herold M, Ivey EA, Keleti J, Kim DY, Kolm P, Kwong RY, Maron MS, Schulz-Menger J, Piechnik S, Watkins H, Weintraub WS, Wu P, Neubauer S. Hypertrophic Cardiomyopathy Registry: The rationale and design of an international, observational study of hypertrophic cardiomyopathy. Am Heart J. 2015;170:223–230. doi: 10.1016/j.ahj.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaman S, Taylor AJ, Stiles M, Chow C, Kovoor P. Programmed Ventricular Stimulation to Risk Stratify for Early Cardioverter-Defibrillator Implantation to Prevent Tachyarrhythmias following Acute Myocardial Infarction (PROTECT-ICD): Trial Protocol, Background and Significance. Heart Lung Circ. 2016;25:1055–1062. doi: 10.1016/j.hlc.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Selvanayagam JB, Hartshorne T, Billot L, Grover S, Hillis GS, Jung W, Krum H, Prasad S, McGavigan AD. Cardiovascular magnetic resonance-GUIDEd management of mild to moderate left ventricular systolic dysfunction (CMR GUIDE): Study protocol for a randomized controlled trial. Ann Noninvasive Electrocardiol. 2017 doi: 10.1111/anec.12420. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Josephson ME. Programmed stimulation for risk stratification for postinfarction sudden cardiac arrest: why and how? Pacing Clin Electrophysiol. 2014;37:791–794. doi: 10.1111/pace.12412. [DOI] [PubMed] [Google Scholar]
- 76.Arevalo HJ, Vadakkumpadan F, Guallar E, Jebb A, Malamas P, Wu KC, Trayanova NA. Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nature communications. 2016;7:11437. doi: 10.1038/ncomms11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldberger JJ, Subacius H, Patel T, Cunnane R, Kadish AH. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2014;63:1879–1889. doi: 10.1016/j.jacc.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X, Cowan BR, Bluemke DA, Finn JP, Fonseca CG, Kadish AH, Lee DC, Lima JA, Suinesiaputra A, Young AA, Medrano-Gracia P. Atlas-based quantification of cardiac remodeling due to myocardial infarction. PLoS One. 2014;9:e110243. doi: 10.1371/journal.pone.0110243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vadakkumpadan F, Trayanova N, Wu KC. Image-based left ventricular shape analysis for sudden cardiac death risk stratification. Heart Rhythm. 2014;11:1693–1700. doi: 10.1016/j.hrthm.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rashid S, Rapacchi S, Shivkumar K, Plotnik A, Finn JP, Hu P. Modified wideband three-dimensional late gadolinium enhancement MRI for patients with implantable cardiac devices. Magn Reson Med. 2016;75:572–584. doi: 10.1002/mrm.25601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leyva F. The Role of Cardiovascular Magnetic Resonance in Cardiac Resynchronization Therapy. Heart Fail Clin. 2017;13:63–77. doi: 10.1016/j.hfc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Bradfield JS, Shivkumar K. Cardiac resynchronization therapy-induced proarrhythmia: understanding preferential conduction within myocardial scars. Circ Arrhythm Electrophysiol. 2014;7:1000–1002. doi: 10.1161/CIRCEP.114.002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schelbert EB, Messroghli DR. State of the Art: Clinical Applications of Cardiac T1 Mapping. Radiology. 2016;278:658–676. doi: 10.1148/radiol.2016141802. [DOI] [PubMed] [Google Scholar]
- 84.Kammerlander AA, Marzluf BA, Zotter-Tufaro C, Aschauer S, Duca F, Bachmann A, Knechtelsdorfer K, Wiesinger M, Pfaffenberger S, Greiser A, Lang IM, Bonderman D, Mascherbauer J. T1 Mapping by CMR Imaging: From Histological Validation to Clinical Implication. JACC Cardiovasc Imaging. 2016;9:14–23. doi: 10.1016/j.jcmg.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 85.Chen Z, Sohal M, Voigt T, Sammut E, Tobon-Gomez C, Child N, Jackson T, Shetty A, Bostock J, Cooklin M, O’Neill M, Wright M, Murgatroyd F, Gill J, Carr-White G, Chiribiri A, Schaeffter T, Razavi R, Rinaldi CA. Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm. 2015;12:792–801. doi: 10.1016/j.hrthm.2014.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.