Abstract
Maternal control of inflammation is essential during pregnancy and an exaggerated response is one of the underlying causes of fetal loss. Inflammatory response is mediated by multiple factors and Toll-like receptors (TLRs) are central. Activation of TLRs results in NALP-3 mediated assembly of apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1 into the inflammasome and production of pro-inflammatory cytokines IL-1β and IL-18. Given that preventing measures are lacking, we investigated PreImplantation Factor (PIF) as therapeutic option as PIF modulates Inflammation in pregnancy. Additionally, synthetic PIF (PIF analog) protects against multiple immune disorders. We used a LPS induced murine model of fetal loss and synthetic PIF reduced this fetal loss and increased the embryo weight significantly. We detected increased PIF expression in the placentae after LPS insult. The LPS induced serum and placenta cytokines were abolished by synthetic PIF treatment and importantly synthetic PIF modulated key members of inflammasome complex NALP-3, ASC, and caspase-1 as well. In conclusion our results indicate that synthetic PIF protects against LPS induced fetal loss, likely through modulation of inflammatory response especially the inflammasome complex. Given that synthetic PIF is currently tested in autoimmune diseases of non-pregnant subjects (clinicaltrials.gov, NCT02239562), therapeutic approach during pregnancy can be envisioned.
Introduction
During pregnancy, allogeneic fetal cells invade the maternal decidua and are protected from the maternal immune system. Controlling the maternal response to inflammation is essential as an exaggerated response is one of the underlying causes of early and even later fetal loss [1]. Fetal loss is associated with multiple causes such as anatomic, genetic, and hematologic disorders but immune defects emerged as central players recently. Not surprisingly, proper immune adaptations play a key role in prevention of pregnancy disorders including preeclampsia, fetal growth restriction, and premature birth [2].
One of the essential players of an immune system during pregnancy is a set of pathogen recognition receptors: toll-like receptors (TLRs) in a trophoblast. Once TLRs are activated by pathogen associated molecular patterns (PAMPs), robust activation of NFκ-B and MAP kinase signaling pathways induce up-regulation of associated genes. These genes are mostly pro-inflammatory and NALP–inflammasome is central [3, 4]. To date four main types of inflammasomes have been described including the NALP-3 subtype. Upon activation of NALP-3, apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1 are assembled into the inflammasome [4, 5]. This multi-protein complex enables the caspase-1-mediated proteolytic processing of the pro-inflammatory cytokines IL-1β, IL-18 and IL-33, thus generating their respective mature secreted forms. All of these events are necessary during inflammatory response [5].
The lipopolysaccharide (LPS) induced inflammation is a well-documented and frequently used model to study induced fetal loss [6, 7]. The bacterial antigen LPS is a PAMP in the extracellular milieu. It induces macrophage-derived TNF-α production which in turn activates NK cells and IFN-γ secretion resulting in a positive feed-back [8]. This pathway leads to activation of the uterine and placental endothelium and the release of embryotoxic cytokines [9, 10]. We hypothesize that counteracting this adverse environment through targeted therapy would constitute important progress in pregnancy management and fetal loss prevention. A potent candidate in fetal loss prevention is PreImplantation Factor (PIF) as PIF regulates the inflammatory response during pregnancy [11]. PIF is a 15-amino acid peptide secreted by the embryo and is associated with favorable pregnancy outcome [12–15]. PIF`s essential role during pregnancy is supported by the ability to promote embryo development, endometrial receptivity, and trophoblast invasion [16–21]. Interestingly, PIF interaction with maternal immune system goes beyond binding to CD14+ T regulatory cells as it targets the adaptive arm of immunity (CD3+ cells) as well [22]. PIF`s mode of action is mediated in part by reducing oxidative stress and protein misfolding, thereby protecting against embryo toxicity [20, 21]. In addition, PIF reduces natural killer cells toxicity by down-regulating the pro-inflammatory CD69 expression [23]. Together, PIF is a unique and pregnancy essential peptide which possesses immune-modulatory and not immune-suppressive properties. Not surprisingly, synthetic PIF (PIF analog) protects against multiple immune disorders [24–30] and received a FAST-Track FDA approval for clinical trial in autoimmune diseases of non-pregnant subjects (clinicaltrials.gov, NCT02239562).
Whether synthetic PIF administration could be effective in improving pregnancy-induced pathologies in vivo is not yet established. In view of its translational potential, we used an intact immune murine model to study the mechanisms of LPS-induced fetal loss and examined synthetic PIF’s potential as a treatment.
Materials and methods
Synthetic PIF15 (MVRIKPGSANKPSDD) was synthesized by solid-phase peptide synthesis (Peptide Synthesizer, Applied Biosystems) employing Fmoc (9-fluorenylmethoxycarbonyl) chemistry at Bio-Synthesis, Inc. (Lewisville, TX, USA). Final purification was carried out by reversed-phase HPLC and identity was verified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and amino acid analysis at >95% purity. Anti-PIF monoclonal antibody against MVRIKPGSANKPSDD was generated in (Genway, SanDiego, CA, USA).
Animals and treatments
Female Swiss mice (7–8 weeks old) were supplied from Centro Ricerche Sperimentali (CENRIS), Università Cattolica del S. Cuore, Roma and were paired with adult Swiss mice; the day of appearance of post-coitum vaginal plug was considered as day 0 of gestation. Animals were housed in accordance with Ethics Committee and Veterinary Department guidelines. Acclimatization of animals to the laboratory environment was allowed prior to surgery. Aseptic rodent survival surgery guidelines were followed. Animals received food and water ad libitum and were housed under controlled conditions of light (12h light/12h dark) and temperature (23–25°C). In preliminary experiments we tested several doses of LPS (from 0.01 μg/g to 1.0 μg/g; from Escherichia Coli serotype 0111:B4; Sigma-Aldrich, St Louis MA, USA) to delineate its ability to induce fetal demise. In our model, we choose the LPS concentration that was able to induce ~ 80% of fetal loss (0.1μg/g).
The experimental protocol included 4 groups (n = 18 pregnant animals each group; Fig 1A). Two groups of pregnant mice were treated with synthetic PIF (1μg/g mouse /day) or with phosphate buffered solution, PBS (200 μl, control group) using micro-osmotic pumps from day 0 until day 15 of gestation. Briefly, mice were anesthetized via intraperitoneal injection of Ketamine and Xylazine (ketamine 80–100 mg/kg, xylazine 10–12.5 mg/kg). Once the animal has lost its righting reflex we proceed with surgical preparation of implantation site, pumps were implanted subcutaneously on the back of mice by making a small cut in the mid-scapulary region and incision was closed with wound clip. After recovery from anesthesia, mice were monitored for several signs including bleeding, discomfort, or pain. If needed, to alleviate postoperative pain, local anesthesia was used successfully (lidocaine, 4 mg/kg, 0.4 mL/kg of a 1% solution). Notably, the dosage of synthetic PIF was used in multiple animal studies previously [24, 25, 27, 28, 30]. Additionally, on day 7 of gestation, each of these two groups was injected, intra-peritoneum, with LPS (0.1 μg/g mouse/200 μl PBS) or PBS (200 μl). Thus, following 4 groups were investigated (Control, synthetic PIF, LPS, and LPS+synthetic PIF). All mice were sacrificed on day 15 of pregnancy, the uteri were dissected and placentae were harvested. Briefly, mice were anesthetized via intraperitoneal injection of Ketamine and Xylazine (ketamine 80–100 mg/kg, xylazine 10–12.5 mg/kg) and sacrificed by cervical dislocation. The number of viable and resorbed embryos was recorded. The fetal loss rate was calculated as follows: Loss rate = (number of demised fetuses/number of total fetuses) x 100. The weight of viable fetuses and placentae were recorded and placental/fetal weight ratio calculated. The placentae were further used for western blot, immunohistochemistry, and cytokine analysis. The blood samples were centrifuged and the serum was stored at -80°C.
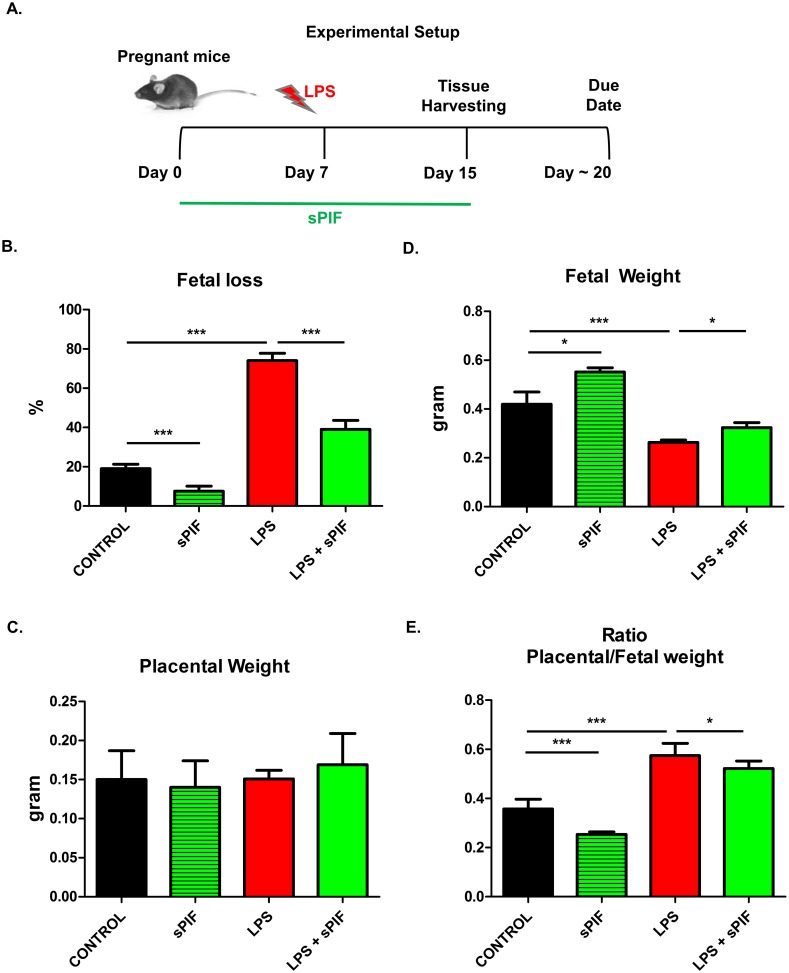
Fig 1. Experimental setup and fetal outcomes after LPS induced insult and synthetic PIF treatment.
(A) Experimental setup: We used 4 experimental groups (n = 18 pregnant animals each group). Control group received PBS (200ul/day from postnatal day 0 until day 15 (P0-15) and 200ul PBS on P7. Synthetic PIF group received synthetic PIF (1ug/g mouse/day) from P0-15 and PBS on P7. LPS group received PBS from P0-15 and LPS (0.1ug/g mouse) on P7. LPS+sPIF group received synthetic PIF and LPS (as above). Fetal outcomes are presented in (B) fetal loss, (C) placental weight, and (D) fetal weight, and (E) Ratio placental/fetal weight. *p<0.05, **p<0.01, and ***p<0.001. sPIF: synthetic PreImplantation Factor; LPS: Lipopolysaccharides. Data are mean ± SD.
All procedures followed the requirements of Commission Directive 86/609/EEC concerning the protection of animals used for experimental and other scientific purposes. All the experimental procedures were approved by the local ethical committee on preclinical studies [n° 5647/14 (A13 D)] Universita`Cattolica del Sacro Cuore Roma, Italy.
Placental PIF immunofluorescence
Endogenous PIF was detected in placental tissue using immunofluorescence. Briefly, placental samples were fixed in formalin and processed through embedded paraffin for the histological evaluation. Paraffin sections (3 μm) were dewaxed in Histosol (Sigma Chemical Co; St Louis, MO) and rehydrated through descending grade of alcohol (95–70%) to distilled water (dH2O). PIF was detected with a Biotin conjugated antibody against PIF, (Biosynthesis Code: AB1473-1) dil. 1:100 and a secondary reagent Alexa Fluor® 633 streptavidin (biotin-binding protein) (Thermo Fisher Scientific Code: S-21375, Rockford IL. USA) dil. 1: 200 and counterstained using hematoxylin and eosin (H&E). Finally, samples were mounted with EMS Shield Mount (Electron Microscopy Sciences Code: 17985–150). All images were obtained with a DM6000 B microscope (Leica Microsystems) at 20x magnification in a blinded fashion.
SDS–PAGE and immunoblotting
Placental tissues were collected (approximately 13 from each pregnant mouse), washed with PBS, minced, and lysed using 1% NP40 in the presence of protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA). Protein concentrations were calculated by the BCA assay (Pierce Biotechnology, Rockford, IL, USA). For Western blotting 18–20 placentae were taken randomly from each group and 50 μg of total lysates were separated by 10% SDS-PAGE electrophoresis under reducing conditions. After gel electrophoresis and transfer of proteins to a nitrocellulose membrane, nitrocellulose sheets were blocked at room temperature for 1 h in 5% non-fat dry milk, and incubated overnight at +4°C with a specific primary antibody (anti-NALP-3, or anti- apoptosis-associated speck-like protein containing a CARD (ASC), 1:200, ThermoFisher Scientific, Rockford, IL, USA). The membranes were washed with PBST and incubated in specific horseradish peroxidase-conjugated IgG diluted 1:2000 in 5% non-fat dried milk in PBST. Bound secondary antibody was detected by chemiluminescence. Bands were analyzed with the use of a Gel Doc 200 Image Analysis System and quantified with the use of Quantity One Quantitation Software (both from BioRad). The level of NALP-3 or ASC was estimated versus the constant level of a 42-kDa protein present in the cytosolic extract (β-actin), which was identified with the use of a mouse monoclonal anti-human β-actin antibody (Sigma-Aldrich, St Louis, MO, USA).
ELISA assay
Caspase-1 levels were measured in lysates obtained from placentae (18–20 placentae taken randomly from each group) by an enzyme-linked immunoassay (ELISA) according to manufacturer’s instructions (USCN Life Science Inc. and Cloud-Clone Corp. Houston, TX, USA). Briefly, samples or standard (100 μl) were added to each well coated with monoclonal anti-caspase-1 antibody. After 2h of incubation at 37°C, wells were washed and incubated with a specific enzyme-linked polyclonal antibody, horseradish peroxidase. Then, tetramethyl-benzidine substrate solution was added to each well, and the color developed in proportion to the amount of the proteins bound in the initial step. The plate was read on a Titertek Multiscan plus Mk II plate reader (ICN Flow Laboratories, Irvine, CA) measuring the absorbance at wavelengths of 450 nm.
Multiplex bead array analysis
Placental tissues (18–20 placentae taken randomly from each group) were washed with PBS, minced and lysed using 1% NP40 in the presence of protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA). The placental extract supernatant and serum inflammatory cytokines and chemokines (TNF-α, IFN-γ, IL-1b, IL-18, GM-CSF, GRO, Eotaxin, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL12p70, IL-13, IL17a, IL-22, Il-23, IL27, IP10, MCP1, MCP3, MIP1α, MIP2, MIP1β, RANTES) were analyzed using a Multiplex Bead Array System, Procarta® Immunoassay Kit (eBioscience). Briefly, 50 μl of sample or standard solution were added to 25 μl of the bead mixture in a well on the plate. Serum or placental lysates samples or recombinant standard were then allowed to bind to their respective primary antibodies on the spheres during the 2 hours of incubation. After washing, a mixture of biotinylated secondary antibodies was added to each well and allowed to bind to the captured analyte on the beads. After removal of excess antibodies, streptavidin-RPE (fluorochrome) was added and allowed to bind to the biotin on the secondary antibodies during the third incubation step. The excess streptavidin-RPE was washed away and the fluorescence of the bead (which identifies the immunological marker) and the RPE fluorescence were quantitated. The RPE fluorescence was directly proportional to the concentration of each analyte present in the original sample. The analyses of levels of cytokines were made using the Luminex 100 instrument (Luminex Corp., Austin, TX, USA) and STarStation software (V1.1, Applied Cytometry Systems, Sheffield, UK).
Statistical analysis
The results are presented as the mean ± standard deviation (SD). The data were analyzed using one-way analysis of variance (ANOVA) followed by a post–hoc test (Bonferroni test). Statistical significance was determined at p<0.05.
Results
Synthetic PIF prevents fetal loss
Given the endogenous PIF pro-pregnancy properties in vitro [14], we examined whether synthetic PIF can replicate its functions in vivo as well. We used a well-established model of LPS induced fetal loss (Fig 1A) [6, 7]. We decided to use this model because of the known optimal reproductive outcome in these mice. Therefore, changes in fetal survival can be related to synthetic PIF treatment. We administered LPS on day 7 of gestation and analyzed pregnancy outcome on day 15 of gestation (expected time of delivery day 18–22) mimicking early inflammatory insult during pregnancy (Fig 1A) [7, 31]. We detected an increased fetal loss rate in mice treated with LPS compared with mice injected with PBS alone (Fig 1B compare red to black bars) and synthetic PIF treatment reduced the LPS induced fetal loss significantly (Fig 1B compare green to red bars). Interestingly, PIF reduced fetal loss as compared to control mice as well (Fig 1B compare green to black bars). We concluded that in an inflammatory compromised pregnancy synthetic PIF treatment results in increased fetal survival and decided to evaluate fetal changes next.
To determine the PIF`s effect on the fetuses, we evaluated placental and fetal weights next. Although we did not detect significant differences in placental weight (Fig 1C), synthetic PIF treatment increased the embryo weight compared to LPS treated or control animals (Fig 1D compare green to black and red bars). In further analysis, we determined the ratio of placental to fetal weight in each group. We detected an increased ratio after LPS insult (Fig 1E compare red to black bars) and decreased after synthetic PIF treatment (Fig 1E compare green to black and red bars). This observation is quite intriguing as it suggests that although the placental weight did not change the LPS induced fetal loss was due to placental alterations [32]. To further understand PIF’s effect on the LPS induced insult in the placenta, we tested PIF expression next.
LPS leads to PIF expression in the placenta
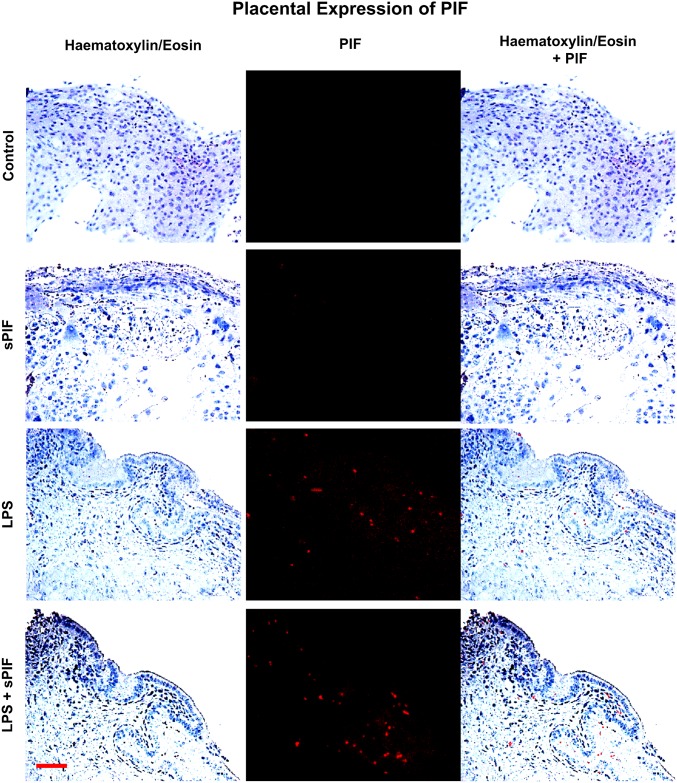
We examined the presence of PIF positive cells by specific anti-PIF monoclonal antibody at day 15 of gestation in the placentae. In line with previous studies [19], we detected minimal to no PIF expression in synthetic PIF or control mice in late gestation (Fig 2: compare two upper panels). Surprisingly, LPS treatment resulted in increased endogenous PIF expression in the placenta and this expression was not additionally amplified by synthetic PIF administration (Fig 2: compare two lower panels). Notably, PIF expression was noticed predominantly in the cytotrophoblast compartment which is in line with previous reports [15]. We postulate that increased endogenous PIF expression may reflect a protective response to the LPS induced inflammatory insult. Further experiments will address the LPS-induced changes in the placenta during embryo development but are beyond the scope of the manuscript. As this endogenous PIF expression did not result in prevention of fetal loss (Fig 1B) and cytokines play a critical role in prevention of fetal loss, we focused on local (placenta) and global (serum) cytokine/chemokine levels next.
Fig 2. Placental PIF expression.
Images of representative placental section (we evaluated 3 consecutive placental sections from 8–10 mice per group) stained using hematoxylin and eosin (left panels) and examined the presence of PIF by specific anti-PIF antibody (red immunofluorescence: middle panels). Merged images are the right panels. We detected no PIF positive cells in Control and synthetic PIF groups (two upper panels). LPS induced inflammatory insult during pregnancy resulted in PIF expression and additional synthetic PIF treatment did not further increase this expression (two lower panels). sPIF: synthetic PreImplantation Factor; LPS: Lipopolysaccharides. Scale bar: 50 μm.
PIF attenuates LPS induced inflammatory signature and inflammasome activation
Given that cytokines play a critical role in the inflammatory response and the placenta is a well-defined source of cytokine production, we tested the placental cytokine/chemokine profile first. We used a multiplex bead array assay to detect levels of 22 cytokines/chemokines. As shown in Table 1 (placental cytokines), LPS administration resulted in increased levels of Tumor necrosis factor (TNF)-α (prime pro-inflammatory cytokine), IL-18 (an inflammasome-related cytokine), and growth related oncogene (GRO: neutrophil-attractive chemokine) and synthetic PIF prevented these increase significantly. To further test the hypothesis of divergent local (placenta) and systemic (serum) inflammatory control, we tested serum cytokine profile as well (Table 2). Indeed, the global inflammatory response was much stronger than in the placenta (Table 2: 14/22 circulating cytokines increased after LPS) and synthetic PIF restored 11 of the 14 LPS induced cytokines/chemokines (Table 2: IFN-γ, IL-18, GM-CSF, GRO, IL-4, IL-5, IL-12p70, IL-17a, IL-22, IL-27, and MIP-1β). Notably, we detected changes after synthetic PIF administration independent of LPS insult as well. For example synthetic PIF up-regulated eotaxin, a chemokine which is involved in placental implantation process [33].
Table 1. Placental inflammatory signature.
| Cytokine | Control | sPIF | LPS | LPS+sPIF |
|---|---|---|---|---|
| TNFα | 9.6±1 | 8.2±0.9 | 14.4±0.8* | 12.1±1*§ |
| IFNγ | 1.2±1.5 | 1.42±1.2 | 1.8±1.5 | 1.7±1.5 |
| IL-1β | 4.7±0.5 | 2.8±0.4* | 5.2±0.3 | 4.5±1.2 |
| IL-18 | 203±23.2 | 190±21.6 | 335±20.4* | 223±24.4§ |
| GM-CSF | ND | ND | ND | ND |
| GRO | 1060±159 | 1255±218* | 1686±401* | 1335±550§ |
| EOTAXIN | 12.5±1.4 | 20.2±1.2 | 11.6±6 | 26.6±19.8 |
| IL-2 | ND | ND | ND | ND |
| IL-4 | ND | ND | ND | ND |
| IL5 | 2.3±0.73 | 4.41±0.5* | 5.2±1.2* | 4.05±0.9 |
| IL-6 | ND | ND | ND | ND |
| IL-9 | ND | ND | ND | ND |
| IL10 | 13.9±1.9 | 5.9±5.4 | 12.08±7.2 | 22.7±17.9 |
| IL12p70 | 0.13±0.06 | 0.82±0.7* | 0.35±0.54 | 0.2±0.12 |
| IL-13 | ND | ND | ND | ND |
| IL-17a | 0.67±0.5 | 0.83±0.4 | 0.75±0.5 | 0.92±0.7 |
| IL-22 | ND | ND | ND | ND |
| IL-23 | 279.7±58.8 | 96.7±28.3* | 208.7±48.7 | 204.7±61.8 |
| IL-27 | 20.7±2.4 | 17.7±3.4 | 18.5±1.5 | 17.3±3.2 |
| IP10 | 14.3±1.5 | 16.3±3.5 | 13.1±7 | 14.4±6.3 |
| MCP1 | 97.4±88 | 214.1±34 | 139.3±78 | 105±86 |
| MCP3 | 173.4±6.7 | 191±19 | 152±10 | 186±16 |
| MIP1α | 7.6±0.6 | 8.5±4.4 | 8.1±3.01 | 8.5±2.7 |
| MIP2 | 13.6±1.16 | 14.6±2.3 | 15.8±1.36 | 17.7±1.53 |
| MIP1β | 3.3±1.3 | 4.9±0.7 | 3.5±2.4 | 4.6±0.8 |
| RANTES | 11.7±1.6 | 11.4±10.1 | 13.8±10.2 | 12.6±39.1 |
Cytokine levels were measured in placental lysates obtained from mice treated with PBS (Control), synthetic PreImplantation Factor (sPIF), Lipopolysaccharides (LPS), and LPS+sPIF by multiplex bead array assay. The results are expressed as ng/ml (Mean ± SD). Statistical significance:
* P<0.05 vs CTR;
§ P<0.05 vs LPS.
Significant changes are marked in bold.
Table 2. Serum inflammatory signature.
| Cytokine | Control | sPIF | LPS | LPS+sPIF |
|---|---|---|---|---|
| TNFα | 6.6±0.4 | 5.6±1.1 | 8.6±0.9* | 7.7±1.0 |
| IFNγ | 3.16±0.2 | 3.39±1.4 | 16.6±1.6* | 5.92±1.1§ |
| IL-1β | 3.4±1 | 2.5±1.1 | 5.2± 1.3* | 4±1.3 |
| IL-18 | 266±65 | 150±25* | 369±26* | 268±64§ |
| GM-CSF | 6.4±1 | 5.3±0.7 | 11.06±1.6* | 8.2±1.5§ |
| GRO | 15.26±1.8 | 18.06±2.2 | 24.9±2.9* | 17.07±1.2§ |
| EOTAXIN | 278±61 | 486±46* | 226±37 | 430±20§ |
| IL-2 | ND | ND | ND | ND |
| IL-4 | 2.53±0.48 | 1.74±0.9 | 7.72±0.2* | 3.95±0.3*§ |
| IL-5 | 3.98±0.5 | 2.66±0.4* | 13.7±0.11* | 6.8±0.5*§ |
| IL-6 | ND | ND | ND | ND |
| IL-9 | ND | ND | ND | ND |
| IL-10 | 7.68±1.1 | 6.65±1.1 | 11.69±2.2 | 8.23±1.3 |
| IL12p70 | 1.36±0.49 | 0.94±0.7* | 3.14±1.73* | 2.07±1.2*§ |
| IL-13 | ND | ND | ND | ND |
| IL-17a | 5.52±0.06 | 4±0.7 | 13.6±0.54* | 10.4±0.12* |
| IL-22 | 35.89±2.1 | 15.9±27* | 47.3±5.6* | 36.2±2.6§ |
| IL-23 | 36.29±8.6 | 21.6±2.3* | 66.9±3.2* | 50.9±5.2 |
| IL-27 | 22.7±1.1 | 13.4±1.08* | 54.5±2.7* | 38.7±3.1§ |
| IP10 | 28.6±11.2 | 41.5±12.8 | 22.5±12.7 | 35.8±14.6 |
| MCP1 | 47.04±3.3 | 34.1±2.7* | 49.01±1.9 | 41.8±3.9 |
| MCP3 | 176.8±71 | 146.4±35 | 168.4±58 | 167.3±93.2 |
| MIP1α | 2.6±1 | 3.08±0.62 | 3.1±0.96 | 3.3±1 |
| MIP2 | 24.7±2.9 | 24.9±3.4 | 27.6±4.1 | 26.3±1.5 |
| MIP1β | 3.95±0.3 | 1.9±0.7* | 6.4±0.4* | 3.6±0.8§ |
| RANTES | 14.2±8.4 | 22.2±8.5 | 21.6±7.7 | 26.5±11.7 |
Cytokine levels were measured in serum obtained from mice treated with PBS (Control), synthetic PreImplantation Factor (sPIF), Lipopolysaccharides (LPS), and LPS+sPIF by multiplex bead array assay. The results are expressed as ng/ml (Mean ± SD). Statistical significance:
* P<0.05 vs CTR;
§ P<0.05 vs LPS.
Significant changes are marked in bold.
We hypothesize that increased endogenous PIF expression in the placenta (Fig 2) results in the divergent inflammatory response at placental and peripheral levels (Tables 1 and 2). However, as in case of progesterone supplementation [34], additional synthetic PIF supplementation is necessary to prevent both the local and peripheral LPS-induced response (Tables 1 and 2) and therefore provide a protective effect (Fig 1).
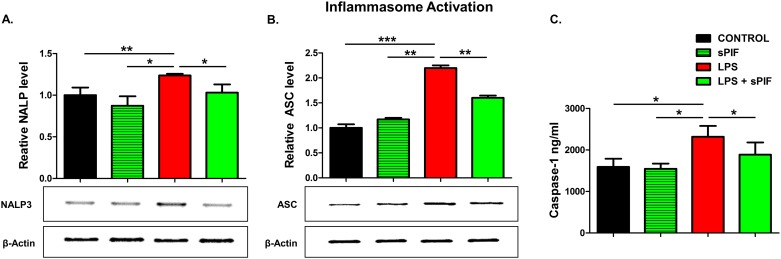
Given that synthetic PIF can reduce major inflammasome signaling cytokines such as IL-1β and IL-18 [35], we decided to test key components of this pathway in the placentae next. Notably, IL-18 contributes to preterm birth in part by modulating TLR4 signaling [36] and TLR4 activation is necessary for PIF effects [27, 28]. We focused on NALP-3 and ASC proteins expression in the placenta as these are the key components of inflammasome signaling [4, 5]. Notably, this multi-protein complex enables caspase-1-mediated production of pro-inflammatory cytokines such as IL-1β and IL-18. We detected increased placental expression of both NALP-3 and ASC proteins in LPS-treated mice (Fig 3A and 3B compare red to black bars). In line with our hypothesis, synthetic PIF reduced this activation significantly (Fig 3A and 3B compare green to black and red bars). We tested downstream inflammasome activation (caspase-1) as well. Again, we detected increased levels of caspase-1 in the placenta after LPS exposure and the addition of PIF reduced this protein’s levels significantly (Fig 3C compare green to red and black bars). Together, we document increased endogenous PIF expression after an LPS insult (Fig 2). Importantly, only application of synthetic PIF results in prevention of increase in cytokine levels after the LPS challenge including the inflammasome pathway. We hypothesize that as in case of progesterone supplementation [34], a combined local and systemic inflammatory control is necessary to reduce LPS-induced fetal loss.
Fig 3. Inflammasome pathway analysis (18–20 placental lysates for each group).
Representative gel images of (A) relative NALP-3 and (B) relative ASC protein levels in placental tissue lysates. The downstream cytokine caspase-1 levels in lysates were measured using enzyme-linked immunoassay (C). Data are mean ± SD. β-Actin was the control. *p<0.05. sPIF: synthetic PreImplantation Factor; LPS: Lipopolysaccharides.
Discussion
Our current findings are in line with the notion of PIF`s trophic and protective action on the embryo, decidua, and trophoblast [12]. Synthetic PIF administration alone decreased fetal loss and improved fetal weight without modulating the inflammasome pathway (Figs 1B, 1D and 3). These favorable effects suggest a beneficial role in optimization of the implantation process. In the current study, we did not detect changes in the number of implanted embryos and plug/pregnancy rate (S1 Table). However, the current experimental setup is not suitable to answer this specific question as this model does not share features with human early recurrent miscarriage as the CBA/J x DBA/2 mouse model (mating of CBA/J females (H2k) with DBA/2J males (H2d)) [37–39]. On the other hand, synthetic PIF increased serum levels of eotaxin independent of the LPS insult (Table 2). Eotaxins regulate extravillous throphoblast function during uterine decidual vessel remodeling [33]. Furthermore, eotaxin has been shown to promote eosinophil adhesion to vascular cell adhesion molecule-1, a major ligand for the integrins [40]. As trophoblast differentiate into an endovascular phenotype a4 integrin is up-regulated [33] and up-regulation of integrins by PIF during throphoblast invasion was reported previously [15, 18]. Thus, current and previous data support the use of synthetic PIF in early recurrent pregnancy loss [20, 23, 41] and studies are in preparation.
Synthetic PIF`s effects on placental/fetal weight ratio after LPS insult are of interest as well (Fig 1E). LPS was reported to reduce uterine blood flow to the fetus and increase uterine resistance without affecting the placental size thereby leading to fibrin deposits and disseminated intravascular coagulation [42]. Thus, growth retardation is expected and was observed in our study (Fig 1D: compare red to black bars). The role of the inflammasome specifically NALP-3 in LPS induced pathology in placental cells has been previously described [35]. Importantly, we report that the LPS induced activation of placental inflammasome can be prevented by synthetic PIF administration (Fig 3). This is supported by the fact that PIF`s effects dependent on TLR4 signaling [27, 28] and TLR4 is the main receptor of inflammasome activation [43]. Furthermore, synthetic PIF targets Kv1.3b channel to reduce K+ flux which activates NALP-3 expression [29, 43]. We hypothesize that synthetic PIF`s effects on placental caspase-1 levels protect against apoptosis and compromise critical oxygen and nutrients transfer to the fetus [44].
Furthermore, the placental/fetal weight ratio relevance for pregnancy outcome was addressed by large population studies previously and an increased ratio was noted in premature birth and fetal demise [32, 45, 46]. Various pathologic and inflammatory conditions are associated with abnormal placental/birthweight ratios including chronic hypertension, preeclampsia, and chorioamnionitis. The present study underscores the fact that the placenta (a primitive organ, supplied by maternal circulation) and the fetus (complex, more evolved with higher oxygen and nutrient demands) are coupled entities. Herein, inflammatory insult affects placental function (but not weight/growth) and still results in reduced fetal weight. We hypothesize that the noted improved fetal-placental exchange is partially due to PIF’s role in promoting implantation [20, 23] and/or reducing vascular inflammation [29]. However, it is still not understood how synthetic PIF increases fetal weight despite of LPS insult (Fig 1D). Although hypothetical, the modulation of the H19/insulin-like growth factor 2 (Igf2) imprinted locus is intriguing. H19 is a long non-coding RNA and placental H19 misregulation is associated with the imprinting disorder such fetal overgrowth disorder (Beckwith–Wiedemann syndrome) and intrauterine growth retardation (Silver–Russell syndrome) [47]. Notably, we recently reported the close functional interactions of H19 and microRNA let-7 [48, 49] and let-7 expression is modulated by synthetic PIF [27]. Thus, synthetic PIF`s role on H19 modulation in intrauterine growth restriction models is currently investigated but beyond the scope of this manuscript. Notably, women with unexplained infertility and women with endometriosis show decreased H19 expression in eutopic endometrium suggesting a direct clinical link [50, 51].
Synthetic PIF`s effect on local and peripheral cytokine level are of interest as well (Tables 1 and 2). Altered balance of pro- and anti-inflammatory cytokines forms the basis of multiple pregnancy disorders and results in fetal and maternal pathological changes [12, 52–56]. For example, IL-18 is significantly elevated at the onset of labor [57] and contributes to preterm birth [36, 57]. Preterm birth or placental hypoxia is associated with high concentrations of granulocyte macrophage-colony stimulating factor (GM-CSF) [58, 59]. GRO a well-defined neutrophil-attractive chemokine increases in case of chorioamnionitis [60]. Besides maternal morbidities, prenatal LPS insult results in sustained inflammation in fetus and newborn, which is associated with an increased risk for adverse outcomes such as brain damage and pulmonary or intestinal complications [61–64]. Given that synthetic PIF prevented the increase of those cytokines/chemokines in the serum (Table 2) and partially in the placenta (Table 1), we envision synthetic PIF treatment in pregnancies at risk as in case of progesterone [52]. This is supported by the fact that synthetic PIF reduces the inflammasome response (Fig 3) and NALP-3 induced IL-18 and IL-1β impact the pathogenesis of preeclampsia, preterm birth, and perinatal brain injury [3, 65, 66].
The simple notion of TH2 overbalance during pregnancy is not specific enough. More recent insights into immunological operative mechanisms in pregnancy favor the TH1/TH2 cooperation [67]. The dual role of IL-4 is an examples [68]. Synthetic PIF prevented the LPS induced Th2-type cytokine IL-4 in the serum (Table 2). Notably, IL-4–induced natural killer cells produce higher levels of IFN-γ, IL-10, and GM-CSF while exhibiting high cytotoxicity [68]. Not surprisingly, synthetic PIF prevented the increase of those cytokines in the serum (Table 2) in line with natural killer cells being PIF`s target [23]. Finally, synthetic PIF modulated IL-22, a member of IL-10 family (Table 2) and increased IL-22 levels are an inflammatory marker of placental dysfunction and early pregnancy loss [56, 69].
In conclusion, we provide evidence supporting synthetic PIF as a targeted therapy in inflammatory induced pregnancy loss. This study is in line with PIF`s efficacy previously observed in several preclinical models of immune disorders [24–30]. Additionally, this is the first description of placental expression of PIF after LPS-induced inflammatory insult. Synthetic PIF is currently in first-in-human Phase Ib clinical trial (NCT02239562) for adult patients with autoimmune disease. Upon completion the use of synthetic PIF as treatment for perinatal brain injuries is planned [27, 28]. Synthetic PIF therapy in pregnancy disorders such as preeclampsia, preterm birth, and early pregnancy loss are in preparation as well [12, 19, 41].
Supporting information
Detailed results of the performed experiments and groups (Control, sPIF, LPS, and LPS+sPIF) are summarized including the number of implanted embryos and plug/pregnancy rate.
(PDF)
Acknowledgments
We thank A. Carter and S. Zinn for editorial assistance.
Data Availability
Data are available from https://figshare.com/s/73ea9df023bbe848e298.
Funding Statement
The work was supported by a research grant from the Università Cattolica del Sacro Cuore (D1, 2014) and by Istituto Scientifico Internazionale, Paolo VI Institute, Università Cattolica del Sacro Cuore, Rome, Italy. Dr. Eytan R. Barnea is the (uncompensated) Chief Scientist for BioIncept, LLC. BioIncept, LLC did not have any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘author contributions’ section.
References
- 1.Christiansen OB. Reproductive immunology. Molecular immunology. 2013;55(1):8–15. doi: 10.1016/j.molimm.2012.08.025 . [DOI] [PubMed] [Google Scholar]
- 2.Clark DA. The importance of being a regulatory T cell in pregnancy. J Reprod Immunol. 2016;116:60–9. doi: 10.1016/j.jri.2016.04.288 . [DOI] [PubMed] [Google Scholar]
- 3.D'Ippolito S, Tersigni C, Marana R, Di Nicuolo F, Gaglione R, Rossi ED, et al. Inflammosome in the human endometrium: further step in the evaluation of the "maternal side". Fertil Steril. 2016;105(1):111–8.e1- 4 doi: 10.1016/j.fertnstert.2015.09.027 . [DOI] [PubMed] [Google Scholar]
- 4.Dunne A. Inflammasome activation: from inflammatory disease to infection. Biochemical Society transactions. 2011;39(2):669–73. doi: 10.1042/BST0390669 . [DOI] [PubMed] [Google Scholar]
- 5.Nold-Petry CA, Nold MF, Nielsen JW, Bustamante A, Zepp JA, Storm KA, et al. Increased cytokine production in interleukin-18 receptor alpha-deficient cells is associated with dysregulation of suppressors of cytokine signaling. J Biol Chem. 2009;284(38):25900–11. doi: 10.1074/jbc.M109.004184 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silver RM, Edwin SS, Umar F, Dudley DJ, Branch DW, Mitchell MD. Bacterial lipopolysaccharide-mediated murine fetal death: the role of interleukin-1. Am J Obstet Gynecol. 1997;176(3):544–9. . [DOI] [PubMed] [Google Scholar]
- 7.Robertson SA, Care AS, Skinner RJ. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol Reprod. 2007;76(5):738–48. doi: 10.1095/biolreprod.106.056143 . [DOI] [PubMed] [Google Scholar]
- 8.Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31(5):379–446. . [DOI] [PubMed] [Google Scholar]
- 9.Clark DA. Immunological factors in pregnancy wastage: fact or fiction. Am J Reprod Immunol. 2008;59(4):277–300. doi: 10.1111/j.1600-0897.2008.00580.x . [DOI] [PubMed] [Google Scholar]
- 10.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2(9):656–63. Epub 2002/09/05. doi: 10.1038/nri886 . [DOI] [PubMed] [Google Scholar]
- 11.Barnea ER. Applying embryo-derived immune tolerance to the treatment of immune disorders. Ann N Y Acad Sci. 2007;1110:602–18. doi: 10.1196/annals.1423.064 . [DOI] [PubMed] [Google Scholar]
- 12.Barnea ER, Almogi-Hazan O, Or R, Mueller M, Ria F, Weiss L, et al. Immune regulatory and neuroprotective properties of preimplantation factor: From newborn to adult. Pharmacol Ther. 2015;156:10–25. doi: 10.1016/j.pharmthera.2015.10.008 . [DOI] [PubMed] [Google Scholar]
- 13.Stamatkin CW, Roussev RG, Stout M, Absalon-Medina V, Ramu S, Goodman C, et al. PreImplantation Factor (PIF) correlates with early mammalian embryo development-bovine and murine models. Reprod Biol Endocrinol. 2011;9:63 doi: 10.1186/1477-7827-9-63 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramu S, Stamatkin C, Timms L, Ruble M, Roussev RG, Barnea ER. PreImplantation factor (PIF) detection in maternal circulation in early pregnancy correlates with live birth (bovine model). Reprod Biol Endocrinol. 2013;11:105 doi: 10.1186/1477-7827-11-105 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moindjie H, Santos ED, Loeuillet L, Gronier H, de Mazancourt P, Barnea ER, et al. Preimplantation factor (PIF) promotes human trophoblast invasion. Biol Reprod. 2014;91(5):118 doi: 10.1095/biolreprod.114.119156 . [DOI] [PubMed] [Google Scholar]
- 16.Paidas MJ, Krikun G, Huang SJ, Jones R, Romano M, Annunziato J, et al. A genomic and proteomic investigation of the impact of preimplantation factor on human decidual cells. Am J Obstet Gynecol. 2010;202(5):459.e1–8. doi: 10.1016/j.ajog.2010.03.024 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duzyj CM, Barnea ER, Li M, Huang SJ, Krikun G, Paidas MJ. Preimplantation factor promotes first trimester trophoblast invasion. Am J Obstet Gynecol. 2010;203(4):402.e1–4. doi: 10.1016/j.ajog.2010.06.060 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnea ER, Kirk D, Paidas MJ. Preimplantation factor (PIF) promoting role in embryo implantation: increases endometrial integrin-alpha2beta3, amphiregulin and epiregulin while reducing betacellulin expression via MAPK in decidua. Reprod Biol Endocrinol. 2012;10:50 doi: 10.1186/1477-7827-10-50 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnea ER, Vialard F, Moindjie H, Ornaghi S, Dieudonne MN, Paidas MJ. PreImplantation Factor (PIF*) endogenously prevents preeclampsia: Promotes trophoblast invasion and reduces oxidative stress. J Reprod Immunol. 2016;114:58–64. doi: 10.1016/j.jri.2015.06.002 . [DOI] [PubMed] [Google Scholar]
- 20.Stamatkin CW, Roussev RG, Stout M, Coulam CB, Triche E, Godke RA, et al. Preimplantation factor negates embryo toxicity and promotes embryo development in culture. Reprod Biomed Online. 2011;23(4):517–24. doi: 10.1016/j.rbmo.2011.06.009 . [DOI] [PubMed] [Google Scholar]
- 21.Barnea ER, Lubman DM, Liu YH, Absalon-Medina V, Hayrabedyan S, Todorova K, et al. Insight into PreImplantation Factor (PIF*) mechanism for embryo protection and development: target oxidative stress and protein misfolding (PDI and HSP) through essential RIKP [corrected] binding site. PLoS One. 2014;9(7):e100263 doi: 10.1371/journal.pone.0100263 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnea ER, Kirk D, Ramu S, Rivnay B, Roussev R, Paidas MJ. PreImplantation Factor (PIF) orchestrates systemic antiinflammatory response by immune cells: effect on peripheral blood mononuclear cells. Am J Obstet Gynecol. 2012;207(4):313.e1–11. doi: 10.1016/j.ajog.2012.07.017 . [DOI] [PubMed] [Google Scholar]
- 23.Roussev RG, Dons'koi BV, Stamatkin C, Ramu S, Chernyshov VP, Coulam CB, et al. Preimplantation factor inhibits circulating natural killer cell cytotoxicity and reduces CD69 expression: implications for recurrent pregnancy loss therapy. Reprod Biomed Online. 2013;26(1):79–87. doi: 10.1016/j.rbmo.2012.09.017 . [DOI] [PubMed] [Google Scholar]
- 24.Weiss L, Bernstein S, Jones R, Amunugama R, Krizman D, Jebailey L, et al. Preimplantation factor (PIF) analog prevents type I diabetes mellitus (TIDM) development by preserving pancreatic function in NOD mice. Endocrine. 2011;40(1):41–54. doi: 10.1007/s12020-011-9438-5 . [DOI] [PubMed] [Google Scholar]
- 25.Weiss L, Or R, Jones RC, Amunugama R, JeBailey L, Ramu S, et al. Preimplantation factor (PIF*) reverses neuroinflammation while promoting neural repair in EAE model. J Neurol Sci. 2012;312(1–2):146–57. doi: 10.1016/j.jns.2011.07.050 . [DOI] [PubMed] [Google Scholar]
- 26.Azar Y, Shainer R, Almogi-Hazan O, Bringer R, Compton SR, Paidas MJ, et al. Preimplantation factor reduces graft-versus-host disease by regulating immune response and lowering oxidative stress (murine model). Biol Blood Marrow Transplant. 2013;19(4):519–28. doi: 10.1016/j.bbmt.2012.12.011 . [DOI] [PubMed] [Google Scholar]
- 27.Mueller M, Zhou J, Yang L, Gao Y, Wu F, Schoeberlein A, et al. PreImplantation factor promotes neuroprotection by targeting microRNA let-7. Proc Natl Acad Sci U S A. 2014;111(38):13882–7. doi: 10.1073/pnas.1411674111 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller M, Schoeberlein A, Zhou J, Joerger-Messerli M, Oppliger B, Reinhart U, et al. PreImplantation Factor bolsters neuroprotection via modulating Protein Kinase A and Protein Kinase C signaling. Cell death and differentiation. 2015;22(12):2078–86. doi: 10.1038/cdd.2015.55 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YC, Rivera J, Fitzgerald M, Hausding C, Ying YL, Wang X, et al. PreImplantation factor prevents atherosclerosis via its immunomodulatory effects without affecting serum lipids. Thromb Haemost. 2016;115(5):1010–24. doi: 10.1160/TH15-08-0640 . [DOI] [PubMed] [Google Scholar]
- 30.Shainer R, Almogi-Hazan O, Berger A, Hinden L, Mueller M, Brodie C, et al. PreImplantation factor (PIF) therapy provides comprehensive protection against radiation induced pathologies. Oncotarget. 2016. doi: 10.18632/oncotarget.10635 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su MT, Lin SH, Chen YC. Association of sex hormone receptor gene polymorphisms with recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2011;96(6):1435–44 e1. doi: 10.1016/j.fertnstert.2011.09.030 . [DOI] [PubMed] [Google Scholar]
- 32.Haavaldsen C, Samuelsen SO, Eskild A. Fetal death and placental weight/birthweight ratio: a population study. Acta Obstet Gynecol Scand. 2013;92(5):583–90. doi: 10.1111/aogs.12105 . [DOI] [PubMed] [Google Scholar]
- 33.Chau SE, Murthi P, Wong MH, Whitley GS, Brennecke SP, Keogh RJ. Control of extravillous trophoblast function by the eotaxins CCL11, CCL24 and CCL26. Hum Reprod. 2013;28(6):1497–507. doi: 10.1093/humrep/det060 . [DOI] [PubMed] [Google Scholar]
- 34.Aisemberg J, Vercelli CA, Bariani MV, Billi SC, Wolfson ML, Franchi AM. Progesterone is essential for protecting against LPS-induced pregnancy loss. LIF as a potential mediator of the anti-inflammatory effect of progesterone. PLoS One. 2013;8(2):e56161 doi: 10.1371/journal.pone.0056161 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pontillo A, Girardelli M, Agostinis C, Masat E, Bulla R, Crovella S. Bacterial LPS differently modulates inflammasome gene expression and IL-1beta secretion in trophoblast cells, decidual stromal cells, and decidual endothelial cells. Reprod Sci. 2013;20(5):563–6. doi: 10.1177/1933719112459240 . [DOI] [PubMed] [Google Scholar]
- 36.Li L, Yang J, Jiang Y, Tu J, Schust DJ. Activation of decidual invariant natural killer T cells promotes lipopolysaccharide-induced preterm birth. Mol Hum Reprod. 2015;21(4):369–81. doi: 10.1093/molehr/gav001 . [DOI] [PubMed] [Google Scholar]
- 37.Clark DA, Manuel J, Lee L, Chaouat G, Gorczynski RM, Levy GA. Ecology of danger-dependent cytokine-boosted spontaneous abortion in the CBA x DBA/2 mouse model. I. Synergistic effect of LPS and (TNF-alpha + IFN-gamma) on pregnancy loss. Am J Reprod Immunol. 2004;52(6):370–8. doi: 10.1111/j.1600-0897.2004.00237.x . [DOI] [PubMed] [Google Scholar]
- 38.Clark DA, Ding JW, Yu G, Levy GA, Gorczynski RM. Fgl2 prothrombinase expression in mouse trophoblast and decidua triggers abortion but may be countered by OX-2. Mol Hum Reprod. 2001;7(2):185–94. . [DOI] [PubMed] [Google Scholar]
- 39.Redecha P, van Rooijen N, Torry D, Girardi G. Pravastatin prevents miscarriages in mice: role of tissue factor in placental and fetal injury. Blood. 2009;113(17):4101–9. doi: 10.1182/blood-2008-12-194258 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodside DG, Kram RM, Mitchell JS, Belsom T, Billard MJ, McIntyre BW, et al. Contrasting roles for domain 4 of VCAM-1 in the regulation of cell adhesion and soluble VCAM-1 binding to integrin alpha4beta1. J Immunol. 2006;176(8):5041–9. . [DOI] [PubMed] [Google Scholar]
- 41.Goodale LF, Hayrabedran S, Todorova K, Roussev R, Ramu S, Stamatkin C, et al. PreImplantation factor (PIF) protects cultured embryos against oxidative stress: relevance for recurrent pregnancy loss (RPL) therapy. Oncotarget. 2017;8(20):32419–32. doi: 10.18632/oncotarget.16028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renaud SJ, Cotechini T, Quirt JS, Macdonald-Goodfellow SK, Othman M, Graham CH. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol. 2011;186(3):1799–808. doi: 10.4049/jimmunol.1002679 . [DOI] [PubMed] [Google Scholar]
- 43.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell death and differentiation. 2007;14(9):1583–9. doi: 10.1038/sj.cdd.4402195 . [DOI] [PubMed] [Google Scholar]
- 44.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215(1 Suppl):S1–S46. doi: 10.1016/j.ajog.2016.03.001 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Jongh BE, Mackley A, Jain N, Locke R, Paul DA. Effects of advanced maternal age and race/ethnicity on placental weight and placental weight/birthweight ratio in very low birthweight infants. Matern Child Health J. 2015;19(7):1553–8. doi: 10.1007/s10995-014-1662-1 . [DOI] [PubMed] [Google Scholar]
- 46.McNamara H, Hutcheon JA, Platt RW, Benjamin A, Kramer MS. Risk factors for high and low placental weight. Paediatr Perinat Epidemiol. 2014;28(2):97–105. doi: 10.1111/ppe.12104 . [DOI] [PubMed] [Google Scholar]
- 47.Hur SK, Freschi A, Ideraabdullah F, Thorvaldsen JL, Luense LJ, Weller AH, et al. Humanized H19/Igf2 locus reveals diverged imprinting mechanism between mouse and human and reflects Silver-Russell syndrome phenotypes. Proc Natl Acad Sci U S A. 2016;113(39):10938–43. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kallen AN, Zhou XB, Xu J, Qiao C, Ma J, Yan L, et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell. 2013;52(1):101–12. doi: 10.1016/j.molcel.2013.08.027 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y, Wu F, Zhou J, Yan L, Jurczak MJ, Lee HY, et al. The H19/let-7 double-negative feedback loop contributes to glucose metabolism in muscle cells. Nucleic Acids Res. 2014;42(22):13799–811. doi: 10.1093/nar/gku1160 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korucuoglu U, Biri AA, Konac E, Alp E, Onen IH, Ilhan MN, et al. Expression of the imprinted IGF2 and H19 genes in the endometrium of cases with unexplained infertility. European journal of obstetrics, gynecology, and reproductive biology. 2010;149(1):77–81. doi: 10.1016/j.ejogrb.2009.12.007 . [DOI] [PubMed] [Google Scholar]
- 51.Ghazal S, McKinnon B, Zhou J, Mueller M, Men Y, Yang L, et al. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO molecular medicine. 2015;7(8):996–1003. doi: 10.15252/emmm.201505245 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lissauer D, Eldershaw SA, Inman CF, Coomarasamy A, Moss PA, Kilby MD. Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile. Eur J Immunol. 2015;45(10):2858–72. doi: 10.1002/eji.201445404 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nahum R, Brenner O, Zahalka MA, Traub L, Quintana F, Moroz C. Blocking of the placental immune-modulatory ferritin activates Th1 type cytokines and affects placenta development, fetal growth and the pregnancy outcome. Hum Reprod. 2004;19(3):715–22. doi: 10.1093/humrep/deh099 . [DOI] [PubMed] [Google Scholar]
- 54.Abumaree MH, Chamley LW, Badri M, El-Muzaini MF. Trophoblast debris modulates the expression of immune proteins in macrophages: a key to maternal tolerance of the fetal allograft? J Reprod Immunol. 2012;94(2):131–41. doi: 10.1016/j.jri.2012.03.488 . [DOI] [PubMed] [Google Scholar]
- 55.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178(9):5949–56. . [DOI] [PubMed] [Google Scholar]
- 56.O'Hern Perfetto C, Fan X, Dahl S, Krieg S, Westphal LM, Bunker Lathi R, et al. Expression of interleukin-22 in decidua of patients with early pregnancy and unexplained recurrent pregnancy loss. J Assist Reprod Genet. 2015;32(6):977–84. doi: 10.1007/s10815-015-0481-7 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ida A, Tsuji Y, Muranaka J, Kanazawa R, Nakata Y, Adachi S, et al. IL-18 in pregnancy; the elevation of IL-18 in maternal peripheral blood during labour and complicated pregnancies. J Reprod Immunol. 2000;47(1):65–74. . [DOI] [PubMed] [Google Scholar]
- 58.Basraon SK, Menon R, Makhlouf M, Longo M, Hankins GD, Saade GR, et al. Can statins reduce the inflammatory response associated with preterm birth in an animal model? Am J Obstet Gynecol. 2012;207(3):224 e1-7. doi: 10.1016/j.ajog.2012.06.020 . [DOI] [PubMed] [Google Scholar]
- 59.Hayashi M, Hamada Y, Ohkura T. Elevation of granulocyte-macrophage colony-stimulating factor in the placenta and blood in preeclampsia. Am J Obstet Gynecol. 2004;190(2):456–61. doi: 10.1016/j.ajog.2003.07.032 . [DOI] [PubMed] [Google Scholar]
- 60.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol Hum Reprod. 2002;8(4):399–408. . [DOI] [PubMed] [Google Scholar]
- 61.Kuypers E, Willems MG, Jellema RK, Kemp MW, Newnham JP, Delhaas T, et al. Responses of the spleen to intraamniotic lipopolysaccharide exposure in fetal sheep. Pediatr Res. 2015;77(1–1):29–35. doi: 10.1038/pr.2014.152 . [DOI] [PubMed] [Google Scholar]
- 62.Smit AL, Lambermont VA, Stokroos RJ, Anteunis LJ, Chenault MN, Schaefer SM, et al. Intrauterine Lipopolysaccharide-Induced Chorioamnionitis in a Sheep: Does It Affect the Auditory System? Reproductive sciences (Thousand Oaks, Calif). 2016;23(2):257–63. doi: 10.1177/1933719115602759 . [DOI] [PubMed] [Google Scholar]
- 63.Al-Amin MM, Alam T, Hasan SM, Hasan AT, Quddus AH. Prenatal maternal lipopolysaccharide administration leads to age- and region-specific oxidative stress in the early developmental stage in offspring. Neuroscience. 2016;318:84–93. doi: 10.1016/j.neuroscience.2016.01.002 . [DOI] [PubMed] [Google Scholar]
- 64.Rose DR, Careaga M, Van de Water J, McAllister K, Bauman MD, Ashwood P. Long-term altered immune responses following fetal priming in a non-human primate model of maternal immune activation. Brain Behav Immun. 2017;63:60–70. doi: 10.1016/j.bbi.2016.11.020 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci. 2008;15(2):121–7. doi: 10.1177/1933719107310992 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xue P, Zheng M, Gong P, Lin C, Zhou J, Li Y, et al. Single administration of ultra-low-dose lipopolysaccharide in rat early pregnancy induces TLR4 activation in the placenta contributing to preeclampsia. PLoS One. 2015;10(4):e0124001 doi: 10.1371/journal.pone.0124001 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sykes L, MacIntyre DA, Yap XJ, Teoh TG, Bennett PR. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediators Inflamm. 2012;2012:967629 doi: 10.1155/2012/967629 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kiniwa T, Enomoto Y, Terazawa N, Omi A, Miyata N, Ishiwata K, et al. NK cells activated by Interleukin-4 in cooperation with Interleukin-15 exhibit distinctive characteristics. Proc Natl Acad Sci U S A. 2016;113(36):10139–44. doi: 10.1073/pnas.1600112113 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bersani I, De Carolis MP, Foell D, Weinhage T, Rossi ED, De Carolis S, et al. Interleukin-22: biomarker of maternal and fetal inflammation? Immunol Res. 2015;61(1–2):4–10. doi: 10.1007/s12026-014-8568-2 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed results of the performed experiments and groups (Control, sPIF, LPS, and LPS+sPIF) are summarized including the number of implanted embryos and plug/pregnancy rate.
(PDF)
Data Availability Statement
Data are available from https://figshare.com/s/73ea9df023bbe848e298.