Abstract
Immunofluorescence was used to determine the relative percentages of T and B lymphocytes found in the lungs of normal and Mycoplasma pulmonis-infected F344 rats. Lymphocytes recovered from controls were approximately 25% T, 25% B, and 50% unclassified mononuclear cells. Infected animals had a 2.6-fold greater number of T cells and IgA-bearing cells, and a 1.6-fold greater number of unclassified mononuclear cells. These studies show that M. pulmonis infection significantly alters lung lymphocyte populations both quantitatively and in subpopulation distribution. Therefore, future studies of rat lung lymphocytes should utilize animals known to be free of this ubiquitous respiratory pathogen.
Full text
PDF
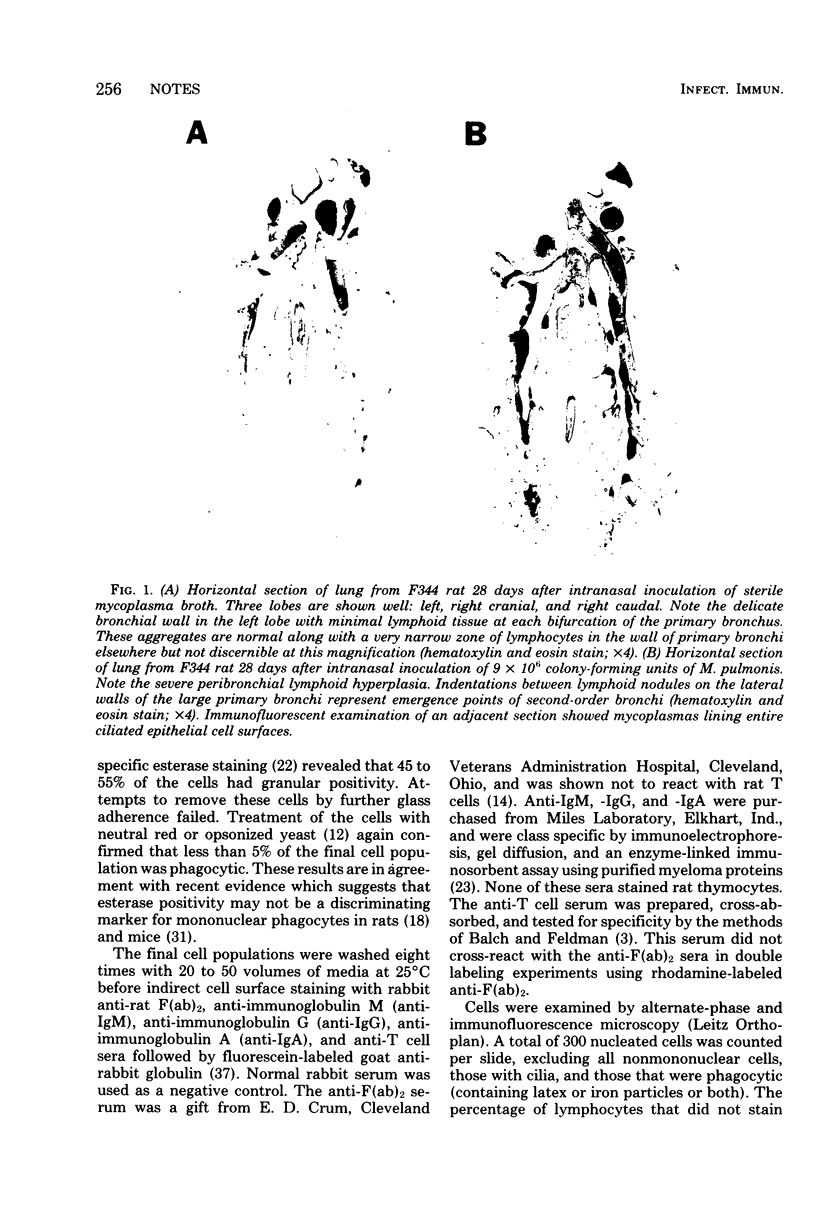



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. L., Titus J. A., Segal D. M. Quantitation of Fc receptors and surface immunoglobulin is affected by cell isolation procedures using plasmagel and ficoll-hypaque. J Immunol Methods. 1978;22(3-4):263–272. doi: 10.1016/0022-1759(78)90034-0. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Feldman J. D. Thymus-dependent (T) lymphocytes in the rat. J Immunol. 1974 Jan;112(1):79–86. [PubMed] [Google Scholar]
- Bienenstock J., Johnston N. A morphologic study of rabbit bronchial lymphoid aggregates and lymphoepithelium. Lab Invest. 1976 Oct;35(4):343–348. [PubMed] [Google Scholar]
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973 Jun;28(6):686–692. [PubMed] [Google Scholar]
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. II. Functional characterisitics. Lab Invest. 1973 Jun;28(6):693–698. [PubMed] [Google Scholar]
- Bienenstock J., Rudzik O., Clancy R. L., Perey D. Y. Bronchial lymphoid tissue. Adv Exp Med Biol. 1974;45(0):47–56. doi: 10.1007/978-1-4613-4550-3_6. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain D. W., Nopajaroonsri C., Simon G. T. Ultrastructure of the pulmonary lymphoid tissue. Am Rev Respir Dis. 1973 Sep;108(3):621–631. doi: 10.1164/arrd.1973.108.3.621. [DOI] [PubMed] [Google Scholar]
- Crum E. D., McGregor D. D. Functional properties of T and B cells isolated by affinity chromatography from rat thoracic duct lymph. Cell Immunol. 1976 May;23(2):211–222. doi: 10.1016/0008-8749(76)90187-8. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., Altose M. D., Rowlands D. T., Jr Immunocompetent cells from the lower respiratory tract of normal human lungs. J Clin Invest. 1975 Oct;56(4):986–995. doi: 10.1172/JCI108179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele R. P., Beacham C. H., Gorenberg D. J. The bronchoalveolar lymphocyte. Studies on the life history and lymphocyte traffic from blood to the lung. Cell Immunol. 1977 Jun 1;31(1):48–54. doi: 10.1016/0008-8749(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Fournier M., Vai F., Derenne J. P., Pariente R. Bronchial lymphoepithelial nodules in the rat: morphologic features and uptake and transport of exogenous proteins. Am Rev Respir Dis. 1977 Oct;116(4):685–694. doi: 10.1164/arrd.1977.116.4.685. [DOI] [PubMed] [Google Scholar]
- Gorenberg D. J., Daniele R. P. Characterization of immunocompetent cells recovered from the respiratory tract and tracheobronchial lymph node of normal guinea pigs. Am Rev Respir Dis. 1976 Dec;114(6):1099–1105. doi: 10.1164/arrd.1976.114.6.1099. [DOI] [PubMed] [Google Scholar]
- Higgy K. E., Burns G. F., Hayhoe F. G. Discrimination of B, T and null lymphocytes by esterase cytochemistry. Scand J Haematol. 1977 May;18(5):437–448. doi: 10.1111/j.1600-0609.1977.tb02098.x. [DOI] [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung. I. A guinea pig model for the study of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):89–97. doi: 10.1016/0008-8749(76)90350-6. [DOI] [PubMed] [Google Scholar]
- Kazmierowski J. A., Fauci A. S., Reynolds H. Y. Characterization of lymphocytes in bronchial lavage fluid from monkeys. J Immunol. 1976 Mar;116(3):615–618. [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- Lobo P. I., Westervelt F. B., Horwitz D. A. Identification of two populations of immunoglobulin-bearing lymphocytes in man. J Immunol. 1975 Jan;114(1 Pt 1):116–119. [PubMed] [Google Scholar]
- Naot Y., Merchav S., Ben-David E., Ginsburg H. Mitogenic activity of Mycoplasma pulmonis. I. Stimulation of rat B and T lymphocytes. Immunology. 1979 Mar;36(3):399–406. [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Tully J. G., Ginsburg H. Lymphocyte activation by various Mycoplasma strains and species. Infect Immun. 1977 Nov;18(2):310–317. doi: 10.1128/iai.18.2.310-317.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Asofsky R., Terry W. D. Characterization of the nonphagocytic adherent cell from the peritoneal cavity of normal and BCG-treated mice. J Immunol. 1977 May;118(5):1612–1621. [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. II. Interaction of respiratory antibodies with Pseudomonas aeruginosa and alveolar macrophages. J Immunol. 1973 Aug;111(2):369–380. [PubMed] [Google Scholar]
- Rudzik O., Clancy R. L., Perey D. Y., Bienenstock J., Singal D. P. The distribution of a rabbit thymic antigen and membrane immunoglobulins in lymphoid tissue, with special reference to mucosal lymphocytes. J Immunol. 1975 Jan;114(1 Pt 1):1–4. [PubMed] [Google Scholar]
- Vai F., Fournier M., Pariente R. Bronchial lympho-epithelial nodules in the rat: definition and morphological characteristics in optical and electron microscopy. Biomedicine. 1977 Apr;26(2):130–137. [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Plasma membrane synthesis in the macrophage following phagocytosis of polystyrene latex particles. J Biol Chem. 1972 Apr 25;247(8):2439–2446. [PubMed] [Google Scholar]