Abstract
Objectives
Tuberculous meningitis is characterized by elevated levels of matrix metalloproteinase 9 (MMP9) in the cerebrospinal fluid (CSF). However, it is unclear whether elevated MMP9 levels are associated with poor treatment outcome. We tested the hypothesis that pretreatment MMP9 levels in the CSF would be higher in tuberculous meningitis patients experiencing a poor treatment outcome.
Methods
We prospectively assessed the treatment outcome in a consecutive sample of human immunodeficiency virus-negative patients with tuberculous meningitis. We defined good outcome as survival without severe neurological disability (modified Rankin scale scores 0–2). We estimated levels of MMP9 and its tissue inhibitor (TIMP1) on pretreatment CSF samples. We used albumin index to assess blood-brain barrier permeability.
Results
We studied 40 patients (23 males [58%]) with tuberculous meningitis. Sixteen patients (40%) had stage 3 disease. On follow-up, 18 (45%) patients had a poor treatment outcome—15 patients died and 3 had severe neurological disability. Pretreatment MMP9 levels were not associated with treatment outcome (median [interquartile range], 254 [115–389] vs. 192 [60–383] ng/mL in good vs. poor outcome groups; P = 0.693). MMP9 levels did not correlate with the albumin index (Spearman’s rho = 0.142; P = 0.381). However, MMP9 levels significantly correlated with CSF glucose levels (rho = −0.419; P = 0.007) and admission Glasgow coma scale score (rho = 0.324; P = 0.032). Likewise, TIMP1 levels also did not differ by treatment outcome (1239 [889–1511] vs. 1522 [934–1949] ng/mL; P = 0.201). MMP9/TIMP1 ratio that reflects net proteolytic activity was also not different between the two groups (0.191 [0.107–0.250] vs. 0.163 [0.067–0.34]; P = 0.625).
Conclusion
Our findings do not support the hypothesis that pretreatment levels of MMP9 would be higher in tuberculous meningitis patients experiencing a poor treatment outcome. Further, MMP9 levels in the CSF did not correlate with blood-brain barrier permeability in patients with tuberculous meningitis.
Introduction
Tuberculous meningitis is the most lethal form of tuberculosis (TB), affecting about 1% of all people with TB [1]. About 40% of adults with tuberculous meningitis succumb to the disease despite early treatment with standard anti-TB treatment and corticosteroids [2]. Intensified anti-TB treatment also did not reduce the mortality in a recent clinical trial on adults with tuberculous meningitis [3]. Thus, there is a pressing need to develop host-directed therapies to improve treatment outcome in tuberculous meningitis. Of late, the role of matrix metalloproteinases (MMPs) in the pathogenesis of TB has generated considerable interest [4,5]. MMP9 plays an important role in macrophage recruitment, and destruction of extracellular matrix by MMPs could damage the blood-brain barrier (BBB) in patients with tuberculous meningitis [6,7]. Hence, MMP9 has been proposed as a potential therapeutic target in TB [4–8].
While earlier studies had found that MMP9 levels are elevated in tuberculous meningitis [9,10], very few studies have looked at the relation between the elevated MMP9 levels and treatment outcome in patients with tuberculous meningitis. Before embarking on clinical trials of MMP inhibitors such as doxycycline, it is necessary to empirically demonstrate that elevated MMP9 levels are associated with unfavorable treatment outcome. Therefore, we conducted the present study to test the hypothesis that pretreatment CSF levels of MMP9 would be higher in tuberculous meningitis patients with unfavorable treatment outcome. We also attempted to correlate the MMP9 levels with BBB permeability.
Materials and methods
This is a case-control study nested in a treatment cohort, conducted from December 2012 to April 2014 at the Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) hospital, Puducherry, India. The Institute Ethics Committee (Human studies) at JIPMER reviewed and approved the study protocol (approval no. IEC/SC/2012/4/127). We prospectively enrolled consecutive adolescents and adults aged >12 years admitted with a clinical diagnosis of tuberculous meningitis, after obtaining informed written consent from the patient or their guardian. We excluded patients if they did not satisfy the consensus diagnostic criteria for tuberculous meningitis [11] or tested positive for human immunodeficiency virus (HIV) infection. We excluded patients who had received anti-TB treatment or corticosteroids for more than 2 days.
We collected the following clinical and laboratory data using a standardized data collection form—demographic information, duration of symptoms, Glasgow coma scale (GCS) score, presence of focal neurological deficit, papilledema, cranial nerve palsy and BCG vaccination scar, laboratory variables such as total leukocyte count and serum albumin, and CSF analysis findings such as opening pressure, total and differential leukocyte counts, glucose, protein, Gram stain and culture, smear for acid-fast bacilli, mycobacterial culture, India ink/latex agglutination for cryptococcal antigen and adenosine deaminase (ADA) levels. We scored the severity of meningitis as per the modified British Medical Research Council (MRC) grading as follows—Grade 1, GCS score 15/15 and no focal neurological deficit; Grade 2, GCS score 15/15 with focal neurological deficit or GCS score 11–14; and Grade 3, GCS score 10 or less [12]. We performed computed-tomographic (CT) imaging of the brain at baseline in all patients, and repeated the imaging if there was any worsening of sensorium or new-onset neurological deficit. We also evaluated for evidence of TB elsewhere in the body and tested for HIV infection.
We measured the CSF opening pressure in lateral recumbent position by lumbar puncture and conventional manometry. In patients who underwent a CSF drainage procedure such as ventriculo-peritoneal shunt or external ventricular drainage before lumbar puncture, the opening pressure could not be measured, and we imputed their data with a value of 60 cm of H2O. In addition to routine analysis, at enrollment, we stored 2 mL of the CSF at −80°C for measurement of albumin, MMP9, and its tissue inhibitor (TIMP1) levels.
Laboratory methods
We used commercially available solid phase sandwich ELISA kits to measure MMP9 and TIMP1 levels in the CSF (Quantikine®, Human MMP9 [catalog no. DMP900] and TIMP1 [catalog no. DTM100] immunoassays, R&D systems, Minneapolis, USA). The MMP9 assay measures 92 kDa pro- and 82 kDa active forms, but not the 65 kDa form of human MMP9. We tested the samples in duplicate and took the average. We measured CSF albumin levels by an immunoturbidimetric assay (MAL kits, Erba Mannheim, Germany). We calculated the albumin index (AI) as, AI = AlbuminCSF / AlbuminSerum. Albumin index is a standard measure of BBB permeability [13,14]. After the conclusion of the study, we tested the remaining CSF samples for Mycobacterium tuberculosis by an automated cartridge-based nucleic acid amplification test (Xpert MTB/RIF, Cepheid, Sunnyvale, USA).
Assessment of treatment outcome
We followed the patients up to the time of death, treatment completion, or study completion whichever was earlier. The last enrolled patients were followed up for a minimum of 3 months. We obtained the details of patients who died at home after hospital discharge by contacting their kin over telephone. We defined good outcome (Controls) as survival without severe neurological disability (modified Rankin scale scores of 0,1, and 2) [12,15]. We classified the remainder (death or severe neurological disability; scores 3 to 5) as poor outcome (Cases). Two observers independently scored the disability using modified Rankin scale, and resolved any disagreement by consensus before the measurement of MMP9/TIMP1 levels.
Sample size calculation
In a previous study, the difference in MMP9 levels between patients with good vs. poor outcome was 194 ng/mL [13]. To detect a difference of this magnitude between two groups assuming a standard deviation of 186 ng/mL, with 80% power at α = 0.05 significance level, we calculated that 15 patients would be required in each group. Providing an allowance of 30% for nonparametric analysis, we required 20 patients in each group. Thus, we needed to enroll 40 patients with tuberculous meningitis in total, given that about 50% of adults would have a good treatment outcome.
Statistical analysis
We used a statistical software package (Stata/IC 12.1 for Windows, StataCorp LP, College Station, Texas, USA) for all analyses. We divided the study sample into two groups—good and poor outcome groups. We compared the clinical and laboratory variables between good vs. poor outcome groups. We summarize normally distributed continuous variables as mean ± SD and tested between group comparisons by independent t-test. We present continuous variables with a skewed distribution such as MMP9 and TIMP1 levels and ordinal variables such as the GCS score as median (interquartile range, IQR) and compared them by Wilcoxon rank-sum test. We summarize categorical variables as frequency with proportion (n [%]), and compared them using Fisher’s exact test. Further, we tested the correlation of MMP9 and TIMP1 levels with albumin index and other laboratory variables by Spearman’s rank correlation. We did not adjust for multiple comparisons. We also performed a post hoc exploratory analysis comparing the MMP9 levels between patients that died in hospital and the rest who were alive at hospital discharge. All tests were two-sided, and a P < 0.05 was considered statistically significant.
Results
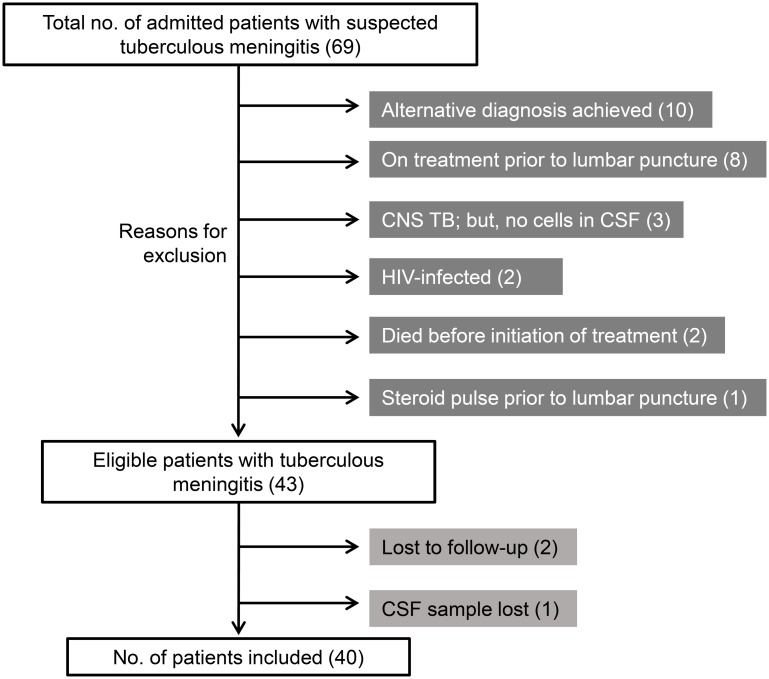
We enrolled 69 patients with clinically suspected tuberculous meningitis over a period of 17 months. After excluding 29 patients for reasons listed in Fig 1, we finally included 40 patients in the present study.
Fig 1. Schematic showing the enrollment of patients and reasons for exclusion.
CNS = central nervous system; CSF = cerebrospinal fluid; HIV = human immunodeficiency virus; TB = tuberculosis.
Clinical characteristics
The mean age of the patients was 36 ± 16 years. Twenty-three (58%) patients were men. Most patients presented after 2 weeks of symptom onset (median [IQR] 20 [14–60] days). Nine (23%) patients had stage 1, 15 (38%) had stage 2, and 16 (40%) had MRC stage 3 disease. In all, 17 (43%) patients required airway intubation and mechanical ventilation. About half the patients (19 [48%]) had systemic symptoms suggestive of TB, and 7 (18%) patients had had TB in the past (Table 1).
Table 1. Correlates of treatment outcome in 40 patients with tuberculous meningitis.
| Characteristic | All patients (N = 40) |
Good outcome (n = 22) |
Poor outcome (n = 18) |
P-value |
|---|---|---|---|---|
| Age, yearsa | 36 ± 16 | 33 ± 13 | 40 ± 17 | 0.169 |
| Male gender | 23 (58) | 10 (45) | 13 (72) | 0.088 |
| Duration of symptoms, daysb | 20 (14–60) | 20 (14–60) | 20 (10–60) | 0.682 |
| Seizures | 10 (25) | 3 (14) | 7 (39) | 0.140 |
| TB in the past | 7 (18) | 4 (18) | 3 (17) | 1.000 |
| BCG scar | 23 (62) | 14 (67) | 9 (56) | 0.517 |
| GCS scoreb | 12 (9–14) | 14 (11–15) | 9 (8–11) | 0.001 |
| Focal neurological deficit | 9 (23) | 4 (18) | 5 (28) | 0.705 |
| Cranial nerve palsy | 15 (38) | 7 (32) | 8 (45) | 0.412 |
| MRC Grade 1 Grade 2 Grade 3 |
9 (23) 15 (38) 16 (40) |
7 (32) 10 (45) 5 (23) |
2 (11) 5 (28) 11 (61) |
0.05 |
| Hydrocephalus | 17 (43) | 8 (36) | 9 (50) | 0.385 |
| CSF drainage procedures | 12 (30) | 4 (18) | 8 (44) | 0.093 |
| Time to treatment initiation, daysb | 4 (2–5) | 3 (1–5) | 4 (2–6) | 0.26 |
| Blood hemoglobin, g/dLa | 11.93 ± 2.2 | 10.8 ± 1.7 | 13.3 ± 1.9 | < 0.001 |
| Total leukocyte count, cells/μLa | 10030 ± 4196 | 8668 ± 2867 | 11694 ± 4990 | 0.031 |
| CSF opening pressure, cm of H2Ob | 30 (16–48) | 25 (16–40) | 39 (25–58) | 0.115 |
| CSF leukocyte count, cells/μLb | 190 (75–315) | 160 (70–280) | 200 (80–330) | 0.540 |
| CSF lymphocyte percentageb | 73 (30–90) | 69 (44–89) | 78 (23–90) | 0.795 |
| CSF protein, mg/dLb | 151 (114–220) | 140 (116–171) | 158 (98–225) | 0.471 |
| CSF glucose, mg/dLb | 29 (21–41) | 29 (17–39) | 29 (21–47) | 0.807 |
| CSF adenosine deaminase, U/Lb | 14 (9–19) | 11 (9–16) | 17 (14–22) | 0.091 |
| CSF albumin, mg/La | 448 ± 120 | 477 ± 83 | 412 ± 147 | 0.106 |
| Serum albumin, g/dLa | 3.6 ± 0.62 | 3.7 ± 0.6 | 3.4 ± 0.50 | 0.116 |
| Albumin indexa | 0.013 ± 0.004 | 0.0132 ± 0.0031 | 0.0125 ± 0.0050 | 0.607 |
a data presented as mean ± SD;
b data presented as median (IQR); all other data presented as n (%).
BCG = bacille Calmette-Guerin; CSF = cerebrospinal fluid; GCS = Glasgow coma scale; MRC = Medical Research Council; TB = tuberculosis
CT scan of the brain was abnormal in 28 (70%) patients—17 (43%) had hydrocephalus; 9 (23%) had basal exudates; 8 (20%) had tuberculomas; and 5 (13%) patients had infarcts. Chest radiographic abnormalities were found in 18 (45%) of 40 patients; 6 (15%) had miliary nodules; and 1 patient had bilateral exudative, lymphocytic pleural effusions with high ADA level. High-resolution CT scan of the chest showed findings suggestive of TB in 5 patients. In total, 19 (48%) patients had evidence of TB elsewhere—18 patients had chest radiographic features of TB; 2 patients had TB lymphadenitis (in one it was confirmed by lymph node biopsy which showed caseating granulomatous inflammation; in the other patient, necrotic lymph nodes were present in the neck, mediastinum and abdomen on imaging); and 1 patient each had encysted ascites and Pott’s spine (numbers not mutually exclusive). M. tuberculosis was detected in CSF by Xpert MTB/RIF in 10 patients. Rifampicin-resistance was not identified in any of the positive samples. Of the 40 patients, 10 (25%) had definite, 19 (48%) had probable and 11 (28%) had possible tuberculous meningitis as per the consensus case-definition.
Treatment outcome
All patients received first-line anti-TB drugs—isoniazid (H), rifampicin (R), pyrazinamide (Z), ethambutol (E) and/or streptomycin (S) along with pyridoxine supplementation. The median time to treatment initiation was 4 (2–5) days. Treatment was started after 1 week in 5 patients. Fifteen (38%) patients received HRZE regimen; 22 (55%) received HRZS regimen; and the remaining 3 patients received HRZES regimen during the intensive phase of treatment. All patients except one received adjunctive dexamethasone.
The median duration of follow-up was 106 (range 5–365) days. Eleven (28%) patients died in hospital. Of the 29 patients discharged alive from the hospital, 4 patients died at home—3 of them died within 1 month and 1 patient died after 3 months of hospital discharge. The total mortality in the study sample was 15 (38%). Of the 25 patients that were alive at study completion, 3 (18%) patients had persistent neurological disability. Thus, the outcome was poor in 18 (45%) patients in total; 22 (55%) patients had a good outcome—4 were totally asymptomatic, 7 had a modified Rankin score of 1, and 11 patients had a score of 2 (S1 Fig).
Clinical correlates of treatment outcome
On comparison, lower GCS score at presentation was significantly associated with a poor outcome (P = 0.001; Table 1). Most patients with a poor outcome had MRC grade 3 meningitis at presentation, whereas a little less than a fourth of patients with good outcome had grade 3 disease (P = 0.05). Blood hemoglobin concentration and total leukocyte count were significantly higher in patients with poor outcome (Table 1). Although the CSF opening pressure was higher in patients with poor outcome, the difference was not statistically significant. There were no significant differences between the two groups with respect to CSF characteristics such as albumin index, total leukocyte count, glucose and protein levels. Median time to treatment initiation was similar in the two groups (Table 1).
Correlation of MMP9 and TIMP1 with CNS inflammation
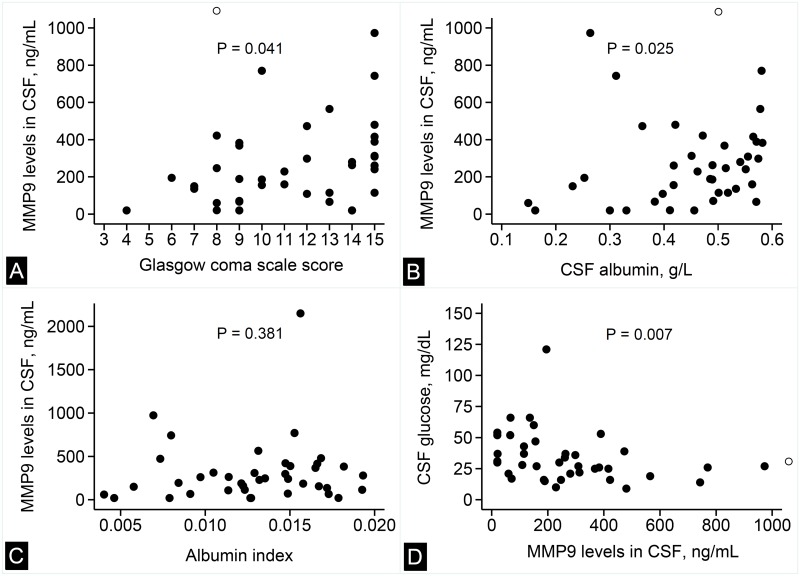
MMP9 levels in the CSF had a significant positive correlation with the GCS score (Spearman’s rho = 0.324; P = 0.041; Fig 2A) and CSF albumin levels (Spearman’s rho = 0.354; P = 0.025; Fig 2B). However, the MMP9 levels did not correlate with albumin index (Spearman’s rho = 0.142; P = 0.381; Fig 2C). The MMP9 levels showed a significant negative correlation with CSF glucose levels (Spearman’s rho = −0.419; P = 0.007; Fig 2D). Further, MMP9 levels did not correlate with neutrophil counts in the CSF (Spearman’s rho = 0.133; P = 0.413; S2 Fig).
Fig 2. Correlation of MMP9 levels with clinical and laboratory variables.
Scatter plots showing the correlation between MMP9 in the CSF with Glasgow coma scale score (panel A), CSF albumin levels (panel B), albumin index (panel C), and CSF glucose levels (panel D). In panels A,B and D, the hollow circle indicates an extreme value of CSF MMP9 level (2150 ng/mL). P-values are for Spearman’s rank correlation.
There was no apparent correlation between MMP9 and TIMP1 levels in the CSF (Spearman’s rho = 0.149; P = 0.358; S3 Fig). TIMP1 levels positively correlated with CSF protein (Spearman’s rho = 0.342; P = 0.031; S4 Fig) and ADA levels (Spearman’s rho = 0.327; P = 0.048; S5 Fig). Although the CSF opening pressure did not show a significant correlation with MMP9 levels or albumin index, it correlated positively with the CSF neutrophil count (Spearman’s rho = 0.368; P = 0.03; S6 Fig).
MMP9, TIMP1, and treatment outcome
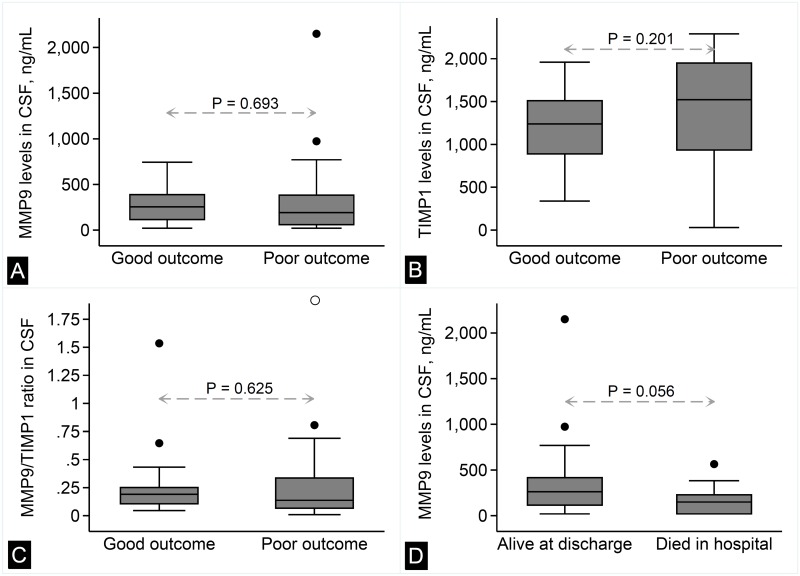
The median MMP9 level in the CSF was 254 (115–389) ng/mL in patients with good outcome and 192 (60–383) ng/mL in patients with poor outcome. This difference, however, was not statistically significant (Fig 3A; P = 0.693). Similarly, there was no significant difference in TIMP1 level between the two groups (Fig 3B; 1239 [889–1511] vs. 1522 [934–1949] ng/mL; P = 0.201). MMP9/TIMP1 ratio that reflects net proteolytic activity was also not different between the two groups (Fig 3C; 0.191 [0.107–0.250] vs. 0.163 [0.067–0.34]; P = 0.625). On exploratory analysis, the apparently lower level of MMP9 in patients with poor outcome was more pronounced when patients that died in hospital (early deaths) were compared to the rest (150 [20–229] vs. 263 [115–416]; P = 0.056; Fig 3D).
Fig 3. Treatment outcome and CSF levels of MMP9, TIMP1, and MMP9/TIMP1 ratio.
Box-whisker plots showing the comparison of MMP9 levels (panel A), TIMP1 levels (panel B), and MMP9/TIMP1 ratio (panel C) between tuberculous meningitis patients with good vs. poor treatment outcome. Panel D depicts the comparison of MMP9 levels between patients that died in hospital and the rest. P-values are for Wilcoxon rank-sum test.
Discussion
We found that tuberculous meningitis patients with a poor treatment outcome did not have higher MMP9 levels in the CSF at baseline. The MMP9 levels did not correlate with markers of BBB permeability such as albumin index and CSF opening pressure. Instead, MMP9 and TIMP1 levels nonspecifically correlated with markers of intrathecal inflammation such as CSF glucose, protein, and ADA levels. We also found that early deaths were associated with a lower level of MMP9, which was quite contrary to our study hypothesis.
There are only a handful of published studies on MMP9 levels in patients with tuberculous meningitis. Rom and colleagues reported for the first time in 1996 that M. tuberculosis upregulated the expression of MMP9 [16]. In the year 2000, Matsurra et al reported that CSF MMP9 levels were elevated in tuberculous meningitis (n = 7) as compared to aseptic meningitis (n = 9), and patients with severe disease had higher levels of MMP9 [9]. Subsequently, Lee et al confirmed that MMP9 levels were significantly higher in tuberculous meningitis (n = 24) even after Day 7 of treatment, and higher levels were associated with neurological complications [10]. However, both these studies did not follow a standard definition of poor outcome. Price et al reported in 2001 that MMP9 concentration per CSF leukocyte was significantly higher in fatal tuberculous meningitis (n = 23) [7]. Further, patients with low GCS score, confusion, or focal neurological deficit had significantly higher levels of MMP9 per CSF leukocyte.
However, a subsequent study from the same setting (n = 21) did not find a statistically significant difference in CSF MMP9 levels between survivors and the rest [13]. On the other hand, in a sub-study of a large clinical trial again from the same setting, patients treated with adjunctive dexamethasone (n = 11) had a significant fall in MMP9 levels by Day 5 of treatment [17]. In the absence of any demonstrable effect on intrathecal immune response [18], the authors ascribed the efficacy of dexamethasone to the fall in MMP9 levels. In an ex vivo experimental model, MMP blockade prevented BBB disruption by M. tuberculosis [19]. Notwithstanding, we found that MMP9 levels did not correlate with albumin index, a standard in vivo measure of BBB permeability. Previously, Thwaites et al and Green et al have also documented a lack of association between MMP9 levels and albumin index in vivo [13,17]. Thus, taken together, available evidence on the relation between MMP9 levels and treatment outcome in tuberculous meningitis is equivocal. Further, previous studies were limited by small sample sizes and insufficient number of patients experiencing unfavorable outcome. Moreover, these studies did not examine the clinically relevant outcome of survival without severe neurological disability, an outcome which has been empirically validated in multiple randomized controlled trials [2,12].
Very recently, a study of 36 HIV-negative tuberculous meningitis patients has been reported from northern India [20]. In this study, MMP9 levels were not associated with treatment outcome similar to ours. However, for unclear reasons, the median MMP9 levels reported in this study were much lower than our findings (4962 pg/mL vs. 235 ng/mL). Moreover, this study did not assess TIMP1 levels and BBB permeability. MMP9 levels observed in our study are comparable to earlier reports by Thwaites et al and Green et al [13,17].
The present study has several strengths—first, it was larger than previous studies, with sufficient number of patients in good as well as poor outcome groups; second, we studied the clinically relevant outcome of survival without neurological disability, and two observers independently scored the disability before estimating MMP9/TIMP1 levels to eliminate bias; third, we followed the recently proposed international consensus case-definition, in order to minimize difficulties in diagnosing tuberculous meningitis; and finally, we also studied the relationship between MMP9 levels and BBB permeability.
On the other hand, there are three possible limitations to this study. First, we imputed the CSF opening pressure values in patients that underwent a CSF drainage procedure before lumbar puncture. This was necessary from a safety perspective, and it seems logical to presume that such patients would have had high opening pressures. Second, drug-resistance could have confounded our results. However, no case of rifampicin-resistance was identified in this study, and as such multidrug-resistance is uncommon among tuberculous meningitis patients in India [21]. Third, we could have used gelatin zymography or a fluorogenic substrate-based assay, which also detect lower molecular weight isoforms of MMP, to measure enzyme activity levels in the CSF and to differentiate active MMP from pro-MMP. We estimated MMP9 levels by ELISA to enable comparison with previous pivotal studies which had used this assay [13,17].
One possible explanation for the lack of association between MMP9 levels and treatment outcome could be that not all deaths in tuberculous meningitis are attributable to CNS inflammation. Of note, in the present study, 4 of the 15 deaths occurred after hospital discharge. It is possible that these late deaths were attributable to poor CNS penetration by anti-TB drugs rather than CNS inflammation. However, our exploratory analysis looking at in-hospital deaths (early deaths) does not lend support to this explanation.
Apart from failing to prove the study hypothesis, the present study has two other important findings. First, the elevated MMP9 levels appear to be a nonspecific reflection of intrathecal inflammation as evidenced by the correlation of MMP9 and TIMP1 levels with CSF glucose, albumin, and ADA levels. The natural history of CSF inflammation in untreated tuberculous meningitis is characterized by progressive increase in protein levels accompanied by decreasing glucose levels on serial analysis [22,23]. Longitudinal changes in CSF protein, glucose, and possibly MMP9 and TIMP1 levels prior to treatment might yield spurious correlations between these variables on a cross-sectional analysis. Although statistically significant, such correlations may not have any biological significance. Second, the current finding that higher MMP9 levels were associated with better GCS scores and in-hospital survival is counterintuitive. Proving the association between elevated levels of MMP9 and treatment outcome is important to justify a clinical trial of adjunctive treatment with already licensed drugs having MMP-inhibitory activity, such as doxycycline. In fact, several authors have made a case for doxycycline as adjunctive treatment in TB [4,6,8,24]. However, our findings temper this enthusiasm, at least in the case of tuberculous meningitis.
One could reasonably argue that it is not necessary to demonstrate a difference in MMP9 levels, since MMP9 is anyway damaging to the tissues. However, the association of higher MMP9 levels with better GCS scores suggests a possibility that MMP9 inhibition could be counterproductive, since altered consciousness is an important prognostic factor in tuberculous meningitis [25,26]. Notwithstanding the fact that MMPs disrupt the BBB, biologically the association of elevated MMP9 levels with better survival is not implausible—a leaky BBB could enhance tissue penetration of anti-TB drugs, many of which poorly penetrate the BBB otherwise [27]. Hence, before embarking on a clinical trial of MMP inhibitors in tuberculous meningitis, it would be prudent to refute or confirm the present findings in a larger cohort of patients with tuberculous meningitis.
Conclusions
We found that pretreatment MMP9 levels were not associated with treatment outcome in patients with tuberculous meningitis. Moreover, we did not find any correlation between MMP9 levels and BBB permeability. On the other hand, MMP9 and TIMP1 levels showed nonspecific correlation with some CSF inflammation variables. Hence, further studies are warranted to justify a clinical trial of MMP9 inhibitors as adjunctive treatment in tuberculous meningitis, which remains the most lethal form of TB.
Supporting information
(JPG)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
Part of this work was presented at the 5th annual conference of the Clinical Infectious Diseases Society, India held at New Delhi (August 21–23, 2015).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by an intramural research grant (No. Edn.7(1)/2013/25) to TK and SM from the Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. In addition, the authors (SM, GVD, TK) used personal funds to procure Xpert MTB/RIF cartridges for this study.
References
- 1.Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J; British Infection Society. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59: 167–187. doi: 10.1016/j.jinf.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 2.Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2016;4: CD002244 doi: 10.1002/14651858.CD002244.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heemskerk AD, Bang ND, Mai NT, Chau TT, Phu NH, Loc PP, et al. Intensified Antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med. 2016;374: 124–134. doi: 10.1056/NEJMoa1507062 [DOI] [PubMed] [Google Scholar]
- 4.Elkington PT, Ugarte-Gil CA, Friedland JS. Matrix metalloproteinases in tuberculosis. Eur Respir J. 2011;38: 456–464. doi: 10.1183/09031936.00015411 [DOI] [PubMed] [Google Scholar]
- 5.Salgame P. MMPs in tuberculosis: granuloma creators and tissue destroyers. J Clin Invest. 2011;121: 1686–1688. doi: 10.1172/JCI57423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327: 466–469. doi: 10.1126/science.1179663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price NM, Farrar J, Tran TT, Nguyen TH, Tran TH, Friedland JS. Identification of a matrix-degrading phenotype in human tuberculosis in vitro and in vivo. J Immunol. 2001;166: 4223–4230. [DOI] [PubMed] [Google Scholar]
- 8.Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol. 2015;15: 255–263. doi: 10.1038/nri3813 [DOI] [PubMed] [Google Scholar]
- 9.Matsuura E, Umehara F, Hashiguchi T, Fujimoto N, Okada Y, Osame M. Marked increase of matrix metalloproteinase 9 in cerebrospinal fluid of patients with fungal or tuberculous meningoencephalitis. J Neurol Sci. 2000;173: 45–52. [DOI] [PubMed] [Google Scholar]
- 10.Lee KY, Kim EH, Yang WS, Ryu H, Cho SN, Lee BI, et al. Persistent increase of matrix metalloproteinases in cerebrospinal fluid of tuberculous meningitis. J Neurol Sci. 2004;220: 73–78. doi: 10.1016/j.jns.2004.02.008 [DOI] [PubMed] [Google Scholar]
- 11.Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10: 803–812. doi: 10.1016/S1473-3099(10)70138-9 [DOI] [PubMed] [Google Scholar]
- 12.Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351: 1741–1751. doi: 10.1056/NEJMoa040573 [DOI] [PubMed] [Google Scholar]
- 13.Thwaites GE, Simmons CP, Than Ha Quyen N, Thi Hong Chau T, Phuong Mai P, Thi Dung N, et al. Pathophysiology and prognosis in vietnamese adults with tuberculous meningitis. J Infect Dis. 2003;188: 1105–1115. doi: 10.1086/378642 [DOI] [PubMed] [Google Scholar]
- 14.Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. II. Relation of the concentration of the proteins in serum and cerebrospinal fluid. Scand J Clin Lab Invest. 1977;37: 391–396. doi: 10.1080/00365517709091497 [DOI] [PubMed] [Google Scholar]
- 15.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19: 604–607. [DOI] [PubMed] [Google Scholar]
- 16.Chang JC, Wysocki A, Tchou-Wong KM, Moskowitz N, Zhang Y, Rom WN. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax. 1996;51: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green JA, Tran CT, Farrar JJ, Nguyen MT, Nguyen PH, Dinh SX, et al. Dexamethasone, cerebrospinal fluid matrix metalloproteinase concentrations and clinical outcomes in tuberculous meningitis. PLoS One. 2009;4: e7277 doi: 10.1371/journal.pone.0007277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmons CP, Thwaites GE, Quyen NT, Chau TT, Mai PP, Dung NT, et al. The clinical benefit of adjunctive dexamethasone in tuberculous meningitis is not associated with measurable attenuation of peripheral or local immune responses. J Immunol. 2005;175: 579–590. [DOI] [PubMed] [Google Scholar]
- 19.Brilha S, Ong CW, Friedland JS. The blood-brain barrier is disrupted by matrix metalloproteinases in tuberculous meningitis. J Infect. 2015;71: 683–684. [Google Scholar]
- 20.Rai D, Garg RK, Mahdi AA, Jain A, Verma R, Tripathi AK, et al. Cerebrospinal fluid cytokines and matrix metalloproteinases in human immunodeficiency seropositive and seronegative patients of tuberculous meningitis. Ann Indian Acad Neurol. 2014;17: 171–178. doi: 10.4103/0972-2327.132617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagarathna S, Rafi W, Veenakumari HB, Mani R, Satishchandra P, Chandramuki A. Drug susceptibility profiling of tuberculous meningitis. Int J Tuberc Lung Dis. 2008;12: 105–107. [PubMed] [Google Scholar]
- 22.Perry TL. Natural history and pathogenesis of miliary and meningeal tuberculosis in children; analysis of 163 cases. Pediatrics. 1950;5: 988–997. [PubMed] [Google Scholar]
- 23.Lincoln EM, Sordillo VR, Davies PA. Tuberculous meningitis in children. A review of 167 untreated and 74 treated patients with special reference to early diagnosis. J Pediatr. 1960;57: 807–823. [DOI] [PubMed] [Google Scholar]
- 24.Walker NF, Clark SO, Oni T, Andreu N, Tezera L, Singh S, et al. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am J Respir Crit Care Med. 2012;185: 989–997. doi: 10.1164/rccm.201110-1769OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasar KK, Pehlivanoglu F, Sengoz G. Predictors of mortality in tuberculous meningitis: a multivariate analysis of 160 cases. Int J Tuberc Lung Dis. 2010;14: 1330–1335. [PubMed] [Google Scholar]
- 26.Erdem H, Ozturk-Engin D, Tireli H, Kilicoglu G, Defres S, Gulsun S, et al. Hamsi scoring in the prediction of unfavorable outcomes from tuberculous meningitis: results of Haydarpasa-II study. J Neurol. 2015;262: 890–898. doi: 10.1007/s00415-015-7651-5 [DOI] [PubMed] [Google Scholar]
- 27.Donald PR. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb). 2010;90: 279–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(JPG)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.