Abstract
BACKGROUND
Recent research in humans and rodents has explored the use of deep brain stimulation (DBS) of the ventral capsule/ventral striatum (VS) as a possible treatment for drug addiction. However, the optimum electrode placement and optimum DBS parameters have not been thoroughly studied. Here we varied stimulation sites and frequencies to determine whether DBS of the VS could facilitate the extinction of morphine-induced conditioned place preference in rats.
Methods
Rats were implanted with DBS electrodes in the dorsal or ventral subregions of the VS and trained to the morphine-CPP. Subsequently, rats received extinction sessions over 9 days, combined with 60 min of either high (130 Hz) or low (20 Hz) frequency DBS. To study circuit-wide activations after DBS of the VS, c-fos immunohistochemistry was performed in regions involved in the extinction of drug seeking behaviors.
Results
High frequency DBS of the dorsal-VS impaired both extinction training and extinction memory, whereas high frequency DBS of the ventral-VS had no effect. In contrast, low frequency DBS of the dorsal-VS strengthened extinction memory when tested 2 or 9 days after the cessation of stimulation. Both DBS frequencies increased c-fos expression in the infralimbic prefrontal cortex, but only low frequency DBS increased c-fos expression in the basal amygdala and the medial portion of the central amygdala.
Conclusions
Our results suggest that low frequency (rather than high frequency) DBS of the dorsal-VS strengthens extinction memory and may be a potential adjunct for extinction-based therapies for treatment-refractory opioid addicts.
Keywords: DBS, ventral striatum, extinction, morphine, CPP, amygdala
Introduction
Deep brain stimulation (DBS) of the ventral capsule/ventral striatum (VC/VS) has been shown to reduce symptoms in treatment-refractory patients with obsessive-compulsive disorder (1), as well as facilitate their responses to extinction-based therapies (2, 3). Recently, DBS of the VC/VS has been suggested as a promising target for treatment-refractory drug addiction (4, 5). Drug addiction is characterized by the persistence of maladaptive behaviors such as compulsion to seek the drug (6), suggesting a deficit in circuits that regulate the extinction of addictive behaviors (7, 8). Thus, given that VC/VS represents a central region of the reward circuit that receives projections from areas that regulate the extinction of drug seeking (7, 9, 10), DBS of this region could represent a clinical tool for understanding the mechanisms of drug addiction.
DBS is usually delivered at frequencies of 90 Hz or more (11). In humans, high frequency DBS of the VC/VS has been shown to reduce symptoms of addiction to alcohol (12–14), nicotine (13, 15), and heroin (16). Preclinical studies in rats have demonstrated that high frequency DBS of the VS is effective in reducing drug seeking behaviors for ethanol (17, 18), cocaine (19, 20), heroin (21), and morphine (22, 23). However, in some patients, addictive symptoms remain unchanged or worsen after high frequency DBS (12, 24, 25). As an alternative to high frequency DBS, low-frequency DBS (≤ 20 Hz) has been recently suggested as a treatment for addiction (26–28). In rats, low-frequency DBS of the VS attenuated cocaine relapse (29), and abolished cocaine sensitization when combined with pharmacological treatments (28).
Although prior studies of DBS have focused on the expression and relapse of addictive behaviors (4, 5), none have examined the effects of DBS on the extinction of addiction, which is the basis of exposure-based therapies for addictive disorders (30, 31). Extinction is a form of learning in which associations between cues and the drug are weakened by repeated exposure to the cues in the absence of the drug (30, 32). Thus, strengthening the extinction of drug-associated memories in addicted patients may reduce the risk of relapse. We therefore assessed the effects of DBS of the VS on extinction of morphine conditioned place preference (CPP) in rats. We applied either high or low frequency DBS to distinct subregions of the VS previously shown to either enhance or impair extinction of conditioned fear (33). Using an immunohistochemical approach, we also assessed the effects of high and low frequency DBS on cellular activity within regions necessary for extinction of drug seeking.
Materials & Methods
Subjects
Eighty-seven adult male Sprague Dawley rats (~350g; Harlan Laboratories) were individually housed with food and water available ad libitum (12:12 hour light/dark cycle; 64°F, 30% humidity). The behavioral experiments were performed during the light phase of the cycle, and all procedures were in accordance with the Institutional Animal Care and Use Committee of the University of Puerto Rico, Medical Sciences Campus, and the Association for Assessment and Accreditation of Laboratory Animal Care.
Surgery
Rats were anesthetized with isofluorane inhalant gas (5% for induction), and positioned in a stereotaxic frame (2–3% for maintenance). Rats were bilaterally implanted with concentric bipolar stimulating electrodes (NEX-100; Rhodes Medical Instruments) as previously described (33, 34). Electrodes in the dorsal-VS site were aimed at −6.5 mm DV, ± 3.5 mm ML and +1.2 mm AP, whereas the ventral-VS site coordinates were −8.0 mm DV, ± 3.5 mm ML and +1.2 mm AP (35). Electrodes were fixed to the skull with anchoring screws, C&B-metabond (Parkell, Inc), and acrylic cement. Finally, a topical antibiotic (Neomycin Sulfate, Certi-Sporyn™) and an analgesic (Ketofen; 5 mg/kg, intramuscular) were applied. Rats were allowed 7–10 days of recovery.
Drug
Morphine sulfate (Sigma Aldrich, St. Louis MO; 5mg/kg) was dissolved in saline (0.2 ml/100g of body weight) and administered to both sham and stimulated groups. The dose, time course (4 days), and route of administration (subcutaneously) were selected based on studies showing efficacy in inducing CPP without affecting locomotion (32, 36).
Conditioned Place Preference (CPP)
The CPP was performed as previously described (37). Briefly, the CPP apparatus consists of an acrylic chamber (42cm long × 30cm high × 42cm high) separated into two compartments with an entry containing a removable guillotine door. Compartments consisted of grated-texture flooring with black and white checker walls, or smooth flooring with black and white lined walls. All CPP protocols were performed at semi-darkness conditions (~10 lux). Behavioral data was acquired using the Any-Maze tracking system (Stoelting Co).
On days 1 (habituation) and 2 (baseline), animals were allowed to move freely between compartments for 20 min, and the preferred and non-preferred sides were noted. During the conditioning phase, rats were injected with morphine and restricted to the non-preferred side (drug-paired side) for 45 min, or injected with saline and restricted to the preferred side (saline-paired side) for the same amount of time. A total of 4 injections of either morphine or saline were administered during the conditioning sessions (days 3–10, alternating the days). Three days after the last conditioning session (day 13, expression test), rats were given a drug-free test of 20 min in which they were allowed to move freely between both compartments of the CPP chamber. The amount of time spent (%) in the drug-paired side was calculated as an index of conditioning.
On the extinction phase, DBS was continuously delivered for 60 min in three blocks of 20 min: the first block in the home cage, the second in the CPP chamber combined with extinction sessions, and the last block in the home cage again. This duration of stimulation was selected based on previous studies showing that 60 min of DBS is sufficient to produce behavioral and neuroplasticity changes in rodents (4, 38, 39). We therefore divided the 60 min of stimulation in three blocks of 20 min between the home cage and the CPP chamber for the following reasons. 1) Our CPP-extinction sessions consisted of 20 min, 2) DBS effects of the VS usually start after a period of adjustment that may take several minutes (~30) (38, 39) and, 3) previous studies in rodents have applied DBS during a range of time that includes not only the behavioral test, but also the pre- and post-test periods (18, 23, 33). A total of 11 or 9 extinction sessions were performed for high (days 14–24) or low (days 14–21) frequency DBS, respectively. Similar to the expression test, rats were allowed to move freely between compartments during each extinction session. The percentage of time spent in the previous drug-paired side was measured as an index of extinction. In the last two days of extinction, the stimulator was turned off to evaluate long-lasting effects of DBS.
Deep Brain Stimulation (DBS)
DBS was delivered as previously reported (33, 34). Briefly, the VS was bilaterally aimed with a concentric bipolar electrode with each polar end measuring 0.5 mm and 0.5 mm apart (NEX-100; Rhodes Medical Instruments). The DBS parameters used for high- and low frequency stimulation were similar to those used in humans and rodents (100–200 μA, 0.1 ms pulse duration, 130 Hz or 20 Hz) (29, 33, 40, 41). The stimulator (S88X) and constant current unit (SIC-C Isolation Unit; Grass Instruments) were connected to a commutator (Plastics One) through a cable that delivered the stimulation to the implanted electrodes.
Histology
Rats were deeply anesthetized with sodium pentobarbital (450 mg/kg, i.p.) and transcardially perfused (10 min) with saline (0.9 %) followed by paraformaldehyde (10%, vol/vol). Brains were stored in a 30 % sucrose-formalin solution for 48 h before sectioning. Coronal sections (40 μm thick) were cut in a cryostat and mounted on gelatin-coated slides. Sections were stained for with cresyl violet, cover slipped with DPX mounting media, and examined in a microscope. Placements outside the ventral striatum were excluded.
Immunohistochemistry
Similar to the behavioral experiments, naïve rats received daily 1 h of high frequency, low frequency or sham stimulation to the dorsal-VS for 6 consecutive days in their home cages. We used naïve rats in order to determine the effects of DBS on neural circuits independent of the effects of morphine exposure or extinction training. Although the effect of high frequency DBS of the VS on the expression of c-fos has been previously evaluated (20, 34), no previous study has investigated the effect of low frequency DBS. We therefore, decided to match the stimulation regimen (i.e. frequencies and duration) used in our behavioral experiments to address this question. On day 6, rats were deeply anesthetized with sodium pentobarbital (450 mg/Kg intraperitoneal) one hour after receiving 1 h of DBS or sham stimulation. They were perfused transcardially with 100 ml of 0.9 % saline followed by 500 ml of 4 % paraformaldehyde in 0.1 M phosphate buffer at pH 7.4. Brains were transferred to a solution of 30 % sucrose in 0.1 M phosphate buffer at 4°C during 48 h for cryoprotection. Brains were frozen and coronal sections (40 μm) were cut on a cryostat (CM 1850; Leica) at the level of frontal cortex, VS and amygdala areas. For c-fos immunohistochemistry, sections were blocked in 2 % normal goat serum (Vector Laboratories) plus 0.3 % triton X-100 (Sigma-Aldrich) in 0.12 M potassium buffer saline for 1 h, as previously described (34). The sections were then incubated overnight at room temperature (RT) with rabbit anti-c-fos serum (Ab-5, Oncogene Science; 1:10,000). Sections were then incubated for 2 h at RT in a solution of biotinylated goat anti-rabbit IgG (Vector Laboratories) and placed in a mixed avidin-biotin horseradish peroxidase complex solution (ABC Elite Kit, Vector Laboratories) for 90 min. Black immunoreactive nuclei labeled for c-fos were visualized after 10 min of exposure to DAB/peroxidase substrate kit (Vector Laboratories). Sections were mounted in coated-gelatin slides, dehydrated and cover slipped. Counter sections were collected, stained, cover slipped and used to determine electrode placement, as well as the anatomical boundaries of each structure analyzed.
Immunoreactivity quantification
Counts of the number of c-fos immunoreactive neurons were carried out at 20X magnification with an Olympus microscope (Model BX51). We restricted our analysis to several areas involved on the extinction of drug seeking behaviors (10). Images were generated for prelimbic (PL) and infralimbic (IL) subregions of the prefrontal cortex (PFC), core and shell subregions of the nucleus accumbens/ventral striatum (NAc/VS), basal nucleus of the amygdala (BA), lateral portion of the central nucleus of the amygdala (CeL), and medial portion of the central nucleus of the amygdala (CeM). Positive c-fos-like immunoreactivity showed brown-black staining distinct from the background. c-fos positive cells were automatically counted and averaged for each hemisphere at 3–4 different rostrocaudal levels of each structure (Metamorph software 6.1). The density of c-fos positive neurons was calculated by dividing the number of c-fos positive neurons by the total area of each region.
Statistical analyses
Student’s t-tests and ANOVA analyses were performed to determine statistical differences in behavioral and immunohistochemistry experiments. Tukey post hoc analyses were used for multiple comparisons. Data is presented as mean ± standard error of the mean (SEM) and statistical significance was established as *p ≤ 0.05. All statistical analyses were performed using the Statistica software (6.0, Statsoft®, Tulsa).
Results
High frequency DBS of the dorsal-VS impairs extinction of morphine place preference
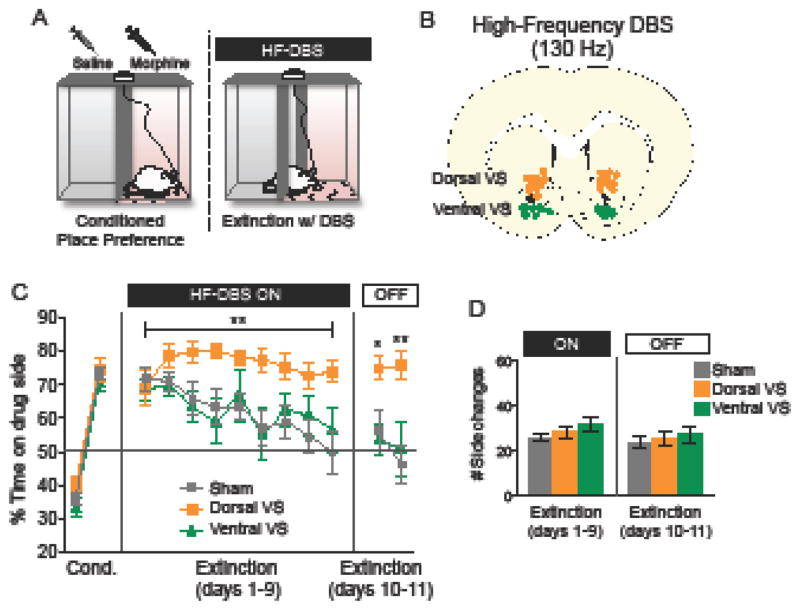
Rats were first conditioned to exhibit place preference for morphine, over 8 days. Morphine was injected and rats were placed in their non-preferred side, similar to previous studies (22, 23). Next, rats were given 9 days of extinction, during which high frequency DBS was delivered to the VS for 60 min (Fig. 1B). DBS was delivered either to the dorsal (n= 11) or ventral (n= 9) -VS, and compared to sham stimulated controls (n= 23). Because control rats that were implanted with electrodes into the dorsal (n= 12) or ventral (n= 11) regions of the VS showed no statistical differences across the 9 days of extinction (F(1, 21)= 0. 33; p= 0.56), they were combined into a single group. Control rats extinguished to chance levels of preference (50%) by day 9 (49.8 ± 6.1) compared with day 1 (71.80 ± 3.36; F(2,40)= 5.31; p= 0.008; Tukey post hoc; p = 0.001). Rats exposed to high frequency DBS of the dorsal-VS maintained a preference to the morphine-paired side throughout the 9 days of extinction training, as revealed by a significant main effect of treatment (Fig. 1C, orange line; repeated-measures ANOVA; F(2,40)= 5.31; p= 0.008). The impairment of extinction in the dorsal-VS group persisted for two days following the cessation of DBS (main effect of treatment; F(2,40)= 5. 24; p= 0.009; Tukey post hoc, Day 1: p= 0.02 for dorsal-VS versus sham; Day 2: p= 0.0002 for dorsal-VS versus sham).
Figure 1. High frequency DBS of dorsal-VS impairs extinction of morphine-CPP.
(A) Diagram showing the CPP-DBS protocol. (B) Representative coronal section of DBS electrode placement in dorsal and ventral-VS (dorsal and ventral to the anterior commissure). (C) High frequency DBS of the dorsal-VS impaired the extinction of morphine-induced CPP, whereas high frequency DBS of the ventral-VS had no effect. (D) There was no effect of DBS on exploratory behavior/locomotion (side changes/transitions) during the CPP-DBS protocol. Sham: n= 23; Dorsal-VS: n= 11; Ventral-VS: n= 9. Data shown as mean and SEM. *p<0.05; **p<0.01.
High frequency DBS of the ventral-VS had no effect on the extinction of morphine-CPP, either during the 9 days of training (with DBS on) or in the two days afterward (with DBS off, Fig. 1C, green line; all p’s > 0.05). High frequency DBS of either the dorsal or the ventral sites did not affect locomotion, as measured by the number of transitions between compartments (Fig. 1D; One-way ANOVA; DBS on: F(2,40)= 1.59 ; p= 0.21; DBS off: F(2,40)= 0.28 ; p= 0.75).
One possible explanation for the apparent impairment in extinction is that DBS of dorsal-VS may itself be rewarding, causing rats to remain on the drug side in the presence of DBS. Arguing against this, however, a separate group of rats showed that high frequency DBS of dorsal-VS was not sufficient to induce a CPP (% time on DBS side during test day - Sham: 47.71 ± 5.78; DBS: 44.76 ± 4.36; p = 0.68). Considering that DBS was continuously applied between the home cage and the CPP chambers during extinction training, it is possible that DBS in the home cage alone would be sufficient to induce extinction impairment. Although we have not tested the effects of high frequency DBS alone on the extinction of morphine-CPP, a previous study found that high frequency DBS of the dorsal-VS in the home cage had no effect on the expression or the extinction of conditioned fear (33). Nonetheless, further studies are needed to investigate the effects of DBS alone on the extinction of drug seeking behavior.
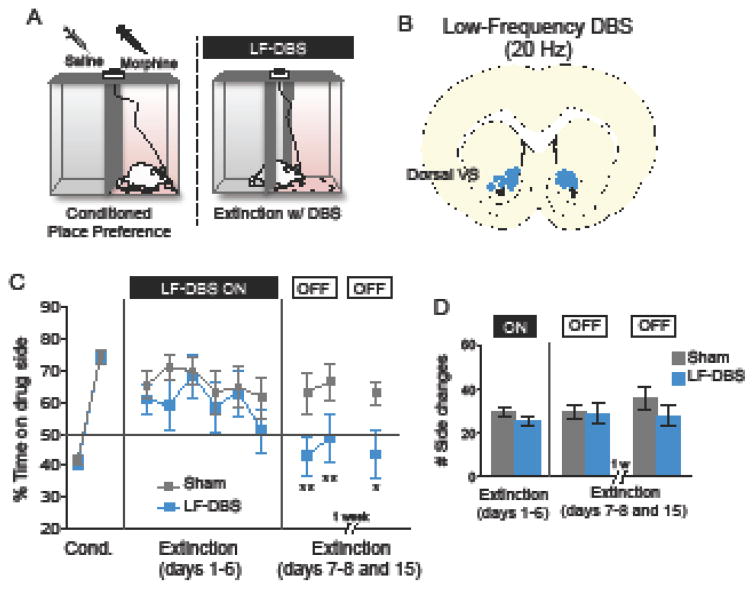
Low frequency DBS of VS strengthens memory for extinction of morphine place preference
Previous studies have demonstrated that low frequency DBS can induce effects opposite to those seen with high frequency DBS in the motor system (11) and in addiction (28, 42). Given that high frequency DBS showed extinction impairment in morphine-CPP, we then hypothesized that low frequency DBS of this same region would have an opposite effect (extinction facilitation). Because high frequency DBS of the ventral portion of the VS had no effect on extinction, rather than investigating the effects of low frequency DBS in this region, we decided to focus on the dorsal-VS (Fig. 2A), the site where extinction was impaired by high frequency DBS (see Fig. 1). Following CPP training, rats were given partial extinction training of 6 days together with low frequency DBS of the dorsal-VS (n= 14), or sham stimulation (n= 15) (Fig. 2B). A partial extinction protocol was used to avoid floor effects caused by complete extinction. Repeated measures ANOVA showed that low frequency DBS of the dorsal-VS had no observable effects across the 6 days of extinction training (Fig. 1C; blue line; F(1,27)= 0.973; p = 0.33). Two days later, however, in the absence of DBS, extinction memory was strengthened as indicated by low levels of side preference (repeated-measures ANOVA; F(1,27) = 5.013; p= 0.033; Tukey post hoc; Day 1: p= 0.003 dorsal-VS versus sham and Day 2: p= 0.009 dorsal-VS versus sham). Enhanced extinction was still observed one week later (extinction day 15; Unpaired t-test; t (12) = 2.27; p = 0.041; DBS: n= 7; Sham: n= 7), suggesting that the DBS-induced enhancement in extinction was long lasting. Low frequency DBS of the dorsal-VS did not affect transitions between CPP compartments (Fig. 2D; Unpaired t-test’s; DBS on: t (27) = 1.49; p = 0.145; DBS off: t (27) = 0.12; p = 0.904), ruling out locomotion effects.
Figure 2. Low frequency DBS of the dorsal-VS strengthens memory for extinction of morphine-induced CPP.
(A) Diagram showing the CPP-DBS protocol. (B) Representative coronal section of DBS electrode placement in the dorsal-VS (dorsal to the anterior commissure). (C) Low frequency DBS of the dorsal-VS reduced the time spent in the drug-paired side during the extinction test (DBS off phase). This effect persisted nine days after stimulation. (D) There was no effect of DBS on exploratory behavior/locomotion (side changes/transitions) during the CPP-DBS protocol. Sham: n= 15; DBS: n= 14. For 1 week test: Sham: n= 7; DBS: n= 7. Data shown as mean and SEM. *p<0.05; **p<0.01.
DBS of VS modulates activity in areas mediating extinction of drug seeking behavior
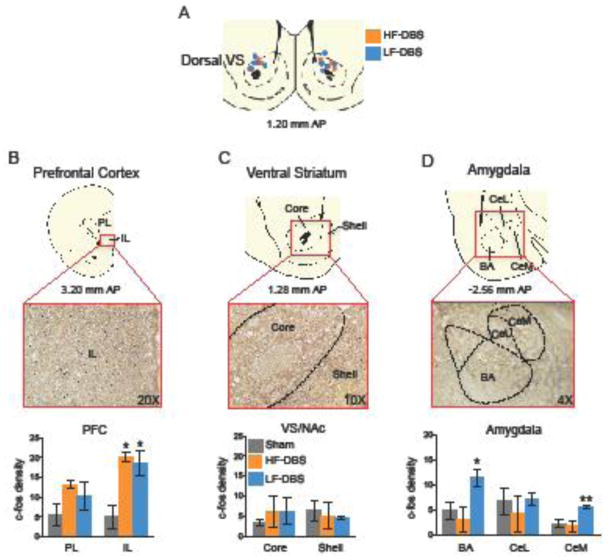
We observed frequency dependent effects of DBS capable of either impairing or enhancing extinction memory. We therefore assessed the effects of both high and low frequency DBS on the activity of reward circuits, as indicated by expression of the immediate early gene c-fos (43–45). To assess the effects of DBS independently from any effects of morphine or CPP training, untrained rats were given high frequency (n= 4), low frequency (n= 5), or sham stimulation (n= 6) in the dorsal-VS for 6 days, 60 min per day (Fig. 3A). Rats were sacrificed 60 min following the last stimulation session.
Figure 3. The effects of high- and low-frequency DBS on c-fos expression in prefrontal, striatal, and amygdala regions.
(A) Diagram showing a representative coronal section of DBS electrode placements in the dorsal-VS, for both high-frequency (HF) and low-frequency (LF) groups. (B–C) Both high and low frequency DBS increased c-fos immunoreactivity in IL, whereas neither affected NAc subregions. (D) Only low frequency DBS increased c-fos labeling in the amygdala (BA and CeM). Abbreviations: PL= prelimbic cortex; IL= infralimbic cortex; core= nucleus accumbens core; shell= nucleus accumbens shell; BA= basal nucleus of the amygdala; CeL= lateral portion of the central nucleus of the amygdala; CeM= medial portion of the central nucleus of the amygdala. Sham: n= 6; high frequency DBS: n= 4; low frequency DBS: n= 5. Data represented as mean SEM. *p<0.05; **p<0.01.
Compared to the sham group, DBS altered c-fos expression in the infralimbic cortex (IL; F (2, 9) = 6.42; p = 0.018, Fig. 3B), basal amygdala (BA; F(2, 10)= 8.53; p= 0.006, Fig. 3D), and medial portion of the central nucleus of the amygdala (CeM; F(2, 10)= 9.73; p= 0.004; Fig. 3D). Tukey post hoc analyses showed that high frequency DBS increased c-fos expression only in IL (p= 0.032), whereas low frequency DBS increased c-fos expression in IL (p= 0.030), BA (p= 0.015) and CeM (p= 0.009). Neither high nor low frequency stimulation altered activity in the NAc (core; F(2, 10)= 0.31; p= 0.734; shell; F(2, 11)= 0.18; p= 0.836, Fig. 3C). Similarly, no changes in c-fos expression were observed in PL (F(2, 9)= 1.45; p= 0.283, Fig. 3B) or in CeL (F(2, 10) = 0.34; p= 0.713, Fig. 3D). Thus, while both high and low frequency DBS activated IL, only low frequency DBS activated the amygdala (BA and CeM).
Discussion
Using a rodent model of DBS treatment of addictive disorders, we demonstrated that facilitation of extinction of morphine-CPP is specific to both the targeted striatal subregion and the frequency of DBS. We observed an impairment in extinction with high frequency DBS of the dorsal-VS, whereas no effect was observed in the ventral-VS. Conversely, low frequency DBS of the dorsal-VS strengthened extinction memory. In the dorsal-VS, low frequency, but not high frequency DBS, increased c-fos expression in the amygdala (BA and CeM), an area commonly associated with the expression and extinction of drug seeking behaviors (10).
Previous studies in rats have investigated the effects of DBS on drug seeking behaviors (4, 5). However, only two studies have tested the effects of DBS on extinction of drug seeking. Levy and colleagues (2007) reported that either high or low frequency DBS of PL, a region associated with drug seeking, facilitated the extinction of cocaine self-administration (26). Friedman and colleagues (2010) demonstrated that a combined pattern of high/low frequency DBS stimulation of the lateral habenula facilitated the extinction of cocaine self-administration (42). In our study, we demonstrated that high frequency and low frequency DBS of the dorsal-VS have opposite effects on the extinction of drug-associated memories. Differences between our study and the previous studies may be due to differences in the region of stimulation (e.g.: PFC vs. VS), the drug studied (e.g. cocaine versus morphine), and/or the behavioral paradigms used (e.g. self-administration versus CPP). Nevertheless, our findings agree with previous work showing that DBS effects on extinction depend on both frequency and placement. Regarding opioids, high frequency DBS of the VS during either morphine conditioning (23) or abstinence of morphine or heroin (21, 22) reduced drug seeking behaviors. Thus, potential differences between these findings and ours may be attributable to the recruitment of important brain regions for the extinction of morphine seeking behaviors (9, 10).
DBS can modulate local and/or distant sites. Locally, high frequency DBS has been shown to induce depolarization blockade due to activation of local interneurons (46, 47), as well as synaptic depression associated with neurotransmitter depletion and inhibition (47). Consistent with this, previous studies have reported that high frequency DBS of the NAc core reduces the acquisition of morphine place preference (23), similar to electrolytic lesions (48). High frequency DBS has also been shown to induce antidromic spikes that activate inhibitory interneurons in cortical areas (38). Because IL projects densely to the dorsal-VS (49, 50), impaired extinction with high frequency DBS could be attributed to distal activation of inhibitory interneurons in IL (5, 38), which is a critical region for the expression of extinction of morphine place preference (10). Consistent with this idea, high frequency DBS of the NAc shell attenuated cocaine reinstatement, an effect that was also observed after pharmacological inactivation of IL (20). Furthermore, pharmacological inactivation of IL impairs extinction of cocaine seeking (51), and extinction of morphine-CPP is enhanced by intra-IL activation of PKM ζ (52) and Narp (53), two proteins involved in long-term potentiation via upregulation of AMPA receptors.
In contrast to high frequency DBS, low frequency DBS (20–60 Hz) has been associated with distal activation rather than distal inhibition (54). In the thalamus, low frequency DBS activates inputs (terminals) from cortical pyramidal neurons (54). Thus, low frequency DBS of the dorsal-VS might be activating IL pyramidal neurons, rather than activating IL inhibitory interneurons. Using a model of cocaine sensitization, it was recently shown that low frequency DBS of the VS/NAc, combined with antagonists of D1 dopamine receptors (D1DR) in the same brain region, produced long-lasting abolishment of behavioral sensitization (28). Although we did not observe changes in c-fos expression in the VS/NAc, our data showed that low frequency DBS increased c-fos expression in the amygdala and IL, both regions that project to VS (49, 55–57) and are critical for the acquisition and expression of drug extinction memories (7, 9, 10, 58). While activity in IL is necessary for the expression of extinction of drug-associated memories (7, 51, 52), plasticity in the basolateral amygdala (BLA) via activation of p-Erk cascade (59) or enhancement of glutamatergic transmission (9, 10) has been correlated with the acquisition of drug extinction.
We also observed that low frequency DBS increased c-fos expression in CeM, suggesting that the central amygdala (CeA) might play a role in the facilitation of extinction memory. Overexpression of GluA1 (Gria1) subunits of AMPA receptors in CeA facilitated the extinction of morphine place preference, whereas downregulation of GluA1 subunits in this same region had the opposite effect (60). In addition, drug seeking behaviors can be attenuated by intra-CeA infusion of agonists of metabotropic glutamate receptors (61), D2/D3 dopaminergic receptors (62), or 5HT-2C serotonergic receptors (63). Thus, the cooperative integration of activity in IL, BA and CeM could underlie the strengthening of extinction memory observed after the cessation of low frequency DBS. Nevertheless, because c-fos findings were obtained from naïve rats, it is possible that additional circuits are recruited in drug-exposed animals receiving extinction training.
In summary, our results demonstrate that stimulation of the dorsal-VS at the frequencies most often used for clinical DBS (high frequency) impaired morphine extinction, whereas low frequency DBS of the same area strengthened extinction memory. Thus, while high frequency DBS of the ventral striatum appears to disrupt the extinction circuitry perhaps by local interruption of extinction afferents and feed-forward inhibition of IL, low frequency DBS might be recruiting brain regions (i.e. amygdala and IL) necessary for extinction behaviors through the stimulation of afferent terminals. However, further studies are needed to determine the effects of low frequency DBS in the ventral portion of the VS, as well as to determine if low frequency DBS could reverse the extinction impairment induced by high frequency DBS.
Because addiction is a chronic relapsing brain disorder (6), DBS could be a promising alternative for treatment-resistant patients who do not respond to conventional therapies. Given that rodent models of drug extinction resemble exposure-based therapies in humans (30), low frequency DBS of dorsal-VS may represent a potential adjunct for extinction-based therapies in opioid addiction. Accordingly, Kuhn and colleagues (2014) reported that DBS intervention combined with pharmacological treatment (levomethadone) reduced the consumption of both, the drug of abuse (heroin) and the prescribed drug (levomethadone) (64). Thus, the incorporation of DBS as a potential therapy for substance-related disorders might represent a valuable tool to enhance the response to standard procedures.
Acknowledgments
This work was supported by R25 GM061838 (to FJM-R), K99-MH105549 (to FHD-M), P50 MH086400 (to GJQ) and NIH Grants G12 RR003051 and G12 MD007600 (to JLB-E). Authors want to thank Janelle M. Miranda-Fajardo, Oscar A. Muñiz-Seda, Ryan D. Rivera-Oyola and Kelvin Quiñones-Laracuente for their help with behavioral experiments and statistical analyses. We also thank Maria E. Santiago-Gascot, Zarkalys Quintero, and Carlos Rodriguez for technical support.
Footnotes
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 2.Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Archives of general psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 3.Mantione M, Nieman DH, Figee M, Denys D. Cognitive-behavioural therapy augments the effects of deep brain stimulation in obsessive-compulsive disorder. Psychological medicine. 2014;44:3515–3522. doi: 10.1017/S0033291714000956. [DOI] [PubMed] [Google Scholar]
- 4.Luigjes J, van den Brink W, Feenstra M, van den Munckhof P, Schuurman PR, Schippers R, et al. Deep brain stimulation in addiction: a review of potential brain targets. Molecular psychiatry. 2012;17:572–583. doi: 10.1038/mp.2011.114. [DOI] [PubMed] [Google Scholar]
- 5.Pierce RC, Vassoler FM. Deep brain stimulation for the treatment of addiction: basic and clinical studies and potential mechanisms of action. Psychopharmacology. 2013;229:487–491. doi: 10.1007/s00213-013-3214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & memory. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behavioural brain research. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 10.Gass JT, Chandler LJ. The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning. Frontiers in psychiatry. 2013;4:46. doi: 10.3389/fpsyt.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McConnell GC, So RQ, Hilliard JD, Lopomo P, Grill WM. Effective deep brain stimulation suppresses low-frequency network oscillations in the basal ganglia by regularizing neural firing patterns. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15657–15668. doi: 10.1523/JNEUROSCI.2824-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J, et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? Journal of neurology, neurosurgery, and psychiatry. 2007;78:1152–1153. doi: 10.1136/jnnp.2006.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn J, Bauer R, Pohl S, Lenartz D, Huff W, Kim EH, et al. Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. European addiction research. 2009;15:196–201. doi: 10.1159/000228930. [DOI] [PubMed] [Google Scholar]
- 14.Muller UJ, Sturm V, Voges J, Heinze HJ, Galazky I, Heldmann M, et al. Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases. Pharmacopsychiatry. 2009;42:288–291. doi: 10.1055/s-0029-1233489. [DOI] [PubMed] [Google Scholar]
- 15.Mantione M, van de Brink W, Schuurman PR, Denys D. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery. 2010;66:E218. doi: 10.1227/01.NEU.0000360570.40339.64. discussion E218. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Xu J, Jiang J. Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: a case report. Biological psychiatry. 2011;69:e41–42. doi: 10.1016/j.biopsych.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Knapp CM, Tozier L, Pak A, Ciraulo DA, Kornetsky C. Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharmacology, biochemistry, and behavior. 2009;92:474–479. doi: 10.1016/j.pbb.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson MB, Green AI, Bradford PS, Chau DT, Roberts DW, Leiter JC. Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats. Neurosurgical focus. 2010;29:E12. doi: 10.3171/2010.4.FOCUS10105. [DOI] [PubMed] [Google Scholar]
- 19.Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8735–8739. doi: 10.1523/JNEUROSCI.5277-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vassoler FM, White SL, Hopkins TJ, Guercio LA, Espallergues J, Berton O, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:14446–14454. doi: 10.1523/JNEUROSCI.4804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L, Zhou H, Wang R, Xu J, Zhou W, Zhang F, et al. DBS of nucleus accumbens on heroin seeking behaviors in self-administering rats. Drug and alcohol dependence. 2013;129:70–81. doi: 10.1016/j.drugalcdep.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Chen N, Wang HM, Meng FG, Zhang JG. Inhibition of the reinstatement of morphine-induced place preference in rats by high-frequency stimulation of the bilateral nucleus accumbens. Chinese medical journal. 2013;126:1939–1943. [PubMed] [Google Scholar]
- 23.Liu HY, Jin J, Tang JS, Sun WX, Jia H, Yang XP, et al. Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addiction biology. 2008;13:40–46. doi: 10.1111/j.1369-1600.2007.00088.x. [DOI] [PubMed] [Google Scholar]
- 24.Smeding HM, Goudriaan AE, Foncke EM, Schuurman PR, Speelman JD, Schmand B. Pathological gambling after bilateral subthalamic nucleus stimulation in Parkinson disease. Journal of neurology, neurosurgery, and psychiatry. 2007;78:517–519. doi: 10.1136/jnnp.2006.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim SY, O’Sullivan SS, Kotschet K, Gallagher DA, Lacey C, Lawrence AD, et al. Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson’s disease. J Clin Neurosci. 2009;16:1148–1152. doi: 10.1016/j.jocn.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:14179–14189. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadid G, Gispan I, Lax E. Lateral habenula deep brain stimulation for personalized treatment of drug addiction. Frontiers in human neuroscience. 2013;7:806. doi: 10.3389/fnhum.2013.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Creed M, Pascoli VJ, Luscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–664. doi: 10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton J, Lee J, Canales JJ. Chronic unilateral stimulation of the nucleus accumbens at high or low frequencies attenuates relapse to cocaine seeking in an animal model. Brain stimulation. 2015;8:57–63. doi: 10.1016/j.brs.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Myers KM, Carlezon WA., Jr Extinction of drug- and withdrawal-paired cues in animal models: relevance to the treatment of addiction. Neurosci Biobehav Rev. 2010;35:285–302. doi: 10.1016/j.neubiorev.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology. 2013;226:659–672. doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinrichs SC, Leite-Morris KA, Carey RJ, Kaplan GB. Baclofen enhances extinction of opiate conditioned place preference. Behavioural brain research. 2010;207:353–359. doi: 10.1016/j.bbr.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8764–8769. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Do-Monte FH, Rodriguez-Romaguera J, Rosas-Vidal LE, Quirk GJ. Deep brain stimulation of the ventral striatum increases BDNF in the fear extinction circuit. Frontiers in behavioral neuroscience. 2013;7:102. doi: 10.3389/fnbeh.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- 36.Mueller D, Perdikaris D, Stewart J. Persistence and drug-induced reinstatement of a morphine-induced conditioned place preference. Behavioural brain research. 2002;136:389–397. doi: 10.1016/s0166-4328(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Rivera FJ, Natal-Albelo EJ, Martinez NA, Orozco-Vega RA, Muniz-Seda OA, Barreto-Estrada JL. The effect of the anabolic steroid, nandrolone, in conditioned place preference and D1 dopamine receptor expression in adolescent and adult mice. Behav Processes. 2015;113:81–85. doi: 10.1016/j.beproc.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12601–12610. doi: 10.1523/JNEUROSCI.3750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:5354–5363. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang JY, Shi LH, Luo F, Zhang WM, Woodward DJ. Studies of the neural mechanisms of deep brain stimulation in rodent models of Parkinson’s disease. Neurosci Biobehav Rev. 2007;31:643–657. doi: 10.1016/j.neubiorev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biological psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 42.Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, et al. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology. 2010;59:452–459. doi: 10.1016/j.neuropharm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 44.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 45.Panagis G, Nomikos GG, Miliaressis E, Chergui K, Kastellakis A, Svensson TH, et al. Ventral pallidum self-stimulation induces stimulus dependent increase in c-fos expression in reward-related brain regions. Neuroscience. 1997;77:175–186. doi: 10.1016/s0306-4522(96)00471-x. [DOI] [PubMed] [Google Scholar]
- 46.Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17(Suppl 3):S69–72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- 47.Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4:142rv148. doi: 10.1126/scitranslmed.3003722. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Zhao Z, Liang Q, Wang X, Chang C, Wang J, et al. The nucleus accumbens core has a more important role in resisting reactivation of extinguished conditioned place preference in morphine-addicted rats. J Int Med Res. 2008;36:673–681. doi: 10.1177/147323000803600408. [DOI] [PubMed] [Google Scholar]
- 49.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Romaguera J, Do-Monte FH, Tanimura Y, Quirk GJ, Haber SN. Enhancement of fear extinction with deep brain stimulation: evidence for medial orbitofrontal involvement. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:1726–1733. doi: 10.1038/npp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He YY, Xue YX, Wang JS, Fang Q, Liu JF, Xue LF, et al. PKMzeta maintains drug reward and aversion memory in the basolateral amygdala and extinction memory in the infralimbic cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1972–1981. doi: 10.1038/npp.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blouin AM, Han S, Pearce AM, Cheng K, Lee JJ, Johnson AW, et al. Role of medial prefrontal cortex Narp in the extinction of morphine conditioned place preference. Learning & memory. 2013;20:75–79. doi: 10.1101/lm.028621.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mina F, Benquet P, Pasnicu A, Biraben A, Wendling F. Modulation of epileptic activity by deep brain stimulation: a model-based study of frequency-dependent effects. Front Comput Neurosci. 2013;7:94. doi: 10.3389/fncom.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson TG, Beart PM. Excitant amino acid projections from rat amygdala and thalamus to nucleus accumbens. Brain Res Bull. 1988;20:467–471. doi: 10.1016/0361-9230(88)90136-0. [DOI] [PubMed] [Google Scholar]
- 56.Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol. 1990;298:40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- 57.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 58.Millan EZ, McNally GP. Accumbens shell AMPA receptors mediate expression of extinguished reward seeking through interactions with basolateral amygdala. Learning & memory. 2011;18:414–421. doi: 10.1101/lm.2144411. [DOI] [PubMed] [Google Scholar]
- 59.Lin X, Wang Q, Cheng Y, Ji J, Yu LC. Changes of protein expression profiles in the amygdala during the process of morphine-induced conditioned place preference in rats. Behavioural brain research. 2011;221:197–206. doi: 10.1016/j.bbr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Cai YQ, Wang W, Hou YY, Zhang Z, Xie J, Pan ZZ. Central amygdala GluA1 facilitates associative learning of opioid reward. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:1577–1588. doi: 10.1523/JNEUROSCI.1749-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biological psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Thiel KJ, Wenzel JM, Pentkowski NS, Hobbs RJ, Alleweireldt AT, Neisewander JL. Stimulation of dopamine D2/D3 but not D1 receptors in the central amygdala decreases cocaine-seeking behavior. Behavioural brain research. 2010;214:386–394. doi: 10.1016/j.bbr.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pockros-Burgess LA, Pentkowski NS, Der-Ghazarian T, Neisewander JL. Effects of the 5-HT2C receptor agonist CP809101 in the amygdala on reinstatement of cocaine-seeking behavior and anxiety-like behavior. Int J Neuropsychopharmacol. 2014;17:1751–1762. doi: 10.1017/S1461145714000856. [DOI] [PubMed] [Google Scholar]
- 64.Kuhn J, Moller M, Treppmann JF, Bartsch C, Lenartz D, Gruendler TO, et al. Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Molecular psychiatry. 2014;19:145–146. doi: 10.1038/mp.2012.196. [DOI] [PubMed] [Google Scholar]