Abstract
Chronic traumatic encephalopathy (CTE) is a neuropathologically defined disease reportedly linked to a history of repetitive brain trauma. As such, retired collision sport athletes are likely at heightened risk for developing CTE. Researchers have described distinct pathological features of CTE as well a wide range of clinical symptom presentations, recently termed traumatic encephalopathy syndrome (TES). These clinical symptoms are highly variable, non-specific to individuals described as having CTE pathology in case reports, and are often associated with many other factors. This review describes the cognitive, emotional, and behavioral changes associated with 1) developmental and demographic factors, 2) neurodevelopmental disorders, 3) normal aging, 4) adjusting to retirement, 5) drug and alcohol abuse, 6) surgeries and anesthesia, and 7) sleep difficulties, as well as the relationship between these factors and risk for developing dementia-related neurodegenerative disease. We discuss why some professional athletes may be particularly susceptible to many of these effects and the importance of choosing appropriate controls groups when designing research protocols. We conclude that these factors should be considered as modifiers predominantly of the clinical outcomes associated with repetitive brain trauma within a broader biopsychosocial framework when interpreting and attributing symptom development, though also note potential effects on neuropathological outcomes. Importantly, this could have significant treatment implications for improving quality of life.
Introduction
One decade ago, the first published case of chronic traumatic encephalopathy (CTE) in an American football player marked the beginning of a significant shift in the understanding, concern, and public awareness of the potential long-term effects of repetitive brain trauma. ”Punch drunk syndrome” appeared first in the medical literature in the late 1920’s (Martland 1928), and was renamed “dementia pugilistica” shortly thereafter (Millspaugh 1937). These terms described the clinical syndrome of prominent cognitive, behavioral, and motor symptoms in boxers. More recently, the modern concept, now referred to as CTE, has garnered substantial attention, stimulated by case studies describing neuropathology identified during the autopsies of former professional football players (Omalu et al. 2011; Omalu et al. 2006; Omalu et al. 2005; Omalu et al. 2010), and followed by similar findings in professional wrestlers, hockey players, and others. The proposed mechanistic link between repetitive brain trauma and neurodegeneration is not well understood, but structural injuries, altered neural signaling, and an “immunoexcitotoxic” cascade have been postulated as likely candidates (Blaylock and Maroon 2011; Giza and Hovda 2014).
Lay interest in CTE has exploded in recent years as a result of coverage and attention paid to it in media, popular culture, and scientific literature. Public awareness of the relationship between repetitive brain trauma and CTE, while a desirable precursor to preventative efforts, may lead to a sense that more is known about this phenomenon than is actually the case. The absence of CTE pathological features in a neurodegenerative brain bank of individuals without documented exposure to repetitive head trauma, compared to presence of CTE pathology in approximately 30% (21 of 66) of individuals with such exposure, further supports the link between repetitive brain trauma and CTE neuropathology (Bieniek et al., 2015). However, when the brain of a deceased athlete is shown to have CTE neuropathology, the temptation is to attribute all reported cognitive, behavioral, or neuropsychiatric problems that may be present in the athlete’s history to CTE neuropathology. We propose that such attributions are premature in that they may neglect other biological, psychological, and social factors that may produce some, or many, of the clinicopathological outcomes associated with in CTE (see Figure). Previous reviews have examined the available evidence linking repetitive brain trauma, neurodegenerative pathology, and late-life clinical outcomes, generally concluding the research remains in its infancy and firm support for cause-and-effect relationships is lacking (McCrory, Meeuwisse, Kutcher, Jordan, & Gardner 2013; Iverson, Gardner, McCrory, Zafonte, & Castellani 2015; Randolph 2014). In this review, we highlight the importance of a broader, biopsychosocial perspective, and describe key factors that may play an important role in producing key behavioral aspects of the CTE phenotype. As efforts to understand, prevent, and treat both the clinical and pathological correlates of CTE advance, we should begin with as broad a conceptual base as possible.
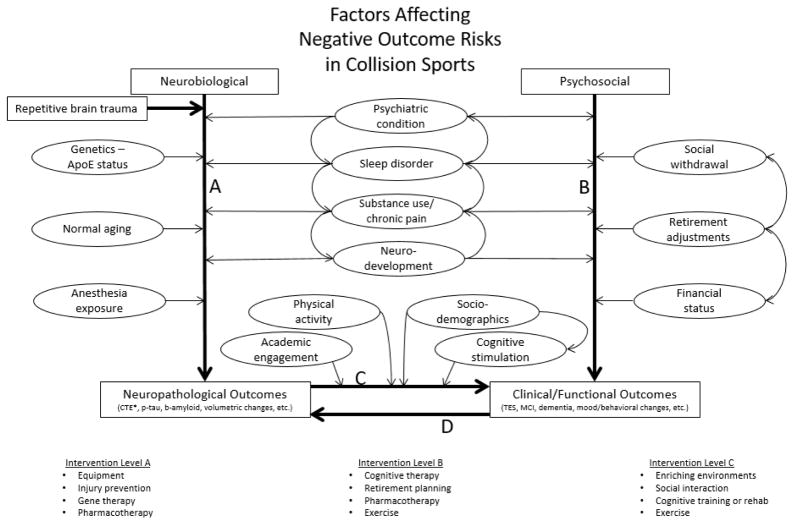
Figure.
Conceptual framework describing a non-exhaustive list of mediators and moderators of neuropathological and clinical outcomes of collision sport exposure. Evidence suggests repetitive brain trauma is a primary risk factor for developing neuropathology unique to CTE. Arrows between factors (ovals) indicate possible uni- and bidirectional influences amongst these variables. Path A includes the potential neurobiological mediators and moderators of pathological burden. Path B includes psychosocial factors directly influencing clinical/functional outcomes which over time, if untreated, may result in underlying neuronal reorganization or compound neuropathological outcomes (Path D). Path C describes factors that may modify the degree to which neuropathological changes ultimately manifest clinically (i.e. “cognitive reserve”). Possible treatment interventions may mitigate neuropathological outcomes by directly targeting neurobiological factors (Intervention Level A), prevent or minimize negative clinical/functional outcomes by directly targeting psychosocial factors (Intervention Level B), or mitigate the degree to which accumulated neuropathology translates to clinical outcomes (Intervention Level C). Research is essential for early identification of at-risk populations and improved understanding of appropriate intervention timing (e.g. before, during, and/or after playing careers).
Abbrev: ApoE – apolipoprotein E, CTE – chronic traumatic encephalopathy, p-tau – phosphorylated tau, b-amyloid – beta amyloid, TES – traumatic encephalopathy syndrome, MCI – mild cognitive impairment
*Research linking CTE to repetitive brain trauma, specifically, suggests mediating/moderating factors are not causally responsible for its unique distribution of neurofibrillary tangles
The Nature of CTE
Pathological CTE is conceptualized as a tauopathy that leads to progressive, degenerative brain changes. Its association with repetitive brain trauma suffered in high-impact sport activities has focused the key question of how repetitive head injury can lead to progressive neurodegenerative disease. After the initial publication by Omalu et al of pathological findings in a deceased American football player (Omalu et al. 2005), there have been a series of publications describing the neuropathology of CTE (Mckee et al. 2009; Omalu et al. 2011, McKee et al. 2013). Pathological studies indicated similar structural changes to those described by Geddes et al. in a small autopsy sample of young men with a recent history of mild chronic head injury; specifically, early presence of neurofibrillary tangles (NFTs) in the neocortex indicative of damaged blood vessels and perivasculature (Geddes, Vowles, Nicoll, & Revesz 1999). Recently, a consensus panel defined the neuropathological criteria for CTE and systematically identified the unique features differentiating CTE from other common tauopathies (McKee et al. 2016). The pathognomonic lesion of CTE includes phosphorylated tau (p-tau) aggregation in neurons, astrocytes, and cell processes around small vessels in an irregular pattern at the depth of the cortical sulci. Other observed pathologies included transactive response DNA binding protein 43 (TDP-43), beta-amyloid plaques, and hippocampal neurofibrillary degeneration. P-tau and neurofibrillary tangles (NFTs) predominantly within neocortex layers II and III differentiated CTE from Alzheimer’s disease (AD), where p-tau is typically found in layers III and V. Hippocampal p-tau deposition in areas CA2 and CA4, as opposed to CA1 and the subiculum, also appeared unique to CTE compared to traditional AD pathology. Defining CTE neuropathological criteria was an essential step towards developing an understanding of incidence rates and clinical correlates.
Of note, CTE is frequently discussed as a clinicopathological entity. Advances in our understanding of underlying postmortem neuropathology, while critically important, are constrained by our suboptimal understanding of a variable and poorly defined clinical symptom picture. Previously proposed clinical stages described progressively debilitating symptoms beginning with headaches and problems in concentration and other vague symptoms (Stage I), then involving depression, explosivity, and memory loss (Stage II), executive dysfunction (Stage III) and finally, dementia, aggression, and language disturbance (Stage IV).
The loci and described progression of pathology are accompanied by cognitive and emotional changes. Stern et al, based on posthumous interviews with 33 informants for cases of “pure CTE” determined to have no comorbid neuropathology, suggested that behavioral and mood changes appear before the onset of neurocognitive decline (Stern et al. 2013). The most commonly-described behavioral features included explosivity, impulsivity, and physical and verbal abuse. The most prominent mood features were depression and hopelessness followed by suicidality and anxiety (Stern et al. 2013). The link between CTE and suicidality has been contested (Iverson 2014; Iverson 2016) and evidence suggests suicide mortality among retired NFL players is significantly lower than that of the general population (Lehman et al. 2016); however other researchers hypothesize that concussions alone appear to increase suicide risk (Fralick, Thiruchelvam, Tien, & Redelmeier 2016). Memory impairment, executive dysfunction (Gavett et al. 2011), and concentration difficulties are the most common cognitive deficits followed by language impairment and visuospatial difficulties (Stern et al. 2013).
Victoroff attempted to define the clinical signs and symptoms of CTE as “clinically probable traumatic encephalopathy” and “clinically possible traumatic encephalopathy” for research diagnostic purposes (Victoroff 2012). Montenigro et al later coined the term “traumatic encephalopathy syndrome” (TES) along with various TES subtypes (Montenigro et al. 2014) to differentiate the clinical syndromes from neuropathologically defined CTE (Jordan 2013; McKee et al. 2016). TES diagnostic criteria initially included five general criteria derived from calculated prevalence of cognitive and emotional deficits across reported cases of CTE, three core clinical features, and nine supportive features (Montenigro et al. 2014). Reams et al. proposed the most recent iteration of TES, but carefully note their diagnostic criteria are not intended to predict underlying CTE neuropathology and such criteria will likely change as research evolves (Reams et al. 2016). The Reams et al. criteria require persistence of symptoms for at least two years, a history of head trauma exposure, delayed onset following head trauma, progressive course, and formal neuropsychological testing corroborating self- or observer-report of cognitive dysfunction in the executive, visuospatial, memory, and/or language domain(s). The clinical features also cannot be better explained by another neurological disorder. Supportive features include emotional dysregulation, behavioral change, and motor disturbance. The researchers further delineate “possible” versus “probable” TES, stating “Possible TES does not require cognitive decline, repetitive head trauma exposure, or delayed onset of symptoms.” Individuals with symptoms better explained by another neurodegenerative or psychiatric disorder qualify for an “Unlikely TES” diagnosis.
The extensive symptom overlap between CTE/TES and other clinicopathological entities, as well as the variability in symptomatic presentation, highlights the need for understanding clinical changes following exposure to repetitive brain trauma within the broader landscape of biological, psychological, and social factors that influence cognitive and emotional changes in at-risk populations. One key question facing CTE researchers concerns why the clinical and pathological features of the disease affect some but not all individuals exposed to repetitive brain trauma. This fact suggests that the links among brain trauma, neuropathology, and clinical symptoms are not obligatory or simple, but that they may be mediated by other factors that, separately or together, elevate or mitigate risk for developing disease characteristics. In this review, we consider other risk factors and biopsychosocial factors that may be important contributors in the development of the CTE syndrome, or TES, and that may moderate the link between trauma, pathology, and clinical changes. We review the effects of 1) developmental and demographic factors, 2) neurodevelopmental disorders, 3) normal aging, 4) adjusting to retirement, 5) drug and alcohol abuse, 6) surgeries and anesthesia, and 7) sleep difficulties. We discuss how each of these factors can be associated with some CTE symptoms in the context of cognitive ability, mood and behavior, and risk for developing neurodegenerative disorders. Such factors may moderate the causal links between repetitive brain trauma and CTE clinicopathology and may themselves produce elements of the CTE clinical syndrome. While we do not detail the impact of genetics or epigenetics, we acknowledge such biological factors add another layer of complexity to the matter, particularly as it relates to degree of accumulated neuropathology, and likely serves as an important risk factor and treatment consideration. We conclude that an understanding of these complex relationships may contribute to our understanding of the mechanisms whereby some individuals develop clinicopathological changes following exposure to repetitive brain trauma and others do not.
Developmental Environment
While professional athletes are a diverse population overall, there are sport-specific demographic biases. Race is a commonly used proxy, albeit imperfect, for socioeconomic status (SES) (Braveman et al. 2005; Manly and Echemendia 2007). The Institute for Diversity and Ethics in Sport (TIDES) tracks and reports racial diversity among collegiate and professional sports leagues (“The Institute for Diversity and Ethics in Sport,” 2015). From 1990 to 2014, African American athletes comprised between 60 and 70 percent of all National Football League (NFL) athletes with significant variability based on player position (e.g. quarterbacks versus running backs). African American and white athletes combined represented at least 97% of all NFL athletes each year during this time frame, indicating minimal overall diversity compared to the general population (“The National Football League Race and Gender Report Card” 2015). Conversely, between 1998 and 2014, Major League Soccer (MLS) consisted of a majority of white athletes (48–65 percent) with significant racial diversity including a growing number of international athletes (“Major League Soccer Racial and Gender Report Card” 2015). Considering both inter- and intra-sport differences in demographic makeup, it would be an oversimplification to call “professional athletes” or “retired athletes” a singular group. Developmental environment and SES are components of a multidimensional construct that, from a methodological perspective, has been inconsistently defined. In addition to race, other commonly-used measures of SES are income, education, and occupation, either individually or in some combination. Studies attempting to covary for these influences or utilize appropriate control groups may benefit from incorporation of a composite variable such as the Hollingshead Four Factor Index, which takes both educational and occupational factors into account (Gottfried 1985). Braveman et al. (2005) provide a comprehensive review of the use of SES in health research, and conclude that observed racial/ethnic disparities in various health outcomes must be considered within the context of potentially “unmeasured aspects of SES,” and not as mutually exclusive constructs.
The relationship between developmental SES factors and expression of neuropsychiatric problems across the lifespan is complex (Dodge and Pettit 2003). Low SES has been linked to emotional and behavioral problems throughout childhood and adolescence (Bradley and Corwyn 2002). Children exposed to economic hardship are more likely to exhibit poor conduct (Lahey et al. 1995). Disadvantaged SES predisposes to increased aggression and higher susceptibility to depression (McLoyd 1997). Some argue these associations are driven primarily by familial stresses and generally maladaptive reactions to adverse circumstances due to the chronic strain of the home environment (Amato and Zuo 1992). Stringaris et al. found the presence of childhood irritability carried a 1.3 to 1.8 times increased risk of developing depression or anxiety in adulthood (Stringaris et al. 2009). Evidence suggests irritability and impulsive aggression are prevalent and nonspecific amongst youth seeking mental health treatment (Van Meter et al. 2016). Considering the violent and aggressive nature of American football and other collision sports, in particular, it would be interesting to investigate how some individuals potentially predisposed to explosivity due to their developmental and early environmental circumstances are able to adapt their aggression when they are no longer able to utilize competitive athletics as a sanctioned outlet.
Demographic variables, and specifically SES, influence the development of cognition throughout the lifespan and represent the “environmental” aspect of the gene-environment interaction model of development. Research assessing the effects of prenatal care has identified conditions associated with low SES during pregnancy, including higher levels of maternal stress, higher infection rates, and poor nutrition, all of which compromise fetal growth and increases the likelihood of premature birth (Spencer et al. 1999). A longitudinal study by Jefferis et al. described an interaction of birth weight and social class such that children with higher birth weight who develop within a higher social class exhibit better math achievement throughout childhood and adolescence and attained higher educational qualifications in adulthood. However, children with lower birth weights developing in higher social classes still outperformed children with higher birth weights in lower social classes, suggesting a strong postnatal environmental influence (Jefferis et al. 2002). The prefrontal cortex, specifically, does not fully develop until early adulthood and is thought to play an important role in the maturation of higher order cognitive abilities such as attentional and inhibitory control (Casey et al. 1997). These results suggest that an interaction of SES and prenatal development, as well as delayed development, could affect cognitive and neural functioning into adulthood.
Further examination of the role of developmental environment on cognition, children from disadvantaged SES backgrounds tend to have poorer cognitive skills. Andersson et al. found both maternal SES and maternal IQ predicted their child’s verbal IQ, but that maternal IQ was nearly twice as strong a predictor when considering the relative beta-weights in their regression model (Andersson et al. 1996). A large and ethnically diverse sample studied by Duncan et al. revaled persistent poverty to be 60–80% more influential on childhood IQ than transient poverty, indicating a cumulative role of impoverished environmental conditions both within the family and the surrounding neighborhood. They also note black children have significantly increased likelihood of exposure to these environments. However, strong interactions of race and environmental poverty show that relying independently on race without accounting for family economic measures will overestimate racial and ethnic differences in intellect; specifically, the 10.7 point IQ-difference found between black and white children was significantly attenuated when considering maternal education, father presence, and individual family income-to-needs ratios (Duncan, Brooks-Gunn, & Klebanov 1994). A review by Evans concluded a child’s physical environment directly impacts development, and both parent-child and other interpersonal interactions are likely strong underlying mechanisms (Evans 2006).
Another explanation underlying SES effects on cognition is early-life cognitive stimulation, which is influenced by SES and likely mediates the association of SES and cognitive development (Conger and Donnellan 2007; Klerman 1991). For example, a lack of cognitive stimulation during childhood development predicts deficits in verbal learning, and math skills in low SES children (Korenman et al. 1995; Bradley and Corwyn 2002). Lower parental education can predict poorer performance on processing speed, verbal fluency, and nonverbal memory in adulthood, further suggesting an interaction of SES factors and cognitive functioning across the lifespan (Kaplan et al. 2001). These findings were corroborated by Turrell et al who indicated individuals between the ages of 58 and 64 from a disadvantaged socioeconomic position during childhood performed statistically worse on neuropsychological tests assessing multiple cognitive domains than those with higher SES backgrounds. However, examination of the study data shows clinically relevant childhood SES group differences for processing speed only, and, in general, broader and more robust differences when grouping subjects according to personal educational attainment and personal income (Turrell et al. 2002). A series of regression analyses based on almost 20,000 adults aged 50 and older found racial and SES factors across the lifespan exerted the strongest influence on general cognitive functioning (R2 = 0.38) relative to other outcomes such as self-rated health (R2 = 0.17), functional limitations (R2 = 0.17), chronic conditions (R2 = 0.08), depressive symptoms (R2 = 0.11), or self-rated memory (R2 = 0.08). Importantly, the researchers found that inclusion of SES factors from the subjects’ adulthood (personally attained education and income) attenuated many of the effects of childhood SES (e.g. parental education levels, parental income), suggesting potential modifiability across the lifespan given appropriately stimulating environments (Luo and Waite 2005).
As individuals age, the quality of their developmental environments can ultimately manifest as differential susceptibility to cognitive difficulties such as mild cognitive impairment or dementia. The mechanism underlying these associations is broadly termed the “cognitive reserve” hypothesis, which states there are individual structural and functional differences that allow the brain to compensate differentially for age- or injury-related pathology (Stern 2009). Variables affecting cognitive reserve are present throughout the lifespan but some of these processes may be particularly influential during development and early in life (Chugani 1998; Meck et al. 2007). Hall et al. examined a sample of African American subjects and the risk of clinically diagnosed AD based on their education level (more or less than seven years) and their childhood neighborhood SES (urban versus rural until age 19). Results showed the combination of both low education and rural childhood residency carried a 6.5 times greater risk of developing clinical AD compared to the high education/urban childhood residency reference group (Hall et al. 2000). Other SES factors such as lower income and bleak developmental environment are also associated with greater risk of cognitive impairment. Specifically, Lynch et al. determined older individuals were over four times more likely to report reduced cognitive functioning if they experienced consistent economic hardship beginning in young adulthood compared to those reporting no periods of economic hardship, and were three times as likely to meet criteria for clinical depression (Lynch et al. 1997). Growing up in a suburban neighborhood, compared to more rural or urban areas with potentially less enriching environments, reportedly carries a 55% reduced risk of developing AD (Moceri et al. 2000), while other findings indicate lower childhood household and community SES do not predict later-life AD (Wilson et al. 2005). Borenstein et al. contend SES risk factors for clinical expression of neurodegenerative conditions like AD are influential across the lifespan as gene-environment interactions consistently impact neurobehavioral development (Borenstein, Copenhaver, & Mortimer 2006). In this regard, it will be useful to know whether developmentally low SES predisposes athletes to expressing symptoms of CTE later in life.
The association between SES and late-life neurobehavioral function is complicated because parental or personal education and income level are often used as a proxy for SES, which may present a number of problems when considering the specific population of professional athletes. As it pertains to American football, this population is considered highly educated relative to the general population since three years removed from high school are required before entering the NFL and the majority who attend college will remain for their fourth or fifth years of eligibility. However, the quality of early education (Manly et al. 2002) and level of engagement in academics is likely variable for many athletes, particularly for those who anticipate playing professionally. Further, many professional athletes experience a drastic increase in income when they become professional relative to their familial income during development; education and income level are likely to be differentially relevant as a predictor of later impairment in this population. Consideration of childhood and developmental influences, such as parental education and income, may more accurately represent SES influence on clinical outcomes across the lifespan in professional athletes, specifically.
Neurodevelopmental Disorders
Neurodevelopmental disorders likely to affect cognitive and behavioral functioning across the lifespan include (among others) learning disabilities (LD) and Attention Deficit Hyperactivity Disorder (ADHD). Chronologically, the presence of LD or ADHD would be one of the first neurobehavioral disorders to manifest. Shifrer et al found that several sociocultural factors predisposed some ethnic groups to be disproportionally diagnosed with learning disability (Shifrer et al. 2011). African American and Hispanic children were 1.5 and 1.6 times more likely to be diagnosed, respectively, than European Americans, which is particularly relevant when considering the previously described demographic and racial makeup of professional athletes. Within the athletic community, LD diagnosis has been reported as high as 13.5% in a sample of male collegiate football players (Collins et al. 1999). This percentage is significantly higher than the 5% prevalence of LD reported in both adolescent and college-aged individuals in the general population (Horn and Nevill 2006; Pastor and Reuben 2008). Nearly 10% of American children meet clinical criteria for ADHD (Barbaresi et al. 2004; Putukian et al. 2011). While ADHD prevalence has not been studied directly in collegiate or professional athletes, incidence in high school athletes is consistent with general population estimates (Iverson et al. 2015). Importantly, evidence suggests many symptoms of neurodevelopmental disorders persist across the lifespan (Biederman et al. 2006; McCann and Roy-Byrne 2000).
Emotional issues may arise as well. Individuals with learning disability and ADHD often develop anxiety and mood disorders consequent to academic difficulties, socialization problems, and poor emotional regulation (Barkley 2010). Females with these learning problems tend to internalize these challenges and develop anxiety disorders (Quinn 2005). However, young males are more likely to externalize and report higher rates of impulsivity, hyperactivity, and poor emotional regulation. Adults with ADHD report substantially higher rates of mood and behavioral problems. Findings from the National Comorbidity Survey – Replication (NCS-R) indicated adults with ADHD reported having a mood disorder 5.0 times as often as adults without ADHD, an anxiety disorder 3.7 times as often, a substance use disorder 3.0 times as often, and intermittent explosive disorder 3.7 times as often (Kessler et al. 2006). Competitive sports provide a structure in which to channel these issues, but many athletes report maladjustment when they step away from the game (described in more detail below). This has been associated with legal issues as well as an increase in psychopathology. Rage, impulsivity, and mood disorders have been frequently associated with individuals later diagnosed with CTE. The relative contribution of repetitive brain trauma on the neurological systems associated with these behaviors is unknown, but the presence of these symptoms in an athletic population where LD and ADHD are common necessitates further investigation of their interaction.
Impaired cognition in individuals with LD is diagnosed based on manifest academic difficulties with reading, writing, and/or arithmetic. ADHD is characterized by chronic inattention and an inability to maintain focus relative to same-aged peers and expected development (“Diagnostic and Statistical Manual of Mental Disorders”, 2003). Adults with ADHD perform poorly both on attentional and memory-based measures, presumably as a result of poor attention and reduced working memory negatively affecting their ability to encode information (Hervey et al. 2004). Individuals with LD-dyslexia also show significant working memory impairments (de Jong 1998). LD affecting both reading and arithmetic results in a broad range of cognitive deficiencies in adulthood. Goldstein et al. reported mean LD group performance one standard deviation below normative group means on assessments of vocabulary (WAIS-R Vocabulary), working memory (WAIS-R Digit Span), processing speed (WAIS-R Digit Symbol), and math skills (WAIS-R Arithmetic) (Goldstein et al. 2001). In athletes, specifically, LD and ADHD are associated with significantly lower scores on measures of verbal and visual memory, slower reaction time, worse impulse control, and more executive dysfunction than athletes without these diagnoses (White et al. 2014; Zuckerman et al. 2013). When coupled with brain trauma, athletes with LD and ADHD who perform worse on cognitive measures at baseline then tend to take longer to recover pre-injury cognitive functioning and be cleared to return to athletic participation following concussion (Waljas et al. 2015).
Some evidence suggests the presence of ADHD predisposes to later diagnosis of neurodegenerative diseases like dementia. Golimstock et al. performed a case-control analysis examining rates of ADHD diagnosis among patients diagnosed with either dementia with Lewy Body (DLB) or AD dementia. They reported 47.8% of DLB cases had preceding ADHD symptoms compared to 15.2% of cases with AD dementia and 15.1% of the control group. Controlling for age, sex, and years of education, preceding ADHD symptoms carried a 5.1 times increased risk of developing DLB compared to controls, but no increased risk of AD dementia (Golimstok et al. 2011). Lule et al proposed mechanisms of symptom overlap and pathophysiological links between ADHD and amyotrophic lateral sclerosis (Lule et al. 2008). However, there are few studies directly relating neurodevelopmental disorders to subsequent risk of neurodegenerative disease. A significant reason is the difficulty in precise symptom attribution, particularly in individuals with previously undiagnosed LD or ADHD who only begin to complain of cognitive symptoms later in life (Ivanchak et al. 2012). While the progressive nature of neurodegenerative disease is considered an important differentiating factor, this is confounded by reports that some individuals with ADHD report progressive worsening of symptoms across the lifespan (Bramham et al. 2012).
Relating these findings to the construct of cognitive reserve initially postulated by Stern (Stern 2002), it is apparent that lifelong neurodevelopmental disorders like LD and ADHD, which conceivably began affecting neural development from an early age, may reduce an individual’s functional capacity to withstand age- or injury-related neurological insults likely to affect cognition. One study examined the effects of repetitive brain trauma during this early developmental window by comparing the neuropsychological profiles of retired American football players who began playing before age 12 to those who began organized play at a later age. Findings suggested those exposed to football before age 12 performed worse in reading accuracy, verbal memory, and executive functioning (Stamm et al. 2015). However, group differences in the reporting of learning disability make these results difficult to interpret and highlight the need for considering the substantial overlap in cognitive symptoms as well as potential interactions between repetitive brain trauma and other comorbid diagnoses. Additionally, the highly comorbid emotional and behavioral problems associated with neurodevelopmental disorders make it important to consider conditions like LD and ADHD when interpreting neurobehavioral change due to any cause across the lifespan.
Normal Aging
Structural brain changes associated with normal aging are well-known and include neurofibrillary tangle (NFT) development, decreased grey and white matter volume, increased ventricular volume, increased white matter hyperintensities on MRI, and default mode network (regions of highly correlated functional activity) dysfunction on MRI as well (Andrews-Hanna et al. 2007; Fjell and Walhovd 2010; Madden et al. 2009; Sambataro et al. 2010). Longitudinal studies of age effects on brain volume have found volume reduction in the prefrontal cortex, caudate, cerebellum, hippocampus, and other associated regions (Fjell and Walhovd 2010). These brain changes presumably lead to age-related cognitive decline, including working memory deficits, behavioral dysregulation, reduced episodic memory, and reductions in procedural learning. Decreased white matter integrity is also associated with slowed processing speed and impaired executive function (Madden et al. 2009). However, aging studies indicate the relationship between clinical changes and underlying pathological changes is complicated. For example, findings from the Honolulu-Asia Aging Study showed only 25% of clinically diagnosed AD dementia patients had pathological confirmation of definite AD (Petrovitch et al. 2001). Beach et al. describe 87.3% sensitivity of a “clinically probable or possible AD” diagnosis to identifying severe AD neuropathology (Braak Stage V or VI), but only 44.3% specificity. Additionally, 39% of subjects without a clinical diagnosis of either probable or possible AD met minimum histopathological criteria for AD (Beach, Monsell, Phillips, & Kukull 2012).
More recent studies have attempted to understand how repetitive brain trauma affects brain aging. While there are several highly publicized cases of young people who have committed suicide and were found to have CTE, it is thought to typically develop later in life for diagnosed former collision sport athletes. Pathologically, NFT deposition accompanies the normal aging process and the recently proposed “primary age-related tauopathy” (PART) describes the continuum of severities with and without associated cognitive changes (Crary et al. 2014). However, the unique distribution of tau deposition in the perivascular spaces of cortical sulci described in CTE differentiates CTE from PART, which is associated with NFT formation in the medial temporal lobe and several subcortical nuclei. Thus, there is strong evidence suggesting the pathological features of CTE do not represent normal or accelerated aging.
Increased cortical thinning and enlarged lateral ventricles have been reported in retired athletes with a history of concussion when compared to controls (Tremblay et al. 2013). Athletes with a history of severe concussion showed smaller hippocampal volumes later in life than both control participants and other athletes with less severe concussions (based on previously used severity grading scales) (Strain et al. 2015). Similar findings of reduced right hippocampal volume have been reported in retired NFL players compared to controls (Coughlin et al. 2015). Retired athletes with a history of concussion show more diffuse white matter abnormalities compared to similarly-aged older adults (Tremblay et al. 2014). These white matter changes were reportedly similar to but worse than those seen in normal aging, suggesting that a history of concussion may accelerate certain aspects of the normal aging process (Tremblay et al. 2014).
Cognitively, episodic memory and verbal fluency have both been reported to be lower in retired athletes with a history of concussion than controls with no concussion history (Strain et al. 2015; Tremblay et al. 2013). Other impairments include performance in delayed visual memory, increased retroactive interference on verbal learning measures (an indication of possible frontal lobe dysfunction), and reduced benefit from motor training on reaction time tasks (Tremblay et al. 2014). However, findings from these studies are qualified by the appropriateness of the control group comparisons. Control groups are often demographically different in racial makeup and age, and inadequate attention is given to neurodevelopmental disorders, such as learning disabilities, as well as premorbid intellectual abilities. These limitations are described later in more detail. In addition to age-equivalent controls with no history of repetitive head trauma, asymptomatic former professional players will be needed for comparison.
Normal aging also brings with it a number of psychosocial life stressors. External stress associated with changes in life roles, threats to competence, and increased negative stereotypes (in a “youth oriented” society) contribute to depression in older adults (Fiske et al. 2009). Late onset depression is associated with cognitive impairments in older adults including executive dysfunction, slowed processing speed, and episodic and semantic memory deficits (Herrmann et al. 2007). Chronic stress and depression can affect cognition by affecting key brain systems. For example, chronic stress can lead to dysfunction in the hypothalamic-pituitary axis which, in turn, can lead to hippocampal atrophy and memory impairment (Butters et al. 2008). Depression is reportedly common in retired athletes, and its appearance may be correlated positively with concussion history (Guskiewicz et al. 2007; Kerr et al. 2012).
Studies have also examined differences between those with and without history of brain trauma on development of neurodegenerative disease or dementia. Pooled odds ratios from meta-analyses indicate a 1.6 to 1.8 times increased risk of developing clinically diagnosed AD, with significantly elevated risk apparently applying to males only (Mortimer et al. 1991; Fleminger, Oliver, Lovestone, Rabe-Hesketh, & Giora 2003). Data also suggest a 1.4 times increased risk of physician-diagnosed Parkinson’s disease (PD) (Jafari, Etminan, Aminzadeh, & Samii 2013). Mortimer et al. highlight the importance of meta-analyses for determining relative risk of developing dementia following brain injury by describing that the seven studies contained in their analysis were not sufficiently powered to address a risk factor hypothesis in isolation. Other large-scale chart review analyses have also examined subsequent risk of neurodegenerative disorder diagnosis following brain injury. Gardner et al. report a 1.46 times increased risk of subsequent dementia diagnosis following brain injury compared to non-brain injured trauma controls (Gardner et al., 2014), and Nordstrom et al. report 3.0 and 8.3 times increased risk following single or multiple mild TBI, respectively (Nordstrom, Michaelsson, Gustafson, & Nordstrom 2014). However, these relative risks are significantly attenuated after controlling for covariates such as depression, drug and alcohol abuse, SES, and other risk factors; the independent effect of brain injury history was rendered clinically negligible and comparatively less predictive than other covariates in both studies. Most recently, Crane et al. reported findings from 7,130 participants across three large prospective datasets relating history of TBI with loss of consciousness to subsequent development of both neurodegenerative pathology and clinically diagnosed dementia. They found no association between TBI history and incident dementia or AD pathology. History of TBI was associated with 3.6 times elevated risk of PD pathology, and 1.6 to 5.7 times elevated risk of Lewy Body presence in various anatomical regions such as the substantia nigra, locus coeruleus, and frontal and temporal cortex (Crane, Gibbons, Dams-O’Connor, & et al. 2016).
The risk of neurodegenerative disorders following a history of repetitive brain trauma warrants separate consideration from history of single or isolated TBI events, and few studies have directly examined this (Gardner & Yaffe 2015). Lehman et al. reported that retired NFL athletes were at a lower risk of mortality compared to same-aged general population counterparts, but carried a three-fold increased risk of neurodegenerative mortality, particularly related to Alzheimer’s disease and amyotrophic lateral sclerosis (Lehman, Hein, Baron, & Gesic 2012). Conversely, a history of participation high school American football did not predict increased risk of subsequent dementia, PD, or ALS diagnosis (Savica, Parisi, Wold, Josephs, & Ahlskog 2012). These competing results may suggest increased risk with longer playing careers and more exposure to repetitive brain trauma.
In summary, normal aging is associated with both structural and functional brain consequences, but likely does not account for the specific neuropathology associated with CTE. Cortical and subcortical volume loss occurs naturally over time, as do cognitive changes, although the latter are milder and more subtle in normal aging compared to individuals who develop dementia. Multifactorial depressive symptoms are common in aging individuals and can impact cognition and brain functioning in older adults. It is important to consider the natural trajectory of these changes to better understand any modifying effects of repetitive brain trauma sustained by collision sport athletes on clinical outcomes across the lifespan. History of brain injury may confer increased risk of subsequent neurodegenerative disease diagnosis, but the clinical significance of the elevated risk in the context of multidimensional covariates is unclear.
Adjusting to Retirement
A career in professional athletics is an option afforded to few and achieved successfully by even fewer. This generally represents a lifetime dedication to a single craft with minimal deviation from childhood/adolescence through adulthood. As a result, professional athletes often possess strong personal identification with their jobs and, subsequently, are subject to many unique challenges upon retirement from athletic careers which often occurs quite early compared to most professions. The typical age of an NFL player, for example, at retirement is in his late 20’s or early 30’s (Ninomiya 2015). The literature on civilian adjustment to retirement in the general population is somewhat limited by reliance on self-reported quality of life measures as outcomes. Additionally, the typical subjects in these studies are significantly older upon retirement than most professional athletes and may face different transitional challenges.
For employed individuals and especially athletes, retirement is an inevitable but important life event that can have profound positive and negative effects on psychological, emotional, and cognitive adaptation. Individuals vary broadly in their ability to adjust to new, post-career roles and there are several contributing and interacting factors that predict success in navigating this transition. A study of retirement community-dwelling adults in both independent and assisted living facilities found that engaging in higher levels of activity was the most substantial predictor of higher functioning based on the Medical Outcomes Study Short-Form Health Survey (SF-36) – being inactive had inverse, negative outcomes (Jenkins et al. 2002). Calvo et al. describe longitudinal findings from over 6,000 participants in the Health and Retirement Study using fixed and random effects regression models. Their results underscored the important influence of retirement timing on quality of life Such that early retirement (prior to age 62) significantly increases the risk of lower self-reported physical and emotional health. The authors observed “work clearly serves to promote better subjective physical and emotional health as it likely serves as a source of identity and resources (Calvo et al. 2013).” Wang et al. provide a comprehensive review of the theoretical and empirically-based influences that highlight the complexities of the retirement process as a dynamic development period in both the short and long term (Wang et al. 2014).
Many people maintain life satisfaction, physical health, self-esteem, and mood beyond retirement (Neuman 2008). However, several mediating relationships exist between retirement and adjustment that may alter these trajectories (Muratore et al. 2014). For example, Dave et al. also reported findings from the Health and Retirement Study showing complete retirement leads to a five to six percent decline in mental health, which is linked with declines in physical activity and social interactions. Further, evidence suggests involuntary retirement amplifies these negative effects (Dave et al. 2006). This is important to note for professional athletes, because retirement in this population is frequently determined by unanticipated events such as injuries or being cut from the team, necessitating a relatively quick retirement decision rather than a carefully planned process. Indeed, Lavallee et al. surveyed 48 elite Australian athletes and found those reporting involuntary retirement had greater difficulty with emotional and social adjustments after retirement (Lavallee, Grove, & Gordon 1997).
In the specific case of professional American football players, retirement from the NFL is vastly different from the retirement experience of most individuals. Compared to an average population retirement age of 62 (Riffkin 2014), NFL players retire on average at the age of 28 (Campbell 2011) and are in surprisingly poor financial shape after retirement. As many as 78% of retired NFL players experience financial distress within two years after retirement (Torre 2009). A recent documentary examined the propensity for athletes to incur financial hardship and described lavish spending, questionable investments, poor financial advising, and feelings of personal responsibility to provide monetary support to many extended family and friends as well as the neighborhood where the athlete grew up (Corben 2012). Such financial stress and an associated lack of access to resources can have a significant negative impact on adjustment to retirement (Wang and Shi 2014).
Aspects of employment duration are likely to have an impact on psychological and cognitive functioning as well. Dufouil et al. performed a chart review of health and pension databases for over 400,000 retired self-employed workers in France and found each additional year of age at retirement was associated with a 3.2% lower risk of subsequent dementia diagnosis (Dufouil et al. 2014). Supporting data from the Health and Retirement Study indicates non-working, retired, and partially retired individuals exhibit steeper declines in immediate memory performance than older adults working full-time (Wickrama and O’Neal 2013). While the effect size for changes in immediate memory performance in retirees is small, which may indicate minimal clinical significance (i.e. mean memory performance decreased by just small fractions of a single word on a 10 word-list retrieval test at each time point), these effects may become more meaningful if they continue to progress over more extended periods of time. The influence of retirement on cognition is particularly relevant for professional athletes since they retire at much earlier ages with variable reengagement in occupational activities. Additionally, the potential moderating effects of repetitive brain trauma history prior to retirement (e.g. a steeper negative change slope) is an important consideration in this population. The relationship between sustained employment and cognition is potentially explained by the “use it or lose it” principle (Vance and Crowe 2006), and the combination of cognitive and social stimulation often provided by productive work activity. Given the average career span of an NFL player is less than four years (Ninomiya 2015) and the young age at retirement from athletics, subsequent career decisions and activity after retirement can significantly impact the quality of adjustment.
In addition to financial impacts, a significant shift in personal identity accompanies sports retirement. Role theory has been applied to retirement to predict adjustment based on the extent to which an individual can successfully redefine his or her self-identity following a work-role transition and the control he or she feels over the process (Carter and Cook 1995). The immersive environment needed to succeed in an athlete’s chosen sport commonly results in social and work roles that are all strongly connected to athlete identity. Depression is reportedly a common outcome following retirement from the NFL (Trotter 2015). However, there is minimal recent or prospective research on athletic populations and their transition to retirement. Schwenk et al. (2007) investigated chronic pain and depression via survey of 3,377 retired members of the NFL Players’ Association, with approximately 50% response rate. Primary outcome questions focused on aspects of the retirement transition, and 27% of all respondents indicated having trouble with transition to life after professional football. Presence of chronic pain and depression exacerbated these difficulties and placed retirees at significantly elevated risk of reporting difficulty finding employment, being dissatisfied with their new employment, and loss of a sense of community and being “part of the team” (Schwenk, Gorenflo, Dopp, & Hipple 2007). Dimoula et al. describe a cohort of 133 elite athletes in Greece and Spain who retrospectively detailed their experiences with leaving professional sports. From their study, the researchers concluded lack of retirement planning and high athletic identity after a sports career underlie difficulties with the retirement transition process (Dimoula, Torregrosa, Psychountaki, & Fernandez 2013). Other sports psychology researchers have proposed similar hypotheses for reduced quality of life in retired athletes (Werthner and Orlick 1986). For more comprehensive reviews specifically related to retirement transitions in athletic populations, we refer readers to Hill and Lowe (1974), Ogilvie and Howe (1986), Svoboda and Vanek (1982), and Lavallee and Wylleman (2000).
Relative to other modifiers described in this review, retirement exerts perhaps the strongest psychosocial influence relevant to the behavioral and emotional symptoms commonly associated with CTE; symptoms which can certainly have an indirect effect on cognitive functioning as well (Blair et al. 2007). As was noted earlier, a specific consideration for retired professional collision sport athletes is the loss of an outlet for aggressive and explosive behavior. Importantly, the wealth of research on retirement and lifestyle adjustments involves individuals who likely did not have careers where they sustained repetitive brain trauma. Exposure history, in combination with significant and abrupt fluctuations in financial status, short careers, loss of self-identity, poor planning, and variable resources conducive to sustained activity after retirement may leave professional athletes particularly vulnerable to reduced quality of life when forced to undergo this shift.
Drug and Alcohol Abuse
Overuse of prescription medications (often associated with chronic pain), alcohol abuse, and use of steroids may all have negative effects on the neurobehavioral profiles of active and aging athletes. Prescription and nonprescription drug use is highly prevalent at many levels of athletic participation (Alaranta et al 2008; Warner et al. 2002). Lawsuits were filed against the NFL pertaining to overprescribing medication in 2011 and 2014 (Belson 2011; “Ex-Players: NFL Illegally Used Drugs” 2014). Specifically, painkillers and anti-inflammatory drugs were reportedly prescribed very frequently and in large quantities so athletes could “play through the pain.” Lawsuits addressing overuse of Toradol, a non-steroidal anti-inflammatory drug which became popular in athletics over the past two decades, have also been levied at the collegiate level (Miles 2012). The misuse of medications like Percocet and Toradol appear to be particularly prevalent in athletic populations. As a result, athletes are likely at greater risk for prolonged misuse which can extend into retirement, at which time chronic pain may persist.
Results from the U.S. National Survey on Drug Use and Health (NSDUH) suggest that 12.7% of the general population aged 26 and older report misuse of prescription pain relievers at some point in their lifetime (SAMHSA 2009). One study examined the rates of opioid use and abuse among retired NFL athletes and found that approximately half (52%) of NFL athletes used prescription opioids during the active portion of their professional career, and the majority of these athletes reported misuse (Cottler et al. 2011). The overall rate of misuse was 37% (238 of 644 athletes studied). The strongest predictors of opioid use during the active portion of the playing career were self-reported undiagnosed concussions, having three or more injuries, and being an offensive lineman.
Chronic pain warrants special consideration in retired athlete populations, and interacts with both substance use and neurobehavioral outcomes. A career in collision sports may have lasting negative effects on the body, predisposing to opioid use, as a staggering 80% of retired NFL athletes report persistent daily joint pain (Weir et al. 2009). Data from the previously described Schwenk et al. survey study found commonly reported retirement problems included difficulty with pain (48% of respondents), loss of fitness and lack of exercise (29%), weight gain (28%), trouble sleeping (28%), difficulty with aging (27%), and trouble transitioning to life after American football (27%). Respondents reporting difficulty with pain were 5.3 times more likely to report “very common” use of prescribed medication, alcohol, or other drugs, 3.0 times more likely to report difficulty transitioning to life after professional football, 4.9 times more likely to have trouble sleeping, 8.5 times more likely to report general difficulty with aging, and elevated risk of many other physical and psychosocial difficulties (Schwenk et al. 2007). These findings highlight the complex clinical outcomes associated with chronic pain and the interrelatedness to other factors described in this review, such as psychosocial adjustments during retirement and sleep disturbances. The contribution of repetitive brain trauma history and resulting CTE pathology must also be considered within the attributional framework. Thus, substance use, chronic pain, and history of repetitive brain trauma likely all confound the path to both neurodegenerative changes and associated clinical outcomes in retired professional athletes.
Relevant to lifelong pain management, individuals with opioid dependence report higher rates of depression, anxiety, apathy, and explosiveness than non-users (Ilyuk et al. 2013). Depression and opioid misuse may have bidirectional influences. Evidence suggests a comorbid diagnosis of depression is itself a risk factor for the development of opioid dependence in patients with chronic pain (Manchikanti et al. 2007). Additionally, Grattan et al. found patients with moderate and severe depression were 1.8 and 2.4 times more likely, respectively, to misuse opioid medication for non-pain symptoms. Presence of depression was also associated with 1.9 to 3.1 times higher likelihood of self-increasing opioid dosage (Grattan et al. 2012). Chronic opioid use can also lead to disordered sleep (Webster et al. 2008), which is associated with a host of neurobehavioral problems outlined in more detail below (and is a side effect of concussive injury). Given the substantial number of retired professional athletes, specifically American football players, reporting daily pain, attention should be paid to current and past history of opioid use.
Acute opioid use has previously been associated with slower performance on timed psychomotor tasks (Chapman et al. 2002). Longer term effects of opioid use on cognition are less clear, but tend to suggest that impairment is stronger among individuals with significant pain compared to healthy volunteers (Chapman et al. 2002). The effects of opioid medication misuse and cognition are influenced by a number of factors including dosage, duration, and pain level. Since substance misuse (i.e. higher doses and prolonged use) is common among athletes, it is important to consider its effects on structural and functional brain changes as they age. Currently, there does not appear to be research that directly investigates opioid use as a risk factor for late-life cognitive impairment, and getting accurate information to do so may be difficult.
Alcohol is commonly misused among adults, and collegiate athletes are particularly at-risk. In 2013, 24.6 percent of people ages 18 or older reported that they engaged in binge drinking and 6.8 percent reported that they had engaged in heavy drinking in the past month (SAMHSA 2013). However, collegiate athletes reported more binge drinking and heavier alcohol use than non-athlete college students (Nelson and Wechsler 2001). Alcohol misuse is associated with a number of neurocognitive and other health consequences both acutely and in the long term. Direct neurotoxic effects of alcohol include depression of central nervous system activity, reduced cerebral blood flow, and blood-brain barrier dysfunction (Alexander et al. 2004; Haorah et al. 2005; Shih et al. 2001). Previous literature suggests that alcohol misuse patterns characterized by binge, heavy, harmful, or hazardous drinking patterns have been associated with executive dysfunction, reduced working memory, problem solving difficulties, and attention impairments (Grant 1987). These symptoms may be attributable to underlying changes to patterns of regional brain activation and reduced grey and white matter volumes (Beresford et al. 2006; Downer et al. 2015). Excessive drinking also exacerbates cognitive issues after a traumatic brain injury, as both pre- and post-injury alcohol use is negatively associated with different aspects of cognitive functioning, such as verbal learning and memory, processing speed, and executive function (Ponsford et al. 2013).
Late-life cognitive impairment is also related to alcohol use. While moderate alcohol intake (1–6 drinks per week) is actually associated with 54% decreased risk of dementia, heavier use (14 or more drinks per week) in an older population led to a 22% greater risk of developing dementia (Mukamal et al. 2003). Similar findings were noted in older males diagnosed with alcohol abuse (Thomas and Rockwood 2001). One possible theory is a reduction in muscarine-like receptors within the hippocampus in alcoholic older adults compared to controls (Nordberg et al. 1983). Lastly, though debate remains regarding the distinctness of its anatomical substrates, diencephalic amnesia associated with alcoholic Korsakoff’s disease, presumably resulting from nutrient deficiencies in chronic alcohol abusers selectively affecting the mammillary bodies and anterior nuclei of the thalamus, is characterized by prominent memory encoding deficits (Butters and Stuss 1989).
Acute and chronic alcohol use also negatively affect mood and behavior. Behaviorally, alcohol is linked strongly to many forms of violence (Hoaken and Stewart 2003). Intoxication is generally associated with sensation seeking and impulsivity as well as reactive aggression (Pihl and Peterson 1995). Similar to opioid dependence, chronic alcohol dependence is commonly comorbid with depression, anxiety, apathy, and explosiveness (Ilyuk et al. 2013). Interestingly, depression has been associated with a past history of alcohol dependence even in the absence of acute intoxication or withdrawal effects (Hasin and Grant 2002), suggesting potentially more complex and long-term interactions between mood regulation and substance use.
Anabolic-androgenic steroids (AAS) are a group of hormones that include the natural male hormone, testosterone, along with synthetic testosterone. Since the 1980’s, there has been a growing use of AAS by healthy individuals in order to gain muscle and lose body fat (Yesalis and Bahrke 1995). Use of AAS in adults has an estimated lifetime prevalence of 3.3%, with much higher use among men (6.4%) than women (1.2%) (Sagoe et al. 2014), and with even greater prevalence among professional football players (9.1%) (Horn et al. 2009). Pathologically, laboratory studies suggest neurotoxic effects of AAS use. Small amounts of testosterone exposure lead to apoptosis (cell death) of human neuroblastoma cells in vitro after only 6–12 hours of exposure (Estrada et al. 2006). AAS exposure has demonstrated neurotoxic effects on mammalian cells (Caraci et al. 2011). Cognitive neuroscience literature suggests that long term AAS use reduces right amygdala resting state functional connectivity with brain areas involved in spatial memory and cognitive control (Kaufman et al. 2015). Acute cognitive impairments like confusion, memory problems, and lack of inhibitory control are commonly reported (Hildebrandt et al. 2014). Longer-term effects of AAS use include reduced visuospatial skills compared to non-users, particularly regarding pattern recognition memory (Kanayama et al. 2013). As was the case with opioid use, there does not appear to be any research as of yet directly investigating chronic AAS use as a risk factor for developing late-life cognitive impairments such as dementia, though one preclinical study indicated no interaction between AAS use and number of beta-amyloid precursor protein-positive axons within brainstem white matter tracts of rats following head injury (Mills et al. 2012).
Acute and chronic AAS use can lead to mood and behavioral disturbances (Hall et al. 2005). Anabolic steroids acutely cause irritability, impulsivity, mood swings, aggression, and violence. Chronic steroid use is associated with increased aggression (Choi et al. 1990), and more severe psychiatric symptoms are seen with higher doses (Pope and Katz 1994). Other common features of chronic AAS use include depression and anhedonia (Kanayama et al. 2003). More direct, longitudinal examinations of the effects of prolonged AAS use on neuropsychiatric symptoms are warranted particularly in the context of repetitive brain trauma.
Substance use and associated comorbidities may be common in athletic populations. Research indicates polysubstance abusers (e.g. both alcohol and opioid dependence) report higher frequencies of irritability and aggression than individuals with single substance abuse (Ilyuk et al. 2013). Substance use produces cognitive, emotional, and behavioral symptoms that show some overlap with CTE symptoms. The interactions among substance use, chronic pain, neurobehavioral outcomes, and repetitive brain trauma deserve special consideration in retired collision sport athlete populations.
Surgeries and Anesthesia
Athletes commonly undergo surgical interventions for injuries sustained during their playing careers. At the high school level, an increasing rate of sport-related surgeries has been seen over the past decade and collision sports like American football pose the highest risk (Rechel et al. 2010). Professional American football players are frequently injured and some of the most common injuries, such as knee ligament sprains, often require surgery (Feeley et al. 2008). Unfortunately, there are also significant lasting orthopedic effects of a career playing collision sports. Retired NFL players are two to five times more likely to be diagnosed with arthritis than general population males, and almost one in four retired NFL players require joint replacement surgery (Weir et al. 2009).
Research investigating the effects of surgeries or anesthesia on mood and behavior is very limited. Some evidence suggests acute increases in depression, fatigue, anger-hostility, and anxiety lasting about one week after administration of general anesthesia compared to unanesthetized controls; only depression symptoms remained slightly elevated after one month (Davison et al. 1975). A systematic review of biopsychosocial predictors of unfavorable post-surgical outcomes identified pre-surgical psychological complaints, pre-surgical pain levels, and lower education levels as consistent risk factors (den Boer et al. 2006). Preoperative anxiety carried a 1.9-fold increased mortality risk following coronary artery surgery (Tully et al. 2008) and has also been associated with slower postoperative recovery, in general (Kiecolt-Glaser, 1998). Both preoperative depression and anxiety are risk factors for persistent pain and decreased functionality following lumbar discectomy (Trief et al. 2000). The complex interactions of preoperative psychological distress, chronic pain, and the effects subsequent exposures to anesthesia on postoperative mood and behavior require further research, but are likely present in former collision sport athletes.
Clinicians and researchers have described cognitive changes following surgery known as post-operative cognitive dysfunction (POCD), though mechanisms underlying POCD have yet to be established (Monk et al. 2008). The cognitive domains affected are highly variable and include learning and memory, verbal abilities, perception, attention, and executive functions (Deiner and Silverstein 2009). Visuospatial deficits have been noted as long as one year post-surgery compared to controls (Ancelin et al. 2010). Anywhere from 30–41% of all adult patients may experience some form of POCD (Monk et al. 2008), though incidence reports differ greatly due to inconsistently defined diagnostic criteria and assessment protocols (Hussain et al. 2014). Risk factors for persistent POCD lasting three months after surgery include increasing age, lower education level, asymptomatic vascular events (i.e. previous stroke with no residual impairment), and POCD at the time of hospital discharge (Monk and Price 2011; Monk et al. 2008). The appearance and course of POCD is likely multifactorial (Monk et al. 2008); physiological stress responses to surgery (e.g. disturbed cortisol secretion) (Lupien et al. 1998), anesthetic effects on anticholinergic and anticatecholeminergic circuitry (Pratico et al. 2005), nitrous oxide-based anesthesias interfering with vitamin B12 metabolism (Myles et al. 2004), sleep deprivation due to extended hospitalization, and postoperative opioid use can all contribute to poor neurocognitive performance postoperatively as well as after hospital discharge. Fong et al. examined preoperative differences on neuropsychological testing between patients who did and did not develop postoperative delirium and found patients in the postoperative delirium group performed worse preoperatively, on average, in cognitive flexibility (Trails B), category fluency (animal naming), new learning and recall (HVLT-R), and visual and sustained attention. However, predicting postoperative delirium based on preoperative performance is likely difficult because, at the individual level, a minority of postoperative delirium patients showed impaired performance preoperatively, ranging from 15% impaired on cognitive flexibility to 27% impaired on visual and sustained attention (Fong et al. 2015).
Although existing POCD findings are most relevant to surgery-induced changes in cognition within a relatively short time window, there is also concern that surgical and anesthesia exposure may increase risk of further cognitive decline later in life. Preclinical studies indicate overlapping risk factors and potential mechanisms of neurodegenerative disorders and POCD (Bilottaa et al. 2010). Links to specific types of surgery or anesthesia have yet to be established, though commonly used anesthetics (e.g. isoflurane) may lead to neuronal apoptosis and accumulation of misfolded proteins (Wei et al. 2008). While some population-based studies show an age-of-exposure and dose-response relationship of general anesthesia and dementia risk (Chen et al. 2014), others have not shown this association (Sprung et al. 2013). Meta-analysis results consolidating data from 15 case-control studies indicated no significant association between prior exposure to general anesthesia and incident AD dementia (Seitz, Reimer, & Siddiqui 2013). Avidan and Evers (2011) also determined there is minimal clinical evidence linking surgery or anesthesia to developing dementia. Neuropathologically, a review by Whittington et al. concluded sufficient evidence exists from preclinical and clinical studies that anesthetics can accelerate tau pathology (Whittington, Bretteville, Dickler, & Planel 2013). Jiang and Jiang (2015) drew similar conclusions from their review, noting that commonly used inhaled anesthetics induce pro-apoptotic signaling, and increase the synthesis and accumulation of beta-amyloid and hyperphosphorylated tau. A randomized trial examining differential effects of anesthesia type found elevated levels of tau in cerebrospinal fluid following surgery, but no difference in degree of elevation based on use of isoflurane versus propofol (Berger et al. 2016). Taken together, evidence indicates exposure to surgery and general anesthesia increases risk for development of neuropathology; however, there is a lack of support for general anesthesia leading to clinically diagnosed dementia.
Surgeries and repeated exposure to anesthesia throughout the lifetime have yet to be studied in relation to clinical or neuropathological changes in retired collision sport athletes. Population-specific characteristics such as preoperative fitness levels, sociodemographic factors, and repetitive brain trauma may modify the risk of POCD or other postsurgical complications. Associations likely exist among surgery frequency, chronic pain, and opioid use in retired athletes, with both direct and indirect neurobehavioral consequences. The bidirectional influences of chronic pain, opioid use, and multiple surgeries/exposures to anesthesia on neurobehavioral outcomes seems particularly relevant in understanding later life risk of cognitive and neuropsychiatric symptoms in retired collision sport athletes.
Sleep Disturbances
As athletes’ careers progress to higher levels, the time dedicated to their sport also increases. Quality and quantity of sleep fluctuate throughout the calendar year as athletic schedules vary throughout the preseason, in season, and postseason (Halson 2014). Efficiency and quality of sleep are reduced in athlete populations, which varies among different sports (e.g. poorer sleep has been reported for individual sports like cycling compared team sports like football and basketball) (Lastella et al. 2015). Concussion has been associated with disrupted sleep, suggesting professional American football players and other collision sport athletes at risk for brain trauma may also be at greater risk of adverse outcomes secondary to sleep deprivation (Jaffee et al. 2015). In the previously described Schwenk survey study of retired American football athletes, individuals reporting both high levels of depression and chronic pain were 32 times more likely to report trouble sleeping as “very common,” which was the most significantly elevated risk of all transition to retirement problems investigated (Schwenk et al 2007). The manner in which disrupted or disordered sleep affects cognition, behavior, and mood is perhaps one of the most important, pervasive, and complicated links within a biopsychosocial interpretation model.
Sleep plays a key role in a wide array of psychological, physiological, and cognitive processes in both younger and older adults. Sleep deprivation leads to increased stress reactivity (Minkel et al. 2012) and decreased quality of life (Farrell-Carnahan et al. 2013). Differential sleep characteristics, such as shorter rapid eye movement (REM) sleep latency (time interval between falling asleep and reaching rapid eye movement sleep) and reduced slow wave sleep (Riemann et al. 2001), as well as reduced frontal lobe activation during both wakefulness and sleep (Germain et al. 2004), have been found in patients diagnosed with depression. Past literature has also reported that depression is highly prevalent in individuals suffering from insomnia, suggesting a common etiology between the two disorders (Tsuno et al. 2005). Disordered sleep and depression have been researched extensively and are the subject of multiple review papers (Antonijevic and Antonijevic 2008; Buysse 2004; Tsuno et al. 2005). Sleep problems during development may carry a 43% increased risk for elevated anxiety/depression symptoms and 51% increased risk of aggressive behavior later in life (Gregory et al. 2008). These relationships between higher emotional reactivity and sleep deprivation have been reported throughout the lifespan. In their review of the literature published on violent and aggressive behavior in sleep deprived children and adults, Kamphuis et al concluded that sleep loss is a potential risk factor for impulsivity and reactive aggression (Kamphuis et al. 2012). Sleep disruptions are also associated with reduced performance on cognitive tasks (Fulda and Schulz 2001), including decreased attention span, slower reaction time, reduced episodic and working memory, and poor executive control (Durmer and Dinges 2005). Sleep appears to be particularly important for memory processes (Diekelmann and Born, 2010; Wagner and Born 2008).
Past literature has also suggested that chronic sleep disorders may be independent risk factors for the later development of neurodegenerative disease. For instance, Marion et al. performed a cross-sectional analysis examining interactions between REM-sleep behavior disorder (RBD) and the presence or absence of clinically diagnosed dementia in 65 Parkinson’s disease patients. The frequency of RBD was significantly higher in those with PD-dementia (10 of 13, 77%) compared to the PD-non demented group (14 of 52, 27%). Further, of 41 PD patients without RBD, only 3 (7.3%) had dementia compared to 10 of the 24 PD patients with RBD (42%), suggesting an association between RBD and the clinical manifestation of dementia in PD patients (Marion et al. 2008). Sleep disordered breathing increased the risk for developing mild cognitive impairment and dementia by 85% in a prospective study of older women (Yaffe et al. 2011). Disordered sleep is also highly comorbid in Alzheimer’s disease (Liguori et al. 2014) and Parkinson’s disease (Porter et al. 2015).
The physiological mechanisms driving the interaction of sleep and neurobehavioral changes are complex. Sleep disturbances in older adults (≥65 years of age) include earlier sleep onset, early morning awakening, decreased total time spent asleep, respiratory disorders (e.g., obstructive sleep apnea syndrome) (Wolkove et al. 2007), increased awakenings throughout the night (Porter et al. 2015), excessive daytime sleepiness (EDS) (Jaussent et al. 2012), and reduced slow wave sleep (SWS) amplitude (Mander et al. 2013). Objective studies have found a disruption in circadian rhythms, reduced time spent in SWS stages (N2 and N3), sleep fragmentation (difficulty staying asleep), and reduced sleep efficiency (time spent in bed versus time spent asleep) in older adults (Cipriani et al. 2015; Porter et al. 2015). Jaussent and colleagues found that EDS is associated with short and long term cognitive decline in non-demented adults (Jaussent et al. 2012). Similarly, reduced SWS is associated with reduced episodic memory recall (Mander et al. 2013).
A number of theories of physiological processes in the brain have been suggested to explain clinical and neuropathological changes in patient populations with sleep disorders, such as region-specific degeneration (Porter et al. 2015) and overall disruption to the ability of the brain to return to synaptic homeostasis following global degenerative changes in the brain (Mander, 2013). Recent evidence indicates that sleep enhances the clearance of metabolic waste products from interstitial space in the brain via the “glymphatic” system (Xie et al. 2013). The glymphatic system is thought to rely on the sleep state to relax interstitial spaces enabling cerebrospinal fluid (CSF) and interstitial fluid (ISF) to move through and clear metabolic waste (Jessen, Munk, Lundgaard, & Nedergaard, 2015). Interstitial space expands by up to 60% during sleep accelerating the flow of fluids (Iliff et al. 2013) and clearance of proteomic waste products (Iliff et al. 2013; Xie et al. 2013). During wakefulness, noradrenergic (NA) signaling in the locus coeruleus (LC), a brainstem structure, facilitates the closing of interstitial space (Mendelsohn and Larrick 2013). During sleep, NA signaling is inhibited, allowing opening of these spaces and the movement of CSF and ISF via aquaporin 4 (AQP4) channels located in highly polarized astrocytic endfeet (Jessen et al. 2015; Mendelsohn and Larrick 2013). Disruption to this system may be particularly relevant to collision sport athletes, who are likely to consistently accumulate metabolic waste from concussive and subclinical brain insults, including beta-amyloid from recurrent trauma (Giza and Hovda 2014).
Volumetric changes to the LC have been found in patient populations with significant sleep problems, including reduced sleep efficiency (more time spent awake) in TBI patients (Sullan et al. 2014) and RBD in Parkinson’s disease (Garcia-Lorenzo et al. 2013), thus indicating a possible role for disruption to the LC-NA system in sleep-related disorders. In TBI patients in particular, astroglial scars, inflammation, the buildup of proteomic waste products such as cleaved-tau and beta-amyloid, as well as astrocytic proteins such as glial fibrillary acidic protein (GFAP), S100 calcium-binding protein B (S100B) and AQP4 re-localization to the soma of the astrocyte following brain trauma (Ren et al. 2013), contribute to reduced glymphatic functioning (Jessen et al. 2015). Taken together, these findings highlight a problem specific to collision sport athletes – a metabolic cascade following brain trauma often results in increased glutamate release, increased metabolic activity, and may lead to a higher level of metabolic waste being produced. With potentially concurrent sleep disturbances (both during and after their playing careers), collision sport athletes may be at a greater risk of glymphatic system dysfunction and an inability to efficiently clear these accumulating metabolic byproducts over time. Since a number of neurodegenerative dementias stem from buildup of proteins such as beta-amyloid and tau, interference with removal may accelerate development of neurodegenerative disease secondary to trauma.
Glymphatic disruption may also help explain some of the variability in TBI outcome (Jessen et al. 2015). Relevant to retired athletes, Kress and colleagues found a 40% reduction in the clearance of metabolic waste from brain parenchyma in older compared to younger brains, with an 80–90% reduction in overall glymphatic functioning (Kress et al. 2014). Thus, the glymphatic system is important for its possible role in the accumulation of proteomic byproducts in the injured and/or aging brain. If exacerbated by a disruption to normal sleep cycles, as is often seen in TBI and older patients, the ability of the brain to repair damage and effectively remove metabolic waste may be progressively impaired.
From a psychosocial perspective, the presence of comorbid illness, medication use, psychological disorders such as depression and anxiety, as well as environmental factors such as stress, diet, exercise, and sleep hygiene practices can significantly affect sleep quality in older adults irrespective of neurodegenerative processes. For instance, depression may alter sleep architecture and disrupts normal SWS cycles (Tabuchi et al. 2015). In the case of neurodegenerative diseases, such as AD, changes to sleep can be profound. In AD patients, reduced sleep quality, sleep fragmentation, and sleep-disordered breathing may lead to an increased burden of structural brain changes, thus increasing the likelihood of neuronal death and cognitive decline (Tabuchi et al. 2015).
Thus, disordered sleep represents a significant factor in the development of a wide range of cognitive, mood, and behavioral problems. Mechanistically, reduced sleep may contribute to increased pathological burden due to decreased glymphatic clearance, as well as increased toxicity to the surrounding tissues, with subsequent deleterious effects on brain health and cognitive function. Given that glymphatic system dysfunction seems to partially explain the reduction in proteomic clearance, its role in the development and progression of CTE symptoms warrants further study. Such research may support the implementation of earlier interventions for sleep disturbances, which could work to delay the time course for clinically significant symptoms of neurodegenerative processes, such as CTE.
Summary and Conclusions
This review focused on factors in addition to repetitive brain trauma that may contribute to symptoms commonly associated with CTE. The factors are not exhaustive but are particularly relevant to retired professional collision sport athletes. Clinicians and researchers must pay careful attention to a career playing collision sports and the associated history of brain trauma, but explanatory models linking such trauma to later-life cognitive, behavioral, and emotional changes must also take these possibly co-occurring factors into account. Understanding the links between brain trauma, neurodegenerative pathology, and clinical changes remains in its infancy, and it is likely that these and other factors serve as complex moderators and mediators in determining who will, and who will not, develop CTE or its related clinical syndrome in their lifetime.
Mood and behavioral changes attributed to CTE pathology include depression, irritability, anxiety, explosivity, aggression, violence, and impulsivity (Montenigro et al. 2014). Such problems can be seen in individuals without repetitive brain trauma who present with neurodevelopmental disorders (e.g. LD and ADHD), normal aging, complicated adjustments to retirement, drug and alcohol abuse, and sleep problems. Suicidality was not addressed as this topic has been covered specifically and more extensively in reviews by Iverson (2014 and 2016).
Memory impairment, executive dysfunction, impaired attention and language abilities, and progressive cognitive decline are all reportedly associated with CTE (McKee et al. 2013, Reams et al. 2016). These same cognitive difficulties may also be associated with developmental socioeconomic factors, neurodevelopmental disorders, normal aging, adjusting to retirement, drug and alcohol abuse, and sleep disorders. The literature is less definitive as it relates to surgeries and repeated exposure to anesthesia, but this warrants additional consideration due to the increased likelihood of a positive surgical history in professional athletes and links between anesthesia exposure and neuropathological outcomes (Weir et al. 2009).
Many of these factors have also been identified as independent risk factors for the development of neurodegenerative disease or dementia. Developmental SES and disordered sleep have ties to dementia or Alzheimer’s disease, while neurodevelopmental disorders, drug/alcohol abuse, and exposure to multiple surgeries/anesthesia have less consistent but notably evident connections to accumulated neuropathology and late-life cognitive impairment. We briefly reviewed the literature examining long-term risk of neurodegenerative disease and pathology following TBI, and results of both individual studies and meta-analyses typically indicate a small but statistically significant increased risk, though no studies to date have linked single-event TBI history to CTE, specifically. The recent report from Crane et al. shows TBI history may specifically influence long-term neuropathological outcomes consistent with PD and Lewy Bodies, but may not elevate risk for clinically diagnosed dementia (2016). Consideration of biopsychosocial influences frequently mitigates the relative risks reported in these studies, and attention must be paid to how these epidemiological investigations define TBI. Varying severities are often included in single analyses, and rarely is repetitive brain trauma specifically evaluated. The two studies reporting results based on repetitive brain trauma history show conflicting results (Lehman et al. 2012; Savica et al. 2012). In order to inform public policy and contribute to our understanding of the true risks of participation in collision sports, more research is needed focusing on overall exposure to repetitive subclinical brain trauma and subsequent risk for both neuropathological and clinical outcomes. Based on available data, it may be concluded that the degree of clinical and neuropathological CTE symptoms in retired athletes is dependent not only on exposure to repetitive brain trauma, but also on the presence of a number of additional risk factors and modifying factors with substantial individual differences. This has important treatment implications.
Incorporating a biopsychosocial approach to the management of CTE increases the complexity of the associated clinical syndrome but also, conceivably, the likelihood of some symptom improvement. Clinicians cannot retroactively eliminate the repetitive brain trauma sustained playing collision sports, but many of the factors described in this paper represent modifiable risks that can be addressed through medical and psychological treatment. Pharmacotherapy for neuropsychiatric or sleep complaints, psychotherapy targeting mood and behavioral disturbances (Hofmann et al. 2012), sleep hygiene or cognitive behavioral therapy for insomnia improving sleep quality (Stepanski and Wyatt 2003; Taylor and Pruiksma 2014), and cognitive training interventions (Ball et al. 2002) offer potentially effective ways of addressing many CTE-related symptoms and, if instituted throughout playing careers or early in the retirement period, may hold some promise for improving quality of life for aging collision sport athletes (see Figure). The Schwenk et al. survey of retired professional American football athletes highlights some of the potential barriers to engaging this population in treatment. Among retirees currently experiencing chronic pain and significant depression, 66% reported they did not recognize their issues as important, 56% reported they felt they would be “weak” if they sought help, and 52% reported they were embarrassed by what friends or family would think if they sought treatment (Schwenk et al. 2007). Education, outreach, and general culture changes are likely necessary for increasing willingness to engage treatment services.
Based on evidence described above, we conclude that a biopsychosocial model should also be applied to research design (McCrea et al. 2015). Arguably the most important piece currently missing from the literature is a lack of clear understanding of the true incidence of CTE pathology in athletes and of athletes expressing clinical manifestation of the disease (i.e. TES). Incidence can only be determined by assessing large samples of both symptomatic and non-symptomatic former athletes. Methodological challenges to determining CTE causality and incidence include, but are not limited to, inconsistent definitions of repetitive brain trauma exposure and mild traumatic brain injury, selection and recall biases, and cohort effects (Gardner and Yaffe 2015). Research to date has examined convenience samples of athletes who donated their brains and were experiencing clinical symptoms prior to their death. This has unfortunately led to misleading headlines about the risk of developing CTE pathology, and estimates of its prevalence which are almost certainly inflated (O’Keefe 2015). As is the case with other neurodegenerative disorders, subsets of the population are prone to express symptoms of the disorder but lack underlying pathology, while others will have underlying pathology but do not express concomitant symptoms. The newly emerging ability to use PET imaging of tau pathology is a potentially invaluable tool that could be utilized to better understand the presence and distribution of tau in vivo.
Identification of CTE pathology in living patients will also substantially contribute to the determination of sensitive and specific clinical correlates. Recently proposed clinical diagnostic criteria for TES are non-specific and non-exclusive. In its current form, any patient receiving a dementia or neurodegenerative disease diagnosis who participated in collision sports at some point in their life, regardless of the affected cognitive domain, would likely be classified as having “Probable TES.” Criteria for “Possible TES” are even more inclusive, and the clinical utility of such a diagnosis is currently unclear. A clinical case series reported by Gardner et al. highlights the complexities of CTE-related phenotypic classification (Gardner et al. 2015).
Biopsychosocial influences are critical when interpreting past results and in the design of future studies. Previous studies have compared retired collision sport athletes to males in the general population in an attempt to determine the additive role of repetitive brain trauma on the natural aging process. However, the control groups used in these studies often fail to control for many of the factors we have discussed. As described, professional athletes are a demographically biased sample with significant inter-sport variability, and various socioeconomic factors can impact cognitive development across the lifespan. Two studies matched controls on age and education level but do not indicate the racial or ethnic diversity of the groups (Tremblay et al. 2013; Tremblay et al. 2014). One study used a control group comprised of approximately 90% (19/21) white and 10% (2/21) African American individuals compared to an athlete sample of 55% (11/20) white and 45% (9/20) African American athletes (Strain et al., 2015). A study with racially similar groups compared a non-athlete group (N=9, mean age 58 years) to an athlete group (N=11, mean age 65 years) (Coughlin et al. 2015) approximately seven years older. Age differences of this magnitude may be significant when considering that the most likely age of onset of dementia is in the mid-60’s (“Alzheimer’s Disease Fact Sheet” 2015).
Another example of the key importance of well-designed control groups can be seen in recent studies that have described length of time playing football as a risk factor for cognitive decline in retired athletes. Stamm et al showed that a group of athletes who began playing football before the age of 12 performed worse on neuropsychological tests (Stamm et al. 2015). This finding makes intuitive sense, but 16% of the before-12 group had a previous diagnosis of learning disability compared to 0% of the athletes who began playing after age 12, thus confounding LD history with the length-of-play grouping. Neurodevelopmental conditions like learning disabilities and attention deficit-hyperactivity disorder significantly affect cognitive and emotional development across the lifespan and must be considered for retired athletes as well (Moss et al. 2000; Putukian et al. 2011). Indeed, a more recent study by Solomon et al utilizing more robust statistical procedures and addressing some of these sample concerns was unable to replicate these findings. They found no neurological, neuroradiological, or neuropsychological associations with exposure to football earlier in life (Solomon et al. 2016). Taken together, this highlights the need to incorporate demographics and comorbid medical conditions (i.e. neurodevelopmental disorders, previous psychiatric conditions, etc.) when attributing causality of clinicopathological outcomes in repetitive brain trauma research.
Clinicians working with individuals exposed to repetitive brain trauma are tasked with the daunting responsibility of consolidating as much relevant information as possible pertaining to the brain that has been injured. The focus of this review is on retired professional athletes, but concerns about CTE are present in younger athletes as well (Mez et al. 2016); some of the findings described here may have relevance to them. Regardless of athlete age, evaluating premorbid neurobehavioral functioning is essential to understanding individual-specific change over time and which factors may modify these changes the most. Importantly, attribution of pathological burden and symptom expression needs to be considered in a broad biopsychosocial explanatory framework that incorporates these factors in the causal model instead of focusing only on a history of repetitive brain trauma as the sole causative factor in the development or degree of CTE symptoms.
Contributor Information
Breton M. Asken, Graduate Student, University of Florida, Department of Clinical and Health Psychology.
Molly J. Sullan, Graduate Student, University of Florida, Department of Clinical and Health Psychology
Aliyah R. Snyder, Graduate Student. University of Florida, Department of Clinical and Health Psychology
Zachary M. Houck, Graduate Student, University of Florida, Department of Clinical and Health Psychology
Vaughn E. Bryant, Graduate Student, University of Florida, Department of Clinical and Health Psychology
Loren P. Hizel, Graduate Student, University of Florida, Department of Clinical and Health Psychology
Molly E. McLaren, Graduate Student, University of Florida, Department of Clinical and Health Psychology
Duane E. Dede, Clinical Professor, University of Florida, Department of Clinical and Health Psychology.
Michael S. Jaffee, Associate Professor, Chief – Divison of Neurology, University of Florida, Department of Neurology
Steven T. DeKosky, Professor, Deputy Director of McKnight Brain Institute, University of Florida, Department of Neurology
Russell M. Bauer, Professor, Graduate Program Director, University of Florida, Department of Clinical and Health Psychology.
References
- Alaranta A, Alaranta H, Helenius I. Use of prescription drugs in athletes. Sports Medicine. 2008;38(6):449–463. doi: 10.2165/00007256-200838060-00002. [DOI] [PubMed] [Google Scholar]
- Alexander S, Kerr ME, Yonas H, Marion DW. The effects of admission alcohol level on cerebral blood flow and outcomes after severe traumatic brain injury. J Neurotrauma. 2004;21(5):575–583. doi: 10.1089/089771504774129900. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Disease Fact Sheet. 2015 from https://www.nia.nih.gov/alzheimers/publication/alzheimers-disease-fact-sheet.
- Amato PR, Zuo J. Rural poverty, urban poverty, and psychological well-being. Sociological Quarterly. 1992:229–240. [Google Scholar]
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders:: DSM-5. ManMag; 2003. [Google Scholar]
- Ancelin ML, De Roquefeuil G, Scali J, Bonnel F, Adam JF, Cheminal JC, … Ritchie K. Long-term post-operative cognitive decline in the elderly: the effects of anesthesia type, apolipoprotein E genotype, and clinical antecedents. Journal of Alzheimer’s Disease. 2010;22:105. doi: 10.3233/JAD-2010-100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson HW, Sommerfelt K, Sonnander K, Ahlsten G. Maternal child-rearing attitudes, IQ, and socioeconomic status as related to cognitive abilities of five-year-old children. Psychol Rep. 1996;79(1):3–14. doi: 10.2466/pr0.1996.79.1.3. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle Marcus E, Buckner RL. Disruption of Large-Scale Brain Systems in Advanced Aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. http://dx.doi.org/10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonijevic I, Antonijevic I. HPA axis and sleep: Identifying subtypes of major depression: Review. Stress. 2008;11(1):15–27. doi: 10.1080/10253890701378967. [DOI] [PubMed] [Google Scholar]
- Avidan MS, Evers AS. Review of clinical evidence for persistent cognitive decline or incident dementia attributable to surgery or general anesthesia. Journal of Alzheimer’s Disease. 2011;24(2):201. doi: 10.3233/JAD-2011-101680. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, … Tennstedt SL. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaresi W, Katusic S, Colligan R, Weaver A, Pankratz V, Mrazek D, Jacobsen S. How common is attention-deficit/hyperactivity disorder? Towards resolution of the controversy: results from a population-based study. Acta Paediatr. 2004;93(s445):55–59. doi: 10.1111/j.1651-2227.2004.tb03058.x. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Differential diagnosis of adults with ADHD: the role of executive function and self-regulation. The Journal of clinical psychiatry. 2010;71(7):1,478–417. doi: 10.4088/JCP.9066tx1c. [DOI] [PubMed] [Google Scholar]
- Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71(4):266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belson K. Ex-Players Suing N.F.L. Over Use of Painkiller. 2011 Retrieved January 18, 2016 http://www.nytimes.com/2011/12/06/sports/football/nfl-sued-by-ex-players-over-painkiller-toradol.html?_r=0.
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, … Davatzikos C. Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res. 2006;30(11):1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Berger M, Nadler JW, Friedman A, McDonagh DL, Bennett ER, Cooter M … MAD-PIA trial team. The Effect of Propofol Versus Isoflurane Anesthesia on Human Cerebrospinal Fluid Markers of Alzheimer’s Disease: Results of a Randomized Trial. J Alzheimers Dis. 2016;52(4):1299–1310. doi: 10.3233/JAD-151190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, … Faraone SV. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychological medicine. 2006;36(02):167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE, … Dickson DW. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130(6):877–889. doi: 10.1007/s00401-015-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilottaa F, Doronzioa A, Stazia E, Fodaleb LTV. Postoperative cognitive dysfunction: toward the Alzheimer’s disease pathomechanism hypothesis. apoptosis. 2010;22:25. doi: 10.3233/JAD-2010-100825. [DOI] [PubMed] [Google Scholar]
- Blair K, Smith B, Mitchell D, Morton J, Vythilingam M, Pessoa L, … Drevets W. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock RL, Maroon J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy—a unifying hypothesis. Surg Neurol Int. 2011;2 doi: 10.4103/2152-7806.83391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2006;20(1):63–72. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Bramham J, Murphy D, Xenitidis K, Asherson P, Hopkin G, Young S. Adults with attention deficit hyperactivity disorder: an investigation of age-related differences in behavioural symptoms, neuropsychological function and co-morbidity. Psychological medicine. 2012;42(10):2225–2234. doi: 10.1017/S0033291712000219. [DOI] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, Iii, … Becker JT. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters N, Stuss DT. Diencephalic amnesia. Handbook of neuropsychology. 1989;3:107–148. [Google Scholar]
- Buysse DJ. Insomnia, depression and aging. Assessing sleep and mood interactions in older adults. Geriatrics. 2004;59(2):47–51. quiz 52. [PubMed] [Google Scholar]
- Calvo E, Sarkisian N, Tamborini CR. Causal effects of retirement timing on subjective physical and emotional health. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2013;68(1):73–84. doi: 10.1093/geronb/gbs097. [DOI] [PubMed] [Google Scholar]
- Campbell L. For retired NFL players, most challening ‘season’ just beginning. 2011 from http://www.cnn.com/2011/OPINION/09/08/nfl.life.after.the.game/
- Caraci F, Pistara V, Corsaro A, Tomasello F, Giuffrida ML, Sortino MA, … Copani A. Neurotoxic properties of the anabolic androgenic steroids nandrolone and methandrostenolone in primary neuronal cultures. J Neurosci Res. 2011;89(4):592–600. doi: 10.1002/jnr.22578. [DOI] [PubMed] [Google Scholar]
- Carskadon MD, WC . Principles and Practice of Slep Medicine, 5th Edition. St. Louis: Elsevier Saunders; 2011. Monitoring and Staging Human Sleep - Normal Human Sleep: An Overview; pp. 16–26. [Google Scholar]
- Carter MAT, Cook K. Adaptation to retirement: Role changes and psychological resources. The Career Development Quarterly. 1995;44(1):67–82. [Google Scholar]
- Casey B, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, … Cohen JD. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of cognitive neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chapman SL, Byas-Smith MG, Reed BA. Effects of intermediate- and long-term use of opioids on cognition in patients with chronic pain. Clin J Pain. 2002;18(4 Suppl):S83–90. doi: 10.1097/00002508-200207001-00010. [DOI] [PubMed] [Google Scholar]
- Chen CW, Lin CC, Chen KB, Kuo YC, Li CY, Chung CJ. Increased risk of dementia in people with previous exposure to general anesthesia: A nationwide population-based case–control study. Alzheimer’s & Dementia. 2014;10(2):196–204. doi: 10.1016/j.jalz.2013.05.1766. [DOI] [PubMed] [Google Scholar]
- Choi P, Parrott A, Cowan D. High dose anabolic steroids in strength athletes: Effects upon hostility and aggression. Human Psychopharmacology: Clinical and Experimental. 1990;5(4):349–356. [Google Scholar]
- Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Preventive medicine. 1998;27(2):184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics. 2015;15(1):65–74. doi: 10.1111/psyg.12069. [DOI] [PubMed] [Google Scholar]
- Collins MW, Grindel SH, Lovell MR, Dede DE, Moser DJ, Phalin BR, … McKeag DB. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;282(10):964–970. doi: 10.1001/jama.282.10.964. joc80880 [pii] [DOI] [PubMed] [Google Scholar]
- Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annu Rev Psychol. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Corben B., Writer . 30 for 30: Broke: Entertainment and Sports Programming Network [ESPN] 2012. 30 for 30: Broke. [Google Scholar]
- Cottler LB, Ben Abdallah A, Cummings SM, Barr J, Banks R, Forchheimer R. Injury, pain, and prescription opioid use among former National Football League (NFL) players. Drug Alcohol Depend. 2011;116(1–3):188–194. doi: 10.1016/j.drugalcdep.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Munro CA, Ma S, Yue C, Chen S, … Pomper MG. Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol Dis. 2015;74:58–65. doi: 10.1016/j.nbd.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Gibbons LE, Dams-O’Connor K, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schnieder JA, Abisambra JF, Abner EL, Alafuzoff I, … Nelson PT. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave D, Rashad I, Spasojevic J. The effects of retirement on physical and mental health outcomes. National Bureau of Economic Research; 2006. [Google Scholar]
- Davison L, Steinhelber J, Eger E, 2nd, Stevens W. Psychological effects of halothane and isoflurane anesthesia. Anesthesiology. 1975;43(3):313–324. doi: 10.1097/00000542-197509000-00008. [DOI] [PubMed] [Google Scholar]
- de Jong PF. Working memory deficits of reading disabled children. Journal of experimental child psychology. 1998;70(2):75–96. doi: 10.1006/jecp.1998.2451. [DOI] [PubMed] [Google Scholar]
- De Ronchi D, Fratiglioni L, Rucci P, Paternico A, Graziani S, Dalmonte E. The effect of education on dementia occurrence in an Italian population with middle to high socioeconomic status. Neurology. 1998;50(5):1231–1238. doi: 10.1212/wnl.50.5.1231. [DOI] [PubMed] [Google Scholar]
- Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(Suppl 1):i41–46. doi: 10.1093/bja/aep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boer JJ, Oostendorp RA, Beems T, Munneke M, Oerlemans M, Evers AW. A systematic review of bio-psychosocial risk factors for an unfavourable outcome after lumbar disc surgery. European Spine Journal. 2006;15(5):527–536. doi: 10.1007/s00586-005-0910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nature Reviews Neuroscience. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dimoula F, Torregrosa M, Psychountaki M, Fernandez MD. Retiring from elite sports in Greece and Spain. Span J Psychol. 2013;16:E38. doi: 10.1017/sjp.2013.18. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Pettit GS. A biopsychosocial model of the development of chronic conduct problems in adolescence. Developmental psychology. 2003;39(2):349. doi: 10.1037//0012-1649.39.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer B, Jiang Y, Zanjani F, Fardo D. Effects of alcohol consumption on cognition and regional brain volumes among older adults. Am J Alzheimers Dis Other Demen. 2015;30(4):364–374. doi: 10.1177/1533317514549411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, Pereira E, Chene G, Glymour MM, Alperovitch A, Saubusse E, … Forette F. Older age at retirement is associated with decreased risk of dementia. European Journal of Epidemiology. 2014;29(5):353–361. doi: 10.1007/s10654-014-9906-3. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Brooks-Gunn J, Klebanov PK. Economic deprivation and early childhood development. Child Dev. 1994;65(2):296–318. [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25(1):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Estrada M, Varshney A, Ehrlich BE. Elevated testosterone induces apoptosis in neuronal cells. J Biol Chem. 2006;281(35):25492–25501. doi: 10.1074/jbc.M603193200. [DOI] [PubMed] [Google Scholar]
- Evans GW. Child development and the physical environment. Annu Rev Psychol. 2006;57:423–451. doi: 10.1146/annurev.psych.57.102904.190057. [DOI] [PubMed] [Google Scholar]
- Ex-Players: NFL Illegally Used Drugs. 2014 from http://espn.go.com/nfl/story/_/id/10958191/nfl-illegally-supplied-risky-painkilling-drugs-former-players-allege-suit.
- Farrell-Carnahan L, Franke L, Graham C, McNamee S. Subjective sleep disturbance in veterans receiving care in the veterans affairs polytrauma system following blast-related mild traumatic brain injury. Mil Med. 2013;178(9):951–956. doi: 10.7205/MILMED-D-13-00037. [DOI] [PubMed] [Google Scholar]
- Feeley BT, Kennelly S, Barnes RP, Muller MS, Kelly BT, Rodeo SA, Warren RF. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597–1603. doi: 10.1177/0363546508316021. [DOI] [PubMed] [Google Scholar]
- Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5:363–389. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural Brain Changes in Aging: Courses, Causes and Cognitive Consequences. Reviews in the Neurosciences. 2010;21:187. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74(7):857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TG, Hshieh TT, Wong B, Tommet D, Jones RN, Schmitt EM, … Inouye SK. Neuropsychological profiles of an elderly cohort undergoing elective surgery and the relationship between cognitive performance and delirium. J Am Geriatr Soc. 2015;63(5):977–982. doi: 10.1111/jgs.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fralick M, Thiruchelvam D, Tien HC, Redelmeier DA. Risk of suicide after a concussion. CMAJ. 2016;188(7):497–504. doi: 10.1503/cmaj.150790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep medicine reviews. 2001;5(6):423–445. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- Garcia-Lorenzo D, Longo-Dos Santos C, Ewenczyk C, Leu-Semenescu S, Gallea C, Quattrocchi G, … Lehericy S. The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain. 2013;136(Pt 7):2120–2129. doi: 10.1093/brain/awt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Iverson GL, McCrory P. British Journal of Sports Medicine. Chronic traumatic encephalopathy in sport: a systematic review. 2013 doi: 10.1136/bjsports-2013-092646. bjsports-2013-092646. [DOI] [PubMed] [Google Scholar]
- Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs. nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71(12):1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Possin KL, Hess CP, Huang EJ, Grinberg LT, Nolan AL, … Rabinovici GD. Evaluating and treating neurobehavioral symptoms in professional American football players: Lessons from a case series. Neurol Clin Pract. 2015;5(4):285–295. doi: 10.1212/CPJ.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Molecular and Cellular Neuroscience. 2015;66:75–80. doi: 10.1016/j.mcn.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett BE, Cantu RC, Shenton M, Lin AP, Nowinski CJ, McKee AC, Stern RA. Clinical appraisal of chronic traumatic encephalopathy: current perspectives and future directions. Current opinion in neurology. 2011;24(6):525–531. doi: 10.1097/WCO.0b013e32834cd477. [DOI] [PubMed] [Google Scholar]
- Geddes JF, Vowles GH, Nicoll JA, Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. [Case Reports] Acta Neuropathologica. 1999;98(2):171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- Germain A, Nofzinger EA, Kupfer DJ, Buysse DJ. Neurobiology of non-REM sleep in depression: further evidence for hypofrontality and thalamic dysregulation. Am J Psychiatry. 2004;161(10):1856–1863. doi: 10.1176/ajp.161.10.1856. [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(Suppl 4):S24–33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G, Beers SR, Siegel DJ, Minshew NJ. A comparison of WAIS-R profiles in adults with high-functioning autism or differing subtypes of learning disability. Appl Neuropsychol. 2001;8(3):148–154. doi: 10.1207/S15324826AN0803_3. [DOI] [PubMed] [Google Scholar]
- Golimstok A, Rojas J, Romano M, Zurru M, Doctorovich D, Cristiano E. Previous adult attention-deficit and hyperactivity disorder symptoms and risk of dementia with Lewy bodies: a case–control study. European Journal of Neurology. 2011;18(1):78–84. doi: 10.1111/j.1468-1331.2010.03064.x. [DOI] [PubMed] [Google Scholar]
- Gottfried AW. Measures of socioeconomic status in child development research: Data and recommendations. Merrill-Palmer Quaterly (1982-) 1985;31(1):85–92. [Google Scholar]
- Grant I. Alcohol and the brain: neuropsychological correlates. Journal of consulting and clinical psychology. 1987;55(3):310. doi: 10.1037//0022-006x.55.3.310. [DOI] [PubMed] [Google Scholar]
- Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. The Annals of Family Medicine. 2012;10(4):304–311. doi: 10.1370/afm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Van der Ende J, Willis TA, Verhulst FC. Parent-reported sleep problems during development and self-reported anxiety/depression, attention problems, and aggressive behavior later in life. Archives of pediatrics & adolescent medicine. 2008;162(4):330–335. doi: 10.1001/archpedi.162.4.330. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K, Marshall SW, Bailes J, Mccrea M, Harding HP, Matthews A, … Cantu RC. Recurrent concussion and risk of depression in retired professional football players. Medicine & Science in Sports & Exercise. 2007;39(6):903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- Hall KS, Gao S, Unverzagt FW, Hendrie HC. Low education and childhood rural residence: risk for Alzheimer’s disease in African Americans. Neurology. 2000;54(1):95–99. doi: 10.1212/wnl.54.1.95. [DOI] [PubMed] [Google Scholar]
- Hall RC, Hall RC, Chapman MJ. Psychiatric complications of anabolic steroid abuse. Psychosomatics. 2005;46(4):285–290. doi: 10.1176/appi.psy.46.4.285. [DOI] [PubMed] [Google Scholar]
- Halson SL. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. 2014;44(Suppl 1):S13–23. doi: 10.1007/s40279-014-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78(6):1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. Major depression in 6050 former drinkers: association with past alcohol dependence. Archives of General Psychiatry. 2002;59(9):794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- Herrmann LL, Goodwin GM, Ebmeier KP. The cognitive neuropsychology of depression in the elderly. Psychological medicine. 2007;37(12):1693–1702. doi: 10.1017/S0033291707001134. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18(3):485. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Langenbucher JW, Flores A, Harty S, Berlin HA. The influence of age of onset and acute anabolic steroid exposure on cognitive performance, impulsivity, and aggression in men. Psychol Addict Behav. 2014;28(4):1096–1104. doi: 10.1037/a0036482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill P, Lowe B. The inevitable metathesis of the retiring athlete. International Review for the Sociology of Sport. 1974;9(3):5–32. [Google Scholar]
- Hoaken PN, Stewart SH. Drugs of abuse and the elicitation of human aggressive behavior. Addictive behaviors. 2003;28(9):1533–1554. doi: 10.1016/j.addbeh.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognitive therapy and research. 2012;36(5):427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn L, Nevill S. National Center for Education Statistics. Washington, DC: US Department of Education; 2006. Profile of undergraduate students in US postsecondary education institutions: 2003–2004: With a special analysis of community college students (NCES 2006–184) [Google Scholar]
- Horn S, Gregory P, Guskiewicz KM. Self-reported anabolic-androgenic steroids use and musculoskeletal injuries: findings from the center for the study of retired athletes health survey of retired NFL players. American Journal of Physical Medicine & Rehabilitation. 2009;88(3):192–200. doi: 10.1097/PHM.0b013e318198b622. [DOI] [PubMed] [Google Scholar]
- Hussain M, Berger M, Eckenhoff RG, Seitz DP. General anesthetic and the risk of dementia in elderly patients: current insights. Clin Interv Aging. 2014;9:1619–1628. doi: 10.2147/CIA.S49680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299–1309. doi: 10.1172/jci67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyuk R, Gromyco D, Kiselev A, Torban M, Krupitsky E. ANS: The Journal for Neurocognitive Research. Hostility and anger in patients dependent on different psychoactive drugs. 2013;54(3–4) [Google Scholar]
- The Institute for Diversity and Ethics in Sport. 2015 from http://www.tidesport.org/home.html.
- Ivanchak N, Fletcher K, Jicha GA. Attention-deficit/hyperactivity disorder in older adults: prevalence and possible connections to mild cognitive impairment. Current psychiatry reports. 2012;14(5):552–560. doi: 10.1007/s11920-012-0305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL. Chronic traumatic encephalopathy and risk of suicide in former athletes. British Journal of Sports Medicine. 2014;48(2):162–164. doi: 10.1136/bjsports-2013-092935. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Gardner AJ, McCrory P, Zafonte R, Castellani RJ. A critical review of chronic traumatic encephalopathy. Neuroscience & Biobehavioral Reviews. 2015;56:276–293. doi: 10.1016/j.neubiorev.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Silverberg ND, Mannix R, Maxwell BA, Atkins JE, Zafonte R, Berkner PD. Factors Associated With Concussion-like Symptom Reporting in High School Athletes. JAMA Pediatr. 2015;169(12):1132–1140. doi: 10.1001/jamapediatrics.2015.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL. Suicide and chronic traumatic encephalopathy. The Journal of neuropsychiatry and clinical neurosciences. 2016;28.1(1):9–16. doi: 10.1176/appi.neuropsych.15070172. [DOI] [PubMed] [Google Scholar]
- Jafari S, Etminan M, Aminzadeh F, Samii A. Head injury and risk for Parkinson disease: a systematic review and meta-analysis. Mov Disord. 2013;28(9):1222–1229. doi: 10.1002/mds.25458. [DOI] [PubMed] [Google Scholar]
- Jaffee MS, Winter WC, Jones CC, Ling G. Sleep disturbances in athletic concussion. Brain injury. 2015;29(2):221–227. doi: 10.3109/02699052.2014.983978. [DOI] [PubMed] [Google Scholar]
- Jaussent I, Bouyer J, Ancelin ML, Berr C, Foubert-Samier A, Ritchie K, … Dauvilliers Y. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35(9):1201–1207. doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis BJ, Power C, Hertzman C. Birth weight, childhood socioeconomic environment, and cognitive development in the 1958 British birth cohort study. Bmj. 2002;325(7359):305. doi: 10.1136/bmj.325.7359.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins KR, Pienta AM, Horgas AL. Activity and health-related quality of life in continuing care retirement communities. Research on Aging. 2002;24(1):124–149. [Google Scholar]
- Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner’s Guide. Neurochem Res. 2015;40(12):2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Jiang H. Effect of the inhaled anesthetics isoflurane, sevoflurane, and desflurane on the neuropathogenesis of Alzheimer’s disease (review) Mol Med Rep. 2015;12(1):3–12. doi: 10.3892/mmr.2015.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nature Reviews Neurology. 2013;9(4):222–230. doi: 10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- Kamphuis J, Meerlo P, Koolhaas JM, Lancel M. Poor sleep as a potential causal factor in aggression and violence. Sleep medicine. 2012;13(4):327–334. doi: 10.1016/j.sleep.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Cohane GH, Weiss RD, Pope HG. Past anabolic-androgenic steroid use among men admitted for substance abuse treatment: an underrecognized problem? The Journal of clinical psychiatry. 2003;64(2):156–160. doi: 10.4088/jcp.v64n0208. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Kean J, Hudson JI, Pope HG., Jr Cognitive deficits in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2013;130(1–3):208–214. doi: 10.1016/j.drugalcdep.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30(2):256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Janes AC, Hudson JI, Brennan BP, Kanayama G, Kerrigan AR, … Pope HG., Jr Brain and cognition abnormalities in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2015;152:47–56. doi: 10.1016/j.drugalcdep.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr ZY, Marshall SW, Harding HP, Jr, Guskiewicz KM. Nine-Year Risk of Depression Diagnosis Increases With Increasing Self-Reported Concussions in Retired Professional Football Players. Am J Sports Med. 2012 doi: 10.1177/0363546512456193. 0363546512456193 [pii] [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, … Secnik K. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. American Journal of Psychiatry. 2006 doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Page GG, Marucha PT, MacCallum RC, Glaser R. Psychological influences on surgical recovery: perspectives from psychoneuroimmunology. American Psychologist. 1998;53(11):1209. doi: 10.1037//0003-066x.53.11.1209. [DOI] [PubMed] [Google Scholar]
- Klerman LV. Alive and Well? A Research and Policy Review of Health Programs for Poor Young Children. ERIC; 1991. [Google Scholar]
- Korenman S, Miller JE, Sjaastad JE. Long-term poverty and child development in the United States: Results from the NLSY. Children and Youth Services Review. 1995;17(1):127–155. [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, … Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76(6):845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Hart EL, Frick PJ, Applegate B, Zhang Q, … Russo MF. Four-year longitudinal study of conduct disorder in boys: patterns and predictors of persistence. Journal of Abnormal Psychology. 1995;104(1):83. doi: 10.1037/0021-843X.104.1.83. [DOI] [PubMed] [Google Scholar]
- Lastella M, Roach GD, Halson SL, Sargent C. Sleep/wake behaviours of elite athletes from individual and team sports. Eur J Sport Sci. 2015;15(2):94–100. doi: 10.1080/17461391.2014.932016. [DOI] [PubMed] [Google Scholar]
- Lavallee D, Grove JR, Gordon S. The causes of career termination from sport and their relationship to post-retirement adjustment among elite-amateur athletes in Australia. Australian Psychologist. 1997;32(2):131–135. [Google Scholar]
- Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79(19):1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. The American journal of sports medicine. 2016 doi: 10.1177/0363546516645093. 0363546516645093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, … Placidi F. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71(12):1498–1505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- Lule D, Ludolph A, Ludolph A. Neurodevelopmental and neurodegenerative diseases–Is there a pathophysiological link? Attention-deficit/hyperactivity disorder and amyotrophic lateral sclerosis as examples. Medical hypotheses. 2008;70(6):1133–1138. doi: 10.1016/j.mehy.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60(2):S93–S101. doi: 10.1093/geronb/60.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, De Santi S, Convit A, Tarshish C, Nair NPV, … Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature neuroscience. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, Shema SJ. Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. N Engl J Med. 1997;337(26):1889–1895. doi: 10.1056/NEJM199712253372606. [DOI] [PubMed] [Google Scholar]
- Madden D, Bennett I, Song A. Cerebral White Matter Integrity and Cognitive Aging: Contributions from Diffusion Tensor Imaging. Neuropsychology Review. 2009;19(4):415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major League Soccer Racial and Gender Report Card. The Institute for Diversity and Ethics in Sport; 2015. from http://www.tidesport.org/mls-rgrc.html. [Google Scholar]
- Manchikanti L, Giordano J, Boswell MV, Fellows B, Manchukonda R, Pampati B. Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. J manag. 2007;5:8. doi: 10.5055/jom.2007.0045. [DOI] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, … Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Echemendia RJ. Race-specific norms: Using the model of hypertension to understand issues of race, culture, and education in neuropsychology. Archives of Clinical Neuropsychology. 2007;22(3):319–325. doi: 10.1016/j.acn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J Int Neuropsychol Soc. 2002;8(3):341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- Marion MH, Qurashi M, Marshall G, Foster O. Is REM sleep behaviour disorder (RBD) a risk factor of dementia in idiopathic Parkinson’s disease? Journal of neurology. 2008;255(2):192–196. doi: 10.1007/s00415-008-0629-9. [DOI] [PubMed] [Google Scholar]
- Martland H. Dementia pugilistica. JAMA. 1928;91 (11031107.9) [Google Scholar]
- McCann B, Roy-Byrne P. Attention-deficit/hyperactivity disorder and learning disabilities in adults; Paper presented at the Seminars in clinical neuropsychiatry; 2000. [DOI] [PubMed] [Google Scholar]
- McCrea M, Broshek DK, Barth JT. Sports concussion assessment and management: future research directions. Brain Inj. 2015;29(2):276–282. doi: 10.3109/02699052.2014.965216. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse WH, Kutcher JS, Jordan BD, Gardner A. What is the evidence for chronic concussion-related changes in retired athletes: behvaioural, pathological, and clinical outcomes? British Journal of Sports Medicine. 2013;47(5):372–330. doi: 10.1136/bjsports-2013-092248. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, … Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, … Baugh CM. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I … TBI/CTE group. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131(1):75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC. The impact of poverty and low socioeconomic status on the socioemotional functioning of African-American children and adolescents: Mediating effects. Social and emotional adjustment and family relations in ethnic minority families. 1997:7–34. [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Frontiers in integrative neuroscience. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. 2007;1 doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn AR, Larrick JW. Sleep facilitates clearance of metabolites from the brain: glymphatic function in aging and neurodegenerative diseases. Rejuvenation Res. 2013;16(6):518–523. doi: 10.1089/rej.2013.1530. [DOI] [PubMed] [Google Scholar]
- Mez J, Solomon T, Daneshvar DH, Stein TD, McKee AC. Pathologically Confirmed Chronic Traumatic Encephalopathy in a 25-Year-Old Former College Football Player. JAMA Neurol, Online First. 2016 doi: 10.1001/jamaneurol.2015.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles K. Armond Armstead, Former USC Football Player, Sues School For Heart Attack. 2012 from http://www.huffingtonpost.com/2012/08/31/armond-armstead-football-player-sues-injections-heart-attack_n_1847871.html.
- Mills JD, Bailes JE, Turner RC, Dodson SC, Sakai J, Maroon JC. Anabolic steroids and head injury. Neurosurgery. 2012;70(1):205–210. doi: 10.1227/NEU.0b013e3182250918. [DOI] [PubMed] [Google Scholar]
- Millspaugh J. Dementia pugilistica. US Naval Med Bull. 1937;35:297–303. [Google Scholar]
- Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, … Dinges DF. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12(5):1015–1020. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moceri VM, Kukull WA, Emanuel I, van Belle G, Larson EB. Early-life risk factors and the development of Alzheimer’s disease. Neurology. 2000;54(2):415–420. doi: 10.1212/wnl.54.2.415. [DOI] [PubMed] [Google Scholar]
- Monk TG, Price CC. Postoperative cognitive disorders. Current opinion in critical care. 2011;17(4) doi: 10.1097/MCC.0b013e328348bece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, Van Der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. ANESTHESIOLOGY-PHILADELPHIA THEN HAGERSTOWN- 2008;108(1):18. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- Montenigro PH, Baugh CM, Daneshvar DH, Mez J, Budson AE, Au R, … Stern RA. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6(5):68. doi: 10.1186/s13195-014-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, … Heyman A, et al. Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S28–35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- Moss S, Emerson E, Kiernan C, Turner S, Hatton C, Alborz A. Psychiatric symptoms in adults with learning disability and challenging behaviour. The British Journal of Psychiatry. 2000;177(5):452–456. doi: 10.1192/bjp.177.5.452. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289(11):1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- Muratore AM, Earl JK, Collins CG. Understanding heterogeneity in adaptation to retirement: a growth mixture modeling approach. Int J Aging Hum Dev. 2014;79(2):131–156. doi: 10.2190/AG.79.2.c. [DOI] [PubMed] [Google Scholar]
- Myles P, Leslie K, Silbert B, Paech M, Peyton P. A review of the risks and benefits of nitrous oxide in current anaesthetic practice. Anaesthesia and intensive care. 2004;32(2):165. doi: 10.1177/0310057X0403200202. [DOI] [PubMed] [Google Scholar]
- The National Football League Race and Gender Report Card. The Institute for Diversity and Ethics in Sport. 2015 from http://www.tidesport.org/nfl-rgrc.html.
- Nelson TF, Wechsler H. Alcohol and college athletes. Med Sci Sports Exerc. 2001;33(1):43–47. doi: 10.1097/00005768-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Neuman K. Quit your job and get healthier? The effect of retirement on health. Journal of Labor Research. 2008;29(2):177–201. [Google Scholar]
- Ninomiya K. How Long is the Average Career of an NFL Player? 2015 May 04; from http://www.livestrong.com/article/15527-long-average-career-nfl-player/
- Nordberg A, Larsson C, Perdahl E, Winblad B. Changes in cholinergic activity in human hippocampus following chronic alcohol abuse. Pharmacology Biochemistry and Behavior. 1983;18:397–400. doi: 10.1016/0091-3057(83)90206-x. [DOI] [PubMed] [Google Scholar]
- Nordstrom P, Michaelsson K, Gustafson Y, Nordstrom A. Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol. 2014;75(3):374–381. doi: 10.1002/ana.24101. [DOI] [PubMed] [Google Scholar]
- Ogilvie BC, Howe M. The trauma of termination from athletics. Applied Sport Psychology: Personal growth to peak performance. 1986:365–382. [Google Scholar]
- O’Keefe M. Study finds 96 percent of former NFL players had CTE. 2015 Retrieved January 7, 2016, from http://www.nydailynews.com/sports/football/study-finds-96-percent-nfl-players-cte-article-1.2365865.
- Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, Robert Fitzsimmons J. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69(1):173–183. doi: 10.1227/NEU.0b013e318212bc7b. [DOI] [PubMed] [Google Scholar]
- Omalu BI, DeKosky ST, Hamilton RL, Minster RL, Kamboh MI, Shakir AM, Wecht CH. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006;59(5):1086–1092. doi: 10.1227/01.NEU.0000245601.69451.27. discussion 1092-1083. 00006123-200611000-00014 [pii] [DOI] [PubMed] [Google Scholar]
- Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–134. doi: 10.1227/01.neu.0000163407.92769.ed. discussion 128–134. [DOI] [PubMed] [Google Scholar]
- Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: Case report and emerging medicolegal practice questions. J Forensic Nurs. 2010;6(1):40–46. doi: 10.1111/j.1939-3938.2009.01064.x. JFN1064 [pii] [DOI] [PubMed] [Google Scholar]
- Ouellet MC, Morin Charles M. Subjective and objective measures of insomnia in the context of traumatic brain injury: A preliminary study. Sleep medicine. 2006;7:486–497. doi: 10.1016/j.sleep.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Pastor PN, Reuben CA. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004–2006. Vital and health statistics. Series 10, Data from the National Health Survey. 2008;(237):1–14. [PubMed] [Google Scholar]
- Petrovitch H, White LR, Ross GW, Steinhorn SC, Li CY, Masaki KH, … Markesbery WR. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology. 2001;57(2):226–234. doi: 10.1212/wnl.57.2.226. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Peterson J. Drugs and aggression: correlations, crime and human manipulative studies and some proposed mechanisms. J Psychiatry Neurosci. 1995;20(2):141–149. [PMC free article] [PubMed] [Google Scholar]
- Ponsford J, Tweedly L, Taffe J. The relationship between alcohol and cognitive functioning following traumatic brain injury. J Clin Exp Neuropsychol. 2013;35(1):103–112. doi: 10.1080/13803395.2012.752437. [DOI] [PubMed] [Google Scholar]
- Pope HG, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use: a controlled study of 160 athletes. Archives of General Psychiatry. 1994;51(5):375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Porter VR, Buxton WG, Avidan AY. Sleep, Cognition and Dementia. Curr Psychiatry Rep. 2015;17(12):97. doi: 10.1007/s11920-015-0631-8. [DOI] [PubMed] [Google Scholar]
- Pratico C, Quattrone D, Lucanto T, Amato A, Penna O, Roscitano C, Fodale V. Drugs of anesthesia acting on central cholinergic system may cause post-operative cognitive dysfunction and delirium. Medical hypotheses. 2005;65(5):972–982. doi: 10.1016/j.mehy.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Putukian M, Kreher JB, Coppel DB, Glazer JL, McKeag DB, White RD. Attention deficit hyperactivity disorder and the athlete: an American Medical Society for Sports Medicine position statement. Clin J Sport Med. 2011;21(5):392–401. doi: 10.1097/JSM.0b013e3182262eb1. [DOI] [PubMed] [Google Scholar]
- Quinn PO. Treating adolescent girls and women with ADHD: Gender-Specific issues. Journal of clinical psychology. 2005;61(5):579–587. doi: 10.1002/jclp.20121. [DOI] [PubMed] [Google Scholar]
- Randolph C. Is chronic traumatic encephalopathy a real diseaes? Current sports medicine reports. 2014;13(1):33–37. doi: 10.1097/OPX.0000000000000170. [DOI] [PubMed] [Google Scholar]
- Reams N, Eckner JT, Ameida AA, Aagesen AL, Giordani B, Paulson H, … Kutcher JS. A Clinical Approach to the Diagnosis of Traumatic Encephalopathy Syndrome: A Review. JAMA Neurol. 2016;73(6):743–749. doi: 10.1001/jamaneurol.2015.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechel J, Collins C, Comstock D. Epidemiology of injuries requiring surgery among US high school athletes. Injury Prevention. 2010;16(Suppl 1):A254–A254. doi: 10.1097/TA.0b013e318230e716. [DOI] [PubMed] [Google Scholar]
- Riemann D, Berger M, Voderholzer U. Sleep and depression--results from psychobiological studies: an overview. Biol Psychol. 2001;57(1–3):67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- Riffkin R. Average U.S. Retirement Age Rises to 62. 2014 from http://www.gallup.com/poll/168707/average-retirement-age-rises.aspx.
- Sagoe D, Molde H, Andreassen CS, Torsheim T, Pallesen S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014;24(5):383–398. doi: 10.1016/j.annepidem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: Impact on working memory performance. Neurobiology of Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. http://dx.doi.org/10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Mental Health Services Administration (2009) Results from the 2008 national survey on drug use and health: national findings. Rockville, MD: 2009. (Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09-4434) [Google Scholar]
- SAMHSA. Table 2.46B—Alcohol Use, Binge Alcohol Use, and Heavy Alcohol Use in the Past Month among Persons Aged 18 or Older, by Demographic Characteristics: Percentages, 2012 and 2013. 2013. National Survey on Drug Use and Health (NSDUH) [Google Scholar]
- Savica R, Parisi JE, Wold LE, Josephs KA, Ahlskog JE. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87(4):335–340. doi: 10.1016/j.mayocp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk TL, Gorenflo DW, Dopp RR, Hipple E. Medicine and science in sports and exercise. Depression and pain in retired professional football players. 2007;39(4):599–605. doi: 10.1249/mss.0b013e31802fa679. [DOI] [PubMed] [Google Scholar]
- Seitz DP, Reimer CL, Siddiqui LN. A review of epidemiological evidence for general anesthesia as a risk factor for Alzheimer’s disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;47:122–127. doi: 10.1016/j.pnpbp.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Shifrer D, Muller C, Callahan R. Disproportionality and learning disabilities: Parsing apart race, socioeconomic status, and language. Journal of Learning Disabilities. 2011;44(3):246–257. doi: 10.1177/0022219410374236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih CL, Chi SI, Chiu TH, Sun GY, Lin TN. Ethanol effects on nitric oxide production in cerebral pial cultures. Alcohol Clin Exp Res. 2001;25(4):612–618. [PubMed] [Google Scholar]
- Solomon GS, Kuhn AW, Zuckerman SL, Casson IR, Viano DC, Lovell MR, Sills AK. Participation in Pre-High School Football and Neurological, Neuroradiological, and Neuropsychological Findings in Later Life: A Study of 45 Retired National Football League Players. Am J Sports Med, OnlineFirst. 2016 doi: 10.1177/0362546515626164. [DOI] [PubMed] [Google Scholar]
- Spencer N, Bambang S, Logan S, Gill L. Socioeconomic status and birth weight: comparison of an area-based measure with the Registrar General’s social class. Journal of Epidemiology and Community Health. 1999;53(8):495–498. doi: 10.1136/jech.53.8.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprung J, Jankowski CJ, Roberts RO, Weingarten TN, Aguilar AL, Runkle KJ, … Hanson AC. Anesthesia and incident dementia: a population-based, nested, case-control study. Paper presented at the Mayo Clinic Proceedings; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm JM, Bourlas AP, Baugh CM, Fritts NG, Daneshvar DH, Martin BM, … Stern RA. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology. 2015;84(11):1114–1120. doi: 10.1212/WNL.0000000000001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep medicine reviews. 2003;7(3):215–225. doi: 10.1053/smrv.2001.0246. [DOI] [PubMed] [Google Scholar]
- Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, … McHale L. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(03):448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JF, Womack KB, Didehbani N, Spence JS, Conover H, Hart J, Jr, … Cullum CM. Imaging Correlates of Memory and Concussion History in Retired National Football League Athletes. JAMA Neurol. 2015;72(7):773–780. doi: 10.1001/jamaneurol.2015.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Psych M, Cohen P, Pine DS, Leibenluft E. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. 2009;166(9):1048–1054. doi: 10.1176/appi.ajp.2009.08121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullan MJ, Bohsali AA, Gullett JM, Goldstein J, Bauer RM, Mareci TH, FitzGerald DB. The Locus Coeruleus and Sleep-Wake Disturbances in Veterans with mTBI. Journal of Sleep Medicine and Disorders 2014 [Google Scholar]
- Svoboda B, Vanek M. Proceedings of the 5th World Congress of Sport Psychology. Ottawa, ON: Coaching Association of Canada; 1982. Retirement from high level competition; pp. 166–175. [Google Scholar]
- Tabuchi M, Lone SR, Liu S, Liu Q, Zhang J, Spira AP, Wu MN. Sleep interacts with abeta to modulate intrinsic neuronal excitability. Curr Biol. 2015;25(6):702–712. doi: 10.1016/j.cub.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, … Fernandez G. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103(3):756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Pruiksma KE. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: A systematic review. International Review of Psychiatry. 2014;26(2):205–213. doi: 10.3109/09540261.2014.902808. [DOI] [PubMed] [Google Scholar]
- Thomas VS, Rockwood KJ. Alcohol abuse, cognitive impairment, and mortality among older people. Journal of the American Geriatrics Society. 2001;49(4):415–420. doi: 10.1046/j.1532-5415.2001.49085.x. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre P. How (and Why) Athletes Go Broke. 2009 from http://www.si.com/vault/2009/03/23/105789480/how-and-why-athletes-go-broke.
- Tremblay S, De Beaumont L, Henry LC, Boulanger Y, Evans AC, Bourgouin P, … Lassonde M. Sports concussions and aging: a neuroimaging investigation. Cereb Cortex. 2013;23(5):1159–1166. doi: 10.1093/cercor/bhs102. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Henry LC, Bedetti C, Larson-Dupuis C, Gagnon JF, Evans AC, … De Beaumont L. Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain. 2014;137(Pt 11):2997–3011. doi: 10.1093/brain/awu236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trief PM, Grant W, Fredrickson B. A prospective study of psychological predictors of lumbar surgery outcome. Spine. 2000;25(20):2616–2621. doi: 10.1097/00007632-200010150-00012. [DOI] [PubMed] [Google Scholar]
- Trotter J. Depression prevalent in ex-players. 2015 from http://espn.go.com/nfl/story/_/page/hotread150225/depression-suicide-raise-issue-mental-health-former-nfl-players.
- Tsuno N, Besset A, Ritchie K. Sleep and depression. Journal of Clinical Psychiatry. 2005 doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Tully PJ, Baker RA, Knight JL. Anxiety and depression as risk factors for mortality after coronary artery bypass surgery. Journal of psychosomatic research. 2008;64(3):285–290. doi: 10.1016/j.jpsychores.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Turrell G, Lynch JW, Kaplan GA, Everson SA, Helkala EL, Kauhanen J, Salonen JT. Socioeconomic position across the lifecourse and cognitive function in late middle age. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57(1):S43–S51. doi: 10.1093/geronb/57.1.s43. [DOI] [PubMed] [Google Scholar]
- Turrell G, Lynch JW, Kaplan GA, Everson SA, Helkala EL, Kauhanen J, Salonen JT. Socioeconomic position across the lifecourse and cognitive function in late middle age. J Gerontol B Psychol Sci Soc Sci. 2002;57(1):S43–51. doi: 10.1093/geronb/57.1.s43. [DOI] [PubMed] [Google Scholar]
- Vance DE, Crowe M. A proposed model of neuroplasticity and cognitive reserve in older adults. Activities, Adaptation & Aging. 2006;30(3):61–79. [Google Scholar]
- Van Meter A, Youngstrom E, Freeman A, Feeny N, Youngstron JK, Findling RL. Impact of Irritability and Impulsive Aggressive Behavior on Impairment and Social Functioning in Youth with Cyclothymic Disorder. J Child Adolesc Psychopharmachol. 2016;26(1):26–37. doi: 10.1089/cap.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoroff J. Traumatic encephalopathy: review and provisional research diagnostic criteria. NeuroRehabilitation. 2012;32(2):211–224. doi: 10.3233/NRE-130839. [DOI] [PubMed] [Google Scholar]
- Wagner U, Born J. Memory consolidation during sleep: Interactive effects of sleep stages and HPA regulation: Review. Stress. 2008;11(1):28–41. doi: 10.1080/10253890701408822. [DOI] [PubMed] [Google Scholar]
- Waljas M, Iverson GL, Lange RT, Hakulinen U, Dastidar P, Huhtala H, … Ohman J. A Prospective Biopsychosocial Study of the Persistent Post-Concussion Symptoms following Mild Traumatic Brain Injury. J Neurotrauma. 2015 doi: 10.1089/neu.2014.3339. [DOI] [PubMed] [Google Scholar]
- Wang M, Shi JQ. Psychological Research on Retirement. Annual Review of Psychology. 2014;65:209–233. doi: 10.1146/annurev-psych-010213-115131. [DOI] [PubMed] [Google Scholar]
- Warner DC, Schnepf G, Barrett MS, Dian D, Swigonski NL. Prevalence, attitudes, and behaviors related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in student athletes. Journal of Adolescent Health. 2002;30(3):150–153. doi: 10.1016/s1054-139x(01)00325-1. [DOI] [PubMed] [Google Scholar]
- Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-Disordered Breathing and Chronic Opioid Therapy. Pain Medicine. 2008;9(4):425–432. doi: 10.1111/j.1526-4637.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, … Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108(2):251–260. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- Weir DR, Jackson JS, Sonnega A. National football league player care foundation study of retired NFL players. Ann Arbor: University of Michigan Institute for Social Research; 2009. [Google Scholar]
- Werthner P, Orlick T. Retirement experiences of successful Olympic athletes. International journal of sport psychology 1986 [Google Scholar]
- White RD, Harris GD, Gibson ME. Attention deficit hyperactivity disorder and athletes. Sports Health: A Multidisciplinary Approach. 2014;6(2):149–156. doi: 10.1177/1941738113484679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington RA, Bretteville A, Dickler MF, Planel E. Anesthesia and tau pathology. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:147–155. doi: 10.1016/pnpbp.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickrama KK, O’Neal CW. The influence of working later in life on memory functioning. Adv Life Course Res. 2013;18(4):288–295. doi: 10.1016/j.alcr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and later life risk of Alzheimer’s disease. Neuroepidemiology. 2005;25(1):8–14. doi: 10.1159/000085307. [DOI] [PubMed] [Google Scholar]
- Wolkove N, Elkholy O, Baltzan M, Palayew M. Sleep and aging: 1. Sleep disorders commonly found in older people. Cmaj. 2007;176(9):1299–1304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, … Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, … Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesalis CE, Bahrke MS. Anabolic-androgenic steroids. Current issues. Sports Med. 1995;19(5):326–340. doi: 10.2165/00007256-199519050-00003. [DOI] [PubMed] [Google Scholar]
- Zuckerman SL, Lee YM, Odom MJ, Solomon GS, Sills AK. Baseline neurocognitive scores in athletes with attention deficit-spectrum disorders and/or learning disability. J Neurosurg Pediatr. 2013;12(2):103–109. doi: 10.3171/2013.5.PEDS12524. [DOI] [PubMed] [Google Scholar]