Abstract
Antineutrophil cytoplasmic autoantibodies (ANCA) are closely associated with systemic small vessel vasculitis characterized by segmental vessel wall necrotizing inflammation and a paucity of immunoglobulin deposition. Clinically, in vitro and experimental animal model observations indicate a direct pathogenic role for ANCA. This review focuses on the results of experiments utilizing a mouse model of ANCA disease induced by transfer of mouse anti-MPO IgG or anti-MPO lymphocytes into recipient mice, which causes small vessel vasculitis and glomerulonephritis that closely mimics human disease. Evidence for the following conclusion about this model, and by implication about human ANCA disease, will be summarized as follows: (1) anti-MPO IgG is sufficient even in the absence of functional T cells to cause disease and anti-MPO T lymphocytes are not sufficient to cause acute injury; (2) neutrophils are required; (3) ANCA antigens in bone marrow-derived cells are sufficient targets; (4) increased circulating pro-inflammatory cytokines and microbial products exacerbate disease, and concurrent viral infection exacerbates and modulates the phenotype of disease; (5) Fcγ receptor engagement is required for disease induction, and Fcγ receptor repertoire modulates the phenotype of disease, especially pulmonary disease; (6) activation of the alternative pathway of complement is required, complement is activated by factors released by neutrophils stimulated by ANCA IgG and engagement of C5a receptors is a primary event in complement-mediated amplification; and (7) genetic background has a marked influence on the severity and outcome of disease, and modified gene expression in bone marrow-derived cells is the primary basis for genetically determined differences in disease susceptibility. Investigations using this animal model of ANCA disease have provided important insights into the cellular, molecular and genetic factors involved in the pathogenesis of ANCA disease which are likely to lead to the identification of improved markers of disease activity and response to therapy, as well as more effective and less toxic therapies.
Antineutrophil cytoplasmic autoantibodies (ANCA) are closely associated with systemic small vessel vasculitis characterized by segmental vessel wall necrotizing inflammation and a paucity of immunoglobulin deposition [1]. Necrotizing and crescentic glomerulonephritis is frequent in patients with ANCA disease, and is the most common form of aggressive (crescentic) glomerulonephritis, especially in adults. Patients with ANCA disease can be classified on the basis of clinical and pathologic features as microscopic polyangiitis, Wegener’s granulomatosis, Churg-Strauss syndrome or renal-limited vasculitis [1]. This chapter will focus on animal models of ANCA-disease that elucidate the pathogenesis of this aggressive form of disease. Current therapy is toxic and not optimally effective, thus a better understanding of the etiology and pathogenesis of this aggressive disease could lead to better diagnosis, prognostication, treatment and even prevention.
The major ANCA antigen specificities are for myeloperoxidase (MPO-ANCA) [2] and proteinase 3 (PR3-ANCA) [3]. The most direct clinical evidence that ANCA are pathogenic is the report of transplacental transfer of MPO-ANCA to a neonate who developed glomerulonephritis and pulmonary hemorrhage [4]. Many laboratories have used in vitro experiments to demonstrate that ANCA IgG causes neutrophils and monocytes, both of which contain MPO and PR3, to become activated and release inflammatory mediators [5]. MPO and PR3 are predominantly in the cytoplasm of unstimulated neutrophils, although there may be small amounts of antigen on the surface. Neutrophils that have been stimulated by cytokines or other pro-inflammatory molecules (e.g. C5a) express more ANCA antigens at their surfaces and undergo activation when exposed to ANCA IgG [6]. Neutrophils in ANCA patients have a unique increased expression of ANCA antigens (MPO and PR3), which may facilitate disease induction [7]. ANCA IgG causes neutrophil activation by ligation of Fc receptors [8, 9] as well as by direct Fab′2 binding to ANCA antigens on or near cell surfaces [10, 11]. Activation of neutrophils by ANCA results in the release of inflammatory mediators that can injure vessel walls [7–15]. Thus, in vitro experimental data clearly demonstrate that ANCA IgG can activate neutrophils and monocytes. If this happens in vivo, pathogenic inflammatory vascular effects will be a likely result, especially in small vessels where leukocytes come in close contact with endothelial cell surfaces.
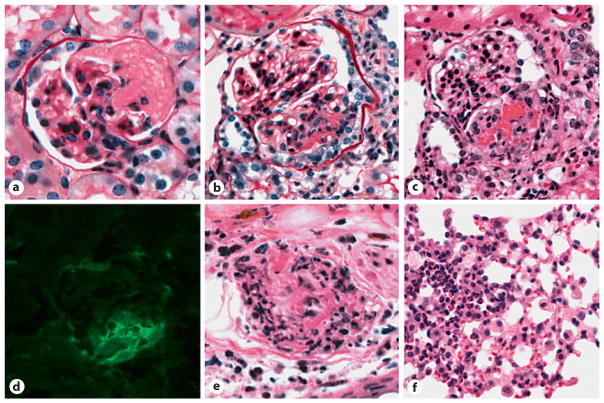
The first convincing animal model of ANCA-disease was reported in 2002 [16]. This model is induced by transfer of mouse anti-MPO IgG or anti-MPO lymphocytes from MPO knockout (Mpo−/−) mice immunized with mouse MPO [16]. All mice develop necrotizing and crescentic glomerulonephritis and some develop systemic vasculitis, e.g. cutaneous leukocytoclastic angiitis, necrotizing arteritis, pulmonary capillaritis and, occasionally, pulmonary necrotizing granulomatous inflammation (fig. 1).
Fig. 1.
Vasculitic lesions in WT B6 mice 6 days after they received anti-MPO IgG. a Glomerulus with segmental fibrinoid neurosis (periodic acid Schiff stain). b Glomerulus with segmental fibrinoid necrosis and crescent formation (periodic acid Schiff stain). c Glomerulus with segmental fibrinoid necrosis and crescent formation (H&E stain). d Immunofluorescence microscopy for fibrin showing prominent staining corresponding to segmental necrosis and crescent formation. e Necrotizing arteritis with leukocytoclasia in the dermis of the ear (H&E stain). f Pulmonary alveolar capillaritis on the left and more normal lung on the right. Reproduced with permission from [16].
A subsequent mouse model was developed by immunizing Mpo−/− mice with mouse MPO, followed by irradiation and transplantation with Mpo+/+ bone marrow (BM) cells [17]. Engraftment of Mpo+/+ cells into Mpo−/− mice with circulating anti-MPO resulted in glomerulonephritis in all mice and pulmonary capillaritis and necrotizing arteritis in some. A third animal model of ANCA disease was reported by Little et al. [18] who produced glomerulonephritis and focal pulmonary capillaritis in rats by immunization with human MPO, which induced anti-MPO antibodies that cross-react with rat MPO. To date, there is no convincing animal model of vasculitic disease induced by anti-PR3 antibodies.
Kain et al. [19] reported the presence of antibodies to lysosomal membrane protein-2 (LAMP-2) in a high proportion of patients with ANCA disease. They reported that anti-LAMP-2 antibodies are present in almost all individuals with ANCA. A human LAMP-2 epitope that is recognized by these antibodies is homologous to peptides of the bacterial adhesin FimH, and human LAMP-2 antibodies cross-react with FimH. Furthermore, anti-LAMP-2 antibodies injected into WKY rats and rats immunized with FimH develop necrotizing and crescentic glomerulonephritis. Kain et al. [19] hypothesize that an immune response to FimH triggers the anti-LAMP-2 immune response by molecular mimicry, and that anti-LAMP-2 antibodies are major players in the pathogenesis of ANCA disease. However, these observations have not been confirmed by additional studies at the time this chapter was written, and this animal model will not be reviewed further.
This chapter will focus primarily on observations made in a mouse model of ANCA disease induced by transfer of mouse anti-MPO IgG or anti-MPO lymphocytes into recipient mice.
Induction of Glomerulonephritis and Vasculitis by Transfer of Mouse Anti-MPO IgG or Anti-MPO Lymphocytes
Glomerulonephritis and vasculitis are induced by injecting mice with anti-MPO IgG or anti-MPO splenocytes derived from MPO−/− mice that have been immunized with mouse MPO [16]. Mouse MPO is purified from WEHI-3 mouse myeloid cells. Mpo−/− mice are immunized intraperitoneally with 10 μg of purified murine MPO in complete Freund’s adjuvant. The IgG fraction is isolated from serum by 50% ammonium sulfate precipitation and protein G affinity chromatography. Anti-MPO lymphocytes are harvested in splenocytes from MPO-immunized Mpo−/− mice by disrupting the spleens into cold RPMI 1640 medium, rinsed, and red blood cells lysed. For anti-MPO antibody transfer experiments, mice are injected intravenously with 50 μg/g body weight of anti-MPO or control IgG in PBS, and sacrificed on day 6. In splenocyte transfer experiments, Rag2−/− mice are injected intravenously with MPO or control splenocytes in PBS and sacrificed on day 13.
In one set of experiments [16], Rag2−/− mice given 1 × 108 or 5 × 107 anti-MPO splenocytes developed renal insufficiency, but those that received 1 × 07 anti-MPO splenocytes or anti-BSA control splenocytes did not. This indicates a dose-dependent pathogenic effect by an anti-MPO immune response, which was further supported by the relative circulating levels of anti-MPO detected by ELISA. However, the urine analysis data were problematic because they showed the induction of proteinuria, hematuria and pyuria not only by anti-MPO splenocytes, but also by anti-BSA and normal control splenocytes.
The basis for abnormal urine findings in all three groups at the higher doses was immune complex localization in glomeruli that resulted from transfer of immune competent splenocytes into Rag2−/− mice, irrespective of any immunization of the donor mice. However, it is important that only the mice which received the two higher doses of anti-MPO splenocytes developed severe necrotizing and crescentic glomerulonephritis and vasculitis that mimicked human ANCA-disease.
In addition to severe glomerulonephritis, mice that received 1 × 108 or 5 × 107 splenocytes developed hemorrhagic pulmonary capillaritis, and necrotizing arteritis in many organs, including the spleen, lymph nodes and skin. Rare mice developed focal necrotizing granulomatous inflammation in spleens and lungs. Thus, not only does the splenocyte transfer model have crescentic glomerulonephritis as in human ANCA-disease, but also small vessel vasculitis.
The greatest drawback of this splenocyte transfer model is the presence of immune complex deposits in control and anti-MPO lymphocyte treated mice. This is problematic because human ANCA-disease is usually pauci-immune, i.e. the inflammatory vascular lesions have a paucity of staining for immunoglobulins. This problem was overcome by injecting purified anti-MPO IgG rather than splenocytes.
Pauci-immune crescentic glomerulonephritis and vasculitis is induced by intravenous infusion of anti-MPO IgG either into Rag2−/− mice that have no functioning T or B cells of their own, or into wild-type mice [16]. The disease in these mice is pathologically identical to ANCA-associated pauci-immune crescentic glomerulonephritis and vasculitis in humans. All mice (100%) that received a nephritogenic dose of anti-MPO IgG developed necrosis and crescents in glomeruli (fig. 1), whereas no control mice that received anti-BSA IgG developed glomerular lesions. Immunofluorescence microscopy demonstrated a paucity of immunoglobulin and complement in the glomeruli of mice with anti-MPO-induced glomerulonephritis, thus modeling the pauci-immune characteristic of human ANCA glomerulonephritis. There is fibrin formation at sites of necrosis and crescent formation (fig. 1).
In addition to pauci-immune glomerulonephritis, mice that received anti-MPO IgG also developed systemic small vessel vasculitis, such as pulmonary alveolar capillaritis and systemic arteritis affecting small arteries (fig. 1), that is pathologically identical to human ANCA vasculitis. As will be discussed in more detail later, the strain of mouse used as recipients of anti-MPO IgG has a major influence on the severity of disease [20, 21]. For example, the same dose of murine anti-MPO induces on average 10% crescents in C57BL/6 (B6) mice and 70% crescents in 129S6 mice.
Neutrophils Are Required
The importance of neutrophils in the induction of glomerulonephritis in mice by anti-MPO IgG was documented by administering cytotoxic anti-murine neutrophil antibody (NIMP-R14) that caused depletion of neutrophils prior to injection of a nephritogenic dose of anti-MPO IgG [22]. NIMP-R14 caused a severe neutropenia that persisted for 5 days. All mice that received anti-MPO IgG without prior neutrophil depletion developed hematuria, proteinuria, leukocyturia and glomerular necrosis and crescents by 6 days. None of the mice that received anti-MPO following depletion of neutrophil developed renal disease.
For documenting glomerular infiltration by neutrophils during lesion induction, wild-type B6 mice were given anti-MPO IgG or anti-BSA IgG intravenously. Immunoenzyme staining for neutrophils was performed on paraffin sections of kidney using a biotin-labeled anti-mouse neutrophil rat monoclonal antibody (Cedarlane Laboratories Ltd., Ontario, Canada). Neutrophil accumulation was quantified by direct counting of stained cells in glomeruli. All B6 mice that received anti-MPO IgG developed hematuria, proteinuria, leukocyturia and elevated blood urea nitrogen in contrast to the mice that received anti-BSA IgG that had no elevation in hematuria, proteinuria, leukocyturia or blood urea nitrogen. By light microscopy, all mice that received anti-MPO IgG had glomerular necrosis (average 18% of glomeruli) and crescents (average 12% of glomeruli), whereas mice that received anti-BSA IgG had no renal histologic abnormalities. Immunoenzyme staining for neutrophils demonstrated that induction of glomerulonephritis by injection of anti-MPO IgG was accompanied by glomerular influx of neutrophils. Neutrophils were concentrated at sites of segmental necrotizing glomerular injury, and also were identified in Bowman’s space in glomeruli with necrosis or crescents, and in a few afferent arterioles. In mice that had received anti-MPO IgG, 30% of glomeruli had positive staining for neutrophils with an average of three neutrophils per glomerular cross-section. In contrast, mice that received anti-BSA IgG had only 6% of glomeruli with neutrophils averaging only one neutrophil per glomerular cross-section [22].
Another set of experiments has shown that BM-derived cells (probably primarily neutrophils) are necessary and sufficient to cause disease, even in the absence of MPO in other cell types (e.g. endothelial cells) [17]. Mpo−/− mice immunized with mouse MPO were exposed to irradiation and transplanted with Mpo+/+ or Mpo−/− BM cells. Engraftment in mice with circulating anti-MPO resulted in development of glomerulonephritis in all mice, and pulmonary capillaritis and splenic necrotizing arteritis in some. Anti-MPO IgG also was introduced intravenously into chimeric mice created by transplanting Mpo+/+ BM into irradiated Mpo−/− mice or Mpo−/− BM into irradiated Mpo+/+ mice. Chimeric Mpo−/− mice with Mpo+/+ neutrophils developed glomerulonephritis, whereas chimeric Mpo+/+ mice with circulating Mpo−/− neutrophils did not, thus indicating that BM-derived cells are not only sufficient, but also necessary, for induction of anti-MPO disease.
In addition, unpublished observations indicate that anti-MPO T lymphocyte are not capable of causing acute vascular inflammation [23]. Rag 2−/− mice injected with unfractionated splenocytes that contained approximately 25% T cells and 65% B cells developed crescents and necrosis in approximately 80% of glomeruli, whereas mice injected with the preparations that contained 80% T cells and 10% B cells developed crescents and necrosis in only 5% of glomeruli, and mice injected with preparations with >99% T cells developed no crescents or necrosis. ELISpot assays documented successful transfer of MPO-reactive T cells into all three experimental groups. These data show that anti-MPO T lymphocytes alone cannot induce glomerulonephritis.
Thus, neutrophils are the primary effector cell of the acute necrotizing and crescentic glomerular injury induced by anti-MPO antibodies. Anti-MPO T cells do not induce acute injury and expression of MPO by BM-derived cells is a sufficient antigen target for the anti-MPO antibodies to be pathogenic.
Influence of Cytokines and Infections
Patients with ANCA disease often have a flu-like illness near the time of onset of vasculitic symptoms, and in vitro studies show a synergistic role for cytokines in the activation of neutrophils by ANCA [5–15]. In accord with these observations, cytokines [24] and viral respiratory tract infection [25] exacerbate murine disease induced by anti-MPO IgG. Huugen et al. [24] demonstrated that systemic administration of bacterial lipopolysaccharide results in increased severity of anti-MPO-induced glomerulonephritis in this model. This is accompanied by increased circulating tumor necrosis factor (TNF)-α, which is known to enhance neutrophil activation by ANCA in vitro. In addition, administration of an antagonist to TNF-α reduced the severity of the lipopolysaccharide-mediated aggravation of anti-MPO IgG-induced glomerulonephritis. These animal model studies confirm the earlier conclusion from in vitro studies that ANCA and pro-inflammatory stimuli act synergistically to induce ANCA disease.
Unpublished studies demonstrate a synergistic effect by concurrent infections in this animal model [25]. B6 mice were inoculated intranasally with influenza A/WSN/33 virus on day 0, and injected with anti-MPO IgG on day 1. Control groups included mice that received virus alone or anti-MPO alone. All mice that received anti-MPO developed glomerular crescents and necrosis. All mice that received both anti-MPO and influenza had significant alveolar capillaritis, but mice that received anti-MPO alone or virus alone did not. Thus, concurrent influenza infection worsened ANCA disease, and concurrent ANCA worsened influenza. These observations provide additional support for the concept that inflammatory stimuli have a synergistic effect on the mediation of vasculitis by ANCA IgG.
Importance of Alternative Pathway Complement Activation
One of the most unexpected and potentially very important discoveries using the anti-MPO mouse model is the major pathogenic role of complement [26–28]. Anti-MPO IgG-induced glomerulonephritis is completely blocked by complement depletion with cobra venom factor, which abrogates all complement pathways [26]. The role of specific complement activation pathways has been investigated using mice with knockout of the final common pathway component C5, classical and lectin binding pathway component C4, and alternative pathway component factor B. After injection of anti-MPO IgG, C4−/− mice developed disease comparable to wild-type disease; however, C5−/− and factor B−/− mice developed no disease, thus implicating the alternative pathway in the pathogenesis of this model [26].
To implicate a role for complement in human ANCA disease and to elucidate the mechanism, IgG was isolated from patients with MPO-ANCA or PR3-ANCA, and from healthy controls. Incubation of MPO-ANCA IgG or PR3-ANCA IgG with normal human neutrophils primed with TNF-α caused the release of factors that activated complement in serum with generation of C3a [26, 28]. IgG from healthy controls did not produce this effect. In vitro experiments using human material also have shown that ANCA-activated neutrophils release factors that activate complement, which in turn primes neutrophils for further activation by ANCA [28].
These effects and other ANCA-mediated pathogenic events depend on generation of C5a by alternative pathway activation and engagement of C5a receptors on neutrophils [27, 28]. Blockade of this critical pathogenic step by blocking C5a or C5aR abrogates disease induction in the mouse model, which suggests a possible novel therapeutic strategy in humans based on currently available complement inhibitors as well as novel therapies soon to be available [29, 30].
The Role of Fcγ Receptors
In mice, engagement by IgG of FcγRI, FcγRIII and FcγRIV activate immunologic and inflammatory processes, whereas engagement of FcγRIIb inhibits immunologic and inflammatory processes. Unpublished data indicate that Fcγ receptors are involved not only in the induction of anti-MPO-induced glomerulonephritis and vasculitis (probably primarily through engagement of the activating receptor FcγRIV), but also in the modulation of disease phenotype (apparently primarily through variable signaling from the inhibitory receptor FcγRIIb) [31]. Preliminary data suggest that the absence of FcγR common γ-chain completely prevents disease induction, the absence of FcγRI or FcγRIII has no effect, the absence of FcγRIV has partial effect, and the absence of FcγRIIb augments induction of pulmonary disease (including granulomatous disease).
An intriguing and potentially very important discovery is that FcγRIIb receptors influence the phenotype of ANCA disease in our anti-MPO model. For example, the absence of inhibitory FcγRIIb receptors results in more severe pulmonary disease with a greater incidence of granulomatous inflammation, and pulmonary disease induced by anti-MPO is more severe in the presence of influenza A infection and even more severe in FcγRIIb−/− mice. Thus, the FcγRIIb gene influences the pulmonary phenotype of ANCA disease in our mouse model. In essence, the absence of FcγRIIb results in a phenotype that is more like Wegener’s granulomatosis, as opposed to the usual phenotype in mice that is more like microscopic polyangiitis or renal-limited disease.
Based on these observations in mice, we hypothesized that pulmonary involvement in human ANCA disease might be modulated by the FcγRIIb gene. To test this hypothesis, we genotyped the −386 promotor site of the FcγRIIb gene in 200 patients with ANCA disease. ANCA disease patients with pulmonary involvement were compared to ANCA disease patients without pulmonary involvement. We observed that the −386C allele was associated with the pulmonary phenotype of ANCA disease (p = 0.02). Carriers of the −386C allele had a 2.8 times greater risk (95% CI: 1.4–6.84) of lung disease compared to those homozygous for the −386G allele. This suggests that polymorphisms in FcγRIIb affect the incidence of pulmonary involvement in ANCA disease in patients [32].
Demonstration for a role for FcγR in ANCA disease is important because there is mounting evidence that blockade of FcγRs can be an effective treatment in autoimmune disease, not only through blockade of activating FcγRs, but also through blockade of complement activation [33, 34], both of which play major roles in the pathogenesis of disease in the anti-MPO mouse model and possibly in ANCA disease patients.
Genetic Control
Anti-MPO IgG induces disease which is much more severe in 129S6 mice compared to B6 mice [20]. In general, a standard batch of our anti-MPO IgG induces crescents in 5–10% of the glomeruli in 100% of B6 mice 6 days after injection. In marked contrast, injection of the same batch of anti-MPO IgG into 129S6 mice induces disease which is much more severe. In preliminary experiments, 129S6 mice (Taconic) and C57BL/6 (B6) mice (Jackson Laboratory), received the same intravenous dose of anti-MPO IgG. 129S6 mice that received anti-MPO IgG developed severe proteinuria and renal failure, but B6 mice that received the same amount of anti-MPO IgG did not. B6 mice that received anti-MPO IgG developed an average of 9% crescents. In contrast, 129S6 mice that received the same amount of anti-MPO IgG developed an average of 69% crescents.
To assess the role of genetic background, 129S6 mice and B6 mice were used to generate F1 mice by B6 backcross with 129S6 mice. F1 (B6 × 129S6) mice that received anti-MPO IgG developed an average of 13.5% crescents. F2 mice were generated by F1 intercross. 60 F2 mice that received anti-MPO IgG had a wide spectrum of severity of glomerulonephritis. 30 mice had <10%, 24 mice 10–25%, 5 mice 26–50% and 1 mouse >50% glomerular crescents.
To determine the importance of genetic difference in BM-derived cells, chimeric mice were produced with genetic differences between donor BM-derived cells and recipients. Rag2−/− B6 or 129S6 mice were lethally irradiated followed by intravenous injection of 129S6 or B6 BM cells, respectively. Four weeks after BM transfer when engraftment was established, anti-MPO IgG was administrated intravenously. Disease induction was assessed at day 6. In these preliminary experiments, Rag2−/− B6 mice that received BM from 129S6 mice and anti-MPO IgG had an average of 79% crescents similar to wild-type 129S6 mice that received anti-MPO antibodies. However, Rag2−/− 129S6 mice that received the BM from B6 mice and the same dose of anti-MPO IgG had an average of 17% crescents, similar to wild-type B6 mice that receive anti-MPO IgG. Thus, genetically determined characteristics of BM-derived cells (most likely neutrophils and monocytes) are responsible for the marked differences in disease onset and outcome among genetically diverse mice.
From these preliminary studies on the influence of genetic factors, we conclude that (1) there is strong genetic influence on the regulation of pathogenic events in this model of ANCA disease; (2) this genetic influence acts primarily through effects on BM-derived cells, including the responsivity of neutrophils to activation by ANCA; (3) the 129S6 model is more appropriate than the B6 model for experimental studies of pathogenesis and therapeutic strategies because of greater severity; and (4) genomic analysis of the basis for the genetic influence should identify genes and gene products that have important roles in the pathogenesis of ANCA disease, would be candidates for markers of disease activity and outcome, and might also be targets for therapy.
Conclusions
Observations with the anti-MPO-induced mouse model indicate that (1) anti-MPO IgG is sufficient even in the absence of functional T cells to cause disease and anti-MPO T lymphocytes are not sufficient to cause acute injury; (2) neutrophils are required, (3) ANCA antigens in BM-derived cells are sufficient targets; (4) increased circulating pro-inflammatory cytokines and microbial products exacerbate disease, and concurrent viral infection exacerbates and modulates the phenotype of disease; (5) Fcγ receptor engagement is required for disease induction, and Fcγ receptor repertoire modulates the phenotype of disease, especially pulmonary disease; (6) activation of the alternative pathway of complement is required, complement is activated by factors released by neutrophils stimulated by ANCA IgG, and engagement of C5a receptors is a primary event in complement-mediated amplification; and (7) genetic background has a marked influence on the severity and outcome of disease, and modified gene expression in BM-derived cells is the primary basis for genetically determined differences in disease susceptibility. These experimental animal observations, which are substantiated by in vitro experimental observations as well, support the pathogenic pathway diagramed in figure 2. This insight on the pathogenesis of ANCA disease is likely to lead to the identification of improved markers of disease activity and response to therapy, as well as more effective and less toxic therapies.
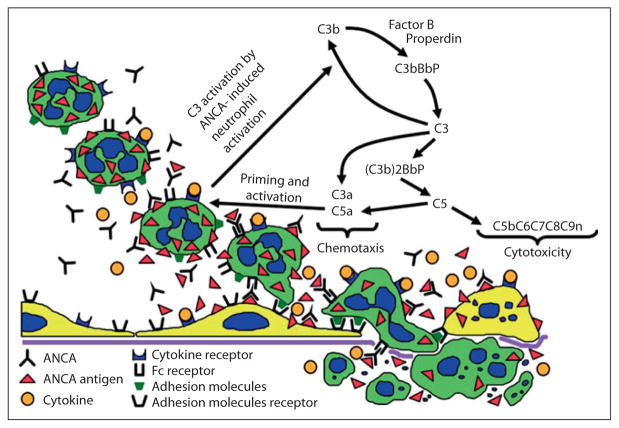
Fig. 2.
Depiction of a hypothesis for the pathogenic events in ANCA vasculitis and glomerulonephritis. Beginning in the upper left, cytokines or other priming factors, such as TNF-α and C5a, induce neutrophils to express more ANCA antigens at the cell surface where they are available for binding to ANCA. ANCA engagement of antigen activates neutrophils by both Fc receptor engagement and direct Fab′2 binding to antigen. Activated neutrophils release toxic factors that cause apoptosis and necrosis of endothelial cells and other vessel wall components. Neutrophils that have been activated by ANCA also release factors that trigger the alternative complement pathway, which generates mediators such as C5a and C3a that amplify the intensity of ANCA-induced inflammation, in part by additional priming additional neutrophils for interaction with ANCA. Modified with permission from [26].
References
- 1.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 2.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 3.Jennette JC, Hoidal JR, Falk RJ. Specificity of anti-neutrophil cytoplasmic autoantibodies for proteinase 3. Blood. 1990;75:2263–2264. [PubMed] [Google Scholar]
- 4.Schlieben DJ, Korbet SM, Kimura RE, Schwartz MM, Lewis EJ. Pulmonary-renal syndrome in a newborn with placental transmission of ANCAs. Am J Kidney Dis. 2005;45:758–761. doi: 10.1053/j.ajkd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Jennette JC, Xiao H, Falk RJ. Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2006;17:1235–1242. doi: 10.1681/ASN.2005101048. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber A, Luft FC, Kettritz R. Membrane proteinase 3 expression and ANCA-induced neutrophil activation. Kidney Int. 2004;65:2172–2183. doi: 10.1111/j.1523-1755.2004.00640.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang JJ, Pendergraft WF, Alcorta DA, Nachman PH, Hogan SL, Thomas RP, Sullivan P, Jennette JC, Falk RJ, Preston GA. Circumvention of normal constraints on granule protein gene expression in peripheral blood neutrophils and monocytes of patients with antineutrophil cytoplasmic autoantibody-associated glomerulonephritis. J Am Soc Nephrol. 2004;15:2103–2114. doi: 10.1097/01.ASN.0000135058.46193.72. [DOI] [PubMed] [Google Scholar]
- 8.Porges AJ, Redecha PB, Kimberly WT, Csernok E, Gross WL, Kimberly RP. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J Immunol. 1994;153:1271–1280. [PubMed] [Google Scholar]
- 9.Mulder AH, Heeringa P, Brouwer E, Limburg PC, Kallenberg CG. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): a Fc gamma RII-dependent process. Clin Exp Immunol. 1994;98:270–278. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kettritz R, Jennette JC, Falk RJ. Crosslinking of ANCA-antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol. 1997;8:386–394. doi: 10.1681/ASN.V83386. [DOI] [PubMed] [Google Scholar]
- 11.Williams JM, Ben-Smith A, Hewins P, Dove SK, Hughes P, McEwan R, Wakelam MJ, Savage CO. Activation of the G(i) heterotrimeric G protein by ANCA IgG F(ab′)2 fragments is necessary but not sufficient to stimulate the recruitment of those downstream mediators used by intact ANCA IgG. J Am Soc Nephrol. 2003;14:661–669. doi: 10.1097/01.asn.0000050223.34749.f4. [DOI] [PubMed] [Google Scholar]
- 12.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charles LA, Caldas ML, Falk RJ, Terrell RS, Jennette JC. Antibodies against granule proteins activate neutrophils in vitro. J Leukoc Biol. 1991;50:539–546. doi: 10.1002/jlb.50.6.539. [DOI] [PubMed] [Google Scholar]
- 14.Ewert BH, Jennette JC, Falk RJ. Antimyeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992;41:375–383. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- 15.Ewert BH, Becker ME, Jennette JC, Falk RJ. Antimyeloperoxidase antibodies induce neutrophil adherence to cultured human endothelial cells. Ren Fail. 1995;17:125–133. doi: 10.3109/08860229509026249. [DOI] [PubMed] [Google Scholar]
- 16.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber A, Xiao H, Falk RJ, Jennette JC. Bone marrow-derived cells are sufficient and necessary targets to mediate glomerulonephritis and vasculitis induced by anti-myeloperoxidase antibodies. J Am Soc Nephrol. 2006;17:3355–3364. doi: 10.1681/ASN.2006070718. [DOI] [PubMed] [Google Scholar]
- 18.Little MA, Smyth L, Salama AD, Mukherjee S, Smith J, Haskard D, Nourshargh S, Cook HT, Pusey CD. Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am J Pathol. 2009;174:1212–1220. doi: 10.2353/ajpath.2009.080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, Davidovits A, Raab I, Jahn R, Ashour O, Spitzauer S, Sunder-Plassmann G, Fukuda M, Klemm P, Rees AJ, Kerjaschki D. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14:1088–1096. doi: 10.1038/nm.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao H, Ciavatta D, Zeng Y, Johnson LA, Pardo-Manuel de Villena F, Falk RJ, Jennette JC. Genetic control of the severity of experimental anti-MPO necotizing and crescentic glomerulonephritis (Abstract) APMIS. 2009;117:90. [Google Scholar]
- 21.Xiao H, Zeng YW, Pardo-Manuel De Villena F, Ciavatta D, Falk R, Jennette JC. Genetic modulation of anti-myeloperoxidase induced murine crescentic glomerulonephritis. Lab Invest. 2010;90:348A. [Google Scholar]
- 22.Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, Falk RJ, Jennette JC. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol. 2005;167:39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao H, Peter P, Hu P, Liu Z, Falk R, Jennette JC. Neutrophils but not T lymphocytes are important in the induction of necrotizing and crescentic glomerulonephritis by antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase (MPO-ANCA) in mice. J Am Soc Nephrol. 2003;14:634A. [Google Scholar]
- 24.Huugen D, Xiao H, van EA, Falk RJ, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-alpha. Am J Pathol. 2005;167:47–58. doi: 10.1016/s0002-9440(10)62952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao H, Morrison TE, Heise MT, Falk RJ, Jennette JC. MPO-ANCA IgG and influenza: a viral infection acts in concert to induce severe hemorrhagic pulmonary capillaritis (Abstract) Clin Exp Rheumatol. 2007;25:S-91. [Google Scholar]
- 26.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huugen D, van EA, Xiao H, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 2007;71:646–654. doi: 10.1038/sj.ki.5002103. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20:289–298. doi: 10.1681/ASN.2008050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellas TC, Wennogle LP. C5a receptor antagonists. Curr Pharm Des. 1999;5:737–755. [PubMed] [Google Scholar]
- 31.Xiao H, Heeringa P, Schreiber A, Falk RJ, Jennette JC. The role of Fcγ receptors in the induction of glomerulonephritis and pulmonary granulomatous inflammation in mice by anti-myeloperoxidase antibodies (Anti-MPO) J Am Soc Nephrol. 2004;15:37A. [Google Scholar]
- 32.Nester CM, Lionaki S, Chin H, Edberg J, Kimberly R, Wilhelmsen K, Jennette JC, Falk RJ. Polymorphisms associated with the pulmonary phenotype of antineutrophil cytoplasmic autoantibody vasculitis (Abstract) J Am Soc Nephrol. 2007;18 [Google Scholar]
- 33.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 34.Jordan SC, Toyoda M, Vo AA. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation. 2009;88:1–6. doi: 10.1097/TP.0b013e3181a9e89a. [DOI] [PubMed] [Google Scholar]