Abstract
High rates of comorbid posttraumatic stress disorder (PTSD) and substance use disorders (SUD) have been noted in veteran populations. Fortunately, there are a number of evidence-based psychotherapies designed to address comorbid PTSD and SUD. However, treatments targeting PTSD and SUD simultaneously often report high dropout rates. To date, only one study has examined predictors of dropout from PTSD/SUD treatment. To address this gap in the literature, this study aimed to 1) examine when in the course of treatment dropout occurred, and 2) identify predictors of dropout from a concurrent treatment for PTSD and SUD. Participants were 51 male and female veterans diagnosed with current PTSD and SUD. All participants completed at least one session of a cognitive-behavioral treatment (COPE) designed to simultaneously address PTSD and SUD symptoms. Of the 51 participants, 22 (43.1%) dropped out of treatment prior to completing the full 12 session COPE protocol. Results indicated that the majority of dropout (55%) occurred after session 6, with the largest amount of dropout occurring between sessions 9 and 10. Results also indicated a marginally significant relationship between greater baseline PTSD symptom severity and premature dropout. These findings highlight inconsistencies related to timing and predictors of dropout, as well as the dearth of information noted about treatment dropout within PTSD and SUD literature. Suggestions for procedural changes, such as implementing continual symptom assessments during treatment and increasing dialog between provider and patient about dropout were made with the hopes of increasing consistency of findings and eventually reducing treatment dropout.
Keywords: PTSD, Dropout, Substance Use, Prolonged Exposure, Veteran, COPE
1. Introduction
A recent meta-analysis estimated that 23% of Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) veterans meet criteria for posttraumatic stress disorder (PTSD; Fulton et al., 2015). PTSD is a chronic disorder characterized by physiological hyperarousal, avoidant behaviors and maladaptive cognitions resulting from directly experiencing or witnessing a life threatening event(s) (American Psychiatric Association, 2013). Although some PTSD symptoms (e.g., hypervigilance) may be considered adaptive in the combat theatre given threat of physical harm, these symptoms can become debilitating once the veteran returns home (Breslau, Lucia, & Davis, 2004). Research has linked PTSD to deficits in occupational performance (Taylor, Wald, & Asmundson, 2006), social functioning (Frueh, Turner, Beidel, & Cahill, 2001), and physical health (Jakupcak, Luterek, Hunt, Conybeare, & McFall, 2008). PTSD is also associated with decreased quality of life (Gill et al., 2014), elevated rates of suicidal ideation (Jakeupcak et al., 2009), and substance use disorders (SUD; Carlson et al., 2010). In fact, veterans with PTSD are roughly three times more likely than veterans without PTSD to develop an SUD than civilians (Petrakis, Rosenheck, & Desai, 2011).
Research on the etiology of comorbid PTSD and SUD suggest that, for most individuals, the onset of the PTSD precedes the onset of the SUD (Hien, Nunes, Levin, & Fraser, 2000, Jacobsen, Southwick, & Kosten, 2001), and that alcohol and drugs are commonly used to self-medicate distressing PTSD symptoms, such as nightmares, intrusive memories, and hyperarousal (Back et al., 2010; 2014; Brady, Back, & Coffey, 2004; Khantzian, 1985; Possemato et al., 2015). Substances such as alcohol reduce physiological arousal, increase disinhibition and provide temporary cognitive distractions (Grosso et al., 2014). Although substance use may result in short-term relief of symptoms, the long-term consequences of addiction can be severe. For instance, veterans diagnosed with PTSD and comorbid SUD tend to display higher rates of homelessness and are more likely to receive VA disability than individuals with PTSD alone (Bowe & Rosenheck, 2015). Veterans with PTSD and SUD also tend to display greater levels of intolerance for uncertainty, lower tolerance for distress (Banducci et al., 2016) and have poorer treatment outcomes (Norman, Tate, Anderson, & Brown, 2007).
Fortunately, a variety of treatments have been developed to address PTSD symptoms. For instance, exposure (e.g., Prolonged Exposure; PE), cognitive (e.g., Cognitive Processing Therapy-Cognitive Therapy; CPT-C) and socially focused treatments (e.g., Interpersonal Psychotherapy; IPT) have shown to be effective in reducing PTSD symptoms (Foa et al., 2013; Markowitz et al., 2015; Walter, Dickstein, Barnes, & Chard 2014). More recently, a number of evidence-based psychotherapies have been developed to address comorbid PTSD and SUD simultaneously (Carrico et al., 2015; McCauley, Killeen, Gros, Brady, & Back, 2012). These treatments seamlessly integrate components for both disorders and have been found to provide significant reduction in symptoms of both conditions. However, treatment dropout is a significant problem in both PTSD and SUD treatment populations. A recent meta-analysis found that the average dropout rate for trauma-focused PTSD treatments was 36% (Imel, Laska, Jakupcak, & Simpson, 2013). Similar dropout rates (30–40%) have been reported in SUD treatments (Rabinowitz & Marjefsky, 1998; Kelly & Moos, 2003). Individuals that dropout of treatment tend to report less symptom reduction and increased future service utilization for both PTSD (Tuerk e t al., 2013) and SUD (Moos & Moos, 2003; Moos, Pettit, & Gruber, 1995). Among individuals seeking treatment for comorbid PTSD and SUD, dropout rates as high as 61% have been reported (Brady, Dansky, Back, Foa, & Carroll, 2001; Mills et al., 2012; Zandberg, Rosenfield, Alpert, McLean, & Foa, 2016). Given this, investigations of predictors of dropout would help inform treatment design and retain patients in treatment longer, which is associated with increased benefits and more favorable treatment outcomes (Tuerk et al., 2013).
Findings from previous studies indicate that the vast majority of subjects dropout from PTSD treatment (Gutner, Gallagher, Baker, Sloan, & Resick, 2016; Szafranski, Gros, Menefee, Norton, & Wanner, 2015) and SUD treatment (McKellar, Kelly, Harris, & Moos, 2006; McMahon, Kouzekanani, & Malow, 1999) early on, and before mid-treatment. Studies examining dropout from PTSD treatment show that demographic and clinical predictors of dropout include younger age (Gros, Yoder, Tuerk, Lozano, & Acierno, 2011), male gender (van Minnen Arntz, & Keijsers, 2002), African American race (Lester, Artz, Resick, & Young-Xu, 2010), lower levels of education (Rizvi et al., 2009), higher military rank (Szafranski et al., 2016), greater concurrent drug use (Szafranski, Gros, Menefee, Wanner, & Norton, 2014), lower income (Galovski, Blain, Mott, Elwood, & Houle, 2012), greater disability status and lower social support (Gros, Price, Yuen, & Acierno, 2013), and higher pretreatment symptom severity (Garcia, Kelley, Rentz, & Lee, 2011). Similarly, studies examining dropout from SUD treatment include younger age, lower income, being unemployed (Mertens & Weisner, 2000), African American race (Milligan, Nich, & Carroll, 2004), lower education level (Mammo & Weinbaum, 1993), female gender (Arfken, Klein, di Menza, & Schuster, 2001) and more frequent drug use (McKellar et al., 2006).
Studies have almost exclusively examined dropout from PTSD and SUD treatments separately. This is surprising given the recent evidence that integrated treatments targeting PTSD and SUD simultaneously are effective in reducing symptoms of both disorders (Foa et al., 2013; McGovern et al., 2015; Roberts, Roberts, Jones, & Bisson, 2015). In fact, only one study to date has examined dropout from concurrent treatments for PTSD and alcohol use disorder (Zandberg, Rosenfield, Alpert, McLean, & Foa, 2016). Zandberg and colleagues (2016) found that accidents and “other” types of trauma were associated with the highest rates of dropout, whereas physical assault was associated with the lowest amount of dropout. Interestingly, both fast and slow rates of PTSD symptom improvement predicted treatment attrition. To address current gaps in the literature, this study aimed to 1) examine when in the course of treatment dropout occurred, and 2) identify predictors of dropout from a concurrent treatment for PTSD and SUD. Consistent with previous studies (Gutner et al., 2016; McKellar et al., 2006; McMahon et al., 1999; Szafranski et al., 2015), it was hypothesized that the majority of dropout would occur prior to mid-treatment. Younger age (Gros et al., 2011; Mertens & Weisner, 2000), African American race (Lester et al., 2010; Milligan et al., 2004), lower education (Mammo & Weinbaum, 1993; Rizvi et al., 2009), greater concurrent drug use (McKellar et al., 2006; Szafranski et al., 2014), lower income (Galovski, Blain, Mott, Elwood, & Houle, 2012) and higher pretreatment symptom severity (Garcia et al., 2011; McKellar et al., 2006) were expected to predict dropout from treatment.
2. Method
2.1. Participants
Participants were 51 veterans diagnosed with current PTSD and SUD. Participants were recruited via newspaper and internet advertisements, clinician referral, and flyers posted in a local hospitals and medical clinics. Baseline inclusion criteria included: 1) status as a veteran, reservist, or member of the National Guard, 2) 18–65 years old, 3) DSM-IV criteria for a current substance use disorder and some substance use in the past 90 days, 4) DSM-IV criteria for current PTSD and a score of > 50 on the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995), and 5) fluency in English. Baseline exclusion criteria included: 1) clinically significant suicidal or homicidal ideation, 2) current or history of psychotic or bipolar affective disorders; 3) current eating disorder or dissociative identity disorder; 4) individuals already participating in ongoing PTSD or SUD treatment; and 5) severe cognitive impairment as indicated by the Mini Mental Status Exam.
2.2. Procedure
Potential participants were initially screened by telephone or in-person, and individuals who were eligible were invited to complete a comprehensive baseline assessment in the office.
Individuals meeting inclusion criteria were randomized (2:1) to receive either COPE or Relapse Prevention as part of a larger, NIDA-sponsored clinical trial. For the purposes of the present study on integrated treatment of PTSD-SUD, only participants in the COPE treatment arm were investigated. The COPE treatment consists of 12 weekly, individual, 90-min sessions which are primarily focused on psychoeducation and methods for coping with cravings to use (sessions 1 – 3), in vivo exposure (sessions 3 – 12), and imaginal exposure (sessions 4 – 11; see Back et al., 2014 for more details on the therapy). All study procedures were IRB-approved and participants read and signed an IRB consent form before any study procedures occurred.
2.3. Measures
2.3.1. Demographics
Demographics were collected via a self-report measure at baseline, which included variables such as age, gender, race, education level, relationship status, employment status, and military history.
2.3.2. PTSD Symptoms
Clinician Administered PTSD Scale
The Clinician Administered PTSD Scale (CAPS; Blake et al., 1995), a semi-structured clinical interview considered the gold standard for PTSD assessment, was used to obtain a current diagnosis of PTSD and ensure a total symptom severity score > 50 at baseline. The CAPS targets the 17 specific PTSD symptoms from the DSM-IV (American Psychiatric Association, 2000) to assess the intensity and frequency of each symptom on a five-point Likert scale. Although a full assessment of past trauma was completed, active combat-related PTSD was the focus of the symptom assessments and related diagnosis. The CAPS has been shown to have adequate internal consistency (αs ranged from .73 to .95), inter-rater reliability on the same interview (rs ranged from .92 to .99), and test-retest reliability over a 2 to 3 day period across different interviewers (rs ranged from .77 to .98; Orsillo, 2002).
2.3.3. Substance Use
Mini International Neuropsychiatric Interview
The Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) was used to diagnose SUD. The MINI is a clinician-rated structured diagnostic interview designed to provide a brief, but accurate, assessment of a wide range of DSM-IV psychiatric disorders, including mood and anxiety disorders, and substance use disorders (Sheehan et al., 1998). The MINI was used to assess all of its targeted disorders with the exception of PTSD, which was assessed via the CAPS. Similar training procedures were used for the MINI as were used for the CAPS. The MINI has demonstrated adequate inter-rater and test-retest reliability across most disorders, and specifically has shown good inter-rater reliability with other structured diagnostic interviews (Sheehan et al., 1998). Timeline Follow-Back. The Timeline Follow-Back (TLFB; Sobell & Sobell, 1992), a calendar-based instrument that obtains retrospective self-report data, was used to assess alcohol and drug use for 60 days prior to baseline. Alcohol use frequency (i.e., percent days using) and quantity (i.e., standard drink units) were assessed weekly during treatment, as was the frequency of other substance use (e.g., cocaine, marijuana, stimulants, sedatives, opioids) and prescription drugs. The TLFB yields consistently high test-retest correlations and correlates well with other self-reports and collateral reports (Carey, 1997).
2.3.4. Depression Symptoms
Beck Depression Inventory-II
The Beck Depression Inventory-II (BDI-II; Beck, Steer & Brown, 1996) is a 21-item, self-report measure of depressive symptoms with higher numbers indicating greater symptom severity. The BDI-II demonstrates excellent one-week test-retest reliability (r = .93), excellent internal consistency (αs < .92), and convergent validity in multiple samples (Beck et al., 1996).
2.4. Data Analytic Plan
Discrete time survival analysis (Muthén & Masyn, 2005; Singer & Willett, 1993, 2003) was used to examine treatment dropout. Survival analysis was conducted in Mplus version 7.4 (Muthén & Muthén, 1998–2012). For all analyses, treatment session was used as the time metric. Remaining in treatment was scored as 0, dropout as 1, and data were coded as missing following treatment dropout. Dropout was calculated based on individuals attending at least the first treatment session. Life tables were first constructed providing information regarding survival probability, hazard ratio, and cumulative survival rates. Predictors of treatment dropout were then examined.
3. Results
3.1. Demographics and clinical characteristics
The majority of participants were male (92.2%), Caucasian (68.6%), unemployed (58%), had a military rank of E-4 or E-5 (58%). The Mage for participants at baseline was 39.9 (SD = 10.8). Baseline PTSD, depression and substance use scores can be found in Table 1.
Table 1.
Baseline PTSD, Depression and Substance Use Scores
| Univariate Predictors | M | SD |
|---|---|---|
| CAPS | 77.77 | 18.85 |
| BDI-II | 29.59 | 12.39 |
| TLFB TDU Any Drugs | 31.45 | 24.67 |
| TLFB TDU Alcohol | 29.16 | 23.48 |
Note. CAPS = Clinician-Administered PTSD Scale. BDI-II = Beck Depression Inventory-II. TLFB TDU = Timeline Followback Total Days Used.
3.2. Predictors of drop-out
Dropout was defined as completing at least one treatment session and discontinuing treatment prior to completion of the full treatment protocol (i.e., 12 sessions). The findings revealed that 56.9% (n = 29) of participants were identified as treatment completers, and 43.1% (n = 22) were identified as treatment dropouts.
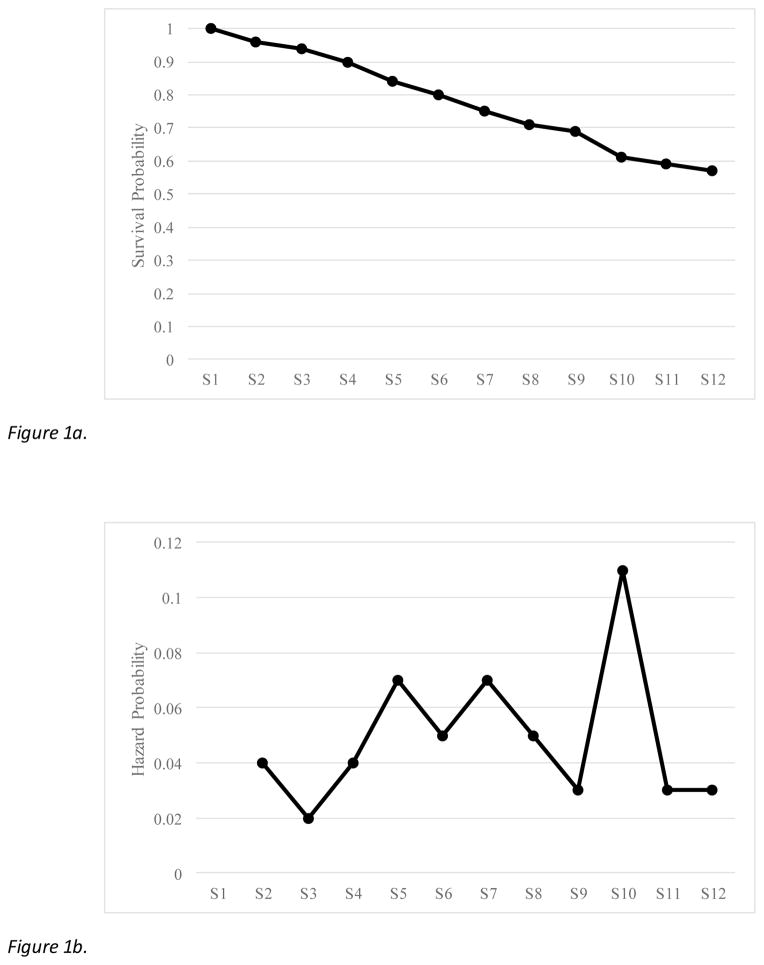
Prior to survival analysis, a life table was constructed to quantify the number of people who dropped out at each session, with corresponding survival and hazard proportions (Table 2). Survival analysis and hazard function plots are provided in Figures 1a and 1b, respectively. Results of the life table indicate that the majority of people completed treatment (57%). In general, rate of dropout did not appear to fluctuate greatly, although the highest hazard probability of dropping out occurred between sessions 9 and 10.
Table 2.
Life Table Displaying Treatment Dropout and Corresponding Survival and Hazard Probabilities
| Session | In Treatment | Dropped Out | Survivor Probability | Hazard Probability |
|---|---|---|---|---|
| 1 | 51 | - | 1.00 | .00 |
| 2 | 49 | 2 | .96 | .04 |
| 3 | 48 | 1 | .94 | .02 |
| 4 | 46 | 2 | .90 | .04 |
| 5 | 43 | 3 | .84 | .07 |
| 6 | 41 | 2 | .80 | .05 |
| 7 | 38 | 3 | .75 | .07 |
| 8 | 36 | 2 | .71 | .05 |
| 9 | 35 | 1 | .69 | .03 |
| 10 | 31 | 4 | .61 | .11 |
| 11 | 30 | 1 | .59 | .03 |
| 12 | 29 | 1 | .57 | .03 |
Note. People are considered to have dropped out of treatment if they failed to return for the indicated session. For example, two people who showed for session 1 did not return for session 2.
Figure 1.
Figure 1a. Survival function demonstrating cumulative proportion of all people who remained in treatment. Proportion at each session reflects proportion of people who returned for that session. S = Session.
Figure 1b. Hazard function demonstrating proportion of people who dropped out at each session, based on those who remained in treatment to that point. S = Session.
Discrete time survival analysis, using a proportional odds Cox ratio, as described by Singer and Willett (2003) was then fit the data, using Mplus version 7.4 (Muthén & Muthén, 1998–2012), to examine treatment dropout. Treatment dropout was coded as 0, continuing in treatment as 1, and the times after a participant dropped out of treatment was coded as missing. Due to the small sample size, baseline predictors were each entered individually to determine impact, and significant predictors were all included in a final model. The impact of predictors on treatment dropout is provided in Table 3. Elevated CAPS scores predicted an increased likelihood of treatment dropout at a trend level (B = .02, SE = .01, p = .08), with an odds ratio of 1.02 (95% confidence interval [1.00, 1.05]). There were no other significant predictors.
Table 3.
Baseline Predictors of Treatment Dropout
| Univariate Predictors | B | SE | p |
|---|---|---|---|
| Military Rank | −.26 | .17 | .14 |
| Age | −.03 | .02 | .21 |
| Race | .09 | .48 | .84 |
| Income | −1.75 | 1.96 | .37 |
| Gender | −.66 | 1.11 | .55 |
| Education | −.17 | .12 | .14 |
| Employment | .37 | .50 | .45 |
| CAPS | .02 | .01 | .08 |
| BDI-II | .02 | .02 | .30 |
| TLFB TDU Any Drugs | .01 | .01 | .32 |
| TLFB TDU Alcohol | .004 | .01 | .70 |
Note. Predictors were first entered individually, with all marginal predictors entered in a final model. Race was coded as 0 = Caucasian, 1 = other. Gender was coded as 1 = male, 2 = female. Employment was coded as 0 = full or regular part-time, 1 = other (student, unemployed, retired/disability). CAPS = Clinician-Administered PTSD Scale. BDI-II = Beck Depression Inventory-II. TLFB TDU = Timeline Followback Total Days Used.
4. Discussion
Recent research indicates that treatments targeting PTSD and SUD concurrently are effective in reducing symptoms of both disorders (Back et al., 2014; Foa et al., 2013; McGovern et al., 2015; Roberts, Roberts, Jones, & Bisson, 2015). However, this area of research remains in the nascent stage, with significant gaps. Despite the support for the efficacy of these treatments, few studies have examined dropout from integrated treatments targeting PTSD and SUD simultaneously. This is particularly problematic when one considers high dropout rates reported in treatments targeting PTSD (Imel et al., 2013) and SUD separately (Rabinowitz & Marjefsky, 1998), added to the increased symptomatology and impairment in PTSD-SUD comorbidity (McCauley et al., 2012). The current study addressed an important gap in the literature by examining the point in treatment at which participants drop out and potential demographic and clinical predictors of treatment dropout.
Contrary to this study’s first hypothesis, the majority of dropout (55%) occurred after session 6, with the largest amount of dropout occurring between sessions 9 and 10. This finding differs from previous research that noted the majority of Veterans dropped out of prolonged exposure prior to session four (Garcia et al., 2011; Mott et al., 2014). An explanation for this finding could be the late implementation of exposure work, which often begins in session 6 of COPE. Other exposure based protocols such as prolonged exposure implement exposure earlier in treatment (e.g., session 3). These protocol differences may explain this finding as previous studies have noted participants who are unwilling to confront trauma relevant stimuli, quickly dropout of treatment (Frye & Spates, 2012). A second possible explanation for this outcome is that motivation to continue treatment decreased as symptoms improved over the course of treatment, leading to dropout later on in treatment (Zandberg et al., 2016; Szafranski, Smith, Gros, & Resick, in press).
Among the investigated variables, greater baseline CAPS scores were identified as a possible predictors of premature dropout. This finding is consistent with previous studies noting that elevated PTSD baseline symptomatology as a risk factor for treatment dropout (Garcia et al., 2011), although this finding has not been consistent across all studies (Gros et al., 2011; 2013) and was only a trend in the present findings. This result could be due to participants with elevated CAPS scores having slower improvements in PTSD symptoms that could lead them to have decreased motivation to attend treatment and therefore drop out. Additionally, individuals with higher CAPS scores may have had faster improvements in PTSD symptoms and therefore think that they do not need to attend treatment anymore and then drop out. Research has found that among participants with elevated baseline PTSD severity, dropout rates in PTSD treatments were higher for individuals with very fast and very slow rates of PTSD improvement (Zandberg et al., 2016). Given the inconsistencies noted across studies and lack of studies examining the role of symptom change as a possible moderating variable, future research is needed to better understand the relationship between baseline severity, symptom change during treatment and treatment dropout.
Interestingly, substance use frequency was not associated with dropout in this sample despite previous research identifying elevated substance use as a risk factor for treatment dropout (McKellar et al., 2006). This finding may be a result of a number of participants being recruited from a Substance Abuse Treatment Clinic at a Veterans Affairs Medical Center. As a result, a large proportion of patients had already taken steps to decrease their substance use prior to the enrollment of this study and may have habituated to possible aversive SUD treatment factors (e.g., material emphasizing individual behavior change). It is also possible that these participants motivation to obtain treatment was high, as they had already taken steps to obtain treatment prior to being recruited for this study.
The findings from the present investigation highlight interesting issues within the dropout literature, including high dropout rates, inconsistent findings across studies related to baseline PTSD severity level (Garcia et al., 2011; Gros et al., 2011), substance use frequency (McKellar et al., 2006), when dropout occurs (Mott et al., 2014) and changes in motivation to continue treatment as symptoms change over time (Szafranski et al., in press; Zandberg et al., 2016). To help reduce inconsistencies within the literature, assessment procedures should be improved surrounding treatment initiation to better understand motivations, commitment, and any resistance to treatment. The implementation of session-by-session probes could be used to continue the assessment process and aid in reducing dropout rates. Post-dropout interviews are another alternative, but generally difficult to obtain after losing contact with participants. Finally, specifically discussing dropout risk throughout the course of treatment would likely improve dialog between participant and provider and possibly decrease dropout.
There are four main limitations to the present study. First, the study had a small sample size that could have impacted the power to detect different predictors of dropout. Second, the majority of participants in the current study were male and all were veterans, which may reduce generalizability of findings to female veteran and citizen populations. Third, participants were from a randomized clinical trial which may not be representative of individuals partaking in treatment in naturalistic settings. Finally, COPE includes exposure treatment for PTSD, which may limit generalizability of findings to non-exposure based treatments for PTSD, such as IPT.
Overall, the findings of the present study highlight problems with dropout such as relatively high rates and inconsistencies across studies. Improved assessment procedures may help reduce these inconsistencies and increase open dialog about dropout, with the hopes of reducing overall rates. To date, our study was the first to look at dropout treatment predictors in a concurrent treatment for PTSD and SUD in a veteran population. Future studies with large sample sizes from both exposure and non-exposure based treatments are needed to evaluate additional pretreatment variables that may predict treatment dropout. Moreover, future studies such as Zandberg and colleagues (2016) are important to examining the role of symptom change on dropout.
Highlights.
To date, one study has examined dropout from treatment targeting PTSD/SUD simultaneously
43% of participants dropped out of treatment
The majority of dropout occurred in the later stages of treatment (session 9 and 10)
Greater baseline PTSD symptom severity was associated with dropout
Procedural changes regarding assessment during treatment are suggested
Acknowledgments
This research was supported by NIDA grants R01 DA030143 (PI: Back) and K02 DA039229 (PI: Back), Department of Veteran Affairs CSR&D Career Development Award CX000845 (PI: Gros), and NIAAA grant K23AA023845 (PI: Flanagan). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of NIDA, Department of Veterans Affairs, or the United States government.
Footnotes
There are no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Arfken CL, Klein C, di Menza S, Schuster CR. Gender differences in problem severity at assessment and treatment retention. Journal Of Substance Abuse Treatment. 2001;20(1):53–57. doi: 10.1016/S0740-5472(00)00155-0. [DOI] [PubMed] [Google Scholar]
- Back SE, Foa EB, Killeen TK, Mills KL, Teesson M, Dansky Cotton B, … Brady KT. Concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE): Therapist guide. New York, NY: Oxford Press; 2014. [Google Scholar]
- Back SE, Killeen T, Foa EB, Santa Ana EJ, Gros DF, Brady KT. Use of prolonged exposure to treat PTSD in an Iraq veteran with comorbid alcohol dependence. American Journal of Psychiatry. 2012;169:688–691. doi: 10.1176/appi.ajp.2011.11091433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Killeen TK, Teer AP, Hartwell EE, Federline A, Beylotte F, Cox E. Substance use disorders and PTSD: An exploratory study of treatment preferences among military veterans. Addictive Behaviors. 2014;39(2):369–373. doi: 10.1016/j.addbeh.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banducci AN, Bujarski SJ, Bonn-Miller MO, Patel A, Connolly KM. The impact of intolerance of emotional distress and uncertainty on veterans with co-occurring PTSD and substance use disorders. Journal Of Anxiety Disorders. 2016;41:73–81. doi: 10.1016/j.janxdis.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck depression inventory: Manual. 2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal Of Traumatic Stress. 1995;8(1):75–90. doi: 10.1002/jts.2490080106. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behaviour Research and Therapy. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Brady KT, Dansky BS, Back SE, Foa EB, Carroll KM. Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: Preliminary findings. Journal Of Substance Abuse Treatment. 2001;21(1):47–54. doi: 10.1016/S0740-5472(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Breslau N, Lucia VC, Davis GC. Partial PTSD versus full PTSD: An empirical examination of associated impairment. Psychological Medicine. 2004;34(7):1205–1214. doi: 10.1017/S0033291704002594. [DOI] [PubMed] [Google Scholar]
- Bowe A, Rosenheck R. PTSD and substance use disorder among veterans: Characteristics, service utilization and pharmacotherapy. Journal Of Dual Diagnosis. 2015;11(1):22–32. doi: 10.1080/15504263.2014.989653. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back S, Coffey SF. Substance abuse and posttraumatic stress disorder. Current Directions in Psychological Science. 2004;13:206–209. doi: 10.1111/j.0963-7214.2004.00309.x. [DOI] [Google Scholar]
- Carlson KF, Nelson D, Orazem RJ, Nugent S, Cifu DX, Sayer NA. Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment-related traumatic brain injury. Journal Of Traumatic Stress. 2010;23(1):17–24. doi: 10.1002/jts.20483. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Nation A, Gómez W, Sundberg J, Dilworth SE, Johnson MO, … Rose CD. Pilot trial of an expressive writing intervention with HIV-positive methamphetamine-using men who have sex with men. Psychology Of Addictive Behaviors. 2015;29(2):277–282. doi: 10.1037/adb0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbes CR, Curry KT, Leskela J. Treatment presentation and adherence of Iraq/Afghanistan era Veterans in outpatient care for posttraumatic stress disorder. Psychological Services. 2009;6(3):175–183. doi: 10.1037/a0016662. [DOI] [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DJ, Oslin D, … Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: A randomized clinical trial. JAMA: Journal Of The American Medical Association. 2013;310(5):488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Frueh BC, Turner SM, Beidel DC, Cahill SP. Assessment of social functioning in combat veterans with PTSD. Aggression And Violent Behavior. 2001;6(1):79–90. doi: 10.1016/S1359-1789(99)00012-9. [DOI] [Google Scholar]
- Frye LA, Spates CR. Prolonged exposure, mindfulness, and emotion regulation for the treatment of PTSD. Clinical Case Studies. 2012;11(3):184–200. doi: 10.1177/1534650112446850. [DOI] [Google Scholar]
- Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, … Beckham JC. The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans: A meta-analysis. Journal Of Anxiety Disorders. 2015;31:98–107. doi: 10.1016/j.janxdis.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Galovski TE, Blain LM, Mott JM, Elwood L, Houle T. Manualized therapy for PTSD: Flexing the structure of cognitive processing therapy. Journal of Consulting and Clinical Psychology. 2012;80(6):968–981. doi: 10.1037/a0030600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia HA, Kelley LP, Rentz TO, Lee S. Pretreatment predictors of dropout from cognitive behavioral therapy for PTSD in Iraq and Afghanistan war veterans. Psychological Services. 2011;8(1):1–11. doi: 10.1037/a0022705. [DOI] [Google Scholar]
- Gill J, Lee H, Barr T, Baxter T, Heinzelmann M, Rak H, Mysliwiec V. Lower health related quality of life in U.S. military personnel is associated with service-related disorders and inflammation. Psychiatry Research. 2014;216(1):116–122. doi: 10.1016/j.psychres.2014.01.046. [DOI] [PubMed] [Google Scholar]
- Gros DF, Price M, Yuen EK, Acierno R. Predictors of completion of exposure therapy in OEF/OIF veterans with posttraumatic stress disorder. Depression and Anxiety. 2013;30(11):1107–1113. doi: 10.1002/da.22207. [DOI] [PubMed] [Google Scholar]
- Gros DF, Yoder M, Tuerk PW, Lozano BE, Acierno R. Exposure therapy for PTSD delivered to veterans via telehealth: Predictors of treatment completion and outcome and comparison to treatment delivered in person. Behavior Therapy. 2011;42(2):276–283. doi: 10.1016/j.beth.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Grosso JA, Kimbrel NA, Dolan S, Meyer EC, Kruse MI, Gulliver SB, Morissette SB. A test of whether coping styles moderate the effect of PTSD symptoms on alcohol outcomes. Journal Of Traumatic Stress. 2014;27(4):478–482. doi: 10.1002/jts.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutner CA, Gallagher MW, Baker AS, Sloan DM, Resick PA. Time course of treatment dropout in cognitive–behavioral therapies for posttraumatic stress disorder. Psychological Trauma: Theory, Research, Practice, And Policy. 2016;8(1):115–121. doi: 10.1037/tra0000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Nunes E, Levin FR, Fraser D. Posttraumatic stress disorder and short-term outcome in early methadone maintenance treatment. Journal of Substance Abuse Treatment. 2000;19(1):31–37. doi: 10.1016/S0740-5472(99)00088-4. [DOI] [PubMed] [Google Scholar]
- Imel ZE, Laska K, Jakupcak M, Simpson TL. Meta-analysis of dropout in treatments for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology. 2013;81(3):394–404. doi: 10.1037/a0031474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: A review of the literature. The American Journal Of Psychiatry. 2001;158(8):1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Jakupcak M, Cook J, Imel Z, Fontana A, Rosenheck R, McFall M. Posttraumatic stress disorder as a risk factor for suicidal ideation in Iraq and Afghanistan war veterans. Journal Of Traumatic Stress. 2009;22(4):303–306. doi: 10.1002/jts.20423. [DOI] [PubMed] [Google Scholar]
- Jakupcak M, Luterek J, Hunt S, Conybeare D, McFall M. Posttraumatic stress and its relationship to physical health functioning in a sample of Iraq and Afghanistan war veterans seeking postdeployment VA health care. Journal Of Nervous And Mental Disease. 2008;196(5):425–428. doi: 10.1097/NMD.0b013e31817108ed. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Moos R. Dropout from 12-step self-help groups: Prevalence, predictors, and counteracting treatment influences. Journal of Substance Abuse Treatment. 2003;24(3):241–250. doi: 10.1016/S0740-5472(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Lester K, Artz C, Resick PA, Young-Xu Y. Impact of race on early treatment termination and outcomes in posttraumatic stress disorder treatment. Journal of Consulting and Clinical Psychology. 2010;78:480–489. doi: 10.1037/a0019551. [DOI] [PubMed] [Google Scholar]
- Mammo A, Weinbaum DF. Some factors that influence dropping out from outpatient alcoholism treatment facilities. Journal Of Studies On Alcohol. 1993;54(1):92–101. doi: 10.15288/jsa.1993.54.92. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Killeen T, Gros DF, Brady KT, Back SE. Posttraumatic stress disorder and co-occurring substance use disorders: Advances in assessment and treatment. Clinical Psychology: Science & Practice. 2012;19:283–304. doi: 10.1111/cpsp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JC, Petkova E, Neria Y, Van Meter PE, Zhao Y, Hembree E, … Marshall RD. Is exposure necessary? A randomized clinical trial of interpersonal psychotherapy for PTSD. The American Journal Of Psychiatry. 2015;172(5):430–440. doi: 10.1176/appi.ajp.2014.14070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern MP, Lambert-Harris C, Xie H, Meier A, McLeman B, Saunders E. A randomized controlled trial of treatments for co-occurring substance use disorders and post-traumatic stress disorder. Addiction. 2015;110(7):1194–1204. doi: 10.1111/add.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar J, Kelly J, Harris A, Moos R. Pretreatment and during treatment risk factors for dropout among patients with substance use disorders. Addictive Behaviors. 2006;31(3):450–460. doi: 10.1016/j.addbeh.2005.05.024. [DOI] [PubMed] [Google Scholar]
- McMahon RC, Kouzekanani K, Malow RM. A comparative study of cocaine-treatment completers and dropouts. Journal Of Substance Abuse Treatment. 1999;16(1):17–22. doi: 10.1016/S0740-5472(97)00317-6. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner CM. Predictors of substance abuse treatment retention among women and men in an HMO. Alcoholism: Clinical And Experimental Research. 2000;24(10):1525–1533. doi: 10.1111/j.1530-0277.2000.tb04571.x. [DOI] [PubMed] [Google Scholar]
- Milligan CO, Nich C, Carroll KM. Ethnic differences in substance abuse treatment retention, compliance, and outcome from two clinical trials. Psychiatric Services. 2004;55(2):167–173. doi: 10.1176/appi.ps.55.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Long-term influence of duration and intensity of treatment on previously untreated individuals with alcohol use disorders. Addiction. 2003;98(3):325–337. doi: 10.1046/j.1360-0443.2003.00327.x. [DOI] [PubMed] [Google Scholar]
- Moos RH, Pettit B, Gruber V. Longer episodes of community residential care reduce substance abuse patients’ readmission rates. Journal Of Studies On Alcohol. 1995;56(4):433–443. doi: 10.15288/jsa.1995.56.433. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- Norman SB, Tate SR, Anderson KG, Brown SA. Do trauma history and PTSD symptoms influence addiction relapse context? Drug And Alcohol Dependence. 2007;90(1):89–96. doi: 10.1016/j.drugalcdep.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Orsillo S. Measures for acute stress disorder and posttraumatic stress disorder. In: Antony MM, Orsillo S, Roemer L, editors. Practitioner’s guide to empirically based measures of anxiety. New York: Kluver Publications; 2002. pp. 255–307. [Google Scholar]
- Petrakis IL, Rosenheck R, Desai R. Substance use comorbidity among veterans with posttraumatic stress disorder and other psychiatric illness. The American Journal On Addictions. 2011;20(3):185–189. doi: 10.1111/j.1521-0391.2011.00126.x. [DOI] [PubMed] [Google Scholar]
- Possemato K, Maisto SA, Wade M, Barrie K, McKenzie S, Lantinga LJ, Ouimette P. Ecological momentary assessment of PTSD symptoms and alcohol use in combat veterans. Psychology Of Addictive Behaviors. 2015;29(4):894–905. doi: 10.1037/adb0000129. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, Marjefsky S. Predictors of being expelled from and dropping out of alcohol treatment. Psychiatric Services. 1998;49(2):187–189. doi: 10.1176/ps.49.2.187. [DOI] [PubMed] [Google Scholar]
- Rizvi SL, Vogt DS, Resick PA. Cognitive and affective predictors of treatment outcome in cognitive processing therapy and prolonged exposure for posttraumatic stress disorder. Behaviour Research and Therapy. 2009;47:737–743. doi: 10.1016/j.brat.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts NP, Roberts PA, Jones N, Bisson JI. Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder: A systematic review and meta-analysis. Clinical Psychology Review. 2015;38:25–38. doi: 10.1016/j.cpr.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Survival analysis. In: Schinka JA, Velicer WF, Schinka JA, Velicer WF, editors. Handbook of psychology: Research methods in psychology. Vol. 2. Hoboken, NJ, US: John Wiley & Sons Inc; 2003. pp. 555–580. [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–73. [Google Scholar]
- Szafranski DD, Gros DF, Menefee DS, Norton PJ, Wanner JL. Treatment adherence: An examination of why OEF/OIF/OND veterans discontinue inpatient PTSD treatment. Military Behavioral Health. 2015;4(1):25–31. doi: 10.1080/21635781.2015.1093976. [DOI] [Google Scholar]
- Szafranski DD, Gros DF, Menefee DS, Wanner JL, Norton PJ. Predictors of Length of Stay Among OEF/OIF/OND Veteran Inpatient PTSD Treatment Noncompleters. Psychiatry: Interpersonal & Biological Processes. 2014;77(3):263–274. doi: 10.1521/psyc.2014.77.3.263. [DOI] [PubMed] [Google Scholar]
- Szafranski DD, Smith BN, Gros DF, Resick PA. High Rates of PTSD Treatment Dropout: A Possible Red Herring? Journal of Anxiety Disorders. doi: 10.1016/j.janxdis.2017.01.002. (in press) [DOI] [PubMed] [Google Scholar]
- Szafranski DD, Talksovsky AM, Little TE, Menefee DS, Wanner JL, Gros DF, Norton PJ. Predictors of inpatient PTSD treatment noncompletion among OEF/OIF/OND veterans. Military Behavioral Health. 2016 doi: 10.1080/21635781.2016.1153536. [DOI] [Google Scholar]
- Taylor S, Wald J, Asmundson GG. Factors associated with occupational impairment in people seeking treatment for posttraumatic stress disorder. Canadian Journal of Community Mental Health. 2006;25(2):289–301. [Google Scholar]
- Tuerk PW, Wangelin B, Rauch SM, Dismuke CE, Yoder M, Myrick H, … Acierno R. Health service utilization before and after evidence-based treatment for PTSD. Psychological Services. 2013;10(4):401–409. doi: 10.1037/a0030549. [DOI] [PubMed] [Google Scholar]
- van Minnen AA, Arntz AA, Keijsers GJ. Prolonged exposure in patients with chronic PTSD: Predictors of treatment outcome and dropout. Behaviour Research and Therapy. 2002;40:439–457. doi: 10.1016/S0005-7967(01)00024-9. [DOI] [PubMed] [Google Scholar]
- Walter KH, Dickstein BD, Barnes SM, Chard KM. Comparing effectiveness of CPT to CPT-C Among U.S. veterans in an interdisciplinary residential PTSD/TBI treatment program. Journal of Traumatic Stress. 2014;27(4):438–445. doi: 10.1002/jts.21934. [DOI] [PubMed] [Google Scholar]
- Zandberg LJ, Rosenfield D, Alpert E, McLean CP, Foa EB. Predictors of dropout in concurrent treatment of posttraumatic stress disorder and alcohol dependence: Rate of improvement matters. Behaviour Research And Therapy. 2016;80:1–9. doi: 10.1016/j.brat.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]