Abstract
Background
Alemtuzumab is a humanized monoclonal antibody directed against CD52, a cell surface antigen on B and T lymphocytes, and used to treat B-cell chronic lymphocytic leukemia and cutaneous T-cell lymphoma. Skin rash is a common adverse reaction following treatment with alemtuzumab. However, the clinicopathologic features and immunologic basis for the reaction have not been previously reported.
Methods
Our hospital's electronic pathology database was searched for cases with documentation of “alemtuzumab” or “anti-CD52” in the clinical history provided by either the ordering physician or the pathologist. Clinical and histopathologic review of the cases was performed.
Results
Five patients with CTCL or CLL were treated with alemtuzumab, and developed pruritic, erythematous papules and plaques. Histopathology of the skin lesions revealed subacute spongiotic dermatitis with multifocal parakeratosis, endothelial activation and perivascular lymphocytic infiltrate. Eosinophils were not a prominent feature.
Conclusions
We describe the clinicopathologic features of a novel hypersensitivity reaction to alemtuzumb, and hypothesize it may be due to an immunologic response precipitated by the persistence of resident memory T-cells (TRM) in the skin. Our findings raise awareness for a novel reaction pattern and guide the histopathologic interpretation of lesions which may clinically mimic residual or recurrent cutaneous lymphoproliferative disorders.
Keywords: Alemtuzumab, CD52, CTCL, CLL, hypersensitivity
Introduction
CD52 is a cell surface antigen that is abundantly expressed on nearly all human lymphocytes as well as most malignant B and T lymphocytes.1,2 Due to the close apposition of the antigen to the cell membrane and lack of modulation upon antibody binding, antibodies directed against CD52 efficiently deplete lymphocytes. In vitro studies have demonstrated that B-cells are depleted by cell lysis via activation of complement-dependent cytotoxicity and induction of apoptosis, while T cells are depleted by antibody-dependent cellular cytotoxicity mediated by neutrophils and natural killer (NK) cells.3,4 Alemtuzumab, a humanized monoclonal antibody directed against the CD52 antigen, has been used successfully in the treatment of B-cell chronic lymphocytic leukemia (B-CLL).5 It has also been shown to augment the treatment of a range of peripheral T-cell malignancies,2 particularly those variants that relapse or follow an aggressive clinical course that is refractory to conventional chemotherapy.
Refractory leukemic cutaneous T-cell lymphoma (L-CTCL) has been successfully treated with alemtuzumab. Our group reported in 2012 that 18 patients with refractory CTCL treated with low dose alemtuzumab (10 mg) showed 50% complete response rate and over 90% partial response rate in both blood and skin diseases. Clearance of skin disease tended to lag behind peripheral blood clearance, however, responses were found to be durable in most patients.4 The reason for the relative persistence of cutaneous disease is that alemtuzumab spares resident memory T-cells (TRM) in the skin due to the absence of significant numbers of neutrophils and NK cells at this tissue site which are required for alemtuzumab-mediated antibody-dependent cellular cytotoxicity.
Alemtuzumab is associated with systemic and cutaneous adverse reactions. The most significant side effect of alemtuzumab therapy is T-cell depletion, requiring the standard prophylactic use of trimethoprim/sulfamethoxazole and valacyclovir to reduce the risk of infection. Early dose intravenous infusion reactions, such as fever and skin rash are common and gradually subside with continued treatment6. Subcutaneous administration of alemtuzumab is also expected to result in transient local skin reactions in most patients6. However, some patients develop persistent cutaneous lesions after receiving multiple doses of alemtuzumab, presenting with recurrent, pruritic, erythematous papules and plaques. The histopathologic features of these lesions have not been previously reported. We present the clinicopathologic features of five patients who developed a cutaneous reaction following treatment with alemtuzumab, and propose a pathophysiologic theory for the cutaneous findings.
Methods
A search of our hospital's electronic pathology database was performed for cases with documentation of “alemtuzumab” or “anti-CD52” in the clinical history provided by either the ordering physician or the pathologist. Histopathologic review of the skin biopsies and clinical review of the patient's electronic medical records were performed for selection of cases for inclusion in the study. Patients with an established history of atopic conditions were excluded.
Results
Five patients were treated with alemtuzumab for chronic lymphoproliferative disorders, two with B-CLL and three with L-CTCL (Table 1). Patients 1, 2, and 3 who were being treated for CTCL had diffuse erythema and pruritus prior to treatment with alemtuzumab, and subsequently developed morphologically different cutaneous lesions of well-demarcated erythematous papules and edematous plaques (Figure 1). Patients 4 and 5 who were being treated for CLL were dermatologically free of disease prior to treatment with alemtuzumab. Both of these patients subsequently developed erythematous and pruritic papules and plaques on the chest, back, and upper extremities. The lesions developed following 1 to 2 months of alemtuzumab (10mg subcutaneous administration). They had been pretreated with an antihistamine prior to the first dose to prevent an infusion reaction and received trimethoprim/sulfamethoxazole and valacyclovir prophylaxis throughout the course of their therapy. The patients did not have a known history of eczema.
Table 1. Summary of Patient Clinicopathologic Characteristics.
| Patient | Age | Gender | Diagnosis | Clinical Findings | Biopsy Site(s) | Histologic Findings |
|---|---|---|---|---|---|---|
| 1 | 77 | Male | L-CTCL | Thin, erythematous, slightly scaly and pruritic patches on upper back, lateral arms and flanks | Right flank, left shoulder | Mixed spongiotic and psoriasiform dermatitis with superficial perivascular lymphohistiocytic infiltrate and intraluminal neutrophils |
| 2 | 83 | Male | L-CTCL | Multiple erythematous papules distributed over lower back, inner arms and proximal thighs | Right thigh | Mixed spongiotic dermatitis with multifocal parakeratosis, and superficial to deep perivascular lymphoid infiltrate |
| 3 | 72 | Female | L-CTCL | Erythematous, pruritic plaques on bilateral forearms and antecubital fossae | Right forearm | Spongiotic dermatitis with superficial perivascular lymphocyte infiltrate with intraluminal neutrophils and endothelial activation. |
| 4 | 71 | Female | B-CLL | Pustular eruption on face, upper chest and back with secondary morbilliform eruption on face, upper chest, and abdomen | Left shoulder, left flank, left thigh, left abdomen | Mixed spongiotic dermatitis with multifocal parakeratosis (thigh) and psoriasiform and spongiotic dermatitis (abdomen) with superficial perivascular lymphohistiocytic infiltrate with neutrophils; florid endothelial activation |
| 5 | 67 | Male | B-CLL | Erythematous, slightly edematous coalescing papules on upper arms, back and chest | Right abdomen | Subacute to chronic spongiotic dermatitis with sparse superficial perivascular lymphocytic infiltrate with endothelial activation |
B-cell chronic lymphocytic leukemia (B-CLL); leukemic cutaneous T-cell lymphoma (L-CTCL)
Figure 1.
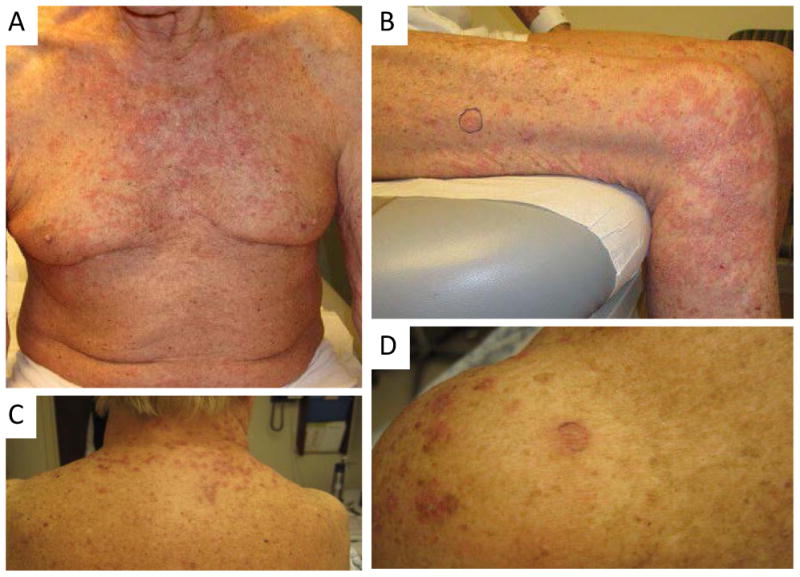
Clinical photographs demonstrating erythematous papules and plaques on the chest (A) and right thigh (B) of patient 2 and the neck (C) and shoulder (D) of patient 3.
Skin biopsies of the lesions were obtained to distinguish between a drug hypersensitivity reaction versus recurrent/residual cutaneous lymphoproliferative disorder. Histopathologically, lesions demonstrated mixed subacute spongiotic and pityriasiform dermatitis, with superficial lymphocytic infiltrate (Figure 2A, B). All cases had prominent endothelial activation of superficial dermal vessels suggestive of a systemic hypersensitivity reaction due to endogenous antigens (Figure 2C). Two cases had scattered interstitial, perivascular, and intravascular neutrophils. An eosinophilic infiltrate, a feature common in hypersensitivity reaction patterns, was not conspicuous in any of the cases. Periodic acid Schiff-diastase and/or Grocott's methenamine silver stains were performed on biopsies from patients 1, 2, 4, and 5, all without evidence of fungal organisms. Immunohistochemistry for lymphoid markers (CD3, CD20, CD4, CD8, CD5, and CD7) was performed on patients 2 and 5 who were being treated for CTCL to rule out an underlying T cell dyscrasia. The immunohistochemical work up was performed because an underlying malignancy could not confidently excluded by cytomorphology alone and/or clinical suspicion was high. Overall, the immunohistochemical findings in conjunction with the histopathology were not diagnostic of an atypical lymphoid infiltrate. Concurrent flow cytometry on peripheral blood or bone marrow aspirate samples was performed on patients 1, 2, 4, and 5 without evidence of an abnormal B or T cell clone. Immunofluorescent studies were performed on the skin biopsies from patients 4 and 5, and demonstrated the persistence of CD8-positive T cells co-expression CD103, consistent with resident memory T-cells (TRM) in the skin (Figure 2D).Overall, the histopathologic features supported the clinicopathologic impression of a novel drug hypersensitivity reaction associated with alemtuzumab.
Figure 2.
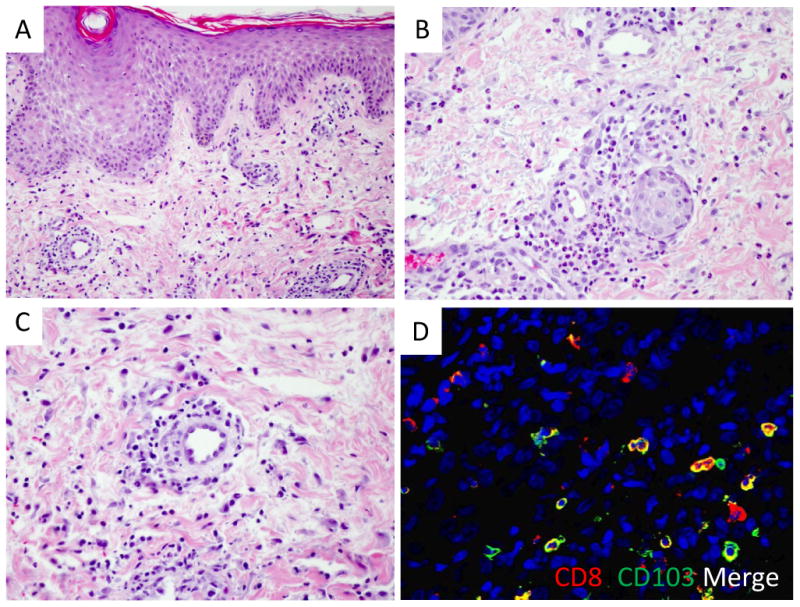
(A) Histopathology showed spongiotic dermatitis with multifocal parakeratosis (H&E, 20×). (B) A superficial perivascular lymphocytic infiltrate was seen in all cases, with the addition of interstitial, perivascular, and intravascular neutrophils in some cases (H&E, 40×). (C) Endothelial activation of superficial dermal vessels was a conspicuous feature (H&E, 40×). (D) Immunofluorescent studies revealed a subset of CD8 T cells are positive for CD103, consistent with TRM phenotype.
In all cases, alemtuzumab therapy was suspended following the skin biopsies. Three of the patients were treated with topical corticosteroids and experienced resolution of the cutaneous eruption. Three of the patients were able to restart alemtuzumab and continued treatment for several months to years while using topical steroids for management of the hypersensitivity reaction.
Discussion
Alemtuzumab is a humanized monoclonal antibody to CD52 antigen that has been used in the treatment of both B-CLL and peripheral T-cell malignancies, including CTCL. We report a novel cutaneous hypersensitivity reaction.
Several etiologies for the cutaneous drug eruptions were considered. In a patient with history of cutaneous involvement by a hematopoietic malignancy and the development of cutaneous lesions resembling those of cutaneous lymphoproliferative disorder, an underling hematopoietic malignancy must be excluded. Histopathologic clues to cutaneous involvement by CTCL include nuclear atypia, epidermotrophism, and background wiry fibrosis in the dermal papillae. An accompanying immunohistochemical profile of atypical CD3+ T cells with increased CD4/CD8 ratio and loss of CD5 expression would support CTCL. Suspicion for CLL may be raised by a predominantly perivascular proliferation of monomorphic CD20+ B cells with co-expression of CD5 and CD23 and light chain restriction. Other entities in the differential diagnosis include phototoxic/photosensitive reaction, hypersensitivity reaction, and urticaria. The location of lesions on the face and upper arms in a photodistributed area raised the possibility of a phototoxic or photosensitivity reaction. However, the presence of lesions on covered areas of the trunk, and the absence of conspicuous dyskeratosis in the histopathology argued against phototoxicity. A hypersensitivity reaction to prophylactic trimethoprim/sulfamethoxazole and valacyclovir regimens was considered; however, the presence of pityriasiform dermatitis with absence of eosinophils is not compatible with the classic drug hypersensitivity reaction pattern. The finding in some cases of intraluminal and perivascular neutrophils associated with endothelial activation (dilated arterioles with plump endothelial cells) and absence of vasculitis suggested an urticarial component. Perivascular and interstitial neutrophils, described in urticaria, occurs by the movement of neutrophils through blood vessels walls into tissue. This process is facilitated by the activation of endothelial cells in response to cytokines that changes the surface of endothelial cells to allow for the adhesion of circulating neutrophils8. While Type 1 hypersensitivity reaction may be part of the underlying mechanism for this unique reaction, it does not fully account for the other findings of spongiotic dermatitis with multifocal parakeratosis.
We propose that the novel hypersensitivity reaction pattern associated with alemtuzumab treatment is due to its anti-CD52 activity. While alemtuzumab effectively depletes circulating central memory T-cells (TCM), its efficacy on non-recirculating resident memory T-cells (TRM) in the skin is limited. The persistence of cutaneous T-RM has been postulated to play a role in driving benign inflammatory dermatoses, such as fixed drug eruptions and recurrent psoriatic plaques,4,9 and it is possible that persistent cutaneous TRM in the setting of alemtuzumab therapy may also contribute to the production of a cutaneous immunological response that uncovers benign inflammatory dermatoses which manifest as a pruritic, erythematous eruption in a subset of patients.
With the increasing use of alemtuzumab in the treatment of B-CLL and refractory peripheral T-cell malignancies, the recognition of this hypersensitivity reaction pattern is important to the clinical management of patients. As the cutaneous manifestation of the hypersensitivity reaction to alemtuzumab may mimic a recurrent/residual cutaneous lymphoproliferative disorder or a drug reaction to prophylactic antibiotic regimens, the histopathologic features reported in our study will help guide the appropriate clinicopathologic correlation.
References
- 1.Dearden CE, Matutes E, Catovsky D. Alemtuzumab in T-cell malignancies. Medical Oncology. 2002;19:S27–S32. doi: 10.1385/mo:19:2s:s27. [DOI] [PubMed] [Google Scholar]
- 2.Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103(8):2920–24. doi: 10.1182/blood-2003-10-3389. [DOI] [PubMed] [Google Scholar]
- 3.Jiang L, Yuan CM, Hubacheck J, et al. Variable CD52 expression in mature T cell and NK cell malignanices: implications for alemtuzumab therapy. British Journal of Hematology. 2009;145:173–79. doi: 10.1111/j.1365-2141.2009.07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark RA, Watanabe R, Teague JE, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4(117):1–20. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundin J, Kimby E, Björkholm M, et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2002;100(3):768–73. doi: 10.1182/blood-2002-01-0159. [DOI] [PubMed] [Google Scholar]
- 6.Österborg A, Karlsson C, Lundin J, et al. Strategies in the management of alemtuzumab-related side effects. Semin Oncol. 2006;33(suppl5):S29–S35. doi: 10.1053/j.seminoncol.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy GA, Seymour JF, Wolf M, et al. Treatment of patients with advanced mycosis fungoides and Sezary syndrome with alemtuzumab. Eur J Haematol. 2003;71:250–56. doi: 10.1034/j.1600-0609.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 8.Hall RP, Takeuchi F, Benbensity KM, et al. Cutaneous endothelial cell activation in normal skin of patients with dermatitis herpetiformis associated with increased serum levels of IL-8, sE-Selectin, and TNF-alpha. J Invest Dermatol. 2006;126:1331–1337. doi: 10.1038/sj.jid.5700277. [DOI] [PubMed] [Google Scholar]
- 9.Clark RA. Skin resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130(2):362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]


