Abstract
Many types of slippery liquid-infused porous surfaces (or ‘SLIPS’) can resist adhesion and colonization by microorganisms. These ‘slippery’ materials thus offer new approaches to prevent fouling on a range of commercial and industrial surfaces, including biomedical devices. However, while SLIPS can prevent fouling on surfaces to which they are applied, they can currently do little to prevent the proliferation of non-adherent (planktonic) organisms, stop them from colonizing other surfaces, or prevent them from engaging in other behaviors that could lead to infection and associated burdens. Here, we report an approach to the design of multi-functional SLIPS that addresses these issues and expands the potential utility of slippery surfaces in antimicrobial contexts. Our approach is based on the incorporation and controlled release of small-molecule antimicrobial agents from the porous matrices used to host infused slippery oil phases. We demonstrate that SLIPS fabricated using nanoporous polymer multilayers can prevent short- and longer-term colonization and biofilm formation by four common fungal and bacterial pathogens (Candida albicans, Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus), and that the polymer and oil phases comprising these materials can be exploited to load and sustain the release of triclosan, a model hydrophobic and broad-spectrum antimicrobial agent, into surrounding media. This approach both improves the inherent anti-fouling properties of these materials and endows them with the ability to efficiently kill planktonic pathogens. Finally, we show that this approach can be used to fabricate dual-action SLIPS on complex surfaces, including the luminal surfaces of flexible catheter tubes. This strategy has the potential to be general; we anticipate that the materials, strategies, and concepts reported here will enable new approaches to the design of slippery surfaces with improved anti-fouling properties and open the door to new applications of slippery liquid-infused materials that host or promote the release of a variety of other active agents.
Keywords: Slippery Surfaces, Multilayers, Controlled Release, Antifungal, Antibacterial
TOC image
We report polymer-based slippery liquid-infused porous surfaces (SLIPS) that can prevent adhesion and colonization by fungal and bacterial pathogens and also kill non-adherent pathogens in surrounding media. Our approach exploits the polymer and liquid oil phases in these slippery materials to sustain the release of a broad-spectrum antimicrobial agent. This controlled release approach improves the inherent anti-fouling properties of SLIPS, has the potential to be general in scope, and expands the potential utility of slippery, non-fouling surfaces in both fundamental and applied contexts.
1. Introduction
Synthetic surfaces that are resistant to fouling by aqueous media, organic fluids, or biological organisms are critical in a broad range of industrial, commercial, and biomedical contexts.[1–7] Surfaces that are superhydrophobic,[1,8] superoleophobic,[7,9] or superomniphobic,[10,11] for example, form a basis for the design of self-cleaning and anti-fogging materials, anti-corrosive interfaces, and stain-resistant textiles, and have enabled new strategies for the transport and manipulation of complex fluids, including approaches to oil recovery and oil/water separation.[1,3,6,7,10] Recent reports on alternative approaches to the development of so-called slippery liquid-infused porous surfaces (or ‘SLIPS’)—fabricated by the infusion of oily liquid lubricants into chemically compatible porous matrices[12,13]—have also enabled the design of new classes of synthetic and highly ‘slippery’ anti-fouling materials that (i) address practical limitations exhibited by conventional non-wetting (e.g., superhydrophobic) surfaces and (ii) introduce new principles for the design of robust, injury-tolerant, and mechanically compliant synthetic anti-fouling surfaces.[12–24]
The work reported here was motivated by recent reports by Aizenberg and co-workers[14,20,25] and Levkin and co-workers[26,27] demonstrating that SLIPS can be designed to resist fouling by bacteria[14,20,25,26] and other marine organisms[25,27] that can colonize and form biofilms on biomedical devices or commercial and industrial equipment. Those past studies suggest that appropriately designed liquid-infused surfaces can resist the attachment, colonization, and organization of communities of these organisms in ways that exceed those exhibited by some conventional anti-fouling surfaces (e.g., surfaces modified with polyethylene glycol and non-wetting superhydrophobic surfaces, etc.),[14,27] even in complex media with proteins, surfactants, or at high ionic strengths typical of environmental conditions encountered in many applied and biologically relevant contexts.[14,20,25–27] While these past studies represent outstanding progress toward the design of new anti-fouling materials with superior functional properties, existing SLIPS do not completely inhibit colonization, and fundamental questions remain regarding both the long-term stabilities of these materials[25,28] and the ability of bacteria or other microorganisms to adapt to or breach the infused liquid barriers that confer slippery character to establish ‘beachheads’ on underlying surfaces[14] that could enable colonization and bio-fouling. In addition, and of particular interest in the context of potential biomedical applications of these new materials, SLIPS can currently do little to influence the behaviors of planktonic microorganisms—that is, while SLIPS can substantially prevent the adhesion of pathogenic microorganisms or the formation of microbial biofilms on treated surfaces, they cannot prevent the growth or proliferation of those organisms in solution, prevent them from colonizing other nearby surfaces, or stop them from engaging in other behaviors (e.g., toxin production) that could lead to infection and other associated burdens.
We reasoned that these fundamental issues could be addressed and, more generally, that the functional properties and potential applications of SLIPS could be significantly expanded, by adopting design strategies that leverage the potential of the infused and lubricating oils in these materials as depots for the storage and subsequent release of bio-active agents. For example, if small-molecule anti-microbial agents could be dissolved and stored in a fugitive oil phase without compromising ‘slippery’ character, this could provide new approaches to the design of multi-functional or dual-action SLIPS with improved antimicrobial properties. Provided that the embedded agent could also diffuse from the oil phase into surrounding aqueous media, this approach would also offer opportunities to design anti-fouling SLIPS that could kill or influence the behaviors of planktonic microorganisms. In a broader and more general context, the ability to store and control the release of small molecules or other agents from SLIPS could open the door to a wide range of other new applications for these liquid-infused materials.
Here, we report a strategy for the design of inherently antifungal and antibacterial polymer-based SLIPS that both inhibit microbial adhesion and promote the sustained release of model small-molecule antimicrobial agents. Our approach is based on SLIPS fabricated by the infusion of a hydrophobic liquid oil into nanoporous polymer multilayers[29] fabricated by reactive/covalent layer-by-layer assembly.[29–32] We demonstrate that (i) these polymer-based SLIPS can substantially prevent surface fouling, including biofilm formation, by several types of common fungal and bacterial human pathogens, and (ii) that biofilm formation on the SLIPS-coated surfaces of planar objects and polymer-based catheter tubes can be reduced further by using porous polymer matrices loaded with triclosan, a model and well-studied broad-spectrum antimicrobial agent.[33,34] We also demonstrate (iii) that the release of triclosan into surrounding media can be used to efficiently and effectively kill planktonic microorganisms present in surrounding media and thereby prevent biofilm formation on neighboring uncoated surfaces. Our results are consistent with a physical picture that involves the gradual partitioning of triclosan from the polymer matrix into the liquid oil phase, and from the liquid oil phase to the surrounding aqueous medium, and introduce new principles that could prove useful for the design of multi-functional slippery surfaces with improved anti-fouling properties.
2. Materials and Methods
Materials
2-Vinyl-4,4-dimethylazlactone (VDMA) was a kind gift from Dr. Steven M. Heilmann (3M Corp., Minneapolis, MN). Poly(2-vinyl-4,4-dimethylazlactone) (PVDMA) was synthesized by free-radical polymerization of VDMA as described previously.[35] Branched poly(ethyleneimine) (BPEI; MW ~25,000), propylamine, n-decylamine (95%), acetone (HPLC grade), tetrahydrofuran (THF, HPLC grade), and silicone oil for melting point and boiling point apparatuses (viscosity = 45–55 cSt at 25 °C) were purchased from Sigma Aldrich (Milwaukee, WI). D-Glucamine (>95%) was purchased from TCI America (Portland, OR). Glass microscope slides were purchased from Fischer Scientific (Pittsburgh, PA). Triclosan (5-chloro-2-(2,4-dichlorophenoxy)phenol) was purchased from Alfa Aesar (Ward Hill, MA). Thin sheets of poly(ethylene terephthalate) film (PET; 0.004 in. thick) were purchased from McMaster Carr (Elmhurst, IL). Aluminum foil was obtained from Reynolds Consumer Products, LLC (Richmond, VA). Polyethylene tubing (PE-100, inner diameter = 0.034 in.) was purchased from Intramedic (Franklin Lakes, NJ). Roswell Park Memorial Institute (RPMI) 1640 powder (with L-glutamine and phenol red, without HEPES and sodium bicarbonate), FUN-1 cell stain, fetal bovine serum (FBS) and 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) were purchased from Invitrogen (Grand Island, NY). 3-(N-Morpholino)propanesulfonic acid (MOPS), Tris-base, phosphate-buffered saline (PBS) liquid concentrate (10×), and NaCl were purchased from Fisher Scientific (Pittsburgh, PA). Menadione, glutaraldehyde, and formaldehyde were purchased from Sigma (St. Louis, MO). Tween-20 was purchased from Acros (Grand Island, NY). Osmium tetroxide (4%) was purchased from Electron Microscopy Sciences (Hatfield, PA). Prof. Barbara Iglewski (University of Rochester) generously donated the Pseudomonas aeruginosa strain (PAO1). Escherichia coli strain ATCC 8739, Staphylococcus aureus strain ATCC10390, and HeLa cells were purchased from American Type Culture Collection (ATTC; Manassas, VA). SYTO-9 staining kits were purchased from ThermoFisher (Waltham, MA). All chemicals, reagents, and solvents were used as received without further purification unless otherwise noted.
General Considerations
Compressed air used to dry samples was filtered through a 0.2 μm membrane syringe filter. Scanning electron micrographs were acquired using a LEO 1530 scanning electron microscope (SEM) at an accelerating voltage of 5 kV. Samples were coated with a thin layer of gold using a gold sputterer operating at 45 mA under a vacuum pressure of 50 mTorr for 40 seconds prior to imaging. Critical point drying was used to prepare fungal biofilm samples for SEM imaging using a Critical Point Dryer (Tousimis Samdri-815). Digital pictures were acquired using a Canon PowerShot SX130 IS digital camera. Water contact angles were measured using a Dataphysics OCA 15 Plus contact angle goniometer at ambient temperature with 10 μL of deionized water. Fluorescence microscopy was performed using an Olympus IX71 microscope and images were obtained using the MetaMorph Advanced version 7.8.1.0 software package. Images were processed using NIH Image J software and Microsoft Powerpoint 2010. In all tube-based experiments (see text), the ends of the tubes were left open. In droplet-based and tube-based experiments, substrates were placed in a humidified microenvironment before incubating at 37 °C. Absorbance measurements used in XTT assays were acquired at 490 nm using a plate reader (EL800 Universal Microplate Reader, Bio-Tek Instruments, Inc.). The M9 buffer used in triclosan release experiments was prepared with the following previously reported composition: 8.6 mM NaCl, 47.7 mM Na2HPO4, 21.7 mM KH2PO4, 18.7 mM NH4Cl, pH 7.35.[36,37]
Covalent Layer-by-Layer Assembly of Porous Polymer Multilayers
Porous PEI/PVDMA multilayers were deposited on glass substrates using a previously reported procedure.[29,32] Briefly: (i) substrates were submerged in a solution of PEI (20 mM in acetone with respect to the polymer repeat unit) for 20 seconds; (ii) substrates were removed and immersed in an initial acetone bath for 20 seconds followed by a second acetone bath for 20 seconds; (iii) substrates were submerged in a solution of PVDMA (20 mM in acetone with respect to the polymer repeat unit) for 20 seconds; and (iv) substrates were removed and rinsed again using the procedure outlined under step (ii). This cycle was repeated 35 times to fabricate porous polymer multilayers consisting of 35 PEI/PVDMA layer pairs (or ‘bilayers’). The concentrations of the polymer solutions were maintained during assembly by the addition of acetone as needed to compensate for solvent evaporation after every dipping cycle. All other substrates used in this study (e.g., catheter tubes, aluminum foil, PET film, and PTFE tubes) were also coated using this general protocol. Multilayers were characterized and used in subsequent experiments immediately or dried under a stream of filtered, compressed air and stored in a vacuum desiccator until use. All films were fabricated at ambient room temperature.
Chemical Functionalization of Multilayers and Infusion of Oils
Porous polymer multilayers containing unreacted azlactone groups, prepared as described above, were functionalized by treatment with solutions of decylamine (20 mM in THF).[29,38] Functionalized films were then rinsed with THF and acetone and dried with filtered air. The resulting hydrophobic multilayers were infused with lubricating liquids (e.g., silicone oil) using the following general protocols.[29] For planar substrates, a droplet of 3 μL of oil was placed onto a film and spread over the surface using weighing paper. For experiments involving coated tubes, film-coated tubes were infused with oil by placing a 4 μL silicone oil droplet at the top end of a vertical tube and allowing gravity-driven spreading of the oil from the top to the bottom of the tube. Excess oil was removed by tapping the bottom end of the tube on a disposable wipe.
Loading and Release of Triclosan
The small-molecule antimicrobial agent triclosan was loaded into porous multilayers prior to the infusion of silicone oil by treating film-coated substrates with a desired number of 10 μL droplets of a solution of triclosan (50 mg/mL) in acetone and allowing the acetone to evaporate under ambient conditions for 5 minutes. All films were then dried under vacuum and infused with silicone oil as described above. Characterization of the release of triclosan from these loaded SLIPS was performed by incubating loaded SLIPS-coated substrates in 750 μL of PBS buffer at 37 °C. The release of triclosan as a function of time was monitored by UV/vis spectrophotometry at a wavelength of 222 nm. At desired time points, buffer was removed for analysis using a pipette and replaced by a fresh 750 μL aliquot.
Characterization of Fungal Cell Adhesion on SLIPS-Coated Substrates
C. albicans SC5314 cells were grown overnight at 30 °C in liquid yeast extract-peptone-dextrose (YPD) medium. Cells were washed with PBS and re-suspended in RPMI 1640 medium buffered with MOPS (pH 7.0). The cell density was adjusted to 107 cfu/mL with RPMI 1640 for experiments with SLIPS that did not contain triclosan, and to 106 cfu/mL for experiments using SLIPS loaded with triclosan. For initial screens, 50 μL droplets of cell suspension were placed on both planar SLIPS-coated and planar bare glass surfaces and incubated at 37 °C. Substrates were removed from the incubator after 3 hours and characterized using either a macroscopic crystal violet stain or a microscopic yeast cell viability dye. The macroscopic cell stain assay was performed by adding 20 μL of a crystal violet solution [CV; 1% (w/w) in deionized water] to droplets of yeast sitting on the surfaces of SLIPS-coated glass or bare glass substrates and incubating at 37 °C for 30 minutes. Substrates were then removed from the incubator and placed in an inclined position at a desired angle (see text) to characterize and compare the anti-fouling properties of the substrates. Microscopic cell viability assays were performed by adding 25 μL of a 10 μM solution of the fungal cell viability probe FUN-1 to the yeast droplets and incubating at 37 °C for at least 30 minutes before imaging using a fluorescence microscope.
Estimation of Biofilm Adhesion on SLIPS-Coated Substrates
C. albicans SC5314 cells were grown overnight at 30 °C in liquid YPD medium. Cells were washed with PBS and re-suspended in RPMI 1640 medium buffered with MOPS (pH 7.0) and supplemented with 5% fetal bovine serum to stimulate biofilm formation. The cell density was manually adjusted to 106 cfu/mL. SLIPS-coated substrates and other control substrates (bare glass, silicone oil-wetted glass, and multilayer-coated glass without silicone oil) were individually submerged in 1 mL of cell suspension (106 cfu/mL) in each well of a 24-well plate. The plate was incubated at 37 °C for 24 hours, after which time either (i) the biofilms formed on the surfaces of the substrates were visualized using the cell stain FUN-1 (200 μL of 10 μM FUN-1, incubated for 30 minutes at 37 °C) or (ii) the metabolic activity of the cells was evaluated. For evaluation of metabolic activity, substrates were carefully removed from the wells, excess liquid was removed and substrates were placed in new wells; 200 μL of XTT solution (0.5 g/L in PBS, pH 7.4, containing 3μM menadione in acetone) was added to every well containing a substrate, including negative control wells that did not have any yeast or substrate in them. After 2 hours of incubation at 37 °C, 75 μL of the supernatant was transferred to a 96-well plate and the absorbance of the solution at 490 nm was measured. Background absorbance from wells containing medium and XTT alone was subtracted from all readings and results were plotted relative to the absorbance values of wells containing samples of solution from control untreated surfaces. Similar sets of experiments were carried out for SLIPS-coated PET film and SLIPS-coated aluminum foil. For studies involving the use of triclosan-loaded SLIPS, the metabolic activity of cells in solution was also quantified (in both triclosan-loaded and non-triclosan-loaded control coatings) in addition to characterization of the metabolic activity of surface-associated cells. For multiple challenge experiments, substrates were incubated with yeast, as described above. At the end of each 24-hour period, substrates were removed from their wells and imaged to characterize the extent to which yeast was adhered on the surface. The SLIPS-coated substrates were then incubated in fresh C. albicans suspensions to perform the next challenge (new bare glass substrates were used in control experiments). Three such 24-hour challenges were performed, and at the end of the third challenge, XTT was used to quantify the reduction in metabolic activity of cells on the surface of the SLIPS substrates compared to glass controls. Similar multiple challenge experiments, consisting of five consecutive challenges, were performed using triclosan-loaded SLIPS. In those experiments, the metabolic activities of both substrate-associated and planktonic cells were quantified at the end of every challenge.
Characterization of Fungal Cell Adhesion in SLIPS-Coated Catheter Tubes
A 15 μL aliquot of a C. albicans suspension (107 cfu/mL) was incubated in 3 cm segments of SLIPS-coated PE tubes at 37 °C. After 4 hours, the amount of attached cells in the tubes was both qualitatively and quantitatively estimated using three different methods: (i) a metabolic XTT assay, (ii) by visualizing the biofilm formed using SEM, and (iii) using the FUN-1 cell stain assay as described above. For the metabolic assay, yeast solution was removed from the tubes and placed in a well containing 50 μL of XTT (0.5g/L in PBS, pH 7.4, containing 3 μM menadione in acetone). A 15 μL aliquot of XTT was also incubated inside the emptied tubes. Both preparations were incubated at 37 °C for 2 hours, after which the absorbance of the solutions was measured at 490 nm to quantify the relative metabolic activity of yeast on the surface of the tubes and inside the tubes. For analysis by SEM, catheter tube segments were placed in a fixative solution [1% (v/v) glutaraldehyde and 4% (v/v) formaldehyde] overnight at 4 °C. The samples were rinsed in PBS (0.1 M) for 10 minutes and then placed in osmium tetroxide (1%) for 30 minutes, followed by 10 minutes in PBS (0.1 M). The samples were subsequently dehydrated in a series of ethanol washes (30%, 50%, 70%, 85%, 95%, and 100% for 20 minutes each). Final desiccation was accomplished by critical point drying. Specimens were mounted on aluminum stubs and sliced open to reveal the inside of the catheter, then sputter coated with gold-palladium. Samples were then imaged in high-vacuum mode at 5 kV. The antifungal and anti-biofilm activities of triclosan-loaded, SLIPS-coated PTFE tubes were characterized by incubating 40 μL of a 106 cfu/mL C. albicans cell suspension in a 2 cm tube segment for 4 hours at 37 °C. After 4 hours, a metabolic XTT assay was performed as outlined above.
Characterization of Bacterial Biofilm Adhesion on SLIPS-Coated Substrates
Freezer stocks of bacterial strains were maintained at −80 °C in 1:1 LB:glycerol. Overnight cultures of bacteria were grown in Luria-Bertani (LB) medium (P. aeruginosa and E. coli) or tryptic soy broth (S. aureus) at 37 °C with shaking at 200 rpm. Biofilms attached to SLIPS-coated glass and bare glass substrates were imaged by fluorescence microscopy. An inoculating subculture of P. aeruginosa was prepared by centrifugation of the overnight culture at 4,000 × g for 10 min followed by resuspension of the cell pellet in an amount of fresh M9+ medium, effecting a 1:10 dilution (v/v) of the overnight culture (M9+ medium consists of the M9 buffer, described above, supplemented with 0.4% arginine, 0.5% casamino acids, 0.2% glucose, 0.2% succinate, 0.2% citrate, 0.2% glutamate, 1 mM MgSO4, and 0.1 mM CaCl2).[37] A subculture of S. aureus was prepared by diluting overnight cultures 1:100 into fresh brain-heart infusion medium supplemented with 1% (w/v) glucose.[39] E. coli subcultures were prepared by diluting overnight cultures 1:1000 into fresh LB medium. Both bare glass substrates and SLIPS-coated substrates were placed individually into the wells of a 12-well microtiter plate (Costar 3737) and sterilized by UV irradiation for 20 minutes in a biological safety cabinet. Bacterial subculture (P. aeruginosa, S. aureus, E. coli) was then added to each well in 2 mL aliquots and the plates were incubated under static conditions at 37 °C for 24 h. Substrates were then removed from the wells using forceps, gently dabbed on a paper towel to remove excess liquid, and placed in the wells of a new 12-well plate to perform biofilm staining with SYTO-9 according to the manufacturer’s protocol. Excess staining solution was removed by dabbing on a paper towel and the substrates were then transferred to the wells of a 24-well plate and covered by 400 μL PBS. Biofilms were then imaged using an Olympus IX71 fluorescence microscope.
Characterization of Mammalian Cell Attachment on SLIPS-Coated Surfaces
All surfaces were sterilized prior to the seeding of cells by exposure to UV light for 15 minutes in a biological safety cabinet. The substrates were then placed individually into the wells of 24-well tissue culture-treated polystyrene culture plates. HeLa cells were cultured in growth medium (MEM supplemented with 10% v/v fetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin), seeded on both SLIPS-coated and bare glass substrates at initial densities of 50,000 cells/mL in 750 μL of growth medium, and incubated at 37 °C for 72 hours. Cytotoxicity measurements were conducted in replicates of three using a commercially available fluorescence live/dead assay kit (Molecular Probes). For imaging, cells were stained with 500 μL of a staining solution containing calcein AM and ethidium homodimer staining solution (1 μg/mL in PBS) for 45 minutes at 37 °C. Following incubation, the staining solution was aspirated and replaced with 1 mL of fresh growth medium and cells were imaged by fluorescence microscopy.
3. Results and Discussion
3.1 Multilayer-Based SLIPS Prevent Adhesion of C. albicans
We recently reported that nanoporous and superhydrophobic polymer multilayers fabricated by the reactive layer-by-layer assembly of PEI and the amine-reactive polymer PVDMA can be infused with hydrophobic oils to design surfaces that are ‘slippery’ to a range of aqueous fluids.[29] The work reported here sought to (i) characterize the ability of these slippery oil-infused surfaces to prevent the short- and longer-term attachment and colonization of fungal and bacterial cells and (ii) test the hypothesis that the porous polymer matrix and liquid oil phases comprising these materials could be exploited as reservoirs for the loading and release of antimicrobial agents that could both (iii) improve the inherent anti-fouling properties of these surfaces and (iv) provide new strategies to kill non-adherent (planktonic) cells in surrounding media. For all of the work reported here, we used SLIPS fabricated by the infusion of silicone oil into nanoporous, decylamine-functionalized PEI/PVDMA films ~3.5 μm thick.[29] Our past studies demonstrate that SLIPS having this general structure and composition exhibit water-droplet sliding angles as low as 1° and can be fabricated on a broad range of objects, including the inner surfaces of tubes.[29] We used silicone oil as a model hydrophobic oil, rather than the highly fluorinated oils used by many other groups to design SLIPS in past studies,[12,14,20,27] on the basis of our past results and because silicone oil can be used as a solvent for a range of different small-molecule (drug-like) agents.
We performed a series of initial experiments to characterize the ability of SLIPS-coated planar glass substrates to prevent fouling by C. albicans, an opportunistic human pathogen that is the leading cause of hospital-acquired systemic fungal infections.[40,41] These experiments were performed by incubating 50 μL droplets of C. albicans yeast inocula onto horizontal bare (control) and SLIPS-coated glass surfaces for 3 hours at 37 °C. The droplets were then stained using crystal violet (a dye used widely to stain microbial biomass and provide a visual means of assessing the extent of macroscopic surface colonization[42,43]), and the substrates were tilted gradually from their initial horizontal positions to identify the angle at which the droplets started to slide down the surfaces of the substrates (Figure 1A). For SLIPS-coated glass substrates, a tilt angle of 10° was sufficient to promote sliding of beaded droplets of inocula, and sliding occurred down the surface without leaving behind a trail of either liquid or yeast cells (Figure 1B). Furthermore, no staining was observed on the surfaces of the SLIPS in the locations where the droplets were initially placed. In contrast, droplets of yeast inocula spread readily on the surfaces of bare glass substrates and required extreme tilt angles of 90° to promote sliding (Figure 1C). On bare glass, sliding left behind a prominent trail of residual liquid, and upon washing the substrate we observed adherent biomass to be present where the droplets of inocula were initially placed. To confirm these observations, we performed a similar series of experiments using the yeast-specific fluorescent dye FUN-1 to characterize the presence or absence of live yeast cells. After tilting the substrates and washing them as described above, we observed bright and uniform green and red fluorescence on the surfaces of bare glass substrates, indicating the presence of substantial numbers of metabolically active yeast cells (Figure 1D–F; green is cytoplasmic staining; red indicates intravacuolar structures in metabolically active cells). In contrast, the surfaces of SLIPS-coated glass slides were dark and almost completely devoid of fluorescence (Figure 1G–I). When combined, these results demonstrate that glass surfaces coated with silicone oil-infused porous PEI/PVDMA multilayers can substantially prevent the adhesion or attachment of C. albicans yeast for short incubation periods.
Figure 1.
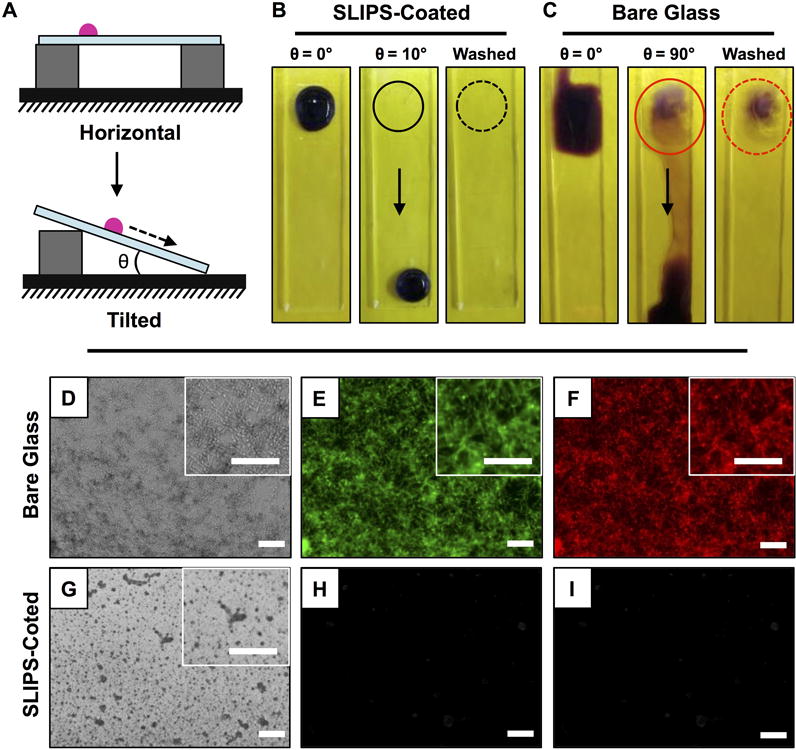
(A) Schematic illustration showing a side-view depiction of the beading and sliding of an aqueous droplet on horizontally placed (top) and tilted (bottom) ‘slippery’ liquid-infused porous surfaces (SLIPS). (B–C) Digital pictures, acquired from a top down vantage point, of a 50 μL droplet of C. albicans inoculum incubated on the surfaces of (B) SLIPS-coated glass substrates and (C) bare glass substrates for 3 hours; droplets are shown after staining with crystal violet and either before or after tilting from horizontal (at the angles indicated) and after washing with DI water (see text); solid and dotted circles mark the original locations of the droplets before sliding; arrows indicate direction of sliding. (D–I) Bright-field (D,G) and fluorescence (E–F, H–I) microscopy images of bare glass (D–F) and SLIPS-coated (G–I) glass substrates after incubation with droplets of C. albicans inocula; droplets were incubated for 3 hours, stained with FUN-1 dye, and tilted to permit aqueous fluids to slide away from the original location of the droplet prior to imaging; green fluorescence indicates cytoplasmic staining, red stain marks intravacuolar structures in metabolically active (live) cells. Scale bars, including insets, are 100 μm.
3.2 Multilayer-Based SLIPS Resist the Formation of C. albicans Biofilms
We performed a second series of experiments to determine whether these PEI/PVDMA-based SLIPS could prevent the attachment and colonization of yeast for longer periods and thus prevent or reduce the formation of C. albicans biofilms. For these experiments, we immersed SLIPS-coated glass substrates into the wells of 24-well plates containing suspensions of C. albicans in media conditioned to stimulate biofilm formation, and incubated them at 37 °C for 24 hours (bare glass substrates, bare glass smeared with silicone oil (no polymer matrix), and multilayer-coated glass substrates (no oil) were also included as controls). After 24 hours, the substrates were then either (i) stained directly with FUN-1 to visualize cells attached to the surface or (ii) carefully removed and placed into empty wells to quantify the metabolic activity of attached cells using an XTT assay. Robust biofilms exhibiting substantial metabolic activity were observed on all three control substrates (bare glass, oil-treated glass, and multilayers without oil) by fluorescence microscopy (Figure 2A–C and 2G–L) and the XTT assay (Figure 2M). In contrast, SLIPS-coated surfaces were almost completely devoid of biofilm (as determined by fluorescence microscopy; Figure 2D–F) and exhibited very low metabolic activity compared to the control surfaces (Figure 2M). These results demonstrate that the individual components of these SLIPS themselves (i.e., the porous polymer matrix and silicone oil) are not substantially cytotoxic, and suggest that the absence of biofilm on SLIPS-coated surfaces arises instead from slipperiness imparted by the fruitful combination of these two components. We note here, however, that while these materials can substantially prevent biofilm formation under these conditions, we occasionally observed small and isolated patches of biofilm along the edges and corners of the coated glass substrates (Figure S1) where defects may prevent uniform coverage by the SLIPS (these small patches of biofilm observed visually likely also account for the small amount of metabolic activity observed in our XTT assays; e.g., ~6% as compared to biofilms formed on bare glass substrates; see Figure 2M). We return to this observation again in the sections below.
Figure 2.
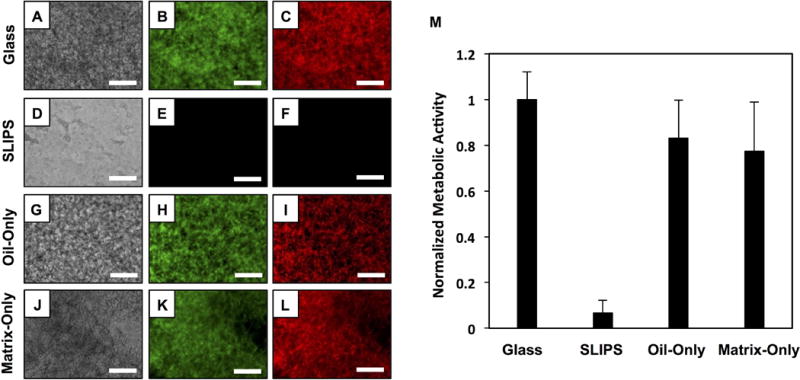
(A–L) Phase contrast and fluorescence microscopy images of bare glass (A–C), SLIPS-coated glass (D–F), oil-smeared glass (oil only; G–I), and porous polymer matrix (matrix only; J–L) surfaces immersed in suspensions of C. albicans for 24 hours; cells were stained with FUN-1 fluorescent dye prior to imaging. (M) Plot showing the quantified metabolic activity of C. albicans on the surfaces of bare glass, SLIPS-coated glass, oil-smeared glass (oil only), and porous polymer multilayers (matrix only) after immersion in suspensions of C. albicans for 24 hours; metabolic activity was quantified using an XTT assay. Scale bars are 200 μm. Error bars represent standard deviation.
To characterize the anti-fouling properties of these SLIPS for longer periods, we performed a ‘multiple challenge’ experiment in which substrates were repeatedly (i) immersed in suspensions of yeast for 24 hours, (ii) removed from the yeast suspensions and characterized, and (iii) immersed again in fresh yeast suspensions for another 24 hours (see Materials and Methods for additional details). This multiple challenge protocol was adopted to circumvent limitations associated with the depletion of nutrients (and resulting cell death) that would occur during prolonged, multiple-day immersion in a single yeast suspension. In addition to the multiple microbial challenges, this protocol also allowed us to assess potential changes in anti-fouling properties that could occur as a result of the physical manipulation and re-use of SLIPS-coated surfaces, including multiple passages through air/water interfaces. After each 24-hour challenge, the substrates were removed to characterize slipperiness and extents of biofilm formation. Figure 3 shows representative results of an experiment involving three consecutive microbial challenges and reveals that SLIPS-coated surfaces maintained their anti-fouling properties, compared to bare glass controls, as determined by bright-field and fluorescence microscopy characterization of FUN-1-stained surfaces (Figure 3A–H). We note that the dark punctate and granular structures observed in the bright-field images of SLIPS-coated surfaces (Figure 3B,D,F) are associated with the textures of the nanoporous multilayer scaffolds, and do not represent yeast cells or biofilm (a result that is confirmed by the lack of green fluorescence in the FUN-1-stained SLIPS-coated surfaces shown in Figure 3H). After the third microbial challenge, we also quantified differences in the metabolic activities of cells present on the surfaces of the substrates (Figure 3I). SLIPS-coated substrates exhibited an ~80% reduction in biofilm formation as compared to bare glass substrates. Based on this result and visual inspection of FUN-1-stained samples, we again attribute the ~20% metabolic activity observed here (relative to controls) to the presence of small patches of biofilm located at the corners and edges of the SLIPS-coated substrates, as described above, and not to the presence of biofilm over the remaining majority of the liquid-infused surfaces.
Figure 3.
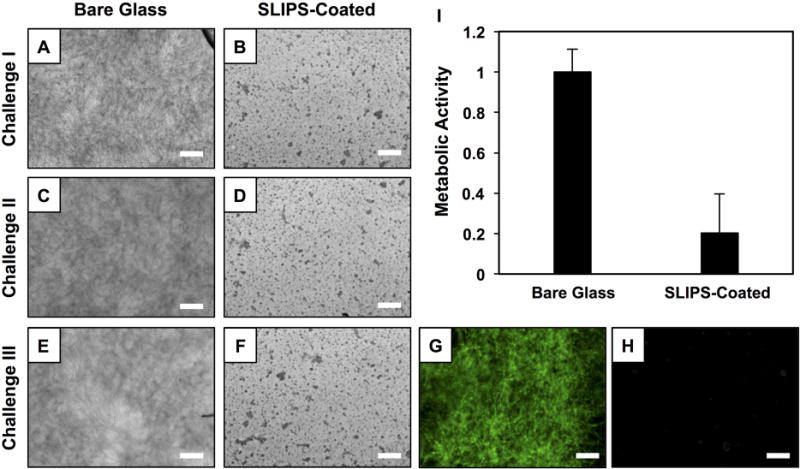
(A–F) Bright-field microscopy images showing the surfaces of bare glass and SLIPS-coated glass substrates after three consecutive 24-hour challenges with C. albicans suspensions. (G–H) Fluorescence microscopy images of the same surfaces shown in (E–F) after staining with the FUN-1 fluorescent dye. (I) Plot showing the quantified metabolic activity of C. albicans on the surfaces of bare glass and SLIPS-coated glass after the third consecutive challenge; metabolic activity was quantified using an XTT assay. Scale bars are 100 μm. Error bars represent standard deviation.
3.3 Characterization of Anti-Fouling Behavior in SLIPS-Coated Catheter Tube Segments
One useful feature of the layer-by-layer process used to fabricate the materials reported here is that it permits the fabrication of SLIPS on a variety of types of surfaces, including plastic and metal surfaces and flexible, curved, or topologically complex surfaces (e.g., tubing) typical of devices or equipment relevant in applied industrial or biomedical contexts[19,29,44] The results of experiments shown in Figure S2 demonstrate that multilayer-based SLIPS can also prevent C. albicans biofilm formation on the surfaces of flexible plastic (PET; Figure S2E–H) and metal (aluminum foil; Figure S2I–L) surfaces. We also performed a series of experiments to characterize the ability of SLIPS to prevent C. albicans adhesion and biofilm formation on the inner surfaces of narrow diameter polymer-based catheter tubes. Figure 4A–B shows low- and high-magnification SEM images of micro/nanoporous PEI/PVDMA multilayers on the inner surfaces of polyethylene tubes ~850 μm in diameter (images were acquired prior to the infusion of silicone oil). The images in panels C-E of Figure 4 demonstrate that the inner surfaces of these tubes become slippery when infused with oil. These images show a 5 μL aliquot of an aqueous solution of the red dye tetramethylrhodamine (TMR) slipping rapidly through a SLIPS-coated catheter tube held at a 10° angle (Figure 4F shows an image of a similar experiment conducted using a bare, uncoated tube; the liquid in this case remains pinned at the top of the tube and does not pass through the tube under these conditions).
Figure 4.
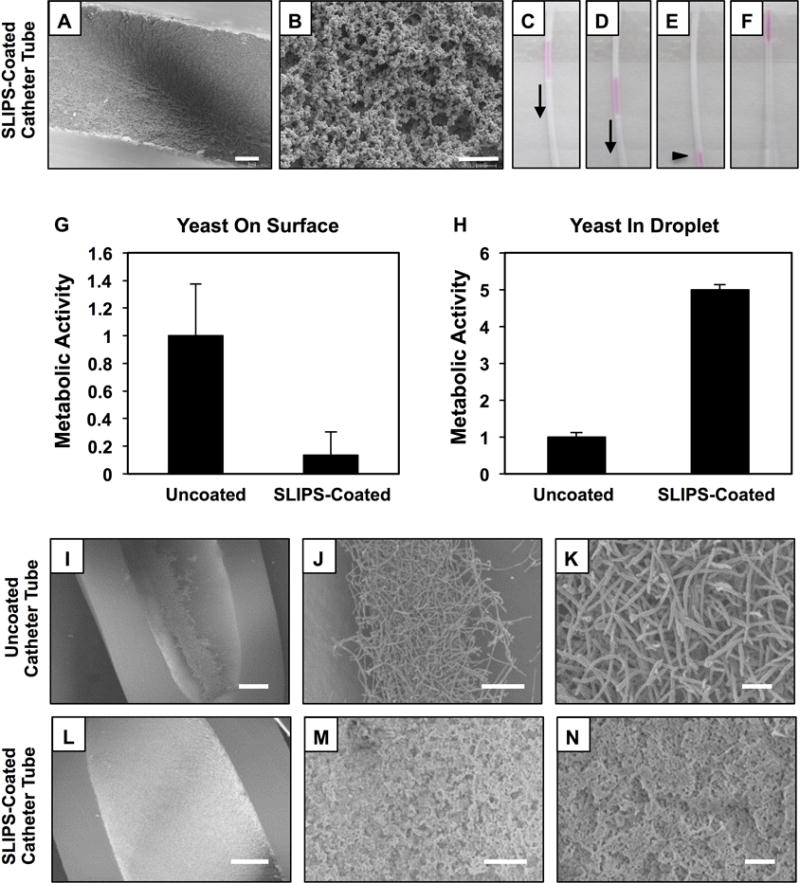
(A–B) Lower- and higher-magnification SEM images showing the surfaces of porous polymer multilayers fabricated on the inner surfaces of catheter tubes prior to the infusion of silicone oil; tube segments were sliced longitudinally prior to imaging (scale bar in A = 100 μm; scale bar in B = 2 μm). (C–E) Digital images showing the sliding of aliquots of aqueous TMR (5 μL) inside SLIPS-coated catheter tubes tilted at an angle of 10°. (F) Digital image showing an aliquot of aqueous TMR at the top end of a bare catheter tube. (G–H) Plots showing the quantified metabolic activity of C. albicans associated with the surfaces (G) of bare and SLIPS-coated catheter tubes and in droplets of yeast inocula (H) collected from bare and SLIPS-coated catheter tubes after 4 hours of incubation. Error bars represent standard deviation. (I–N) Lower- and higher-magnification SEM images of bare (I–K) and SLIPS-coated (I–K) catheter tubes after inoculation with suspensions of C. albicans for 4 hours; samples were prepared by conventional critical-point drying and tubes were sliced longitudinally prior to imaging. Scale bars in panels I,L; panels J,M and panels K,N are 200 μm, 50 μm, and 10 μm, respectively.
We filled segments of uncoated and SLIPS-coated catheter tubes with C. albicans inocula, incubated them at 37 °C for 4 hours, and then used SEM to visualize and characterize the amounts and types of cells adhered to both types of tubes. We observed hyphal and pseudohyphal C. albicans cells on the surfaces of bare control tubes (Figure 4 I–K), as expected in the early stages of biofilm formation.[45,46] The surfaces of SLIPS-coated catheters were largely devoid of C. albicans cells (Figure 4L–N; the roughness and texture apparent in panels M and N are again features associated with the polymer multilayer matrix; e.g., see Figure 4A; Figure S3 shows representative fluorescence microscopy images of FUN-1-stained bare or SLIPS-coated samples). The metabolic activity of cells associated with SLIPS-coated surfaces was also ~85% lower than that measured in bare, uncoated catheter tubes (as determined by an XTT assay; Figure 4G). Furthermore, we estimate that ~5 times more planktonic cells remained in the liquid media contained inside SLIPS-coated tubes in these experiments (Figure 4H) compared to the media inside bare, uncoated tubes that permit or promote cell attachment. This result is to be expected in view of the anti-fouling nature of the SLIPS-coated tubes (to which suspended, planktonic cells cannot attach). This result also illustrates, however, one potential limitation of SLIPS in certain contexts—namely that, as described in the Introduction, SLIPS can prevent or reduce microbial cell attachment and biofilm formation on the surfaces to which they are applied, but can themselves do little to prevent the growth and proliferation of planktonic C. albicans cells and other potentially harmful pathogens in surrounding aqueous environments. The results above also demonstrate that the anti-fouling nature of these SLIPS is not perfect and, in particular, that fungal adhesion and biofilm formation can occur over short periods in locations (e.g., edges and corners) where slippery character may be compromised. We consider it likely that this latter issue could be addressed through the application of SLIPS to substrates that do not contain sharp edges, such as the glass slides used in several of the above experiments. In the sections below, however, we describe a controlled release approach to the design of new multi-functional SLIPS that could help address these broader issues and expand the potential utility of these slippery surfaces.
3.4 Multilayer-Based SLIPS Prevent Adhesion & Colonization by Bacteria & Mammalian Cells
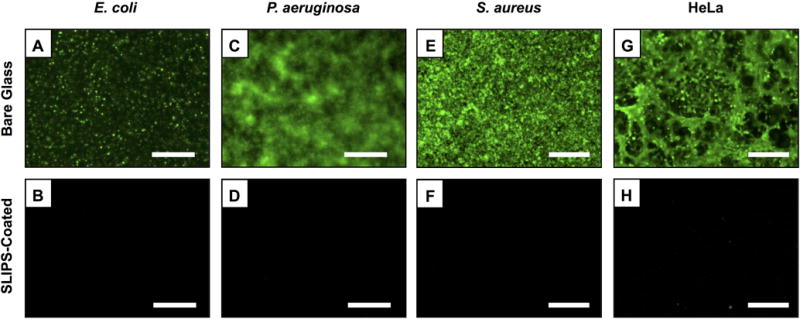
We also investigated the ability of silicone oil-infused, PEI/PVDMA-based SLIPS-coated surfaces to resist attachment and fouling by common bacterial pathogens and mammalian cells. For these experiments, we incubated SLIPS-coated glass substrates and bare glass slides in suspensions of two Gram-negative (P. aeruginosa, E. coli) and one Gram-positive (S. aureus) species of bacteria for 24 hours at 37 °C. All surfaces were then removed from solution and stained using a SYTO-9 biofilm staining solution to identify the presence of bacterial biofilms using fluorescence microscopy. The images in Figure 5A–F clearly indicate the presence of adherent E. coli and dense biofilms of P. aeruginosa and S. aureus on bare glass substrates (Figure 5A,C,E). These images also reveal the lack of bacterial colonization and biofilm formation on SLIPS-coated substrates (Figure 5B,D,F). Finally, Figure 5G–H shows images of SLIPS-coated and bare glass surfaces after incubation with HeLa cells, a human cervical carcinoma cell line widely used as a model in biomedical research, at 37 °C for 72 hours (cells were stained using a calcein AM fluorescent cell stain prior to imaging). The fluorescence microscopy images in Figure 5G–H demonstrate that SLIPS coatings can also substantially prevent attachment and fouling by this mammalian cell line. Figure S4 shows additional representative images associated with these experiments. Overall, our results demonstrate that these multilayer-based SLIPS coatings can prevent surface fouling by fungal, bacterial, and mammalian cells under a range of different conditions and time scales.
Figure 5.
(A–F) Fluorescence microscopy images of the surfaces of bare glass and SLIPS-coated glass substrates after incubation in suspensions of E. coli (A–B), P. aeruginosa (C–D), and S. aureus (E–F) for 24 hours; samples were stained with a SYTO-9 green fluorescent nucleic acid stain prior to imaging. (G–H) Fluorescence microscopy images of bare glass and SLIPS-coated glass substrates after incubation with mammalian HeLa cells for 72 hours; samples were stained with calcein-AM prior to imaging. Scale bars are 100 μm.
3.5 SLIPS Loaded with Antimicrobial Agents Prevent Fouling and Kill Planktonic Cells
One guiding hypothesis of the work reported here was that the infused oil phases of these multilayer-based SLIPS could be used to host and sustain the release of small-molecule antimicrobial agents. We reasoned that if antimicrobial agents could be incorporated without degrading the inherent slippery character of these surfaces, the release of these agents into surrounding liquid media could kill planktonic cells and further prevent or reduce the likelihood of biofilm growth. To explore the feasibility of this approach and establish proof-of-concept, we performed experiments using silicon oil-infused SLIPS and triclosan, a model broad-spectrum antimicrobial agent that can kill both fungal and bacterial cells[33,34] (the molecular structure of triclosan is shown in the inset of Figure 6C). Triclosan is soluble in silicon oil and thus permits the facile design of triclosan-loaded SLIPS by direct infusion of triclosan/silicon oil solutions into nanoporous multilayer matrices. In this study, however, we adopted an alternative approach to loading[47] that involves the solvent-assisted loading of triclosan into the nanoporous multilayers prior to infusion with silicone oil (see Materials and Methods for details). We used this technically straightforward, solvent-assisted method for three reasons: (i) it allows precise control over the amount of triclosan (or any other agent) loaded, (ii) it permits the loading of agents into SLIPS at concentrations that far exceed their solubility in the oil phase and, perhaps most notably, (iii) it has the potential to promote more gradual and sustained release, through a process that involves the partitioning of triclosan from the polymer matrix into the oil and from the oil into the surrounding aqueous phase, than a material in which all of the loaded agent is initially present in the oil phase.
Figure 6.
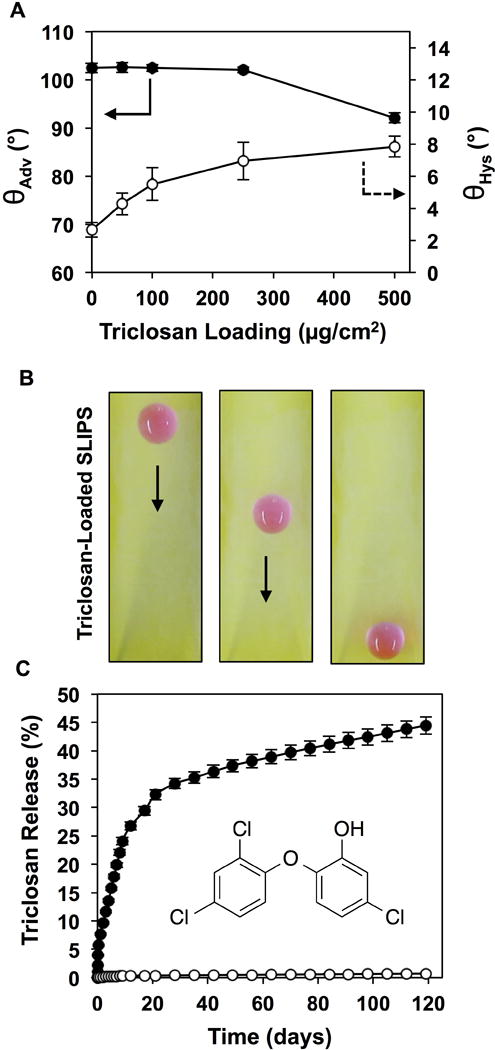
(A) Plot showing the advancing water contact angles, θadv, and contact angle hystereses, θhys, of water droplets (8 μL) on SLIPS loaded with triclosan (at levels ranging from 0 μg/cm2 to 500 μg/cm2). (B) Digital pictures, acquired from a top-down perspective, showing the sliding of a droplet of aqueous TMR (15 μL) on a triclosan-loaded SLIP (loading = 500 μg/cm2; tilt angle = 10°). (C) Plot showing the release of triclosan from triclosan-loaded SLIPS (closed circles; loading = 500 μg/cm2) upon incubation in PBS buffer at 37 °C; open circles correspond to the release profile of otherwise identical films not loaded with triclosan; the inset of panel C shows the molecular structure of triclosan. Error bars represent standard deviation.
Figure 6 shows the results of experiments using triclosan-loaded SLIPS prepared by the pre-oil-infusion treatment of porous PEI/PVDMA multilayers with solutions of triclosan in acetone. As shown in Figure 6A, the loading of triclosan into the polymer matrix did not have a substantial impact on the advancing water contact angle (θadv) for loadings of triclosan up to 250 μg/cm2 (θadv ~103°), and reduced it only slightly at loadings of 500 μg/cm2 (θadv ~93°). The contact angle hysteresis (θhys) of these surfaces increased gradually over a small range (from ~3° to ~8°) as triclosan loading was increased from 0 μg/cm2 to 500 μg/cm2 (Figure 6A). This combination of features allows triclosan to be incorporated into oil-infused SLIPS that maintain their slippery properties, as demonstrated by sliding of a 15 μL droplet of an aqueous TMR solution on the triclosan-loaded SLIPS-coated glass slide shown in Figure 6B (tilt angle = 10°; loading = 500 μg/cm2).
Figure 6C shows the cumulative amount of triclosan released into solution over time for triclosan-loaded SLIPS submerged and incubated in PBS buffer at 37 °C (closed circles; open circles correspond to the release profile of otherwise identical films not loaded with triclosan). Approximately 30% of the loaded triclosan was released over the first 20 days of incubation, with an additional ~15% of the triclosan released over an additional 100 days. This profile is consistent with an initial burst release phase followed by a slower and more sustained phase of release, and is also consistent with concentration dependent diffusion processes involving the gradual partitioning of triclosan from the polymer matrix into the oil phase and into solution. Our results demonstrate that this loading approach can be used to design SLIPS that promote the sustained release of an antimicrobial agent for a period of at least 4 months. We note that only ~45% of the initially loaded triclosan was released over this 4-month period. The results shown in Figure 6C suggest that release would likely continue occur over a substantially longer period, but we did not monitor release for longer than 4 months as part of this proof-of-concept study.
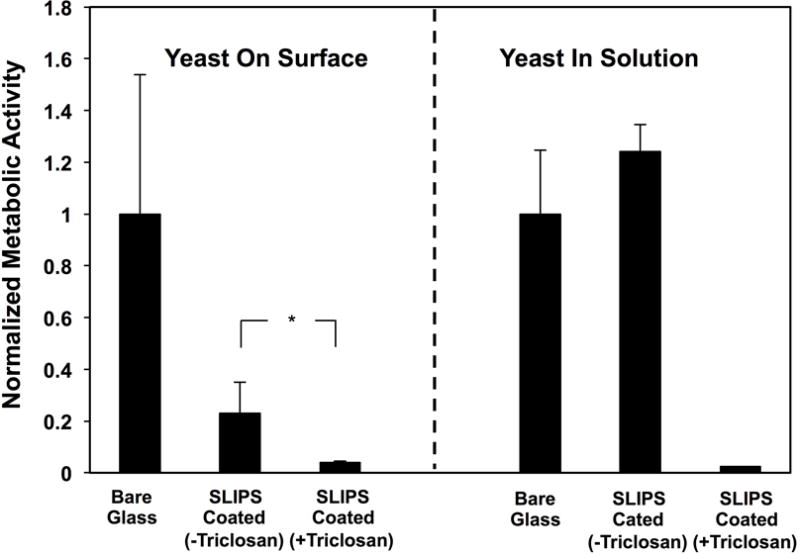
To determine whether the triclosan released from these SLIPS could be released in amounts sufficient to kill planktonic C. albicans and inhibit biofilm formation, triclosan-loaded SLIPS-coated glass slides were immersed in C. albicans inocula and incubated at 37 °C for 24 hours (SLIPS-coated glass substrates without triclosan and bare glass substrates were used as controls). The amounts of metabolically active yeast present both (i) on the surfaces of the substrates and (ii) in the surrounding media in the wells containing the substrates were quantified separately using the XTT assay; the quantitative results of these studies are shown in Figure 7 (representative qualitative visual results are also shown in Figure S5). Triclosan-loaded SLIPS exhibited substantial and significant reductions in the amounts of C. albicans present both in solution and on their surfaces relative to SLIPS without triclosan and bare glass substrates. In particular, the nearly complete lack of metabolically active C. albicans present in solution when triclosan-loaded SLIPS were used (Figure 7; right) demonstrates that the release of triclosan occurred in amounts sufficient to kill nearly all of the planktonic C. albicans cells present in the liquid media. The loading of triclosan also yielded significant reductions in the amount of metabolically active yeast associated with the surfaces of the SLIPS (Figure 7; left), a result that is likely a direct outcome of the ability of those SLIPS to substantially reduce the number of viable planktonic C. albicans cells in their surrounding environments.
Figure 7.
Plot showing the quantified metabolic activity of C. albicans associated with the surfaces (left) of bare glass, SLIPS-coated glass, and triclosan-loaded SLIPS-coated glass and (right) the metabolic activity of planktonic yeast growing in the surrounding medium; metabolic activities were quantified using an XTT assay; all data are normalized with respect to the metabolic activities measured for experiments using bare glass substrates. Substrates were incubated with C. albicans inoculum for 24 hours, removed from wells, and the surfaces and remaining cell suspensions were quantified separately (see text). Error bars represent standard deviation; *indicates p < 0.05 by a two-tailed T-test.
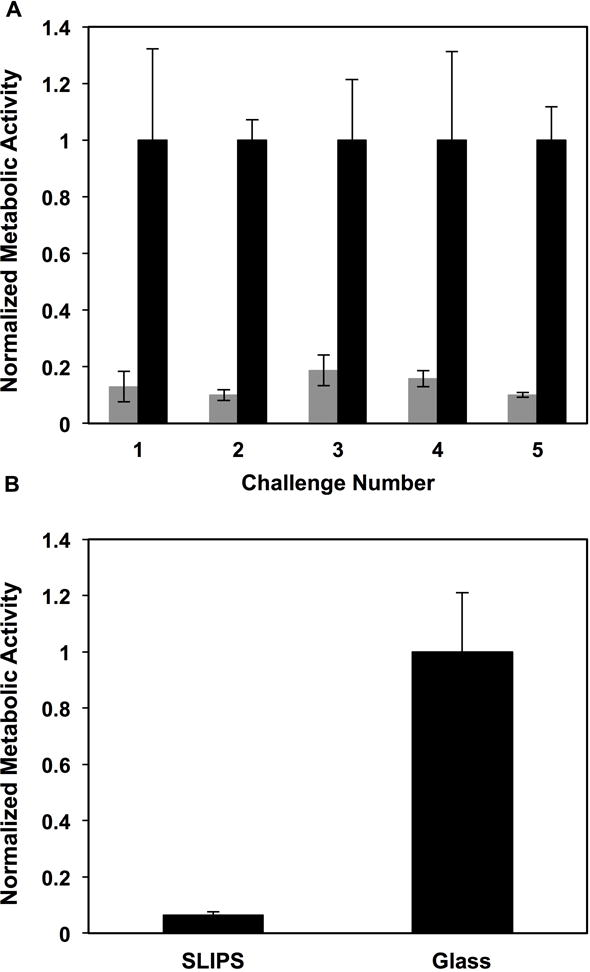
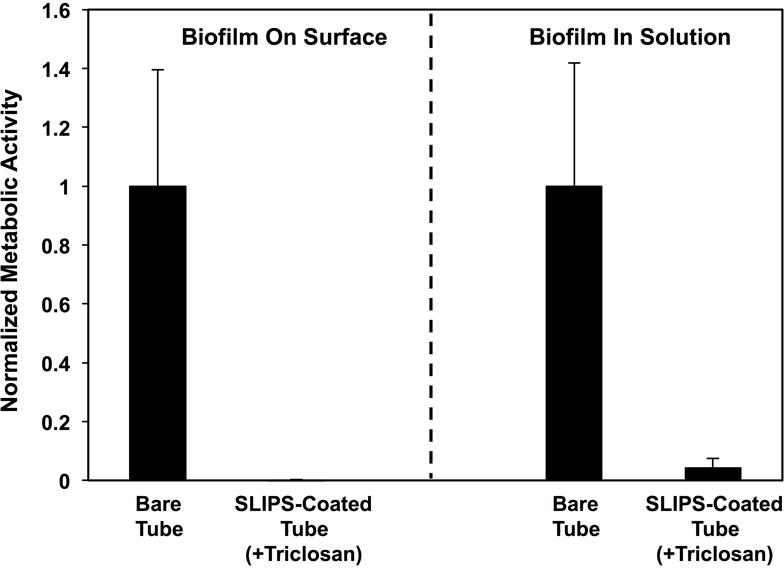
Because triclosan-loaded SLIPS release triclosan for prolonged periods, we performed a multiple challenge experiment similar to that described above and a second, longer-term incubation experiment to determine whether triclosan-loaded SLIPS could exhibit enhanced resistance to C. albicans colonization under longer and more challenging conditions. For multiple challenge experiments, we subjected triclosan-loaded SLIPS (and control bare glass substrates) to five sequential 24-hour immersions in wells containing freshly prepared C. albicans inocula. As shown in Figure 8A, we observed SLIPS loaded with triclosan to prevent biofilm formation on these surfaces (we also observed almost no viable cells in solution at the remainder of each 24-hour challenge, consistent with the results shown in Figure 7; data for multiple challenges not shown here). For the long-term antifungal experiment, substrates were placed in wells containing C. albicans inoculum and removed at the end of one week (with one quarter of the volume of the culture media in the wells gently replaced every two days). We observed triclosan-loaded SLIPS to have substantially reduced amounts of metabolically active C. albicans cells associated with their surfaces after this extended period of incubation (compared to glass controls; Figure 8B). In a final series of experiments, we also fabricated triclosan-loaded SLIPS inside catheter tubes. As shown in Figure 9, the incorporation of triclosan also resulted in substantial reductions in both biofilms and viable cells in the intraluminal solutions of media incubated in these tubes.
Figure 8.
(A) Plot showing the quantified metabolic activity of C. albicans associated with the surfaces of bare glass (black) and triclosan-loaded SLIPS (grey) after each of five consecutive 24-hour challenges in C. albicans inoculum, as determined using an XTT assay. (B) Plot showing the quantified metabolic activity of C. albicans associated with the surfaces of bare glass and triclosan-loaded SLIPS after continuous incubation in C. albicans inoculum for 7 days (see text for details). Error bars represent standard deviation.
Figure 9.
Plot showing the quantified metabolic activity of C. albicans associated with the surfaces (left) of bare catheter tubes and triclosan-loaded SLIPS-coated catheter tubes and (right) the metabolic activity of planktonic cells growing in the surrounding intraluminal medium; metabolic activities were quantified using an XTT assay; all data are normalized with respect to the metabolic activities measured for experiments using bare catheter tubes. Substrates were incubated with C. albicans inoculum for 4 hours, and the surfaces and remaining cell suspensions were quantified separately (see text). Error bars represent standard deviation.
When combined, the results of these experiments demonstrate that these multi-functional, triclosan-loaded SLIPS can exert influences in important ways that extend beyond those that rely on direct interactions with cells at their slippery surfaces. Our results also demonstrate that this approach to killing planktonic microbial cells in surrounding media can improve the anti-fouling properties of these materials. Because triclosan is a broad-spectrum antimicrobial agent that also exhibits substantial activity against bacteria,[33,34] we anticipate that the approach to the loading and release of triclosan described above for the design of SLIPS that kill planktonic fungal cells could also be used to improve the antibacterial properties of these coatings. This approach thus has the potential to be general and provide broader benefits in biomedical contexts by eliminating both planktonic yeast and bacterial cells that could promote downstream infections or produce harmful toxins (e.g., hemolysin, toxic shock syndrome toxin, etc.) through processes that are independent of problems associated with the simple attachment of cells or the formation of biofilms on surfaces.
4. Summary and Conclusions
We have demonstrated an approach to the design of slippery liquid-infused porous surfaces (SLIPS) that can both strongly prevent surface fouling and effectively kill microbial pathogens in surrounding media. This approach addresses a current limitation of SLIPS-based coatings reported by other groups, which can prevent fouling by microorganisms on the surfaces to which they are applied, but cannot prevent the proliferation of or kill nearby non-adherent cells that could colonize nearby surfaces or engage in other behaviors that could lead to infections or other associated burdens. The multi-functional SLIPS reported here address these important issues and thus have the potential to significantly expand the utility and effectiveness of these anti-fouling surfaces in a range of biomedical, industrial, and commercial contexts.
Our results demonstrate that SLIPS fabricated by the infusion of silicon oil into nanoporous polymer multilayers can prevent short- and longer-term colonization and the formation of biofilms by the prevalent and opportunistic fungal pathogen C. albicans. Our results also demonstrate that the porous polymer and hydrophobic oil phases comprising these materials can be exploited to load and then sustain the release of the hydrophobic and broad-spectrum antimicrobial agent triclosan into surrounding media. Using the silicon oil-infused PEI/PVDMA model SLIPS system reported here, we demonstrated that the release of triclosan into surrounding aqueous media can be sustained for extended periods (up to at least 4 months, the longest period investigated in this proof-of-concept study). This approach improves both the inherent anti-fouling properties of these slippery surfaces (e.g., by reducing surface-associated fungi and biofilm growth that can occur at defects present at the edges of planar substrates) and, importantly, endows these coatings with the ability to efficiently kill planktonic C. albicans.
Finally, we demonstrated that these SLIPS coatings can also prevent surface fouling by common bacterial pathogens and a model mammalian cell line and that they can be fabricated on the insides of flexible catheter tubes, suggesting one potential applied biomedical context in which the multi-functionality of this dual-action approach may prove useful. We note, however, that the general strategy reported here for the loading and release of small-molecule agents into slippery surfaces is not likely to be limited to the model system reported here, and has the potential to be general. Provided that the properties of the porous matrix and the infused oil are chosen appropriately, for example, this general approach could be used to host and control the rates of release of a broad range of functional agents into aqueous environments or other surrounding media. As such, the materials, strategies, and concepts reported here have the potential to open the door to many new applications of this new class of slippery liquid-infused materials.
Supplementary Material
Acknowledgments
Financial support for this work was provided by the Office of Naval Research (N00014-07-1-0255), the National Science Foundation through a grant provided to the UW-Madison Materials Research Science and Engineering Center (MRSEC; DMR-1121288), and the National Institutes of Health (R01 AI092225). The authors acknowledge use of instrumentation supported by the NSF through the UW MRSEC (DMR-1121288) and UW NSEC (DMR-0832760), and Michael J. Kratochvil for many helpful discussions.
Footnotes
Supporting Information. Supporting information including characterization of fungal, bacterial, and mammalian cell attachment and biofilm formation on SLIPS-coated substrates is available from the Wiley Online Library or from the author.
Contributor Information
Uttam Manna, Department of Chemical & Biological Engineering, University of Wisconsin—Madison, 1415 Engineering Drive, Madison, WI 53706, USA.
Namrata Raman, Department of Chemical & Biological Engineering, University of Wisconsin—Madison, 1415 Engineering Drive, Madison, WI 53706, USA.
Michael A. Welsh, Department of Chemistry, University of Wisconsin—Madison, 1101 University Avenue, Madison, WI 53706, USA
Yashira M. Zayas-Gonzalez, Department of Chemical & Biological Engineering, University of Wisconsin—Madison, 1415 Engineering Drive, Madison, WI 53706, USA
Prof. Helen E. Blackwell, Department of Chemistry, University of Wisconsin—Madison, 1101 University Avenue, Madison, WI 53706, USA
Prof. Sean P. Palecek, Department of Chemical & Biological Engineering, University of Wisconsin—Madison, 1415 Engineering Drive, Madison, WI 53706, USA
Prof. David M. Lynn, Department of Chemical & Biological Engineering, University of Wisconsin—Madison, 1415 Engineering Drive, Madison, WI 53706, USA Department of Chemistry, University of Wisconsin—Madison, 1101 University Avenue, Madison, WI 53706, USA.
References
- 1.Liu K, Yao X, Jiang L. Chem Soc Rev. 2010;39:3240. doi: 10.1039/b917112f. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee I, Pangule RC, Kane RS. Adv Mater. 2011;23:690. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 3.Yao X, Song Y, Jiang L. Adv Mater. 2011;23:719. doi: 10.1002/adma.201002689. [DOI] [PubMed] [Google Scholar]
- 4.Liu KS, Jiang L. Ann Rev Mater Res. 2012;42:231. [Google Scholar]
- 5.Campoccia D, Montanaro L, Arciola CR. Biomaterials. 2013;34:8533. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 6.Ueda E, Levkin PA. Adv Mater. 2013;25:1234. doi: 10.1002/adma.201204120. [DOI] [PubMed] [Google Scholar]
- 7.Bellanger H, Darmanin T, Taffin de Givenchy E, Guittard F. Chem Rev. 2014;114:2694. doi: 10.1021/cr400169m. [DOI] [PubMed] [Google Scholar]
- 8.Genzer J, Efimenko K. Science. 2000;290:2130. doi: 10.1126/science.290.5499.2130. [DOI] [PubMed] [Google Scholar]
- 9.Tuteja A, Choi W, Ma M, Mabry JM, Mazzella SA, Rutledge GC, McKinley GH, Cohen RE. Science. 2007;318:1618. doi: 10.1126/science.1148326. [DOI] [PubMed] [Google Scholar]
- 10.Chu Z, Seeger S. Chem Soc Rev. 2014;43:2784. doi: 10.1039/c3cs60415b. [DOI] [PubMed] [Google Scholar]
- 11.Deng X, Mammen L, Butt HJ, Vollmer D. Science. 2012;335:67. doi: 10.1126/science.1207115. [DOI] [PubMed] [Google Scholar]
- 12.Wong TS, Kang SH, Tang SKY, Smythe EJ, Hatton BD, Grinthal A, Aizenberg J. Nature. 2011;477:443. doi: 10.1038/nature10447. [DOI] [PubMed] [Google Scholar]
- 13.Grinthal A, Aizenberg J. Chem Mater. 2014;26:698. [Google Scholar]
- 14.Epstein AK, Wong TS, Belisle RA, Boggs EM, Aizenberg J. Proc Natl Acad Sci USA. 2012;109:13182. doi: 10.1073/pnas.1201973109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao X, Hu YH, Grinthal A, Wong TS, Mahadevan L, Aizenberg J. Nat Mater. 2013;12:529. doi: 10.1038/nmat3598. [DOI] [PubMed] [Google Scholar]
- 16.Liu HL, Zhang PC, Liu MJ, Wang ST, Jiang L. Adv Mater. 2013;25:4477. doi: 10.1002/adma.201301289. [DOI] [PubMed] [Google Scholar]
- 17.Smith JD, Dhiman R, Anand S, Reza-Garduno E, Cohen RE, McKinley GH, Varanasi KK. Soft Matter. 2013;9:1772. [Google Scholar]
- 18.Vogel N, Belisle RA, Hatton B, Wong TS, Aizenberg J. Nat Commun. 2013;4 doi: 10.1038/ncomms3176. [DOI] [PubMed] [Google Scholar]
- 19.Huang XY, Chrisman JD, Zacharia NS. ACS Macro Lett. 2013;2:826. doi: 10.1021/mz400387w. [DOI] [PubMed] [Google Scholar]
- 20.Leslie DC, Waterhouse A, Berthet JB, Valentin TM, Watters AL, Jain A, Kim P, Hatton BD, Nedder A, Donovan K, Super EH, Howell C, Johnson CP, Vu TL, Bolgen DE, Rifai S, Hansen AR, Aizenberg M, Super M, Aizenberg J, Ingber DE. Nat Biotechnol. 2014;32:1134. doi: 10.1038/nbt.3020. [DOI] [PubMed] [Google Scholar]
- 21.Glavan AC, Martinez RV, Subramaniam AB, Yoon HJ, Nunes RMD, Lange H, Thuo MM, Whitesides GM. Adv Funct Mater. 2014;24:60. [Google Scholar]
- 22.Wei Q, Schlaich C, Prevost S, Schulz A, Bottcher C, Gradzielski M, Qi ZH, Haag R, Schalley CA. Adv Mater. 2014;26:7358. doi: 10.1002/adma.201401366. [DOI] [PubMed] [Google Scholar]
- 23.Yao X, Ju J, Yang S, Wang JJ, Jiang L. Adv Mater. 2014;26:1895. doi: 10.1002/adma.201304798. [DOI] [PubMed] [Google Scholar]
- 24.Zhang JP, Wang AQ, Seeger S. Adv Funct Mater. 2014;24:1074. [Google Scholar]
- 25.Howell C, Vu TL, Lin JJ, Kolle S, Juthani N, Watson E, Weaver JC, Alvarenga J, Aizenberg J. ACS Appl Mater Inter. 2014;6:13299. doi: 10.1021/am503150y. [DOI] [PubMed] [Google Scholar]
- 26.Li JS, Kleintschek T, Rieder A, Cheng Y, Baumbach T, Obst U, Schwartz T, Levkin PA. ACS Appl Mater Inter. 2013;5:6704. doi: 10.1021/am401532z. [DOI] [PubMed] [Google Scholar]
- 27.Xiao LL, Li JS, Mieszkin S, Di Fino A, Clare AS, Callow ME, Callow JA, Grunze M, Rosenhahn A, Levkin PA. ACS Appl Mater Inter. 2013;5:10074. doi: 10.1021/am402635p. [DOI] [PubMed] [Google Scholar]
- 28.Kim P, Kreder MJ, Alvarenga J, Aizenberg J. Nano Lett. 2013;13:1793. doi: 10.1021/nl4003969. [DOI] [PubMed] [Google Scholar]
- 29.Manna U, Lynn DM. Adv Mater. 2015;27:3007. doi: 10.1002/adma.201500893. [DOI] [PubMed] [Google Scholar]
- 30.Buck ME, Zhang J, Lynn DM. Adv Mater. 2007;19:3951. [Google Scholar]
- 31.Buck ME, Lynn DM. Polym Chem. 2012;3:66. doi: 10.1039/C1PY00314C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manna U, Lynn DM. Adv Funct Mater. 2015;25:1672. [Google Scholar]
- 33.Bhargava HN, Leonard PA. Am J Infect Control. 1996;24:209. doi: 10.1016/s0196-6553(96)90017-6. [DOI] [PubMed] [Google Scholar]
- 34.Jones RD, Jampani HB, Newman JL, Lee AS. Am J Infect Control. 2000;28:184. [PubMed] [Google Scholar]
- 35.Buck ME, Schwartz SC, Lynn DM. Chem Mater. 2010;22:6319. doi: 10.1021/cm102115e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Kievit TR, Gillis R, Marx S, Brown C, Iglewski BH. Appl Environ Microbiol. 2001;67:1865. doi: 10.1128/AEM.67.4.1865-1873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frei R, Breitbach AS, Blackwell HE. Angew Chem Int Ed. 2012;51:5226. doi: 10.1002/anie.201109258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manna U, Broderick AH, Lynn DM. Adv Mater. 2012;24:4291. doi: 10.1002/adma.201200903. [DOI] [PubMed] [Google Scholar]
- 39.Kratochvil MJ, Tal-Gan Y, Yang T, Blackwell HE, Lynn DM. ACS Biomater Sci Eng. 2015;1:1039. doi: 10.1021/acsbiomaterials.5b00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kojic EM, Darouiche RO. Clin Microbiol Rev. 2004;17:255. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mavor AL, Thewes S, Hube B. Curr Drug Targ. 2005;6:863. doi: 10.2174/138945005774912735. [DOI] [PubMed] [Google Scholar]
- 42.Peeters E, Nelis HJ, Coenye T. J Microbiol Meth. 2008;72:157. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Taff HT, Nett JE, Andes DR. Med Mycology. 2012;50:214. doi: 10.3109/13693786.2011.580016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunny S, Vogel N, Howell C, Vu TL, Aizenberg J. Adv Funct Mater. 2014;24:6658. [Google Scholar]
- 45.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Eukaryot Cell. 2005;4:633. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Crit Rev Microbiol. 2009;35:340. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 47.Manna U, Kratochvil MJ, Lynn DM. Adv Mater. 2013;25:6405. doi: 10.1002/adma.201302561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


