Abstract
Lessons Learned.
Trebananib leveraging anti‐angiogenic mechanism that is distinct from the classic sorafenib anti‐vascular endothelial growth factor inhibition did not demonstrate improved progression‐free survival at 4 months in patients with advanced hepatocellular carcinoma (HCC).
In support of previously reported high Ang‐2 levels’ association with poor outcome in HCC for patients, trebananib treatment with lower baseline Ang‐2 at study entry was associated with improved overall survival to 22 months and may suggest future studies to be performed within the context of low baseline Ang‐2.
Background.
Ang‐1 and Ang‐2 are angiopoietins thought to promote neovascularization via activation of the Tie‐2 angiopoietin receptor. Trebananib sequesters Ang‐1 and Ang‐2, preventing interaction with the Tie‐2 receptor. Trebananib plus sorafenib combination has acceptable toxicity. Elevated Ang‐2 levels are associated with poor prognosis in hepatocellular carcinoma (HCC).
Methods.
Patients with HCC, Eastern Cooperative Oncology Group ≤2, and Childs‐Pugh A received IV trebananib at 10 mg/kg or 15 mg/kg weekly plus sorafenib 400 mg orally twice daily. The study was planned for ≥78% progression‐free survival (PFS) rate at 4 months relative to 62% for sorafenib historical control (power = 80% α = 0.20). Secondary endpoints included safety, tolerability, overall survival (OS), and multiple biomarkers, including serum Ang‐2.
Results.
Thirty patients were enrolled sequentially in each of the two nonrandomized cohorts. Demographics were comparable between the two arms and the historical controls. PFS rates at 4 months were 57% and 54% on the 10 mg/kg and 15 mg/kg trebananib cohorts, respectively. Median OS was 17 and 11 months, respectively. Grade 3 and above events noted in ≥10% of patients included fatigue, hypertension, diarrhea, liver failure, palmar‐plantar erythrodysesthesia syndrome, dyspnea, and hypophosphatemia. One death was due to hepatic failure. Serum Ang‐2 dichotomized at the median was associated with improved OS in both cohorts.
Conclusion.
There was no improvement in PFS rate at 4 months in either cohort, when compared with sorafenib historical control.
Abstract
经验总结
•采用与经典的索拉非尼抗血管内皮生长因子抑制作用不同的抗血管生成机制的Trebananib治疗晚期肝细胞癌(HCC)患者未发现4个月时无进展生存率有改善。
•为支持既往报告的高Ang‐2水平与HCC患者不良结局的关联, Trebananib治疗和研究入组时较低水平的基线Ang‐2与总生存期改善至22个月有关, 提示未来的研究需要在低基线Ang‐2背景下进行。
摘要
背景. Ang‐1和Ang‐2属于血管生成素, 被认为通过活化Tie‐2血管生成素受体促进新血管形成。 Trebananib阻断Ang‐1和Ang‐2, 从而阻止其与Tie‐2受体的相互作用。 Trebananib联合索拉非尼组合的毒性可接受。Ang‐2水平升高与肝细胞癌(HCC)的预后不佳有关。
方法.东部肿瘤协作组评分≤2和Childs‐Pugh A级的HCC患者接受了Trebananib (10mg/kg 或15mg/kg, IV, 每周一次)联合索拉非尼(400mg, 口服, 每天两次)的给药。相对于索拉非尼历史对照的62%(把握度= 80%, α= 0.20), 本研究计划使无进展生存(PFS)率在4个月时达到≥78%。次要终点包括安全性、耐受性、总生存期(OS)和多种生物标志物(包括血清Ang‐2)。
结果.依次将三十名患者入组到两个非随机分配的队列中。两组和历史对照之间的人口统计学相当。10mg/kg 和15mg/kg Trebananib队列4个月时的PFS率分别是57% 和 54%, 中位OS分别为17个月和11个月。在≥10%的患者中观察到3级及以上事件, 包括疲劳、高血压、腹泻、肝衰竭、掌足癣红斑综合征、呼吸困难和低磷血症。一名患者因肝衰竭死亡。中位血清Ang‐2与两个队列中的OS改善有关。
结论.与索拉非尼历史对照相比, 任何一个队列中4个月时的PFS率都没有得到改善。
Discussion
High Ang‐2 levels’ association with poor outcome in HCC for patients treated with sorafenib or placebo has been reported [1]. Adding trebananib, which sequesters Ang‐1 and Ang‐2, preventing their interaction with the Tie‐2 receptor [2], to sorafenib treatment on a continuous schedule in two nonrandomized cohorts of two doses of trebananib with comparable demographics between the two arms and the historical control did not show an improvement in progression‐free survival (PFS) rate at 4 months, compared with the estimate of historical control sorafenib in patients with advanced HCC. This is, albeit a favorable median PFS of 7.9 for the 10 mg/kg arm, a reminder of the difficulty of interpreting these endpoints vis‐à‐vis the complexity of HCC and the accompanying cirrhosis.
The combination of trebananib plus sorafenib seems relatively well tolerated; however, the relatively higher than anticipated worsening of liver function is a concern and may add some pretext to the relatively poor outcome of the higher dose of 15 mg/kg cohort compared with the lower dose of 10 mg/kg cohort.
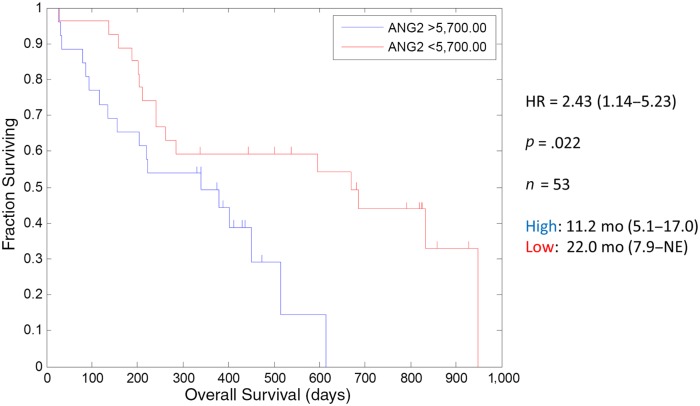
The exploratory biomarker analyses showed several patterns, among which the most intriguing finding is the lower baseline Ang‐2 at study entry, suggesting an association with improved OS to 22 months (Fig. 1). The association between Ang‐2 and survival was previously observed (p < .006) in a phase II trial of trebananib plus sunitinib in renal cancer patients [3].
Figure 1.
Kaplan‐Meier curves depicting overall survival in the Ang‐2 >5,700 ng/mL and <5,700 ng/mL dichotomized 10 mg/kg trebananib arm.
Abbreviations: HR, hazard ratio; NE, not evaluable.
A relatively improved estimate of 17 months median OS of the 10 mg/kg compared with 11 months of the 15 mg/kg trebananib cohort, which is commensurate with the sorafenib single agent historical control of 10.7 months [4], is noted. We do not believe that the biology of trebananib could explain a lower dose improved efficacy or synergy with sorafenib. This may likely be an artifact of the Kaplan‐Meier curve estimation and censoring.
In conclusion, the combination of sorafenib and trebananib did not demonstrate improved control of tumor growth at 4 months, the primary endpoint of this trial. Any further studies of this combination or similar in HCC should be studied within the context of low baseline Ang‐2 and possibly other markers reported herein.
Trial Information
- Disease
Hepatocellular carcinoma
- Stage of Disease/Treatment
Metastatic/Advanced
- Prior Therapy
None
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Randomized
- ORR
RECIST 1.0 objective response rates were 3% and 7%, for the 10 mg/kg and 15 mg/kg cohorts, respectively.
- PFS
PFS rates at 4 months were 57% and 54% for the 10 mg/kg and 15 mg/kg trebananib cohorts, respectively.
- TTP
Median TTP was 9 months (95% CI: 3.4, 16.4) and 6.9 months (95% CI: 3.6, 12.7) in the 10 mg/kg and 15 mg/kg trebananib cohorts, respectively.
- Response Duration
There was no significant difference in the rate of durable stable disease at ≥16 weeks from study day 1 (46.7% and 40% on the 10 mg/kg and 15 mg/kg trebananib cohorts, respectively). This translated into a disease controlled rate of 50% and 46.7% in the 10 mg/kg arm and 15 mg/kg arm, respectively.
- Primary Endpoint
Progression‐free survival at 4 months
- Secondary Endpoint
Toxicity
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Time to progression
- Secondary Endpoint
Overall response rate
- Secondary Endpoint
Pharmacokinetics
- Secondary Endpoint
Correlative endpoint
- Additional Details of Endpoints or Study Design
- The study consisted of two sequentially enrolled cohorts of trebananib 10mg/kg and trebananib 15mg/kg, each dosed weekly in combination with sorafenib given at the standard dose of 400mg twice daily in an every‐4‐weeks dosing schedule. Based on an estimated 4‐month progression‐free survival rate of 62% for sorafenib single agent [5], and assuming a 4‐month progression‐free survival rate of 78% in each cohort, 30 patients in each cohort were required to accrue to satisfy a power of 80% with the one‐sided exact test for single proportion at α = 0.20. Survival curves were estimated using the Kaplan‐Meier methodology. The safety, tolerability, and adverse events were summarized using descriptive statistics. Patients who received at least one dose of trebananib plus sorafenib were evaluable for toxicity.
Drug Information for Phase II Trebananib 10mg/kg + sorafenib
- Drug 1
- Generic/Working name
Trebananib
- Company name
Amgen
- Drug type
Peptibody
- Dose
10 milligrams (mg) per kilogram (kg)
- Route
Intravenous (IV)
- Schedule of Administration
Once a week
- Drug 2
- Generic/Working name
Sorafenib
- Trade name
Nexavar
- Company name
Bayer
- Drug type
Small molecule
- Dose
400 milligrams (mg) per flat dose
- Route
Oral (PO)
Drug Information for Phase II Trebananib 15mg/kg + sorafenib
- Drug 1
- Generic/Working name
Trebananib
- Company name
Amgen
- Drug type
Peptibody
- Dose
15 milligrams (mg) per kilogram (kg)
- Route
Intravenous (IV)
- Schedule of Administration
Once a week
- Drug 2
- Generic/Working name
Sorafenib
- Trade name
Nexavar
- Company name
Bayer
- Drug type
Small molecule
- Drug class
- Dose
400 milligrams (mg) per flat dose
- Route
Oral (PO)
Patient Characteristics for Phase II Trebananib 10mg/kg + sorafenib
- Number of patients, male
50
- Number of patients, female
10
- Stage
Macroscopic Vascular Invasion: trebananib 10mg/kg + sorafenib, n = 30: 8 (26.7)
Macroscopic Vascular Invasion: trebananib 15mg/kg + sorafenib, n = 30: 8 (26.7)
Extrahepatic Spread: trebananib 10mg/kg + sorafenib, n = 30: 10 (33.3)
Extrahepatic Spread: trebananib 15mg/kg + sorafenib, n = 30: 10 (33.3)
- Age
Median (range): 64 and 60 per arm
- Number of prior systemic therapies
Median (range): 0
- Performance Status: ECOG
0 —
1 — 57 (0–1)
2 —
3 —
unknown — 3
Abbreviations: NASH, nonalcoholic steatohepatitis.
Patient Characteristics for Phase II Trebananib 15mg/kg + sorafenib
- Number of patients, male
50
- Number of patients, female
10
- Stage
Macroscopic Vascular Invasion: trebananib 10mg/kg + sorafenib, n = 30: 8 (26.7)
Macroscopic Vascular Invasion: trebananib 15mg/kg + sorafenib, n = 30: 8 (26.7)
Extrahepatic Spread: trebananib 10mg/kg + sorafenib, n = 30: 10 (33.3)
Extrahepatic Spread: trebananib 15mg/kg + sorafenib, n = 30: 10 (33.3)
- Age
Median (range): 64 and 60 per arm
- Number of prior systemic therapies
Median (range): 0
- Performance Status: ECOG
0 —
1 — 57 (0–1)
2 —
3 —
unknown — 3
Abbreviations: NASH, nonalcoholic steatohepatitis.
Primary Assessment Method for Phase II Trebananib 10mg/kg + sorafenib
- Assessment
- Number of patients enrolled
30
- Number of patients evaluable for toxicity
30
- Number of patients evaluated for efficacy
30
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 1 (3%)
- Response assessment SD
n = 14 (47%)
- Response assessment PD
n = 15 (50%)
- Response assessment OTHER
n = 0 (0%)
- (Median) duration assessments PFS
7.9 months, 95% CI: 3.1–12.6
- (Median) duration assessments TTP
9 months, 95% CI: 3.4–16.4
- (Median) duration assessments OS
17 months, 95% CI: 8.6–27.4
- (Median) duration assessments duration of treatment
5.5 months
Primary Assessment Method for Phase II Trebananib 15mg/kg + sorafenib
- Assessment
- Number of patients enrolled
30
- Number of patients evaluable for toxicity
30
- Number of patients evaluated for efficacy
30
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 2 (7%)
- Response assessment SD
n = 12 (40%)
- Response assessment PD
n = 16 (53%)
- Response assessment OTHER
n = 0 (0%)
- (Median) duration assessments PFS
5.5 months, 95% CI: 3.9–9
- (Median) duration assessments TTP
6.9 months, 95% CI: 3.6–12.7
- (Median) duration assessments OS
The median overall survival was 17 months (95% CI: 8.6, 27.4) and 11 months (95% CI: 6.7, not evaluable).
- (Median) duration assessments duration of treatment
3.5 months
- Assessment
- Number of patients enrolled
30
- Number of patients evaluable for toxicity
30
- Number of patients evaluated for efficacy
30
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 2 (7%)
- Response assessment SD
n = 12 (40%)
- Response assessment PD
n = 16 (53%)
- Response assessment OTHER
n = 0 (0%)
- (Median) duration assessments PFS
5.5 months, 95% CI: 3.9–9
- (Median) duration assessments TTP
6.9 months, 95% CI: 3.6–12.7
- (Median) duration assessments OS
11 months, 95% CI: 6.7, not evaluable
- (Median) duration assessments duration of treatment
3.5 months
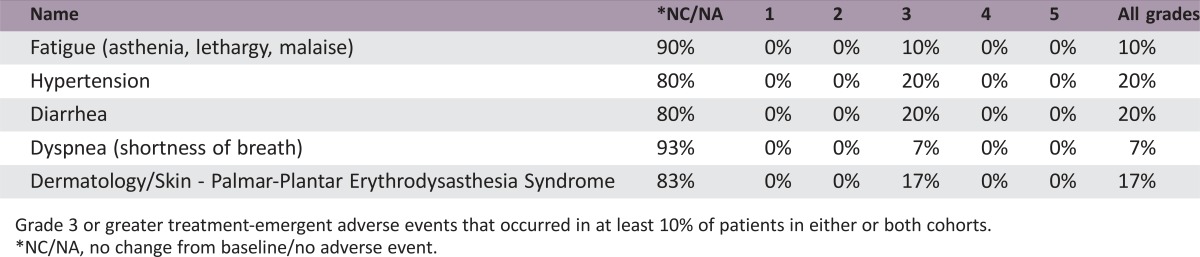
Adverse events: Phase II trebananib 10mg/kg + sorafenib
Grade 3 or greater treatment‐emergent adverse events that occurred in at least 10% of patients in either or both cohorts.
*NC/NA, no change from baseline/no adverse event.
Pharmacokinetics/Pharmacodynamics
- Cmax and Cmin of the trebananib and sorafenib at the end of infusion of week 1, 5 and 9; predose, week 2, 5, and 9, as well as at 48 and 96 hours of week 5, showed no clear dose proportionality in exposure between the 10 and 15 mg/kg dose groups.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Pharmacokinetics/Pharmacodynamics
Correlative endpoints met
- Investigator's Assessment
Correlative endpoints met but not powered to assess activity
Trebananib is a first‐in‐class anti‐angiogenic agent that sequesters Ang‐1 and Ang‐2, preventing their interaction with the Tie‐2 receptor [1], [2]. Elevated serum Ang‐2 levels have been associated with a poor prognosis in hepatocellular carcinoma (HCC) [3]. Furthermore, leveraging an anti‐angiogenic mechanism that is distinct from the classic anti‐vascular endothelial growth factor inhibition (VEGF), trebananib might be expected to provide a synergistic anti‐angiogenic effect when combined with anti‐VEGF therapies. The combination of trebananib plus sorafenib has been previously studied in renal cell carcinoma, where it showed a similar toxicity profile to that of sorafenib as single agent [4]. While sorafenib remains the sole standard treatment of advanced HCC [5], its efficacy is marginal, and better therapies are needed. We therefore evaluated the safety and efficacy of the combination of trebananib plus sorafenib in HCC.
Since the advent of sorafenib as a standard treatment of patients with advanced HCC [5], improved anti‐angiogenic agents remain an attractive approach for the treatment of advanced HCC. Efforts to identify new agents have, however, been rather disappointing, with no evidence so far of improved overall survival beyond the 10.7 months that sorafenib has previously demonstrated [5]. Herein, we studied the novel approach of targeting a non‐VEGF‐associated biological axis in angiogenesis, adding trebananib, which sequesters Ang‐1 and Ang‐2, preventing their interaction with the Tie‐2 receptor, to sorafenib treatment on a continuous schedule [1]. This did not show an improvement in progression‐free survival (PFS) rate at 4 months, compared with the estimate of sorafenib in the historical registration study control, with similar demographics when compared with the present study (Table 1). This is, albeit a favorable median PFS of 7.9 for the 10 mg/kg arm, a reminder of the difficulty of interpreting these endpoints vis‐à‐vis the complexity of HCC and the accompanying cirrhosis. This, add to the length of time on therapy or of observation that may be needed before one may be able to discern any improved efficacy outcome.
The median duration of trebananib therapy given in the 10 mg/kg trebananib cohort was 5.5 months, with a range of 0.3–24.7 months, a median dose of 10.2 mg/kg, and a relative dose intensity of 99%. These figures were similar for the 15 mg/kg trebananib cohort. The median duration of trebananib therapy was 3.5 months (range 1 day to 21 months), with a median dose and relative dose intensity of 15.2 mg/kg and 99%, respectively.
The median duration of sorafenib therapy was 3.7 months (range 0.3–28 months), and the median dose was 744 mg daily with relative intensity of 87% for the 10 mg/kg trebananib cohort. These figures were similar for the 15 mg/kg trebananib cohort: the median duration of therapy was 3.7 months (range 0.13–21 months), with a median daily dose and relative intensity of 781 mg and 95%, respectively.
The outcome of this study may be explained in different ways. An alleged ceiling of benefit from anti‐angiogenic therapy may exist [6]. In order to improve on existing approaches, combination studies that inhibit alternative targets or pathways will be required. The investigation of multiple novel approaches is underway [7], including immunotherapeutic therapies [8].
The combination of trebananib plus sorafenib seems relatively well tolerated. However, within the realm of this small, uncontrolled, sequentially enrolled study, the relatively higher than anticipated worsening of liver function is a concern and may add some pretext to the relatively poor outcome of the 15 mg/kg cohort compared with the 10 mg/kg cohort, raising the question of whether a higher dose would be necessary to achieve the potential synergy between trebananib and sorafenib. In support of this statement, the renal carcinoma study evaluated the combination of trebananib and sorafenib at 10 mg/kg and 3 mg/kg trebananib dose levels [4]. The adverse event profiles of the studies have lot of similarities but differ in the degree of liver toxicity, which is reported at a higher rate in the present study, even at the 10 mg/kg dose. This is another reminder of the dual nature of HCC and the accompanying cirrhosis that may well render subjects more prone to certain toxicities that are not necessarily of concern otherwise. Liver failure was the cause of death in one patient in the HCC study and in none of the four adverse events‐related deaths on the renal study [4].
The biomarker analyses showed several patterns that are exploratory in nature and would require further validation and confirmation. The most intriguing finding is the lower baseline Ang‐2 at study entry, suggesting an association with improved OS to 22 months. High Ang‐2 levels’ association with poor outcome in HCC for patients treated with sorafenib or placebo has already been reported [9]. The association between Ang‐2 and survival was previously observed (p < .006) in a phase II trial of trebananib in combination with sunitinib in renal cancer patients [4]. The higher Ang‐2 levels may indicate greater tumor angiogenic activity or metastatic potential [10].
A relatively improved estimate of 17 months median OS of the 10 mg/kg compared with 11 months of the 15 mg/kg trebananib cohort, which is commensurate with the sorafenib single agent historical control of 10.7 months [5], is noted. These values, however, have to be interpreted with caution given the limited sample size and the fact that these were sequentially accrued cohorts. We do not believe that the biology of trebananib could explain a lower dose improved efficacy or synergy with sorafenib. This may likely be an artifact of the Kaplan‐Meier curve estimation and censoring. The similar 4‐month PFS in the two arms of the study, plus the same duration and dose intensity, argue against any enhanced drug exposure advantage and thus against a treatment effect resulting in improved survival, except a delayed one that is not discernible except beyond 4 months, albeit with lack of any biologic argument to support it. An imbalance that is not accounted for may have influenced the point estimate of OS, which in both arms exceeds the single agent sorafenib estimate of 10.7 months.
In conclusion, the combination of sorafenib and trebananib did not demonstrate improved control of tumor growth at 4 months, the primary endpoint of this trial. Any further studies of this combination or similar in HCC should be studied within the context of low baseline Ang‐2 and possibly other markers reported herein.
Figures and Tables
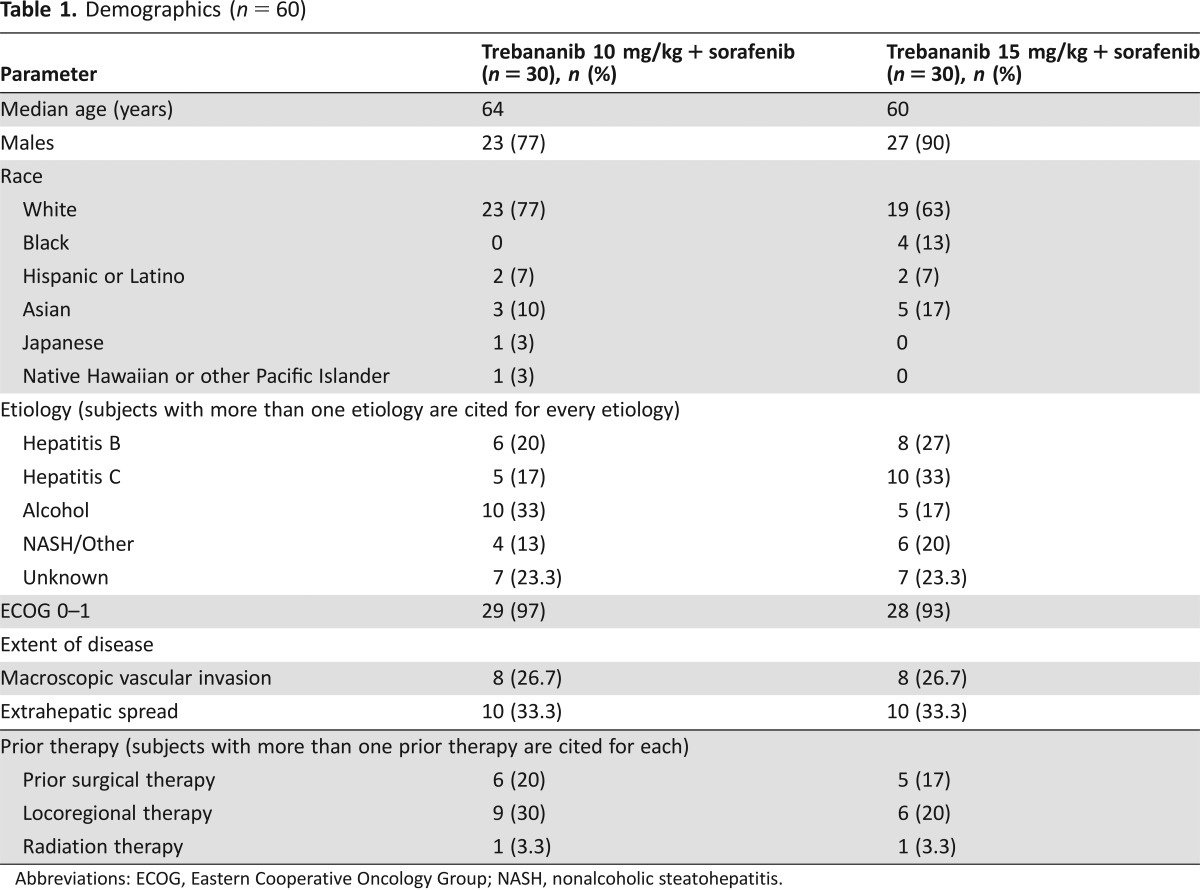
Table 1. Demographics (n = 60).
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NASH, nonalcoholic steatohepatitis.
Footnotes
ClinicalTrials.gov Identifier: NCT00872014
Sponsor(s): Amgen
Principal Investigator: Ghassan K. Abou‐Alfa
IRB Approved: Yes
Disclosures
Ghassan K. Abou‐Alfa: Amgen, Bayer (C/A, RF); Jean‐Frederic Blanc: Bristol‐Myers Squibb, Bayer SP (C/A); Jörg Trojan: Amgen, Bayer, Bristol‐Myers Squibb, Eli Lilly & Co., Merck Serono, Merck Sharp & Dohm, Roche (C/A), Amgen, Bayer, Bristol‐Myers Squibb, Eli Lilly & Co., Merck Serono, Roche (H); Charu Gupta: Amgen (E); Benjamin Wu: Amgen (E, OI); Michael Bass: Amgen (E, OI); Leonard B Saltz: Taiho (RF). The other authors indicated no financial relationships.
References
- 1. Neal J, Wakelee H. AMG‐386, a selective angiopoietin‐1/‐2‐neutralizing peptibody for the potential treatment of cancer. Curr Opin Mol Ther 2010;12:487–495. [PubMed] [Google Scholar]
- 2. Mita AC, Takimoto CH, Mita M et al. Phase 1 study of AMG 386, a selective angiopoietin 1/2‐neutralizing peptibody, in combination with chemotherapy in adults with advanced solid tumors. Clin Cancer Res 2010;16:3044–3056. [DOI] [PubMed] [Google Scholar]
- 3. Torimura T, Ueno T, Kin M et al. Overexpression of angiopoietin‐1 and angiopoietin‐2 in hepatocellular carcinoma. J Hepatol 2004;40:799–807. [DOI] [PubMed] [Google Scholar]
- 4. Rini B, Szczylik C, Tannir NM et al. AMG 386 in combination with sorafenib in patients with metastatic clear cell carcinoma of the kidney: A randomized, double‐blind, placebo‐controlled, phase 2 study. Cancer 2012;118:6152–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 6. Abou‐Alfa GK, Venook AP. The antiangiogenic ceiling in hepatocellular carcinoma: Does it exist and has it been reached? Lancet Oncol 2013;14:e283–e288. [DOI] [PubMed] [Google Scholar]
- 7. Harding JJ, Abou‐Alfa GK. Treating advanced hepatocellular carcinoma: How to get out of first gear. Cancer 2014;120:3122–3130. [DOI] [PubMed] [Google Scholar]
- 8. Harding, JJ, El Dika, I, Abou‐Alfa GK. Immunotherapy in hepatocellular carcinoma: Primed to make a difference? Cancer 2016;122:367–377. [DOI] [PubMed] [Google Scholar]
- 9. Llovet JM, Peña CE, Lathia CD et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2012;18:2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu B, Cheng SY. Angiopoietin‐2: Development of inhibitors for cancer therapy. Curr Oncol Rep 2009;11:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]