A challenge in precision medicine is the identification of actionable driver mutations. Alterations can be identified within the tumor tissue, by small biopsy or fine‐needle aspirates, or by noninvasive methods, such as circulating tumor cells or circulating tumor DNA. This article presents a case of atypical neuroendocrine tumor metastatic to the bone and brain for which circulating tumor DNA analysis found an ALK translocation.
Abstract
A challenge in precision medicine requires identification of actionable driver mutations. Critical to such effort is the deployment of sensitive and well‐validated assays for mutation detection. Although identification of such alterations within the tumor tissue remains the gold standard, many advanced non‐small cell lung cancer cases have only limited tissue samples, derived from small biopsies or fine‐needle aspirates, available for testing. More recently, noninvasive methods using either circulating tumor cells or tumor DNA (ctDNA) have become an alternative method for identifying molecular biomarkers and screening patients eligible for targeted therapies. In this article, we present a case of a 52‐year‐old never‐smoking male who presented with widely metastatic atypical neuroendocrine tumor to the bones and the brain. Molecular genotyping using DNA harvested from a bone metastasis was unsuccessful due to limited material. Subsequent ctDNA analysis revealed an ALK translocation. The clinical significance of the mutation in this particular cancer type and therapeutic strategies are discussed.
Key Points.
To our knowledge, this index case represents the first reported ALK translocation identified in an atypical carcinoid tumor.
Liquid biopsy such as circulating tumor DNA is a feasible alternative platform for identifying sensitizing genomic alterations.
Second‐generation ALK inhibitors represent a new paradigm for treating ALK‐positive patients with brain metastases.
Patient Story
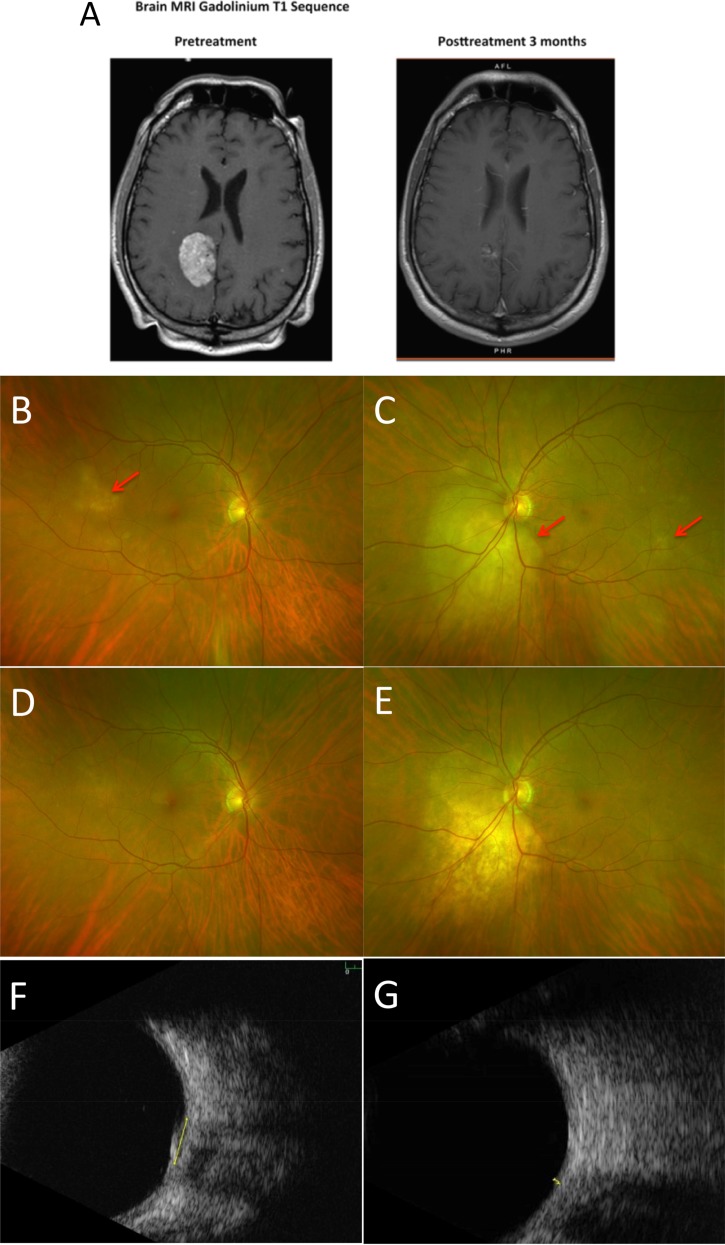
A 52‐year‐old never‐smoking male initially presented with acute‐on‐chronic back pain, which gradually worsened to involve the hips and shoulders. After several months of conservative management without relief, lumbar spine magnetic resonance imaging (MRI) showed multifocal abnormal marrow enhancement suggesting an infiltrative process. Bone scan showed abnormal uptake throughout the axial skeleton, including the skull, sternum, bilateral ribs, and the sacroiliac joints. Computed tomography (CT) of the chest, abdomen, and pelvis identified a large spiculated mass in the right middle lobe of the lung spanning 1.2 × 1.0 cm and a lytic lesion in the manubrium. Brain MRI revealed a 4.3‐cm mass to the right of the falx cerebri with extensive calcifications and vasogenic edema, a rim‐enhancing lesion in the posterior aspect of the left superior frontal gyrus, and numerous punctate foci of enhancement infra‐ and supra‐tentorially and within the calvarium (Fig. 1A). The patient complained of floaters, and fundoscopic examination showed bilateral choroidal metastases (Fig. 1B).
Figure 1.
Regression of brain and choroidal metastases after alectinib. (A): T1 gadolinium enhanced MRI images npre‐ and post‐treatment showing shrinkage of the dominant brain lesion. (B): Right fundus at presentation with a small metastasis in the temporal macula. (C): Left fundus with tumors inferior to the optic disc and in the temporal macula. (D): Right fundus 2 months later with tumor regression. (E): Left fundus 2 months later with regression of the juxtapapillary tumor and disappearance of the temporal metastasis. Ultrasound scans showing left juxtapapillary tumor at presentation, when the thickness was 1.3 mm (F), and after 2 months, when the thickness had diminished to 0.5 mm (G). The metastases are indicated by red arrows.
Abbreviations: MRI, magnetic resonance imaging.
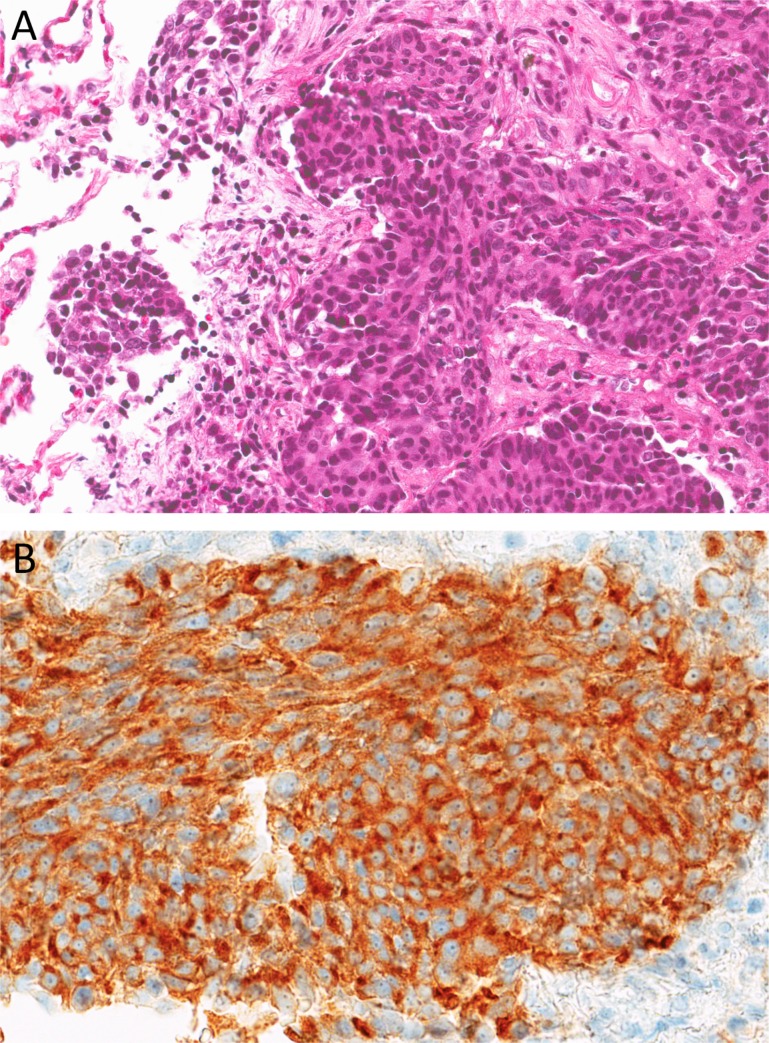
CT‐guided, fine‐needle aspiration of the manubrium lesion and the right middle lobe lesion both revealed monomorphic tumor cells with moderate amount of eosinophilic cytoplasm, forming organoid nests with small foci of coagulative necrosis, that stained diffusely positive for TTF‐1, CK7, synaptophysin, and neural‐specific enolase and focally positive for chromogranin. High‐grade features were not identified in this specimen. Ki67 was approximately 30%. These cells were negative for CK5/6 and p40. The morphological appearance, positive neuroendocrine markers, and intermediate Ki67 all suggest a neuroendocrine tumor of the atypical carcinoid subtype assuming lung origin (Fig. 2). Molecular genotyping could not be completed due to insufficient material.
Figure 2.
Core biopsy findings. (A): Hematoxylin and eosin section shows consolidated lung parenchyma with invasive nests of neoplastic epithelioid cells within a surrounding desmoplastic stroma. The tumor cells have round‐to‐ovoid nuclei with dense chromatin, rare small nucleoli, and moderate amount of eosinophilic cytoplasm. Adjacent normal lung parenchyma is seen on the left (×200). (B): Immunohistochemical stain for synaptophysin demonstrates positive cytoplasmic granular staining (×400).
Planned gamma knife radiotherapy was aborted due to innumerable brain metastases. Because the patient was minimally symptomatic neurologically, whole brain radiation was deferred. Systemic therapy with temozolamide and capecitabine was initiated, with dexamethasone added to control cerebral edema. Restaging scans after two cycles showed stable bone disease but worsening brain metastases. At that time, the patient was referred to our clinic and a hybrid capture‐based circulating tumor DNA (ctDNA) test (FoundationACT, Foundation Medicine, Cambridge, MA, http://www.foundationone.com) revealed an ALK translocation.
Molecular Tumor Board
The Expanding Role of Liquid Biopsies
Liquid biopsies utilizing ctDNA offer an alternate means of detecting genomic alterations to inform treatment. Compared with traditional tissue biopsies, liquid biopsies are faster, more economical, and less invasive, thereby reducing the health risk to patients. Liquid biopsies are most useful when a patient's clinical condition precludes a tissue diagnosis or the lesion in question is anatomically inaccessible. Because tissue biopsies usually sample only a small portion of the tumor, mutations may be missed due to tumor heterogeneity. Liquid biopsies have the potential to sample all metastatic sites, to monitor therapeutic response, and to predict recurrences through serial sampling. Finally, because ctDNA samples do not undergo formalin fixation, background is reduced.
The greatest challenge of liquid biopsies is the false negative rates. Although several series report mutation detection rates between 60%–80% for ctDNA, some cancers may simply shed little DNA into circulation because of small size, limited metastatic spread, or other factors [1]. Liquid biopsies may miss co‐occurring mutations and tumors with mixed histology.
Genotyping Results and Interpretation of the Molecular Results
Molecular profiling was performed in a Clinical Laboratory Improvement Amendments‐certified laboratory using a hybrid capture ctDNA platform. Briefly, 10–20 mL peripheral, whole blood was collected from the patient and spun down to collect 5–10 mL of plasma. Greater than 50 ng of ctDNA was extracted from the sample and quantitated using smear analysis. An adaptor‐ligation library was constructed and hybrid capture was performed using a panel of 2,695 5′ biotinylated single‐stranded DNA oligonucleotides to isolate exonic regions of 62 genes and introns of six genes often involved in genomic rearrangement in cancer. A sequencing library was prepared with short oligonucleotide sequences called fragment‐barcodes inserted between the sequencing adaptor and the sample barcode that allow downstream analysis to uniquely identify the original double‐stranded DNA fragment. Multiplexed sequencing was performed using 2 × 175 paired‐end on the Illumina HiSeq 2500 platform (Illumina, San Diego, CA, https://www.illumina.com). Analyses included error correction across duplicate ctDNA molecules, as identified by the fragment barcodes, to <0.02% and target depth post‐correction of >5,000× unique coverage. Variant calling included indels, substitutions, rearrangements, and amplifications. Reporting of ctDNA was done without a matched normal sample from the patient and compared with known driver alterations in the Catalogue of Somatic Mutations in Cancer (COSMIC) database.
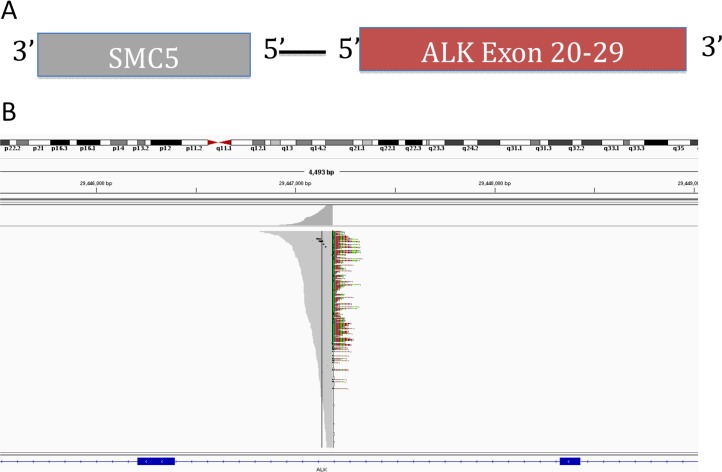
ctDNA allows for sampling across all metastatic sites. ctDNA from this patient detected only two mutations: the ALK translocation and a variant in the PDCD1LG2 gene of unknown significance. The ALK rearrangement in this patient occurred at the canonical intron 19 breakpoint and contained the kinase domain of ALK. This event was a translocation between chromosome 2 and chromosome 9 leading to an out‐of‐strand rearrangement with SMC5 intron 18 where the SMC5 gene was in the opposite transcriptional orientation as ALK (Fig. 3). The median exon coverage for this sample was 5,986. Even though, to our knowledge, ALK translocations have not been reported previously in atypical carcinoid cancer, and SMC5 has never been identified as a fusion partner for ALK, we hypothesized that this fusion was likely the driver mutation because it contained an intact kinase domain capable of downstream signaling. Although we could not rule out the presence of a subclonal population harboring other driver mutations, given the paucity of mutations identified, we believe the patient's tumor was relatively homogeneous and the ALK rearrangement represented a truncal mutation that disseminated early during evolution. Because ctDNA is a new platform, the sensitivity and specificity of the test remains uncertain. Extrapolating from a prior report using circulating tumor cells, investigators were able to identify ALK translocation with 100% sensitivity and specificity [2]. Furthermore, not all tumors shed DNA into the blood stream. Therefore, we would surmise that the actual false negative rate for detecting ALK translocation would be higher than the false positive rate. Being able to detect the translocation at all in the blood sample suggests it was likely an actionable driver mutation in this patient.
Figure 3.
Novel ALK fusion. (A): Circulating tumor DNA sequencing revealed an ALK intron 19 translocation with an out‐of‐strand fusion to SMC5 gene. (B): Integrated Genomic Viewer alignment with a median exon coverage of ∼6,000. Breakpoint denoted in color.
Functional and Clinical Significance of the ALK Mutation
ALK encodes the anaplastic lymphoma kinase, a receptor tyrosine kinase that belongs to the insulin receptor superfamily and induces downstream activation of pathways associated with cell survival, angiogenesis, and proliferation [3]. It was first identified as a fusion with the echinoderm microtubule‐associated protein‐like 4 (EML4) gene in lung cancer in 2007, and this fusion is sufficient to transform 3T3 cells in vitro and induce tumor formation in nude mice in vivo [4]. Since then, other fusion partners have been identified with ALK, including TFG and KIF5B [5], [6]. The rearrangement in this tumor has a breakpoint within ALK intron 19, resulting in separation of the ALK kinase domain (exons 20–29) from the N‐terminal regions (exons 1–19). When expressed in cultured cells, the kinase domain of ALK exhibits oncogenic activity [7], [8]. We hypothesize the fusion in this patient's tumor to be oncogenic because it contains the intact ALK kinase domain. Clinically the EML4‐ALK gene fusion has been observed in ∼3%–7% of non‐small cell lung cancer (NSCLC) cases, and these patients tend to be younger, non‐smoking males of Asian heritage [9]. Notably, ALK mutation has not been observed in 12 atypical lung carcinoid‐endocrine samples analyzed in the COSMIC database to date.
Potential Strategies to Target the Pathway and Implications for Clinical Practice
The ALK inhibitors crizotinib, ceritinib, and alectinib have been approved for the treatment of patients with metastatic NSCLC whose tumors test positive for ALK rearrangement [10], [11], [12], [13], [14]. Several others, such as brigatinib and lorlatinib, are currently in preclinical studies [15], [16]. The first‐generation ALK inhibitor crizotinib is the preferred initial therapy for patients whose tumor harbors the ALK fusion because results of a phase III trial comparing crizotinib with chemotherapy demonstrated improved progression‐free survival (PFS), response rates (RR), and quality of life [17]. The incidence of brain metastases in newly diagnosed stage IV ALK‐positive NSCLC is approximately 20%–30%, comparable to those without the ALK fusion [17], [18]. With improved control of systemic disease using crizotinib, however, approximately 60% of patients will ultimately develop brain metastases [19], [20], [21]. This observation is in part due to the poor central nervous system (CNS) penetration of crizotinib [22]. Until recently, surgery and/or radiation have been the mainstay of treatment for patients with brain metastases, including those with ALK fusions, although next‐generation ALK inhibitors may alter this treatment paradigm because of their impressive intracranial activity (Table 1). A small phase I/II trial of alectinib consisting of 21 crizotinib‐refractory patients showed a complete response in 29% (6), partial response in 24% (5), stable disease in 38% (8), and progression only in 10% (2) [23]. In a subsequent phase II study, objective responses were observed in 67% of the patients (10 complete responses and two partial responses) with baseline brain metastases who had not received prior CNS directed treatment, 28% with stable disease, and only 6% (1 patient) with CNS progression [13]. Similarly, other second‐generation ALK inhibitors, certinib and brigatinib, have demonstrated intracranial overall RR between 36% to 63% in early phase studies, depending on whether the patient has had prior exposure to an ALK inhibitor [24], [25]. A recent interim analysis of the phase III J‐ALEX trial, where 207 treatment‐naïve, Japanese, ALK‐positive patients were randomized either to receive alectinib or crizotinib in the first‐line setting, showed improved PFS and tolerability with alectinib. The median PFS for crizotinib was 10.2 months (HR 0.34, 99% CI 0.17–0.7, p < .0001) but the PFS has not been reached in the alectinib arm [26]. A global phase III study comparing crizotinib with alectininb in the upfront setting (ALEX) has finished accrual with results expected next year. In this case of NSCLC with diffuse CNS disease and a suspected oncogenic ALK translocation, the molecular tumor board recommended initiating therapy with the second‐generation ALK inhibitor alectinib.
Table 1. Treatment options.
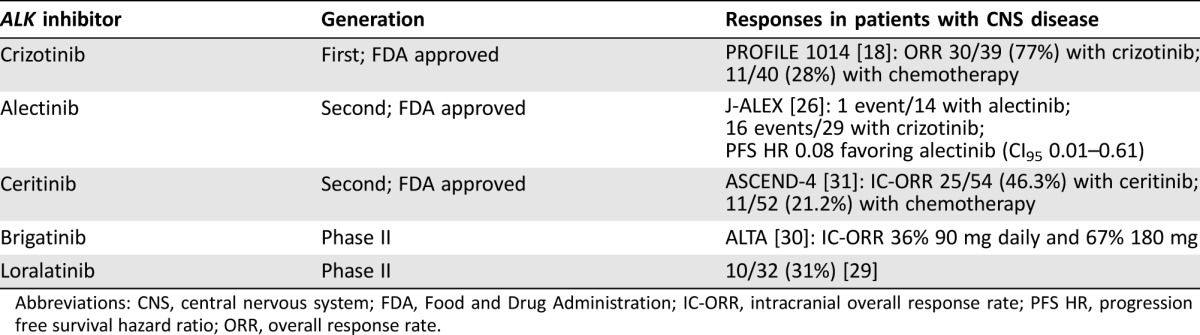
Abbreviations: CNS, central nervous system; FDA, Food and Drug Administration; IC‐ORR, intracranial overall response rate; PFS HR, progression free survival hazard ratio; ORR, overall response rate.
Patient Update
In the case of this 52‐year‐old man with newly diagnosed metastatic ALK‐positive atypical carcinoid tumor, the patient was started on alectinib with rapid shrinkage of his disease after 1 month, notably the brain and choroidal metastases (Fig. 2). He achieved a partial response with approximately 60% shrinkage of his dominant brain metastatic lesion and resolution of many smaller lesions. All the choroidal lesions resolved. The impressive clinical response supports that the novel ALK translocation identified using ctDNA is the driver mutation in this patient's tumor. His disease remains stable after 5 months and the patient continues on alectinib.
Conclusion
Given the dramatic response to alectinib, the question was raised whether to pursue stereotactic radiation to the remaining brain lesions. Evidence on this topic is limited, although case reports have shown an increased risk of radionecrosis with alectinib [27]. Therefore, we recommended expectant management with close surveillance. At progression, efforts will be made to identify resistance mechanisms via direct biopsy of accessible sites of progression or by utilization of ctDNA. Treatment options directed at CNS progression include alectinib dose escalation (from 600 mg p.o. b.i.d to 900 mg p.o. b.i.d) to re‐induce CNS response versus the third‐generation ALK inhibitor lorlatinib, which has shown high CNS penetration and response in a heavily pre‐treated patient cohort [28], [29]. Therapies for systemic progression include chemotherapy such as platinum and pemetrexed combination versus lorlatinib.
Glossary of Genomic Terms and Nomenclature
Circulating tumor cells: tumor cells identified circulating in the patient's blood thought to be derived from tumor shedding
Circulating tumor DNA: tumor DNA identified in the patient's blood thought to be released upon tumor cell death
Hybrid capture‐based comprehensive genomic profiling: method of sequencing DNA using probes to pull down exons and fusions of interest
Indel: insertions or deletions of bases in the DNA
Median exon coverage: average number of times an exon is read during sequencing
Pair‐end sequencing: sequencing from both ends of a DNA as opposed to just from one end (single‐end)
Substitution: a DNA mutation that exchanges one base for another
Target depth correction: an error‐correction process where fragment barcodes are employed to identify polymerase chain reaction duplicates of unique ctDNA molecules and merge them into a single consensus read
Translocation or rearrangement: a chromosome abnormality whereby parts of non‐homologous chromosomes join together
Footnotes
Editor's Note: See the related commentary, “ALK Fusion Detection in Circulating Free DNA: Finding an Important Needle in the Haystack,” by Meghan J. Mooradian and Justin F. Gainor on pages 759–761 of this issue.
Author Contributions
Conception/Design: Victoria Wang
Provision of study material or patients: Victoria Wang, Anatoly Urisman, John Wolfe, Bertil Damato, Shannon Fogh, Emily K. Bergsland
Collection and/or assembly of data: Victoria Wang, Lauren Young, Anatoly Urisman, Trever G. Bivona, Bertil Damato, Shannon Fogh, Emily K. Bergsland
Data analysis and interpretation: Victoria Wang, Lauren Young, Siraj Ali, Vincent A. Miller, Bertil Damato, Shannon Fogh, Emily K. Bergsland
Manuscript writing: Victoria Wang, Lauren Young, Bertil Damato, Shannon Fogh, Emily K. Bergsland
Final approval of manuscript: Victoria Wang, Lauren Young, Anatoly Urisman, John Wolfe, Trever G. Bivona, Bertil Damato, Shannon Fogh, Emily K. Bergsland
Disclosures
Siraj Ali: Foundation Medicine (E, OI); Emily K. Bergsland: Lexicon, Ipsen, Exelexis (C/A), Merck, Lexicon, Novartis (RF), UpToDate (royalties); Vincent A. Miller: Foundation Medicine (E, OI); Trever G. Bivona: Revolution Medicines (RF, C/A), Novartis, AstraZeneca, Array Biopharma (RF), Ignyta (C/A); Lauren Young: Foundation Medicine (E, OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Oxnard GR, Thress KS, Alden RS et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non‐small‐cell lung cancer. J Clin Oncol 2016;34:3375–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pailler E, Adam J, Barthélémy A et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK‐positive non‐small‐cell lung cancer. J Clin Oncol 2013;31,2273.–. [DOI] [PubMed] [Google Scholar]
- 3. Soda M, Takada S, Takeuchi K et al. A mouse model for EML4‐ALK‐positive lung cancer. Proc Natl Acad Sci USA 2008;105:19893.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soda M, Choi YL, Enomoto M et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature 2007;448:561.–. [DOI] [PubMed] [Google Scholar]
- 5. Choi YL, Takeuchi K, Soda M et al. Identification of novel isoforms of the EML4‐ALK transforming gene in non‐small cell lung cancer. Cancer Res 2008;68:4971.–. [DOI] [PubMed] [Google Scholar]
- 6. Takeuchi K, Choi Yl, Togashi Y et al. KIF5B‐ALK, a novel fusion oncokinase identified by an immunohistochemistry‐based diagnostic system for ALK‐positive lung cancer. Clin Cancer Res 2009;15:3143.–. [DOI] [PubMed] [Google Scholar]
- 7. Simonitsch I, Polgar D, Hajek M et al. The cytoplasmic truncated receptor tyrosine kinase ALK homodimer immortalizes and cooperates with ras in cellular transformation. FASEB J 2001;15:1416.–. [DOI] [PubMed] [Google Scholar]
- 8. Wiesner T, Lee W, Obenauf AC et al. Alternative transcription initiation leads to expression of a novel ALK isoform in cancer. Nature 2015;526:453.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shaw, A. T. et al. Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol 2009;27:4247.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kwak EL, Bang YJ, Camidge DR et al. Anaplastic lymphoma kinase inhibition in non‐small‐cell lung cancer. N Engl J Med 2010;363:1693.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friboulet L, Li N, Katayama R et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non‐small cell lung cancer. Cancer Discov 2014;4:662.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ou SH, Ahn JS, De Petris L et al. Alectinib in Crizotinib‐Refractory ALK‐Rearranged Non‐Small‐Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661.–. [DOI] [PubMed] [Google Scholar]
- 13. Shaw AT, Gandhi L, Gadgeel S et al. Alectinib in ALK‐positive, crizotinib‐resistant, non‐small‐cell lung cancer: A single‐group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaw AT, Engelman JA. Ceritinib in ALK‐rearranged non‐small‐cell lung cancer. N Engl J Med 2014;370:1189.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaw AT, Friboulet L, Leshchiner I et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N Engl J Med 2016;374:54.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang S, Anjum R, Squillace R et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first‐ and second‐generation ALK inhibitors in preclinical models. Clin Cancer Res 2016;22:5527–5538. [DOI] [PubMed] [Google Scholar]
- 17. Solomon BJ, Mok T, Kim DW et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med 2014;371:2167.–. [DOI] [PubMed] [Google Scholar]
- 18. Solomon BJ, Cappuzzo F, Felip E et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK‐positive non‐small‐cell lung cancer: Results from PROFILE 1014. J Clin Oncol 2016;34:2858.–. [DOI] [PubMed] [Google Scholar]
- 19. Johung KL, Yeh N, Desai NB et al. Extended survival and prognostic factors for patients with ALK‐rearranged non‐small‐cell lung cancer and brain metastasis. J Clin Oncol 2016;34:123.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rusthoven CG, Doebele RC. Management of brain metastases in ALK‐positive non‐small‐cell lung cancer. J Clin Oncol 2016;34:2814.–. [DOI] [PubMed] [Google Scholar]
- 21. Shaw AT, Yeap BY, Soloman BJ et al. Effect of crizotinib on overall survival in patients with advanced non‐small‐cell lung cancer harbouring ALK gene rearrangement: A retrospective analysis. Lancet Oncol 2011;12:1004.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costa DB, Kobayashi S, Pandya SS et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443.–. [DOI] [PubMed] [Google Scholar]
- 23. Gadgeel SM, Gandhi L, Riely GJ et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib‐resistant ALK‐rearranged non‐small‐cell lung cancer (AF‐002JG): Results from the dose‐finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119.–. [DOI] [PubMed] [Google Scholar]
- 24. Kim DW, Mehra R, Tan DSW et al. Activity and safety of ceritinib in patients with ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐1): Updated results from the multicentre, open‐label, phase 1 trial. Lancet Oncol 2016;17:452.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camidge DR, Bazhenova L, Salgia R et al. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non‐small cell lung cancer (NSCLC). J Clin Oncol 2015;33:8062a. [Google Scholar]
- 26. Nokihara H, Hida T, Kondo M et al. Alectinib (ALC) versus crizotinib (CRZ) in ALK‐inhibitor naive ALK‐positive non‐small cell lung cancer (ALK+ NSCLC): Primary results from the J‐ALEX study. J Clin Oncol 2016;34:9008a. [Google Scholar]
- 27. Ou SH, Weitz M, Jalas JR et al. Alectinib induced CNS radiation necrosis in an ALK+NSCLC patient with a remote (7 years) history of brain radiation. Lung Cancer 2016;96:15.–. [DOI] [PubMed] [Google Scholar]
- 28. Gainor JF, Chi AS, Logan J et al. Alectinib dose escalation reinduces central nervous system responses in patients with anaplastic lymphoma kinase‐positive non‐small cell lung cancer relapsing on standard dose alectinib. J Thorac Oncol 2016;11:256.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solomon BJ, Bauer TM, Felip E et al. Safety and efficacy of lorlatinib (PF‐06463922) from the dose‐escalation component of a study in patients with advanced ALK+ or ROS1+ non‐small cell lung cancer (NSCLC). J Clin Oncol 2016;34:9009a. [Google Scholar]
- 30. Gettinger SN, Bazhenova LA, Langer CJ et al. Activity and safety of brigatinib in ALK‐rearranged non‐small‐cell lung cancer and other malignancies: A single‐arm, open‐label, phase 1/2 trial. Lancet Oncol 2016;17:1683.–. [DOI] [PubMed] [Google Scholar]
- 31. Soria JC, Tan DS, Chiari R et al. First‐line ceritinib versus platinum‐based chemotherapy in advanced ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐4): A randomised, open‐label, phase 3 study. Lancet 2017;4:917–929. [DOI] [PubMed] [Google Scholar]