Abstract
Lessons Learned.
The combination of everolimus and low‐dose prednisone administered daily was hypothesized to prevent noninfectious pneumonitis (NIP) and mucositis, two common adverse events related to everolimus. Although mucositis was detected in only one case, all‐grade NIP occurred in four of eight cases (50%), and this was considered enough to stop accrual of the study.
These data suggest the need for careful monitoring of patients receiving everolimus who are treated with corticosteroids.
Background.
Everolimus is standard of care in the treatment of patients affected by metastatic renal cell carcinoma (mRCC) that has progressed after at least one previous line of treatment. Stomatitis and noninfectious pneumonitis (NIP) are common adverse events (AEs) in patients treated with everolimus. Prednisone could reduce the incidence of stomatitis, and it is commonly used to treat NIP. We hypothesized that low doses of prednisone could reduce the incidence and/or the severity of everolimus‐induced NIP and stomatitis.
Methods.
We have conducted an open‐label, single‐arm, phase II trial of prednisone 5 mg b.i.d. added to everolimus 10 mg/day in patients with mRCC. We planned to evaluate the safety, tolerability, and activity of this combination in mRCC patients. We aimed to reduce incidence of drug discontinuations due to stomatitis or NIP from 25% to 10%.
Results.
Three (38%) of the first eight patients enrolled experienced grade ≥2 pneumonitis and stopped treatment. Grade 1 stomatitis occurred in only one patient (13%). Five of eight patients experienced disease progression at the 2‐month evaluation. Two patients (25%) were reported free of disease progression at 1 year of treatment.
Conclusion.
The incidence of NIP in these patients was considered too high for completing accrual of this study. These results may be of interest for investigating the pathogenesis of NIP and suggest that patients should be carefully followed if treated with chronic corticosteroids while receiving everolimus.
Abstract
经验总结
• 假设依维莫司联合低剂量泼尼松每日给药可预防与依维莫司相关的两种常见不良事件, 即非感染性肺炎(NIP)和黏膜炎。虽然仅在单个病例中观察到黏膜炎, 但8例病例中有4例(50%)出现了各种级别的NIP, 认为足以根据该结果停止研究招募。
• 这些数据表明, 需要密切监测使用皮质类固醇的依维莫司治疗患者。
摘要
背景. 依维莫司是既往至少接受一线治疗后疾病进展的转移性肾细胞癌(mRCC)患者的标准治疗药物。口腔炎和非感染性肺炎(NIP)是依维莫司治疗患者中的常见不良事件(AE)。泼尼松可降低口腔炎的发生率, 并且其也常用于治疗NIP。我们假设低剂量泼尼松可降低依维莫司引起的NIP和口腔炎的发生率和/或严重程度。
方法. 我们进行了一项开放性、单臂、II期临床试验, mRCC患者在试验中接受泼尼松5mg b.i.d与依维莫司10mg/日联合治疗。计划在mRCC患者中评价这一联合治疗方案的安全性、耐受性和疗效。本试验的目标是将口腔炎或NIP导致的停药率从25%降至10%。
结果. 最先入组的8例患者中有3例(38%)出现≥2级肺炎并停止治疗。仅1例患者(13%)出现1级口腔炎。2个月时的评价结果显示, 8例患者中有5例出现疾病进展。报告称治疗1年时2例患者(25%)无疾病进展。
结论. 这8例患者中的NIP发生率过高, 故无法按计划完成研究招募。这些结果对于NIP发病机制的研究可能具有重要意义, 且提示我们应密切随访在依维莫司治疗期间长期使用皮质类固醇的患者。
Discussion
The treatment of metastatic renal cell carcinoma (mRCC) has dramatically changed over the last 10 years with the approval of several drugs that have significantly improved the prognosis of these patients. Everolimus is a mammalian target of rapamycin inhibitor that prolongs progression‐free survival compared to placebo in pretreated patients with mRCC.
Noninfectious pneumonitis (NIP) was reported in up to 19% of patients treated with everolimus in clinical trials; mouth ulcers, stomatitis, and oral mucositis have occurred at an incidence ranging from 44% to 78%. In the literature, grade 2–3 stomatitis and NIP requiring everolimus discontinuation or dose reductions are reported in approximately 25% of cases (15%–16% for stomatitis, 9%–10% for pneumonitis).
Management of NIP consists of dose reduction or drug discontinuation and treatment with corticosteroids. Prednisone has a role in the treatment of recurrent oral aphthae nonresponsive to local corticosteroid therapy.
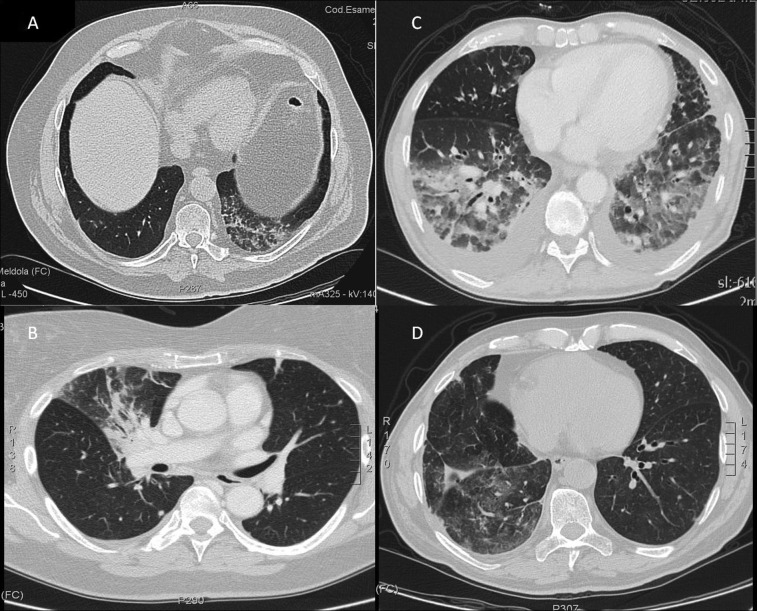
Figure 1.
Radiologic images. (A): Ground glass areas and reticular opacification in the lower left lobe. Asymptomatic patient (grade 1 NIP). (B): Ground glass opacification in the right upper lobe. Patient hospitalized due to severe dyspnea and fever (grade 3 NIP). (C): Consolidations and ground glass opacification in the lower lobes. Pleural efffusion in both lungs. Patient hospitalized with severe dyspnea and desaturation (grade 3 NIP). (D): Pulmonary consolidation with reticular opacifications in the middle lobe of the right lung; patient with fever and dyspnea (grade 2 NIP).
The rationale of our study was to provide evidence‐based literature on the use of oral prednisone 5 mg b.i.d. associated with everolimus 10 mg/day in mRCC to reduce the incidence and/or the severity of everolimus‐induced NIP and stomatitis.
Our study enrolled eight patients who had previously received at least one previous systemic treatment. Five patients were treated in a second‐line setting and three patients were treated in third line.
At first radiological evaluation after 2 months, three of eight patients (38%) developed NIP and discontinued everolimus (one grade 2 and two grade 3). Infective etiologies were excluded. Radiologic images were reviewed by a radiologist expert in lung pathology. Two of these patients had concomitant progressive disease and one had stable disease. All patients were treated with high‐dose corticosteroids and oxygen as needed. In one further case, a routine computed tomography scan showed drug‐related ground‐glass areas in the lung, although the patient was completely asymptomatic.
Grade 1 mucositis was found only in one case (13%). One patient experienced grade 3 diabetes, and three patients had grade 2 asthenia.
Five patients (63%) experienced disease progression at the first radiological evaluation after a median of 2 months. One patient discontinued study treatment due to drug‐related grade 3 NIP after 2 months of treatment, whereas two patients are continuing treatment free of progression after 12+ months. Four patients of the five with disease progression died. Median overall survival was 11.6 months (95% confidence interval 2.7 to not reached).
In conclusion, we stopped the accrual of the study due to a high incidence of NIP with the impossibility to reach the preplanned target to reduce drug discontinuation to 10%. The results of this study suggest a need to better investigate the real pathogenesis of NIP and to carefully monitor for toxicities in patients treated with everolimus and concomitant chronic corticosteroids taken for any clinical reason.
Trial Information
- Disease
Renal cell carcinoma—clear cell
- Stage of disease/treatment
Metastatic/advanced
- Prior therapy
One prior regimen
- Type of study ‐ 1
Phase II
- Type of study ‐ 2
Single arm
- Primary endpoint
Safety
- Secondary endpoint
Tolerability
- Additional details of endpoints or study design
- Incidence of noninfectious pneumonitis and stomatitis is the rate or range of occurrence or influence of these two adverse events related to standard treatment with everolimus. From data reported in literature, the treatment with everolimus induces an incidence of pneumonitis and stomatitis of grade ≥2 of 25%: we assume that the association of everolimus and prednisone will reduce these toxicity events to approximately 10%. Evaluation method: RECIST 1.1.
- Investigator's Analysis
Poorly tolerated/not feasible
Drug Information for Phase II Control Arm
- Drug 1
- Generic/working name
Everolimus
- Trade name
Afinitor
- Company name
Novartis
- Drug type
Biological
- Drug class
mTOR
- Dose
10 milligrams (mg) per flat dose
- Route
Oral (PO)
- Schedule of administration
Everolimus 10mg daily, oral administration
- Drug 2
- Generic/working name
Prednisone
- Drug type
Corticosteroid
- Dose
5mg b.i.d. milligrams (mg) per flat dose
- Route
Oral (PO)
- Schedule of administration
Prednisone 5mg b.i.d., oral administration
Patient Characteristics for Phase II Control Arm
- Patient Characteristics for Phase II Control Arm
- Number of patients, male
6
- Number of patients, female
2
- Stage
Metastatic renal cell carcinoma, stage IV
- Age
Median (range): 44–77
- Number of prior systemic therapies
Median (range): 2–3
- Performance status: ECOG
0 — 7
1 — 1
2 — 0
3 — 0
unknown —
- Cancer types or histologic subtypes
Clear cell carcinoma, 8
Primary Assessment Method for Phase II Control Arm
- Assessment
- Number of patients screened
10
- Number of patients enrolled
8
- Number of patients evaluable for toxicity
8
- Number of patients evaluated for efficacy
8
- Evaluation method
RECIST 1.1
- Response assessment CR
n = 0
- Response assessment PR
n = 0
- Response assessment SD
n = 2
- Response assessment PD
n = 6
- Response assessment OTHER
n = 0
- (Median) duration assessments PFS
2 months, 95% CI: 1.8 to not reached
- (Median) duration assessments OS
12 months, 95% CI: 1.8 to not reached
Adverse Events: Phase II Control Arm
Maximum Common Terminology Criteria for Adverse Events grade adverse events reported during the treatment with everolimus plus prednisone.
Abbreviations: NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated reason
Toxicity
- Pharmacokinetics/Pharmacodynamics
Not collected
- Investigator's Assessment
Poorly tolerated/not feasible
The treatment of metastatic renal cell carcinoma (mRCC) has dramatically changed over the last 10 years with the approval of several drugs that have significantly improved the prognosis of these patients. Most of them are tyrosine kinases inhibitors (TKIs), such as sunitinib, pazopanib, axitinib, sorafenib, cabozantinib, and lenvatinib. Some of them are mammalian target of rapamycin (mTOR) inhibitors, such as everolimus and temsirolimus, and a newly approved agent, nivolumab, is an immune checkpoint inhibitor [1], [2], [3], [4], [5], [6], [7], [8], [9].
Everolimus is an mTOR inhibitor that prolongs progression‐free survival compared with placebo in pretreated patients with mRCC [7]; it is actually also approved for treatment of advanced hormone receptor‐positive, human epidermal growth receptor 2‐negative breast cancer, along with exemestane, in postmenopausal women and for treatment of advanced gastrointestinal, pancreatic, and lung neuroendocrine tumors [10], [11], [12].
Noninfectious pneumonitis (NIP) is a nonmalignant infiltration of the lungs and is a class effect of mTOR inhibitors such as everolimus and temsirolimus. In the investigator brochure, NIP was reported in up to 19% of patients treated with everolimus [13]. In a phase III study by Motzer et al., NIP was reported in 14% of patients, but a reanalysis of this study's chest imaging reported clinical pneumonitis in 13.5% but also radiological evidence of pneumonitis in 39% of patients on everolimus [14], [15]. The mechanism by which mTOR inhibitors induce NIP is not well understood.
Clinical findings of NIP are quite varied. Patients may be completely asymptomatic or have nonspecific respiratory symptoms, such as cough, dyspnea, and hypoxia. Fever may be present, making differential diagnosis from bacterial pneumonia even more difficult. The most typical radiographic changes observed with mTOR inhibitor‐associated pneumonitis are ground‐glass areas and focal consolidations [16].
Stomatitis is an inflammation of mucosal surfaces of the oral cavity, often associated with erythema, edema, burning sensation, and occasionally bleeding [17]. In the RECORD‐1 trial, the incidence of stomatitis in the everolimus arm was 42%, and 3% of cases were grade 3. The investigator brochure reports that mouth ulcers, stomatitis, and oral mucositis have occurred at an incidence ranging from 44% to 78% of patients treated with everolimus [13].
In the literature, grade 2–3 stomatitis and NIP requiring everolimus discontinuation or dose reduction are reported in approximately 25% of cases (15%–16% for stomatitis, 9%–10% for pneumonitis) [13].
Management of NIP consists of dose reduction or drug discontinuation and the use of corticosteroids. Prednisone has a role in the treatment of recurrent oral aphthae nonresponsive to local corticosteroid therapy [18], [19].
Prednisone at low doses is considered a week inducer of CYP3A4; however, no data on the influence of prednisone on everolimus trough levels are available [13].
We designed a proof‐of principle open‐label, single‐arm, phase II trial of prednisone 5 mg b.i.d. added to everolimus 10 mg/day in patients with mRCC who had disease progression following at least one prior vascular endothelial growth factor receptor TKI. The study was designed to enroll 42 patients. The rationale for our study was to provide evidence‐based literature on the use of prednisone associated with everolimus in mRCC to reduce the incidence and/or the severity of everolimus‐induced NIP and stomatitis. As a parameter of efficacy, we sought a reduction in the incidence of NIP to 10% due to the combination of everolimus and prednisone.
Our study enrolled eight patients treated with everolimus for mRCC pretreated with at least one previous systemic treatment. All patients were treated for stage IV renal cell carcinoma. Histology was clear cell carcinoma for all patients. Five patients were treated with everolimus after one previous TKI (sunitinib in four cases and pazopanib in one case), and three patients were treated in third line after two previous lines (sunitinib‐axitinib, sunitinib‐cabozantinib, and pazopanib‐nivolumab). Patients were treated according to clinical practice until disease progression or unacceptable toxicity occurred. Compliance to the treatment was optimal. Toxicities were evaluated and registered according to the National Cancer Institute Common Toxicity Criteria version 4.0.
At first radiological evaluation after a median of 56 days (range 53–58), three of eight patients (38%) developed NIP and discontinued everolimus (one grade 2 and two grade 3). Infective etiologies were excluded. Radiologic images were reviewed by a radiologist expert in lung pathology. Two of these patients had concomitant progressive disease and one had stable disease. All patients were treated with high‐dose corticosteroids and oxygen as needed. In one further case, a routine computed tomography scan showed drug‐related ground‐glass areas, but the patient was completely asymptomatic, so no specific treatment was administered. This patient underwent disease progression at the first evaluation, and treatment was discontinued.
Grade 1 mucositis was found only in one case (13%), and it was resolved in few days without additional treatments. Regarding other toxicities, one patient experienced grade 3 diabetes, and three patients experience grade 2 fatigue.
Five patients (63%) experienced disease progression at the first radiological evaluation after a median of 2 months. One patient discontinued study treatment due to drug‐related grade 3 NIP after 2 months of treatment with stable disease, whereas two patients are still continuing treatment free of progression after 12+ months. Four patients among the five with disease progression have died. Median overall survival is 11.6 months (95% confidence interval 2.7 to not reached).
The study was stopped due to a high incidence of NIP with the impossibility to reach the preplanned target to reduce drug discontinuation to 10%.
In conclusion, the results of this study suggest better investigating the real pathogenesis of NIP and carefully monitoring for toxicities in patients treated with everolimus and concomitant chronic corticosteroids taken for any clinical reason. The low incidence of mucositis may suggest a protective role of systemic corticosteroids, but further studies are necessary.
Footnotes
ClinicalTrials.gov Identifier: NCT02479490
Sponsor(s): Istituto Scientifico Romagnolo per lo Studio e la cura dei Tumori
Principal Investigator: Ugo De Giorgi
IRB Approved: Yes
Disclosures
Ugo De Giorgi: Bristol‐Meyers Squibb, Pfizer, Pierre‐Fabre, Astellas, Sanofi (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Motzer RJ, Hutson TE, Tomczak P et al., Sunitinib versus interferon alfa in metastatic renal‐cell carcinoma. N Engl J Med 2007;356:115–124. [DOI] [PubMed] [Google Scholar]
- 2. Sternberg CN, Davis ID, Mardiak J et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol 2010;28:1061–1068. [DOI] [PubMed] [Google Scholar]
- 3. Rini BI, Escudier B, Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 4. Escudier B, Eisen T, Stadler WM et al. Sorafenib in advanced clear‐cell renal‐cell carcinoma. N Engl J Med 2007;356:125–134. [DOI] [PubMed] [Google Scholar]
- 5. Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Motzer RJ, Hutson TE, Glen H et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open‐label, multicentre trial. Lancet Oncol 2015;16:1473–1482. [DOI] [PubMed] [Google Scholar]
- 7. Motzer RJ, Escudier B, Oudard S et al. Efficacy of everolimus in advanced renal cell carcinoma: A double‐blind, randomised, placebo‐controlled phase III trial. Lancet 2008;372:449–456. [DOI] [PubMed] [Google Scholar]
- 8. Hudes G, Carducci M, Tomczak P et al. Temsirolimus, interferon alfa, or both for advanced renal‐cell carcinoma. N Engl J Med 2007;356:2271–2281. [DOI] [PubMed] [Google Scholar]
- 9. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baselga J, Campone M, Piccart M et al. Everolimus in postmenopausal hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2012;366:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yao JC, Shah MH, Ito T et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao JC, Fazio N, Singh S et al. Everolimus for the treatment of advanced, non‐functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT‐4): A randomised, placebo‐controlled, phase 3 study. Lancet 2016;387:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everolimus Investigator Brochure, Novartis, January 2015. version.
- 14. Motzer RJ, Escudier B, Oudard S et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors. Cancer 2010;116:4256–4265. [DOI] [PubMed] [Google Scholar]
- 15. White DA, Camus P, Endo M et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med 2010;182:396–403. [DOI] [PubMed] [Google Scholar]
- 16. Duran I, Siu LL, Oza AM et al. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer 2006;42:1875–1880. [DOI] [PubMed] [Google Scholar]
- 17. Köstler WJ, Hejna M, Wenzel WC et al. Oral mucositis complicating chemotherapy and/or radiotherapy: Options for prevention and treatment. CA Cancer J Clin 2001;51:290–315. [DOI] [PubMed] [Google Scholar]
- 18. Femiano F, Buonaiuto C, Gombos F et al. Pilot study on recurrent aphthous stomatitis (RAS): A randomized placebo‐controlled trial for the comparative therapeutic effects of systemic prednisone and systemic montelukast in subjects unresponsive to topical therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:402–407. [DOI] [PubMed] [Google Scholar]
- 19. Femiano F, Gombos F, Scully C. Recurrent aphthous stomatitis unresponsive to topical corticosteroids: A study of the comparative therapeutic effects of systemic prednisone and systemic sulodexide. Int J Dermatol 2003;42:394–397. [DOI] [PubMed] [Google Scholar]