This article summarizes the evidence for the impact of BRAF mutations on treatment outcome of anti‐EGFR monoclonal antibodies. Based on a review of literature, eight meta‐analyses were included in this study, which consistently show that patients with BRAF mutations have a lack of treatment benefit of anti‐EGFR monoclonal antibodies. Considering the quality and quantity of available evidence, current guidelines may be revised.
Keywords: Pembrolizumab, Squamous cell carcinoma of the head and neck, U.S. Food and Drug Administration, Programmed cell death 1 receptor, Immunotherapy
Abstract
On August 5, 2016, the U.S. Food and Drug Administration granted accelerated approval to pembrolizumab (KEYTRUDA injection, Merck Sharp & Dohme Corp., Kenilworth, NJ) for treatment of patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) with disease progression on or after platinum‐containing chemotherapy. Approval was based on the objective response rate (ORR) and duration of response (DoR) in a cohort of patients in a nonrandomized multi‐cohort trial (KEYNOTE‐012) that included 174 patients with recurrent or metastatic HNSCC who had disease progression on or after platinum‐containing chemotherapy. Patients received either intravenous pembrolizumab 10 mg/kg every 2 weeks or 200 mg every 3 weeks. ORR was determined by independent review according to Response Evaluation Criteria in Solid Tumors 1.1. ORR was 16% (95% confidence interval 11, 22) with a complete response rate of 5%. DoR ranged from 2.4+ months to 27.7+ months. Twenty‐three of 28 responding patients (82%) had response durations of ≥6 months. Safety was evaluated in 192 patients with HNSCC receiving at least one dose of pembrolizumab. Frequent (≥2%) serious adverse reactions were pneumonia, dyspnea, confusional state, vomiting, pleural effusion, and respiratory failure. Clinically significant immune‐mediated adverse reactions included pneumonitis, colitis, hepatitis, adrenal insufficiency, diabetes mellitus, skin toxicity, myositis, and thyroid disorders. The benefit‐risk profile of pembrolizumab was considered acceptable in this patient population. As a condition of accelerated approval, Merck is required to conduct a confirmatory trial; this trial, KEYNOTE‐040, is ongoing.
Implications for Practice.
This accelerated approval expands the U.S. Food and Drug Administration‐approved indications for pembrolizumab, providing health care providers with new information regarding pembrolizumab for the treatment of patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) with disease progression on or after platinum‐containing chemotherapy. Pembrolizumab is the first drug to receive approval for treatment of patients with HNSCC since cetuximab was approved for this indication in 2006.
Introduction
Recurrent, unresectable locally advanced or metastatic head and neck squamous cell carcinoma (HNSCC) is a serious and life‐threatening disease. Squamous cell cancers of the head and neck are heterogeneous tumors with variable prognosis depending on the site of origin. The Surveillance, Epidemiology, and End Results Program database reports statistics for two broad categories relevant to HNSCC: (a) oral cavity and pharynx and (b) larynx. Of the cancers arising in these areas, 90%–95% are squamous cell carcinoma. It is estimated there will be 48,330 new cases of oral cavity and pharyngeal cancer and 13,430 cases of laryngeal cancer diagnosed in the U.S. in 2016, with approximately 13,190 deaths due to these cancers [1], [2]. This represents approximately 2% of all cancer deaths in the U.S.
At the time of initial diagnosis, distant metastases are present in approximately 18% of patients with oral cavity/pharyngeal cancer [1] and 19% of patients with laryngeal cancer [2]. In addition, regional lymph node involvement (without distant metastasis) is present in approximately 47% of patients with oral cavity/pharyngeal cancer [1] and 22% of patients with laryngeal cancer [2] at the time of initial diagnosis; for patients with such locally advanced disease, 20%–30% will recur locally, while another 10%–15% can be expected to develop distant metastases. Median survival for patients with recurrent or metastatic HNSCC in most clinical series is 6–10 months.
Standard treatment for locally advanced HNSCC includes platinum‐containing chemotherapy given in conjunction with radiation (e.g., as induction therapy, as concurrent therapy with radiation, or as part of adjuvant therapy with radiation following surgical resection). First‐line chemotherapy for metastatic HNSCC consists of a multi‐agent platinum‐containing chemotherapy regimen, such as cisplatin or carboplatin plus 5‐fluorouracil plus cetuximab [3]. The only drug specifically approved by the U.S. Food and Drug Administration (FDA) for the treatment of HNSCC that has progressed following a platinum‐containing regimen is cetuximab, which was approved in 2006 based on an objective response rate (ORR) of 13% (95% confidence interval [CI]: 7, 21), with a median duration of response (DoR) of 5.8 months (range 1.2–5.8 months) [4]. The FDA approved methotrexate for the treatment of HNSCC in the 1950s, prior to the widespread use of cisplatin for this disease. Methotrexate is still used in the treatment of patients with recurrent or metastatic HNSCC who have progressed on platinum‐based therapy. Contemporary data indicate ORRs in the range of 5% when methotrexate is used in this setting [5], [6].
While docetaxel is FDA‐approved for the treatment of locally advanced HNSCC as induction therapy in combination with cisplatin and fluorouracil, both docetaxel and paclitaxel are commonly used in the U.S. for the treatment of patients with recurrent or metastatic HNSCC who have progression on or after platinum‐containing chemotherapy. For reference, a single‐arm trial of docetaxel in 23 patients with recurrent or metastatic HNSCC who had progressive disease following treatment with platinum‐based chemotherapy reported an ORR of 13% (95% CI: 8, 26) and median DOR of 4.4 months (range 1.8–5.5 months) [7].
Pembrolizumab is a humanized monoclonal antibody that binds to the programmed death receptor‐1 (PD‐1) and blocks its interaction with programmed death‐ligand 1 (PD‐L1) and programmed death‐ligand 2, releasing PD‐1 pathway‐mediated inhibition of the immune response, including the antitumor immune response. The FDA approved pembrolizumab for the treatment of patients with unresectable or metastatic melanoma, for the first‐line treatment of patients with metastatic non‐small cell lung cancer (NSCLC) whose tumors have high PD‐L1 expression (Tumor Proportion Score [TPS] ≥50%) as determined by an FDA‐approved test, and for the treatment of patients with metastatic NSCLC whose tumors express PD‐L1 (TPS ≥1%) as determined by an FDA‐approved test, with disease progression on or after platinum‐containing chemotherapy. The primary basis of the supplemental biologics license application (sBLA) for pembrolizumab for treatment of recurrent or metastatic HNSCC was the KEYNOTE‐012 trial.
Materials and Methods
Trial Design
KEYNOTE‐012 (NCT01848834) was a multicenter, nonrandomized, open‐label, activity‐estimating trial that included several disease‐specific cohorts. Cohorts B and B2 of KEYNOTE‐012 enrolled 192 patients with recurrent or metastatic HNSCC. The efficacy population supporting this supplement was limited to 174 patients from Cohorts B and B2 with recurrent or metastatic HNSCC who had disease progression on or after platinum‐containing chemotherapy administered for recurrent or metastatic HNSCC or as part of induction, concurrent, or adjuvant therapy for HNSCC. This led to the exclusion of 18 patients who had never received platinum‐containing chemotherapy. The trial excluded patients with active autoimmune disease or a history of severe autoimmune disease, a medical condition requiring immunosuppression, evidence of interstitial lung disease, or Eastern Cooperative Oncology Group performance status ≥2.
Patients received pembrolizumab either at 10 mg/kg every 2 weeks (Cohort B, n = 60; Cohort B efficacy population, n = 53) or 200 mg every 3 weeks (Cohort B2, n = 132; Cohort B2 efficacy population, n = 121) as a 30‐minute intravenous (IV) infusion until unacceptable toxicity, disease progression, or death. Patients without disease progression were treated for up to 24 months. The major efficacy outcome measures supporting this supplement were ORR according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, as assessed by blinded independent central review, and DoR.
Clinical Pharmacology
The proposed dosage regimen for the HNSCC indication was 200 mg every 3 weeks.
Based on population pharmacokinetic (PK) analysis, exposure using pembrolizumab 200 mg every 3 weeks is approximately 30% higher than with a dosage regimen of 2 mg/kg every 3 weeks. Exposure with the 10 mg/kg every 2 weeks dosage regimen is approximately fourfold higher than exposure using the 200 mg every 3 weeks fixed dose regimen. Only 25% of patients in the KEYNOTE‐012 HNSCC efficacy population had PK data available, so analysis of the exposure‐response relationship in this population was inconclusive. Exploratory dose‐response analyses that pooled data from KEYNOTE‐012 and KEYNOTE‐055 (NCT02255097) suggested a flat dose‐response relationship for ORR in patients with HNSCC for doses of pembrolizumab 200 mg every 3 weeks and pembrolizumab 10 mg/kg every 2 weeks.
Results
Demographic and disease characteristics for the 174 patients included in the efficacy population are presented in Tables 1 and 2. Of the 192 patients with HNSCC enrolled in Cohorts B and B2 of KEYNOTE‐012, 65% had PD‐L1 positive tumors as determined by an investigational assay. Among the 174 patients in the efficacy population, 49% received chemotherapy as part of adjuvant or neoadjuvant therapy, and 85% received prior systemic therapy for recurrent or metastatic disease. The median number of prior lines of therapy administered for the treatment of recurrent or metastatic HNSCC was two. All 26 patients (15%) in the efficacy population who did not receive systemic therapy for recurrent or metastatic disease had received a platinum agent as part of either induction, concurrent, or adjuvant chemotherapy. In addition, 63% of the efficacy population had received cetuximab and 72% had received a taxane.
Table 1. Baseline characteristics for patients in the KEYNOTE‐012 head and neck squamous cell carcinoma efficacy population.
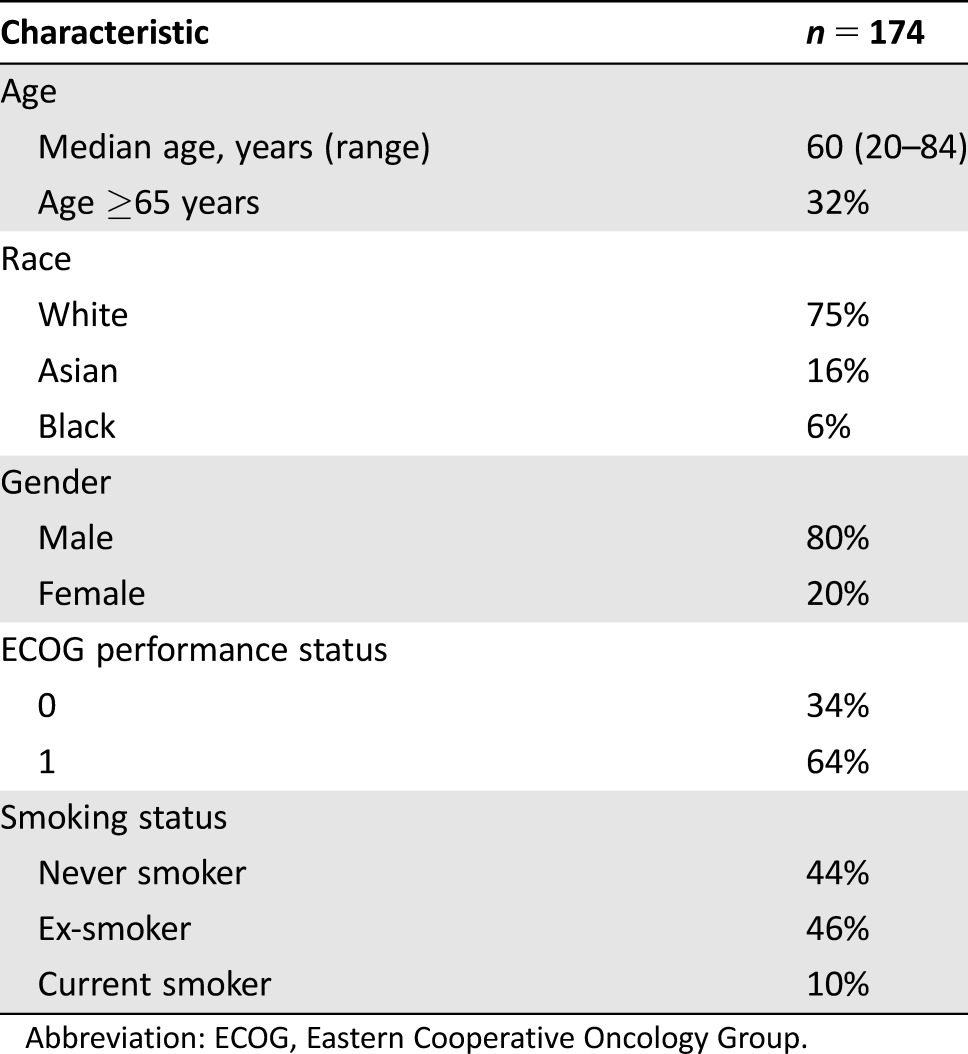
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Table 2. Disease characteristics for patients in the KEYNOTE‐012 head and neck squamous cell carcinoma efficacy population.
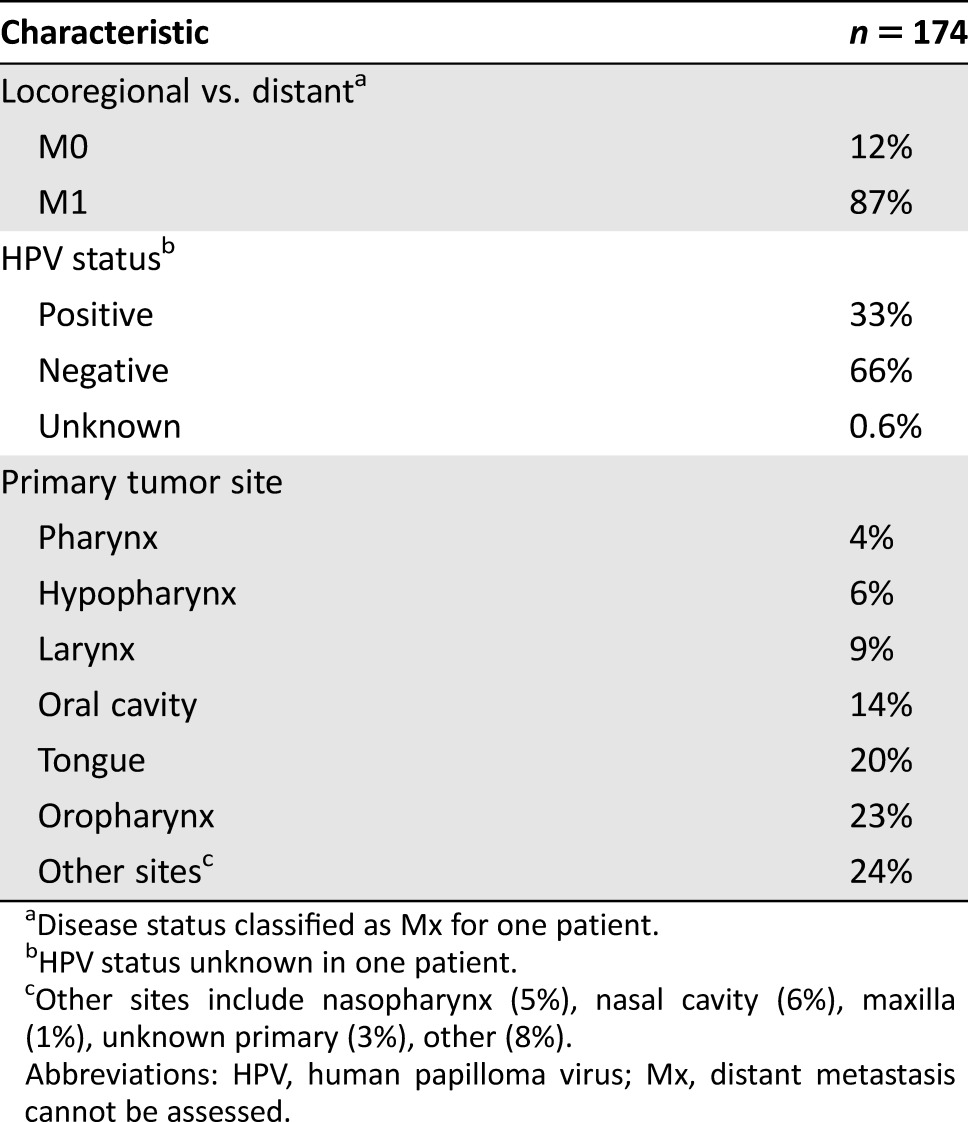
Disease status classified as Mx for one patient.
HPV status unknown in one patient.
Other sites include nasopharynx (5%), nasal cavity (6%), maxilla (1%), unknown primary (3%), other (8%).
Abbreviations: HPV, human papilloma virus; Mx, distant metastasis cannot be assessed.
Efficacy
The ORR was 16% (95% CI: 11, 22) with a complete response rate of 5%. The median DoR had not been reached among the 28 responding patients; DoR ranged from 2.4+ to 27.7+ months (“+” indicates ongoing response; the low end of the range for DoR without an ongoing response was 4.2 months). Among the 28 responders, 23 patients (82%) had responses lasting 6 months or longer. The ORR and DoR appeared similar across subgroups defined by age, gender, race (white vs. non‐white), dosage regimen (10 mg/kg every 2 weeks or 200 mg every 3 weeks), or human papilloma virus status.
The efficacy results in the subset of patients enrolled in KEYNOTE‐012 were supported by an interim analysis of efficacy in the first 50 patients with ≥6 months of follow‐up who were enrolled in another study, KEYNOTE‐055, which enrolled a similar patient population and administered pembrolizumab at a dose of 200 mg every 3 weeks. To be eligible for enrollment in KEYNOTE‐055, patients must have received both a platinum and cetuximab as prior therapy for HNSCC. In this interim analysis, the confirmed ORR as assessed by independent central radiology review according to RECIST 1.1 was 18% (95% CI: 8.6, 31.4). The median DoR was 6.9 months, with response duration ranging from 3.0–8.3+ months. The difference in the estimated median DoR between KEYNOTE‐055 and KEYNOTE‐012 may be attributable to the difference in duration of follow‐up between the trials, with a maximum follow‐up duration of 8.3 months for KEYNOTE‐055 versus 30 months in KEYNOTE‐012.
Safety
The primary safety analysis for this application was based on safety information from all 192 patients enrolled in Cohorts B and B2 of KEYNOTE‐012. Median duration of exposure was 3.3 months (range 1 day to 27.9 months). Of these patients, 132 (69%) received pembrolizumab at a dose of 200 mg every 3 weeks, the proposed recommended dosage regimen for this indication.
The exposure with the 10 mg/kg every 2 weeks dosage regimen was approximately fourfold higher than the exposure with the 200 mg every 3 weeks fixed dose. However, the incidence of adverse reactions, including serious adverse reactions, was similar between dosage regimens. Based on the flat exposure‐toxicity relationship, the safety data from patients receiving these two regimens were pooled. Serious adverse reactions occurred in 45% of HNSCC patients receiving pembrolizumab in KEYNOTE‐012. The most frequent serious adverse reactions (reported in ≥2% of HNSCC patients) were pneumonia (3.6%), dyspnea (3.1%), confusional state (2.6%), vomiting, pleural effusion, and respiratory failure (2.1% each). Pembrolizumab was discontinued due to adverse reactions in 17% of patients. The most common adverse reactions (occurring in ≥20% of patients) were fatigue/asthenia, decreased appetite, and dyspnea.
The risks of pembrolizumab have been well characterized from the results of randomized clinical trials enrolling patients with metastatic melanoma or NSCLC [4]. Adverse reactions, including immune‐mediated adverse reactions, occurring in patients with HNSCC were generally similar to those observed in patients with melanoma or NSCLC treated with pembrolizumab, with the exception of increased incidences of facial edema (10% all grades; 2.1% grades 3–4) and new or worsening hypothyroidism (14.6%) reported in KEYNOTE‐012 (Table 3). Among the 28 patients reported as having new or worsening hypothyroidism, only 15 had no prior history of hypothyroidism.
Table 3. Potentially immune‐mediated adverse events (reviewer table).
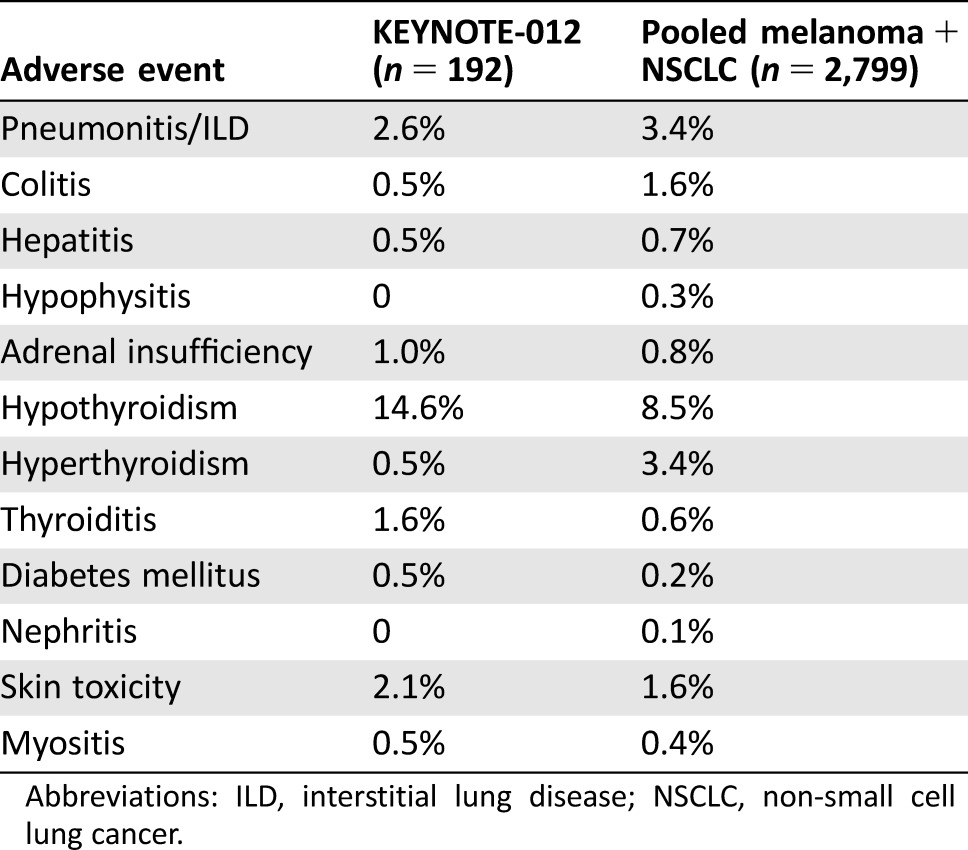
Abbreviations: ILD, interstitial lung disease; NSCLC, non‐small cell lung cancer.
Discussion
On August 5, 2016, the FDA granted pembrolizumab accelerated approval for the treatment of patients with recurrent or metastatic HNSCC with disease progression on or after platinum‐containing chemotherapy. This approval was based on determination of a favorable benefit‐risk profile, which weighted the prolonged DoR in considering the surrogate endpoint of ORR and the safety profile of pembrolizumab observed in patients with recurrent or metastatic HNSCC enrolled in two cohorts in KEYNOTE‐012, a multi‐cohort trial of pembrolizumab (Table 4). Supportive efficacy and safety data came from an analysis of the first 50 patients with ≥6 months of follow‐up treated on KEYNOTE‐055, an ongoing, single‐arm trial of pembrolizumab 200 mg IV every 3 weeks in patients with recurrent metastatic HNSCC with progression following previous treatment with platinum and cetuximab.
Table 4. U.S. Food and Drug Administration benefit‐risk assessment.
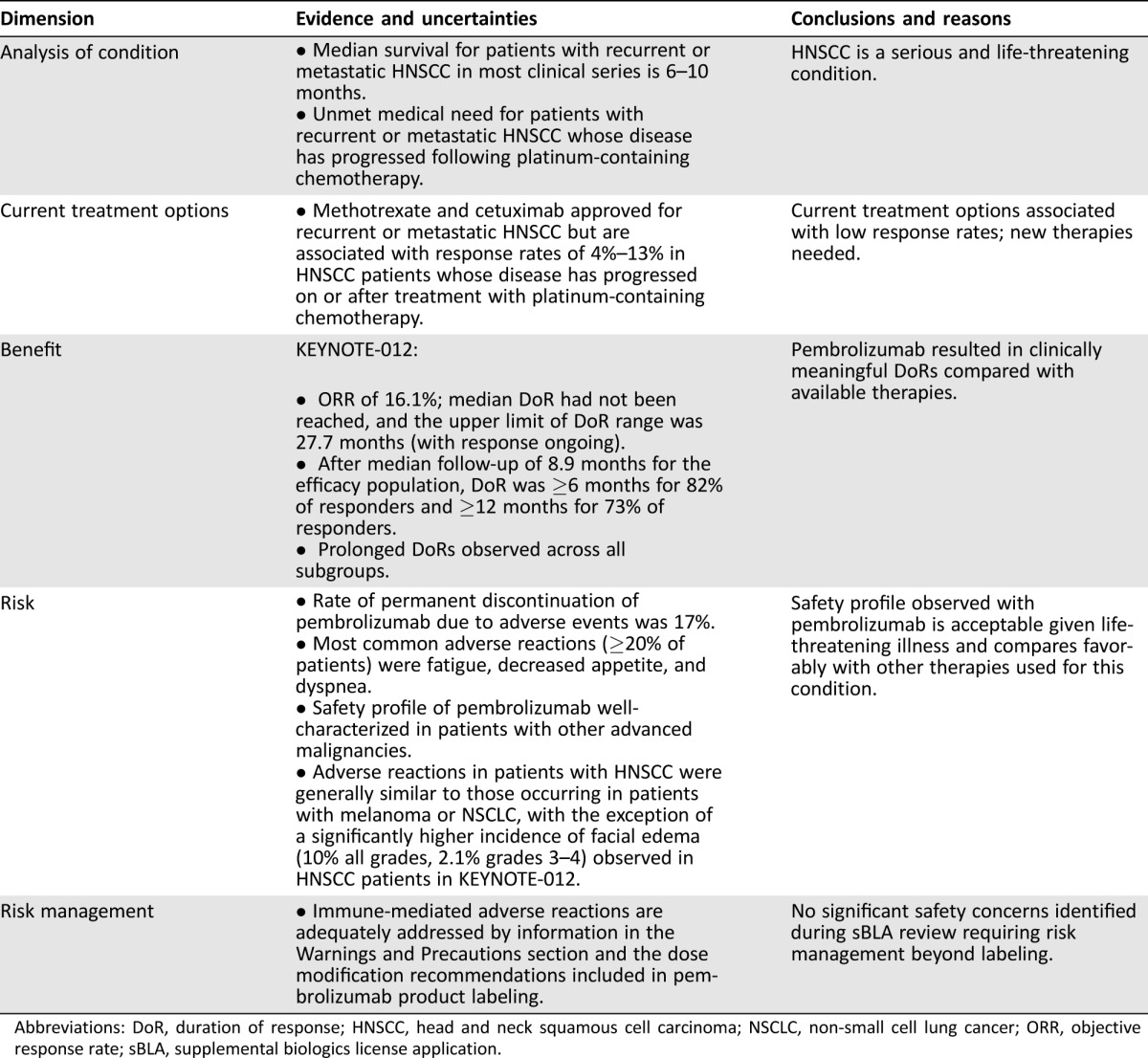
Abbreviations: DoR, duration of response; HNSCC, head and neck squamous cell carcinoma; NSCLC, non‐small cell lung cancer; ORR, objective response rate; sBLA, supplemental biologics license application.
Limitations of trials lacking an internal control arm include the potential for known and unknown patient selection bias, confounding comparisons to historical and external controls, and the lack of controlled safety data. Continued approval for this indication requires verification of clinical benefit in a confirmatory trial. The confirmatory trial, KEYNOTE‐040 (NCT02252042), assessing pembrolizumab versus single agent therapy (i.e., methotrexate, docetaxel, or cetuximab) in a patient population similar to that in the approved indication, is ongoing.
The key issue considered during the review was whether the observed response rate provided a meaningful advantage over available therapy and was likely to predict clinical benefit. The ORR observed in KEYNOTE‐012 patients with recurrent or metastatic HNSCC with disease progression on or after platinum‐containing chemotherapy was 16% with a 95% CI that does not exclude response rates reported with currently available treatments; however, the durability of responses in KEYNOTE‐012 are notable in that 82% of responders had DoR of ≥6 months. Prolonged durations of response were observed across various subgroups of patients. These observed durations of response demonstrated clinically meaningful improvement compared with available therapies in this patient population.
Another major consideration for FDA review of this supplement was whether the clinical pharmacology data were sufficient to support the proposed new dosage regimen of pembrolizumab, 200 mg IV every 3 weeks. Based on FDA Clinical Pharmacology review, this new dosage regimen was supported by population PK analyses using pooled data from KEYNOTE‐012 and KEYNOTE‐055, as well as a larger pooled dataset that also included information from clinical trials in patients with melanoma and NSCLC. The proposed new dosage regimen was determined to be adequately supported by the clinical pharmacology data package, when considered along with the safety and efficacy data for this dosage regimen from KEYNOTE‐012 and the interim analysis of KEYNOTE‐055.
Overall, the safety profile of pembrolizumab appears to be acceptable in this patient population in light of its effects on ORR, specifically the prolonged durations of response observed in KEYNOTE‐012, and due to unsatisfactory alternative therapies for an incurable, life‐threatening disease. As expected, based on the known safety profile of pembrolizumab, immune‐mediated adverse reactions were observed in patients with HNSCC treated with pembrolizumab. There was no suggestion of an increased incidence of these immune‐mediated reactions compared with the incidence observed in the melanoma and NSCLC populations, except in the case of hypothyroidism. Hypothyroidism is a common finding among previously treated patients with advanced HNSCC, largely due to the fact that treatment of locally advanced HNSCC frequently includes radiation therapy targeting the head and neck area. Among the 28 patients from KEYNOTE‐012 with HNSCC and the reported adverse event of hypothyroidism, 27 had received radiation as part of prior therapy for HNSCC.
Other adverse reactions occurring in patients with HNSCC were generally similar to those occurring in patients with other advanced malignancies, specifically melanoma and NSCLC, with the exception of a significantly higher incidence of facial edema (10% in HNSCC from KEYNOTE‐012), which has been a rare event (0.6%) in melanoma and NSCLC studies. The incidence of facial edema (6%) was somewhat lower among the 50 patients from KEYNOTE‐055 with safety data available for review. Facial edema is not an unexpected occurrence in patients with advanced HNSCC, many of whom have persistent tumor in the head and neck area and who may also be predisposed to this event due to prior treatment for HNSCC (i.e., surgery and/or radiation). However, facial edema could potentially be a manifestation of an inflammatory or an immune‐mediated reaction associated with pembrolizumab treatment. Since the available safety data are from single‐arm trials, it is difficult to determine to what extent, if any, facial edema is related to pembrolizumab treatment. The ongoing randomized controlled trials of pembrolizumab in HNSCC will clarify the extent to which pembrolizumab causes facial edema.
Conclusion
This accelerated approval expands the FDA‐approved indications for pembrolizumab and describes the risks and benefits of pembrolizumab for the treatment of patients with recurrent or metastatic HNSCC with disease progression on or after platinum‐containing chemotherapy. The prolonged durations of response observed in the HNSCC population from KEYNOTE‐012 are clinically meaningful when considering the intended patient population and currently available therapies, and the clinical benefits outweigh the risks associated with pembrolizumab identified during the review of this sBLA. KEYNOTE‐040, the ongoing, randomized confirmatory trial, will help to more accurately define the incidence of facial edema and hypothyroidism in patients with previously treated HNSCC associated with pembrolizumab and will allow for direct comparison with a similar patient population being treated with standard single agent therapy.
Footnotes
For Further Reading: Jessica M. Moskovitz, Jennifer Moy, Tanguy Y. Seiwert et al. Immunotherapy for Head and Neck Squamous Cell Carcinoma: A Review of Current and Emerging Therapeutic Options. The Oncologist 2017;22:680‐693.
Implications for Practice: This review article summarizes recently developed agents that harness the immune system to fight head and neck squamous cell carcinoma. A brief review of the immune system and its role in cancer development is included. Recently completed and emerging therapeutic trials centering on the immune system and head and neck cancer are reviewed.
Author Contributions
Conception/design: Erin Larkins, Gideon M. Blumenthal, Kirsten B. Goldberg, Patricia Keegan, Richard Pazdur
Collection and/or assembly of data: Erin Larkins, Gideon M. Blumenthal, Weishi Yuan, Kun He, Rajeshwari Sridhara, Sriram Subramaniam, Hong Zhao, Chao Liu, Jingyu Yu, Amy E. McKee
Data analysis and interpretation: Erin Larkins, Gideon M. Blumenthal, Weishi Yuan, Kun He, Rajeshwari Sridhara, Sriram Subramaniam, Hong Zhao, Chao Liu, Jingyu Yu, Amy E. McKee, Patricia Keegan, Richard Pazdur
Manuscript writing: Erin Larkins, Gideon M. Blumenthal, Kirsten B. Goldberg, Amy E. McKee, Patricia Keegan, Richard Pazdur
Final approval of manuscript: Erin Larkins, Gideon M. Blumenthal, Weishi Yuan, Kun He, Rajeshwari Sridhara, Sriram Subramaniam, Hong Zhao, Chao Liu, Jingyu Yu, Kirsten B. Goldberg, Amy E. McKee, Patricia Keegan, Richard Pazdur
Disclosures
The authors indicated no financial relationships.
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program. SEER stat fact sheets: Oral cavity and pharynx cancer. Available at http://seer.cancer.gov/statfacts/html/oralcav.html. Accessed April 24, 2017.
- 2.Surveillance, Epidemiology, and End Results (SEER) Program. SEER stat fact sheets: Larynx cancer. Available at http://seer.cancer.gov/statfacts/html/laryn.html. Accessed April 24, 2017.
- 3.National Comprehensive Cancer Network. NCCN guidelines: Head and neck cancer, version 1.2016. Available at https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed April 24, 2017.
- 4.USPI cetuximab (revision date 3/2006). Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/125084s046LBL.pdf. Accessed April 24, 2017.
- 5. Machiels JP, Haddad RI, Fayette J et al. Afatinib versus methotrexate as second‐line treatment in patients with recurrent or metastatic squamous‐cell carcinoma of the head and neck progressing on or after platinum‐based therapy (LUX‐Head & Neck 1): An open‐label, randomised phase 3 trial. Lancet Oncol 2015;16:583–594. [DOI] [PubMed] [Google Scholar]
- 6. Stewart JS, Cohen EE, Licitra L et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 2009;27:1864–1871. [DOI] [PubMed] [Google Scholar]
- 7. Cho BC, Keum KC, Shin SJ et al. Weekly docetaxel in patients with platinum‐refractory metastatic or recurrent squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 2009;65:27–32. [DOI] [PubMed] [Google Scholar]


