Abstract
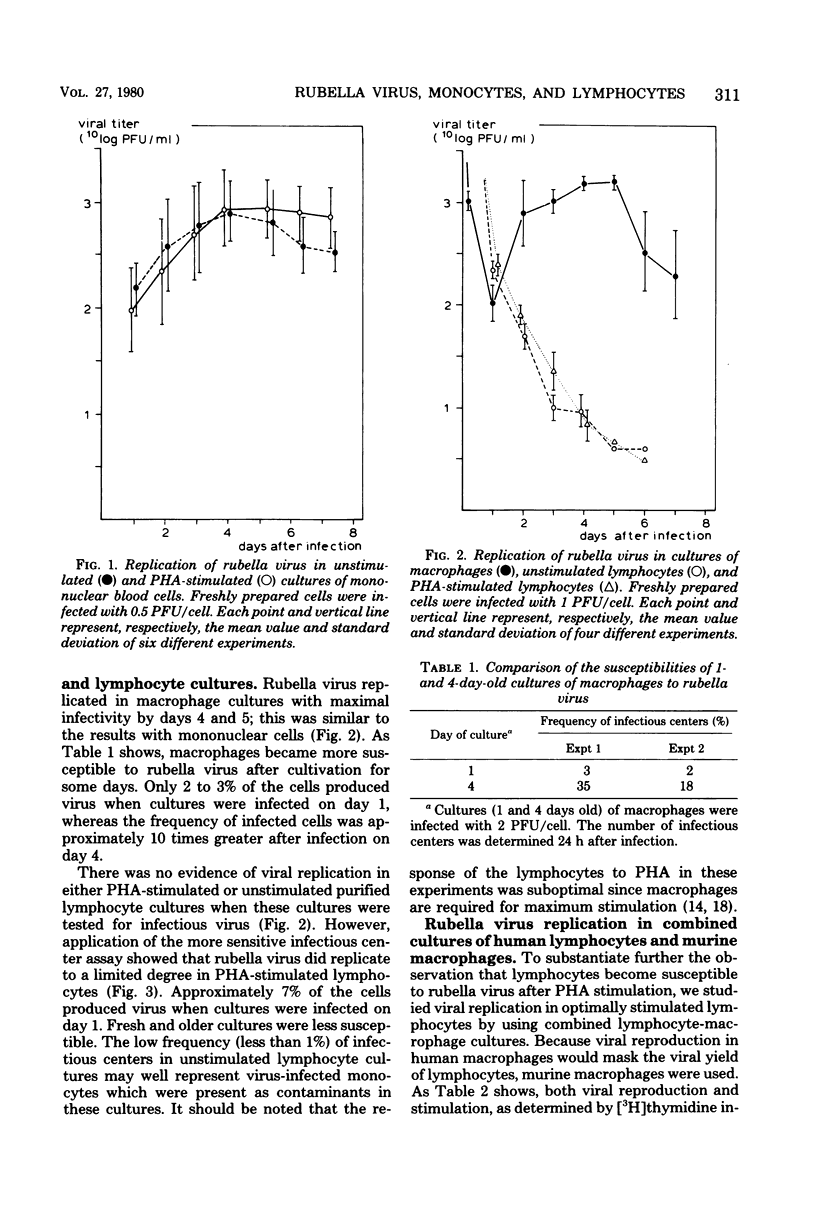
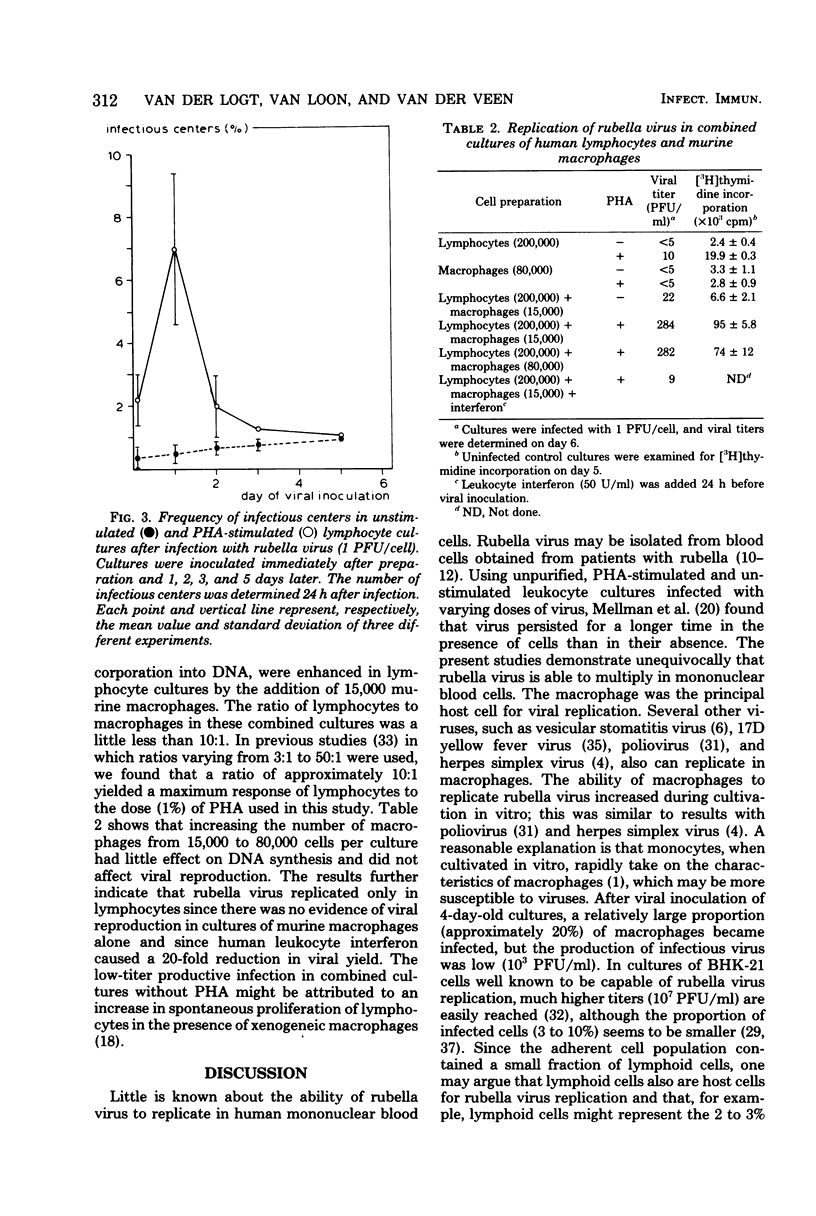
Rubella virus was capable of replicating in both unstimulated and phytohemagglutinin-stimulated cultures of human mononuclear blood cells. Monocyte-derived macrophages were the main cell type responsible for viral replication. The susceptibility of macrophages increased during cultivation. Phytohemagglutinin-stimulated lymphocytes were able to support replication to a limited degree. No viral replication was detected in unstimulated lymphocytes. Both stimulation and viral replication in phytohemagglutinin-treated lymphocyte cultures were enhanced by the addition of murine macrophages. Human leukocyte interferon depressed the production of virus in these combined cultures. The finding that rubella virus is able to replicate in human lymphocytes as well as in macrophages may contribute to understanding the mechanisms of the suppressive effect of the virus on in vitro lymphocyte phytohemagglutinin responsiveness and in vivo immune functions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. A., Kleinerman E. S., Snyderman R. Abortive and productive infections of human mononuclear phagocytes by type I herpes simplex virus. Am J Pathol. 1978 Apr;91(1):119–136. [PMC free article] [PubMed] [Google Scholar]
- Edelman R., Wheelock E. F. Specific role of each human leukocyte type in viral infections. I. Monocyte as host cell for vesicular stomatitis virus replication in vitro. J Virol. 1967 Dec;1(6):1139–1149. doi: 10.1128/jvi.1.6.1139-1149.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman R., Wheelock E. F. Vesicular stomatitis virus replication in human leukocyte cultures: enhancement by phytohemagglutinin. Science. 1966 Nov 25;154(3752):1053–1055. doi: 10.1126/science.154.3752.1053. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Lipsky P. E., Rosenthal A. S. Phytohemagglutinin-induced proliferation of guinea pig thymus-derived lymphocytes. II. Accessory cell function. J Immunol. 1976 Mar;116(3):876–880. [PubMed] [Google Scholar]
- Ganguly R., Cusumano C. L., Waldman R. H. Suppression of cell-mediated immunity after infection with attenuated rubella virus. Infect Immun. 1976 Feb;13(2):464–469. doi: 10.1128/iai.13.2.464-469.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGGIE A. D., ROBBINS F. C. RUBELLA IN NAVAL RECRUITS; A VIROLOGIC STUDY. N Engl J Med. 1964 Jul 30;271:231–234. doi: 10.1056/NEJM196407302710504. [DOI] [PubMed] [Google Scholar]
- Jack I., Grutzner J. Cellular viraemia in babies infected with rubella virus before birth. Br Med J. 1969 Feb 1;1(5639):289–292. doi: 10.1136/bmj.1.5639.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman L. F., Kibrick S., Ennis F., Polgar P. Herpes simplex virus replication in human lymphocyte cultures stimulated with phytomitogens and anti-lymphocyte globulin. Proc Soc Exp Biol Med. 1972 Dec;141(3):1095–1099. doi: 10.3181/00379727-141-36941. [DOI] [PubMed] [Google Scholar]
- Levis W. R., Robbins J. H. Effect of glass-adherent cells on the blastogenic response of 'purified' lymphocytes to phytohemagglutinin. Exp Cell Res. 1970 Jul;61(1):153–158. doi: 10.1016/0014-4827(70)90269-7. [DOI] [PubMed] [Google Scholar]
- Maller R., Frydén A., Sörén L. Mitogen stimulation and distribution of T- and B-lymphocytes during natural rubella infection. Acta Pathol Microbiol Scand C. 1978 Jun;86C(3):93–98. doi: 10.1111/j.1699-0463.1978.tb02564.x. [DOI] [PubMed] [Google Scholar]
- Maller R., Sörén L. In vitro effects of rubella virus, strain RA 27/3, on human lymphocytes. I. Viral inhibition of mitogen stimulation in relation to Rubella Haemagglutination inhibition antibodies. Acta Pathol Microbiol Scand C. 1977 Feb;85(1):49–56. doi: 10.1111/j.1699-0463.1977.tb03610.x. [DOI] [PubMed] [Google Scholar]
- McCombs C., Michalski J. P., Talal N. Cellular interactions in lymphocyte proliferation: effect of syngeneic and xenogeneic macrophages. Cell Immunol. 1976 May;23(2):283–296. doi: 10.1016/0008-8749(76)90194-5. [DOI] [PubMed] [Google Scholar]
- McMorrow L. E., Vesikari T., Wolman S. R., Giles J. P., Cooper L. Z. Suppression of the response of lymphocytes to phytohemagglutinin in rubella. J Infect Dis. 1974 Nov;130(5):464–469. doi: 10.1093/infdis/130.5.464. [DOI] [PubMed] [Google Scholar]
- Mellman W. J., Plotkin S. A., Moorhead P. S., Hartnett E. M. Rubella infection of human leukocytes. Chromosomal and viral studies. Am J Dis Child. 1965 Oct;110(4):473–476. doi: 10.1001/archpedi.1965.02090030493021. [DOI] [PubMed] [Google Scholar]
- Miller G., Enders J. F. Vaccinia virus replication and cytopathic effect in cultures in phytohemagglutinin-treated human peripheral blood leukocytes. J Virol. 1968 Aug;2(8):787–792. doi: 10.1128/jvi.2.8.787-792.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J. R., South M. A., Rawls W. E., Melnick J. L., Olson G. B., Dent P. B., Good R. A. Viral inhibition of lymphocyte response to phytohemagglutinin. Science. 1967 Sep 1;157(3792):1068–1070. doi: 10.1126/science.157.3792.1068. [DOI] [PubMed] [Google Scholar]
- Olson G. B., Dent P. B., Rawls W. E., South M. A., Montgomery J. R., Melnick J. L., Good R. A. Abnormalities of in vitro lymphocyte responses during rubella virus infections. J Exp Med. 1968 Jul 1;128(1):47–68. doi: 10.1084/jem.128.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. B., South M. A., Good R. A. Phytohaemagglutinin unresponsiveness of lymphocytes from babies with congenital rubella. Nature. 1967 May 13;214(5089):695–696. doi: 10.1038/214695a0. [DOI] [PubMed] [Google Scholar]
- Rawls W. E. Viral persistence in congenital rubella. Prog Med Virol. 1974;18(0):273–288. [PubMed] [Google Scholar]
- Roberts N. J., Jr, Steigbigel R. T. Effect of in vitro virus infection on response of human monocytes and lymphocytes to mitogen stimulation. J Immunol. 1978 Sep;121(3):1052–1058. [PubMed] [Google Scholar]
- Schmidtke J. R., Hatfield S. Activation of purified human thymus-derived (T) cells by mitogens. II. Monocyte- macrophage potentiation of mitogen-induced DNA synthesis. J Immunol. 1976 Feb;116(2):357–362. [PubMed] [Google Scholar]
- Sedwick W. D., Sokol F. Nucleic acid of rubella virus and its replication in hamster kidney cells. J Virol. 1970 Apr;5(4):478–489. doi: 10.1128/jvi.5.4.478-489.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M. J., Jack I. Lymphocyte viraemia in congenital rubella. Lancet. 1968 Nov 2;2(7575):953–954. doi: 10.1016/s0140-6736(68)91174-4. [DOI] [PubMed] [Google Scholar]
- Soontiëns F. J., van der Veen J. Evidence for a macrophage-mediated effect of poliovirus on the lymphocyte response to phytohemagglutinin. J Immunol. 1973 Nov;111(5):1411–1419. [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A., Maes R. Cytopathic effect of rubella virus in RHK21 cells and growth to high titers in suspension culture. Virology. 1965 Oct;27(2):239–241. doi: 10.1016/0042-6822(65)90170-4. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Buimovici-Klein E. Lymphocyte responses to rubella antigen and phytohemagglutinin after administration of the RA 27/3 strain of live attenuated rubella vaccine. Infect Immun. 1975 Apr;11(4):748–753. doi: 10.1128/iai.11.4.748-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock E. F., Edelman R. Specific role of each human leukocyte type in viral infections. 3. 17D yellow fever virus replication and interferon production in homogeneous leukocyte cultures treated with phytohemagglutinin. J Immunol. 1969 Sep;103(3):429–436. [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Replication of poliovirus in phytohemagglutinin-stimulated human lymphocytes. J Virol. 1969 May;3(5):451–457. doi: 10.1128/jvi.3.5.451-457.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. T., Robinson W. S., Merigan T. C. Synthesis of viral-specific ribonucleic Acid in rubella virus-infected cells. J Virol. 1969 Dec;4(6):901–903. doi: 10.1128/jvi.4.6.901-903.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- van Loon A. M., van der Logt J. T., van der Veen J. Poliovirus-induced suppression of lymphocyte stimulation: a macrophage-mediated effect. Immunology. 1979 May;37(1):135–143. [PMC free article] [PubMed] [Google Scholar]


