Abstract
Purpose
Racial minority cancer patients may experience underuse of antiemetic medications to prevent chemotherapy-induced nausea and vomiting (CINV). In addition to its adverse implications for quality of life, antiemetic underuse may contribute to observed disparities in acute illness during chemotherapy. To understand the potential contribution of CINV prophylaxis to breast cancer disparities, we assessed racial variation in potent antiemetic use and post-chemotherapy utilization related to CINV, and the relationship between the two.
Methods
We used SEER-Medicare data to evaluate health care utilization in the 14 days following chemotherapy initiation among black and white women receiving highly emetogenic chemotherapy for breast cancer. We used modified Poisson regression to assess the relationship between: 1) race and CINV-related utilization; and 2) NK1 use and CINV-related utilization, overall and stratified by race. We report adjusted risk ratios (aRR) and 95% confidence intervals (CI).
Results
The study included 1,130 women. Black women were 11% less likely than white women to use neurokinin-1 receptor antagonists (NK1s) for CINV prophylaxis (p=0.02); however, they experienced fewer CINV-related encounters following chemotherapy (unadjusted RR:0.63, 95%CI:0.40–0.99; p=0.05). After adjustment for clinical covariates, estimates were similar but no longer statistically significant (p=0.07). Among white women NK1 use was associated with increased CINV-related utilization (aRR NK1 users vs. non-users: 1.35, 95%CI: 1.07–1.69, p=0.01), likely resulting from unmeasured confounders.
Conclusion
Black women were less likely to use NK1s and CINV-related services. Racial variation in CINV-related services use may be partly explained by differential symptom reporting or access to care.
Introduction
In the United States, breast cancer is the most common malignancy among women.[1] Advancements in early detection and treatment have improved breast cancer outcomes, leading to five-year survival rates of 99% for local-stage disease and 85% for regional-stage disease.[2] Thus the goals of breast cancer care have expanded from treating the disease to preserving women’s quality of life (QOL) during treatment. This includes managing symptoms related to breast cancer and its treatment, an area increasingly recognized as critical to high-quality breast cancer care.[3–5]
Research suggests that cancer patients of minority race may receive inadequate symptom management. Studies have documented racial/ethnic disparities in outcomes related to symptom burden and severity, [6,7] adequacy of pain treatment, [8–10] and patients’ perceived unmet need for supportive care.[11] Other studies have demonstrated that minority patients may underuse medications to control treatment-related symptoms. In particular, evidence suggests that black patients may be more likely than white patients to experience underuse of guideline-recommended antiemetic medications to prevent chemotherapy-induced nausea and vomiting (CINV), which cancer patients have consistently cited as a major and fearful concern.[12] Specifically, Samuel and colleagues found that among colorectal, lung, and prostate cancer patients being treated with chemotherapy in the Veterans Affairs system, black patients were less likely than white patients to use 5HT3 receptor antagonists.[13] Gomez and colleagues also found racial and income disparities in use of both 5HT3 antagonists and dexamethasone among lung cancer patients in Texas.[14] More recently, we documented disparities in use of neurokinin-1 receptor antagonists (NK1s), a newer and more potent class of antiemetics recommended for use with highly emetogenic chemotherapy regimens, among women with early-stage breast cancer, a population that frequently receives highly emetogenic chemotherapy.[15]
In addition to the known implications for patients’ QOL, racial disparities in CINV prophylaxis may perpetuate well-documented disparities in other dimensions of breast cancer care. Namely, research has demonstrated that black women may be less likely to adhere to recommended chemotherapy regimens and schedules[16–19] and more likely to experience hospitalizations and acute illness during treatment with chemotherapy.[19] Others have suggested that minority women’s difficulty accessing medications to control treatment-related side effects may help to explain differential treatment experiences.[20] However, the link between disparities in side effect control and treatment experiences of breast cancer patients has not been empirically studied.
With the objective of furthering understanding of how racial disparities in CINV management may contribute to racial disparities in breast cancer treatment experiences, we assessed racial differences in post-chemotherapy health care utilization related to CINV, including use of inpatient, emergency department or outpatient services. We also assessed the role of prophylactic NK1 use in potentially attenuating these differences. Finally, we assessed racial differences in any post-chemotherapy health care utilization overall, and for other common breast cancer treatment-induced side effects to determine whether any potential differences in utilization for CINV could be explained by differential use of services more broadly.
Methods
Data
We used the National Cancer Institute’s Surveillance Epidemiology and End Result (SEER) database linked with Medicare fee-for-service claims from 2006–2012. The SEER program consists of population-based cancer registries and represents 28% of the population with cancer. SEER data are merged with fee-for-service Medicare claims.[21] Data for our analysis came from the Prescription Drug Event records, Medicare Provider Analysis and Review (MEDPAR) file for inpatient services, the Hospital Outpatient Standard Analytic file for outpatient facility services, the 100% Physician/Supplier file for physicians’ services, and the Durable Medical Equipment (DME) File.
Our study was conducted in accordance with a SEER-Medicare data use agreement and was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Sample
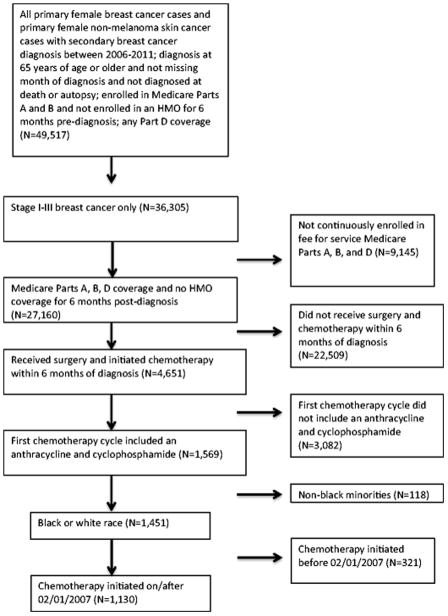
We included women aged 65 years and older who were diagnosed with stage I, II, or III breast cancer between January 1, 2007 and December 31, 2011. Eligible women were: (1) not diagnosed at autopsy or death; (2) continuously enrolled in Medicare Parts A and B for 6 months before and 12 months after diagnosis; (3) continuously enrolled in Medicare Part D for 12 months after diagnosis; and (4) not enrolled in an HMO for 6 months before and 12 months after diagnosis. There were 27,160 women meeting these criteria. From this sample, we restricted our analysis to women who received surgery (mastectomy or breast conserving surgery) and initiated chemotherapy within 6 months of diagnosis (n=4,651). The analysis was further restricted to women whose first cycle of adjuvant chemotherapy included an anthracycline and cyclophosphamide (n=1,569), as guidelines have consistently recommended use of an NK1 for these regimens throughout the study period.[22–26] Because of the small proportion of non-black minorities (n=118), the study was restricted to black and white women (n=1,451). Finally, because Part D claims are available starting on 01/01/2007, women in our sample initiated chemotherapy on or after February 1, 2007 (n=1,130). This enabled us to observe Part D claims for antiemetics in the 30 days before chemotherapy initiation. A CONSORT diagram is displayed in Figure 1.
Figure 1.
CONSORT Diagram
Measures
The primary outcome was post-chemotherapy health care utilization, measured as any inpatient or outpatient claims (including emergency department claims) in the 14 days after the first chemotherapy infusion. We were specifically interested in CINV-related utilization, identified by claims with an associated diagnosis of nausea and vomiting (ICD-9 codes 787.0–787.02), volume depletion (ICD-9 code 276.5), dehydration (ICD-9 code 276.51), or hypovolemia (ICD-9 code 276.52) in the 14 days after the first chemotherapy infusion. We chose 14 days as the window of observation because adjuvant chemotherapy regimens for breast cancer should be given no more frequently than every 14 days.[27]
The main independent variables in our analysis were race (black or white), as reported by patients at the time of diagnosis and included in the SEER data, and prophylactic NK1 use. NK1 users were defined as having a Medicare Part D claim for aprepitant (oral formulation), as identified by the drug name, in the 30 days before or on the day of chemotherapy initiation. Alternatively, they had a Part B claim for aprepitant in the 30 days before or on the day of chemotherapy initiation, as identified using Health Care Common Procedure Coding System codes (J8501) and as recorded in the outpatient, physician services or durable medical equipment claims files. Finally, NK1 users could have a claim for fosaprepitant (IV formulation) (C9242, J1453) on the day of chemotherapy initiation, as recorded in the outpatient or physician services files. We focused on the first cycle of chemotherapy because we were interested in measuring use of NK1s for CINV prophylaxis rather than use that may be in response to symptoms experienced during a previous cycle.
Covariates included patients’ demographic and clinical characteristics: age at diagnosis, American Joint Committee on Cancer stage, tumor grade, hormone receptor status, lymph node involvement, comorbid illness (calculated using the Klabunde modification of the Charlson score based on patients’ Medicare Part A and B claims pre-diagnosis)[28], and year of chemotherapy initiation. We also included information on patients’ marital status. Area-level measures of socioeconomic status (SES) included census tract-level high school completion rate, and median income, obtained from the 2000 census. Geographic variables were U.S. region of residence, and extent of urbanization in patients’ neighborhoods.
Statistical Analysis
We examined the distribution of patient characteristics between racial groups using chi-squared tests. To directly estimate risk ratios with robust error variance, we used modified Poisson regression[29] to assess the relationships between race, NK1 use, and post-chemotherapy health care utilization, controlling for relevant patient characteristics. We present unadjusted risk ratios (RR) and adjusted risk ratios (aRR) with 95% confidence intervals (CI) for post-chemotherapy health care utilization, comparing black and white women and NK1 users versus non-users.
Accounting for SES
The Institute of Medicine (IOM) defines racial healthcare disparities as differences in quality of care provided to patients of different racial groups that are not justified by clinical need or preferences of patients. [30] Analytic approaches to implement this definition use statistical models that control for differences in health status (e.g. comorbidity, age) and clinical need (e.g., tumor characteristics) and, if available, preferences for care, between racial groups.[31,32,13] This approach recognizes the mediating role of an individual’s SES and SES-related factors, that is, minorities tend to have lower SES profiles than whites, and such differences can impact care received. Excluding SES-related factors from estimates of the effect of race on care may more accurately reflect minority patients’ experiences of receiving care.
Consistent with the IOM definition of health care disparities, our primary models adjusted for clinical characteristics (age at diagnosis, year of chemotherapy initiation, tumor characteristics, and medical comorbidity).[32] We did not adjust for census tract-level measures of SES or in our primary models; neither did we adjust for other potential mediators of disparities, namely geographic factors (U.S. region of residence and metropolitan versus non-metropolitan residence) and marital status. However, because it is important to understand where disparities in care might arise, we conducted secondary analyses to assess whether differences in census tract-level SES, marital status, or geography attenuated potential racial differences in utilization.
Sensitivity Analyses
To ensure we were not incorrectly classifying claims associated with CINV prophylaxis (i.e., NK1 administration) for a women’s second chemotherapy cycle as outpatient utilization for the treatment of CINV, in sensitivity analyses, we restricted the outcome measurement window to 7 days post-chemotherapy administration. In addition, we restricted CINV-related utilization to claims with a primary or secondary diagnosis related to CINV.
Results
Among the 1,130 women who met our eligibility criteria, 1,015 (90%) were white and 115 (10%) were black. Compared to white women, black women were less likely to be married (25% versus 53%, p<0.0001). There were also racial differences in census tract-level income and education and U.S. region of residence (p<0.0001). Regarding CINV prophylaxis, black women were 13% less likely to use an NK1 (p<0.05) (Table 1). Overall, 91% of women had outpatient visits in the 14 days following their first chemotherapy infusion and 23% of women were treated for CINV. CINV-related utilization consisted largely of claims for outpatient visits (22%); only 2% of women had ED or inpatient claims related to CINV.
Table 1.
Sample Characteristics and NK1 Use, by Race
| White | Black | |
|---|---|---|
| Number of Patients | 1,015 | 115 |
| Demographic Characteristics (%) | ||
| Age at Cancer Diagnosis | ||
| 65–66 | 20.7 | 25.2 |
| 67–68 | 24.7 | 24.4 |
| 69–71 | 25.1 | 22.6 |
| 72–91 | 29.4 | 27.8 |
| Marital Status at Diagnosisa | ||
| Married/Partnered | 52.9 | 25.2 |
| Non Married/Partnered | 42.5 | -- |
| Unknown | 4.6 | -- |
| Median Household Income in Census Tract of Residencea | ||
| $0–32,791 | 21.6 | 53.0 |
| $32,972–44,039 | 25.5 | -- |
| $44,040–58,436 | -- | 13.0 |
| $58,437–188,340 | 27.1 | -- |
| Unknown | -- | 0 |
| Proportion of Adult Residents with No High School Degree in Census Tract of Residencea | ||
| 1.22–9.69% | 27.4 | -- |
| 9.70–16.57% | 26.7 | -- |
| 16.58–27.88% | -- | 30.4 |
| 27.89–75.17% | 20.3 | 57.4 |
| Unknown | -- | 0 |
| Residence | ||
| Metropolitan County | 74.8 | 82.6 |
| Non-Metropolitan County | 25.1 | 16.4 |
| U.S. Region | ||
| Northeast | 19.4 | 20.0 |
| Midwest | 18.0 | 14.8 |
| West | 37.4 | 14.8 |
| South | 25.1 | 50.4 |
| Clinical Characteristics | ||
| Year of Chemotherapy Initiationa,b | ||
| 2007 | 28.9 | 31.3 |
| 2008 | 20.1 | 20.9 |
| 2009 | 17.0 | 18.3 |
| 2010 | 14.8 | 11.3 |
| 2011 | 15.4 | -- |
| 2012 | 3.8 | -- |
| Charlson Comorbidity Scorea | ||
| 0 | 78.4 | 75.7 |
| 1 | 17.2 | -- |
| >1 | 4.3 | -- |
| Cancer Stage | ||
| Stage I | 12.8 | 10.4 |
| Stage II | 53.6 | 56.5 |
| Stage III | 33.6 | 33.0 |
| Hormone Receptor Statusa | ||
| HR positive | 67.0 | 62.6 |
| HR negative | 28.7 | -- |
| Unknown | 3.7 | -- |
| Tumor Gradea | ||
| Low | 10.3 | -- |
| Intermediate | 40.1 | 33.9 |
| High | 45.9 | 56.5 |
| Unknown | 4.3 | -- |
| Lymph Node Involvementa | ||
| Yes | 70.9 | 67.0 |
| No | 27.7 | -- |
| Unknown | 1.4 | -- |
| CINV Prophylaxis | ||
| NK1 Receptor Antagonist Use | ||
| Yes | 41.0 | 27.8 |
| No | 59.0 | 72.2 |
Cells containing proportions that reflect Ns<11 or information that would allow Ns<11 to be derived have been suppressed (--) to protect patients’ identities
A small proportion of patients initiated chemotherapy in 2012 because we only have SEER data on patients diagnosed through December 2011. Thus, only patients who received chemotherapy within the first 6 months of 2012 are included in our sample.
CINV-Related Utilization
In unadjusted analysis, compared to white women, black women had a 37% decreased risk of experiencing any CINV-related utilization (RR: 0.63, 95% CI: 0.40–0.99) (Table 2). Racial differences in CINV-related utilization did not persist in our primary model adjusting for clinical characteristics (aRR: 0.66, 95% CI: 0.42–1.04, p=0.07) or in the secondary model adjusting for clinical characteristics along with SES, marital status, and geographic variables (aRR: 0.69, 0.42–1.14, p=0.12; data not shown). In both adjusted models, estimates were similar to the unadjusted results, but they were no longer statistically significant due to widening confidence intervals.
Table 2.
Unadjusted and Adjusted Associations of Race with Post-Chemotherapy Utilization
| Risk and 95% Confidence Intervals | Risk Ratios and 95% Confidence Intervals, Black v. White | |||||
|---|---|---|---|---|---|---|
| Unadjusted models | Models adjusted for clinical characteristics | Unadjusted models | Models adjusted for clinical characteristics | |||
| White | Black | White | Black | |||
| CINV-Related Utilization | ||||||
| Any | 0.23 (0.21–0.26) | 0.15 (0.10–0.23) | 0.24 (0.14–0.42) | 0.16 (0.08–0.32) | 0.63a (0.40–0.99) | 0.66 (0.42–1.04) |
| Outpatient visits | 0.23 (0.21–0.26) | 0.15 (0.09–0.23) | 0.23 (0.13–0.41) | 0.15 (0.08–0.311) | 0.64a (0.41–1.01) | 0.67 (0.43–1.06) |
| ED visits & inpatient admissionsb | 0.03 (0.02–0.05) | 0.03 (0.01–0.09) | -- | -- | 1.07 (0.39–2.97) | -- |
| Any Post-Chemotherapy Utilization | 0.91 (0.89–0.93) | 0.87 (0.81–0.93) | 0.88 (0.80–0.96) | 0.84 (0.74–0.94) | 0.95 (0.89–1.03) | 0.95 (0.89–1.03) |
| Utilization For Other Side Effects | ||||||
| Fatigue | 0.06 (0.05–0.08) | 0.03 (0.01–0.08) | 0.02 (0.00–0.09) | 0.01 (0.00–0.07) | 0.43 (0.14–1.36) | 0.45 (0.14–1.44) |
| Neutropenia | 0.41 (0.38–0.44) | 0.37 (0.30–0.47) | 0.35 (0.24–0.52) | 0.32 (0.21–0.50) | 0.92 (0.72–1.18) | 0.90 (0.71–1.16) |
Estimates in bold are marginally statistically significant (p=0.05)
Only unadjusted models for ED visits and inpatients admissions were run due to very low frequency of this outcome, resulting in insufficient cell sizes
Unexpectedly, compared to women who did not receive an NK1 for the prevention of CINV, women who did experienced higher CINV-related utilization as measured through post-chemotherapy inpatient or outpatient visits for nausea and vomiting, volume depletion, dehydration or hypovolemia (aRR: 1.34, 95%CI: 1.07–1.68, p=0.01). This positive relationship persisted among white women (aRR: 1.33, 95%CI: 1.06–1.69, p=0.01), but it was not statistically significant among black women (aRR: 1.08, 95% CI: 0.34–3.41, p=0.90) (data not shown).
In the sensitivity analysis restricting CINV-related utilization to claims with a primary or secondary (versus any) diagnosis code related to CINV, the racial difference in utilization was larger than in the primary model, but still statistically non-significant (aRR: 0.52, 95% CI: 0.26–1.05; p=0.07). In an additional sensitivity analysis restricting the window of observation for CINV-related claims to 7 days post-chemotherapy initiation, estimates were similar to those of the main model (aRR: 0.61, 95% CI: 0.25–1.47, p=0.30).
Other Post-Chemotherapy Utilization
In analyses examining racial differences in any post-chemotherapy health care utilization, we found no differences in either unadjusted or adjusted models (aRR: 0.95, 95%CI: 0.89–1.03, p=0.21) (Table 2). There were also no statistically significant racial differences in women’s outpatient utilization for other common chemotherapy-induced side effects. Specifically, black women were no less likely than white women to receive treatment for neutropenia (aRR: 0.90, 95% CI: 0.71–1.16, p=0.42) or fatigue (aRR: 0.45, 95% CI: 0.14–1.44, p=0.18).
Discussion
We observed possible racial variation in use of outpatient services for CINV during the first cycle of highly emetogenic chemotherapy, with black women being less likely to receive CINV-related care in the post-chemotherapy period. This finding was counter to our hypothesis that black women would be more likely to experience CINV-related utilization because of evidence of potential underuse of NK1s for CINV prophylaxis among black patients. Instead, in this SEER-Medicare sample, black women were at lower risk for both using an NK1 and for receiving treatment for CINV. Although the racial difference in CINV-related utilization was not statistically significant after adjustment for covariates, estimates were still consistent with lower utilization among black patients. There are several potential explanations for our findings.
One explanation for racial variation in cancer-related health services use is that black cancer patients are less likely to access care in general, [30] for example, adjuvant treatment for breast cancer.[33] Similar patterns have been observed in other cancers, with black patients more likely to refuse lung cancer treatment.[34] However, it seems unlikely that racial differences in care-seeking behavior fully explain the variation we observed, because our sample is limited to women who underwent surgery and initiated adjuvant chemotherapy. Moreover, we observed no racial variation in the use of any outpatient services during the 14 days following chemotherapy initiation. This suggests that even among women who chose to undergo multi-model therapy, differences in CINV-related health care utilization exist.
Because racial differences in general or cancer care-seeking behavior do not appear to explain racial variation in CINV-related services use, it may be that the variation we observed is specific to CINV or symptom management. There are two reasons black women may be less likely to have claims with diagnosis codes related to CINV. First, black and white women may be at equal risk of experiencing CINV, but black women may be less likely to report this experience to their providers.[35] Differential reporting could result from differential thresholds for reporting symptoms across demographic or cultural characteristics.[36] Alternatively, minority and low-income women may have competing health or social concerns that affect their likelihood of reporting symptoms and/or prioritizing their management.[36] Others have suggested that vulnerable populations, including minorities, may receive suboptimal care due to decreased self-efficacy, defined as patients’ perceived ability in obtaining needed information and attention regarding their medical concerns.[37] In a study by Maly and colleagues, perceived self-efficacy was positively associated with nausea resolution in a cohort of low-income women with breast cancer.[38] In any case, it is ultimately physician’s awareness of symptoms that leads to discussion of treatment options with the patient, thereby facilitating symptom resolution. Thus, if minority patients are less likely to mention symptoms for any reason, they may be less likely to receive treatment for their symptoms, which could explain the lower incidence of CINV-related claims for black women in our data. A second potential explanation is that the black women in our sample may differ from white women with respect to unmeasured factors (e.g., body mass index), which could affect the incidence of treatment-induced side effects like CINV.[36,39,40] Higher rates of obesity could also lead to chemotherapy under-dosing among black women, [41] which could decrease the incidence of side effects like CINV.
Reporting bias could also occur at the provider level. Our measures of health care utilization rely on providers’ coding of diagnoses. If providers are less likely to code nausea and related conditions among black patients, for example, due to competing or more pressing health concerns, rates of CINV could appear artificially low in black patients.
We did not observe statistically significant racial differences in patients’ receipt of treatment for fatigue or neutropenia, side effects commonly experienced among breast cancer patients including those in our sample. Our lack of observation of a statistically significant relationship between race and fatigue-related services use may be due to our small sample size, as estimated risk ratios were consistent with substantial racial variation. Specifically, black patients were 55% less likely than white patients to have claims related to fatigue. Racial differences in use of services related to neutropenia were smaller (10%), however, neutropenia is often not symptomatic and thus more commonly diagnosed through routine post-chemotherapy blood testing. Therefore, it is not clear whether neutropenia-related claims capture testing for the condition or patients’ experience of neutropenia-related infection.
The positive association between prophylactic NK1 use and CINV-related utilization was also surprising. A possible explanation is that we inadvertently captured claims with associated CINV diagnosis codes used to justify the prophylactic administration of antiemetics. This seems unlikely, however, as our observation window begins the day after chemotherapy initiation, and extends for 14 days. Adjuvant chemotherapy regimens for breast cancer should be given no more frequently than every 14 days; thus, we should not have captured claims for antiemetic administration during a women’s second cycle of chemotherapy. Therefore, we suspect confounding – specifically confounding by indication - may account for this relationship. For example, patients’ (or their providers’) level of concern about CINV might help to explain why patients who receive NK1s are also more likely to subsequently receive care related to the side effect. Our data suggest that white patients may be more likely to both use NK1s to prevent CINV and be treated for CINV, raising the question of whether black women are not being identified as being in need of CINV prevention and treatment. It is also possible that black women are concentrated within providers or systems where it may be more difficult to access high quality cancer care, [42] including medications to prevent side effects and services to address them. Black women may also experience access barriers that make both obtaining NK1s and side effect-related care more difficult.
Our study has several limitations. First, we restricted our cohort to fee-for-service Medicare beneficiaries with Part D coverage. It is unknown whether our findings generalize to younger women, Medicare beneficiaries enrolled in an HMO or women without prescription drug coverage through Part D. In particular, disparities may be even larger in samples that are more diverse with respect to insurance coverage. [43] Second, we focused on NK1 receptor antagonist use as an indicator of CINV prophylaxis. We did so because NK1s are, according to guidelines, effective only in combination with two other less potent antiemetics (5HT3 antagonists and dexamethasone). However, our measure did include less potent antiemetics without an NK1. It is possible that patients who used an NK1 did not use it combination with less potent antiemetics. Third, we were unable to account for patients’ need for CINV related-care (i.e., their clinical experience with CINV). Fourth, our use of claims data prevented our ability to measure other clinically meaningful outcomes, for example, early termination of or withdrawal from chemotherapy, as it is not possible to determine a woman’s intended chemotherapy regimen or duration. Finally, only 115 black women met our study inclusion criteria, which might have resulted in our lacking statistical power for some comparisons.
These limitations not withstanding, we present novel data about possible racial variation in receipt of CINV-related care following the first cycle of highly emetogenic adjuvant chemotherapy for early-stage breast cancer. This variation may point to racial differences in women’s experience with CINV and their need for its treatment, their preferences for seeking care related to CINV, or their ability to obtain needed care for CINV and, potentially other symptoms. Thus, our data suggest that there may be a need for increased awareness and assessment of common side effects during post-treatment visits to ensure patients’ supportive care needs are met. Future research should assess whether black women’s relatively lower use of CINV-related medications and services is consistent with their informed preferences, or whether they may be experiencing barriers to access of needed services. In addition, determining the role of women’s side effect experiences in contributing to disparities in important breast outcomes, for example patient-reported QOL and treatment adherence represents a novel area for future research.
Acknowledgments
Dr. Check is supported by the National Cancer Institute under Award Number R25CA116339. Dr. Dusetzina is supported by National Institutes of Health Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) K12 Program and the North Carolina Translational and Clinical Sciences Institute (UL1TR001111). Drs. Zullig and Weinberger are supported by the Department of Veterans Affairs Office of Health Services Research and Development (Grant No. RCS 91-408 to MW and Grant No. CDA 13-025 to LLZ).
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The database infrastructure used for this project was funded by the CER Strategic Initiative of UNC’s Clinical & Translational Science Award (UL1TR001111); and the UNC School of Medicine.
Footnotes
Disclosures
None.
References
- 1.National Cancer Institute. Surveillence Epidemiology and End Results Program. [Accessed 26 January 2016];SEER Stat Fact Sheets: All Cancer Sites. http://seer.cancer.gov/statfacts/html/all.html.
- 2.National Cancer Institute. Surveillence Epidemiology and End Results Program. [Accessed 26 January 2016];SEER Stat Fact Sheets: Female Breast Cancer. http://seer.cancer.gov/statfacts/html/breast.html.
- 3.Nadler NE, Page AEK. Cancer care for the whole patient: Meeting psychosocial health needs. Institute of Medicine; 2008. [PubMed] [Google Scholar]
- 4.Smith TJ, Temin S, Alesi ER, Abernethy AP, Balboni TA, Basch EM, Ferrell BR, Loscalzo M, Meier DE, Paice JA, Peppercorn JM, Somerfield M, Stovall E, Von Roenn JH. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(8):880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 5.Surbone A. Cultural competence in oncology: where do we stand? Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2010;21(1):3–5. doi: 10.1093/annonc/mdp546. [DOI] [PubMed] [Google Scholar]
- 6.Reyes-Gibby CC, Anderson KO, Shete S, Bruera E, Yennurajalingam S. Early referral to supportive care specialists for symptom burden in lung cancer patients: a comparison of non-Hispanic whites, Hispanics, and non-Hispanic blacks. Cancer. 2012;118(3):856–863. doi: 10.1002/cncr.26312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez KA, Snyder CF, Malin JL, Dy SM. Is race/ethnicity related to the presence or severity of pain in colorectal and lung cancer? Journal of pain and symptom management. 2014;48(6):1050–1059. doi: 10.1016/j.jpainsymman.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisch MJ, Lee JW, Weiss M, Wagner LI, Chang VT, Cella D, Manola JB, Minasian LM, McCaskill-Stevens W, Mendoza TR, Cleeland CS. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(16):1980–1988. doi: 10.1200/JCO.2011.39.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeill JA, Reynolds J, Ney ML. Unequal quality of cancer pain management: disparity in perceived control and proposed solutions. Oncology nursing forum. 2007;34(6):1121–1128. doi: 10.1188/07.ONF.1121-1128. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson N, Dalton JA, Carlson J, Youngblood R, Bailey D. Racial and ethnic disparities in cancer pain management. Journal of National Black Nurses’ Association: JNBNA. 2009;20(1):11–18. [PubMed] [Google Scholar]
- 11.John DA, Kawachi I, Lathan CS, Ayanian JZ. Disparities in perceived unmet need for supportive services among patients with lung cancer in the Cancer Care Outcomes Research and Surveillance Consortium. Cancer. 2014;120(20):3178–3191. doi: 10.1002/cncr.28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickok JT, Roscoe JA, Morrow GR, King DK, Atkins JN, Fitch TR. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: a University of Rochester James P. Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer. 2003;97(11):2880–2886. doi: 10.1002/cncr.11408. [DOI] [PubMed] [Google Scholar]
- 13.Samuel CA, Landrum MB, McNeil BJ, Bozeman SR, Williams CD, Keating NL. Racial disparities in cancer care in the Veterans Affairs health care system and the role of site of care. American journal of public health. 2014;104(Suppl 4):S562–571. doi: 10.2105/AJPH.2014.302079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez DR, Liao KP, Giordano S, Nguyen H, Smith BD, Elting LS. Adherence to national guidelines for antiemesis prophylaxis in patients undergoing chemotherapy for lung cancer: a population-based study. Cancer. 2013;119(7):1428–1436. doi: 10.1002/cncr.27899. [DOI] [PubMed] [Google Scholar]
- 15.Check DK, Reeder-Hayes KE, Basch EM, Zullig LL, Weinberger M, Dusetzina SB. Investigating Racial Disparities in Use of NK1 Receptor Antagonists to Prevent Chemotherapy-Induced Nausea and Vomiting Among Breast Cancer Patients. Breast Cancer Research and Treatment. 2016;156(2):351–359. doi: 10.1007/s10549-016-3747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith K, Wray L, Klein-Cabral M, Schuchter L, Fox K, Glick J, DeMichele A. Ethnic disparities in adjuvant chemotherapy for breast cancer are not caused by excess toxicity in black patients. Clinical breast cancer. 2005;6(3):260–266. doi: 10.3816/CBC.2005.n.029. discussion 267–269. [DOI] [PubMed] [Google Scholar]
- 17.Hershman D, Weinberg M, Rosner Z, Alexis K, Tiersten A, Grann VR, Troxel A, Neugut AI. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. Journal of the National Cancer Institute. 2003;95(20):1545–1548. doi: 10.1093/jnci/djg073. [DOI] [PubMed] [Google Scholar]
- 18.Hershman DL, Unger JM, Barlow WE, Hutchins LF, Martino S, Osborne CK, Livingston RB, Albain KS. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(13):2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast cancer research and treatment. 2003;81(1):21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 20.Hassett MJ, Griggs JJ. Disparities in breast cancer adjuvant chemotherapy: moving beyond yes or no. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(13):2120–2121. doi: 10.1200/JCO.2008.21.1532. [DOI] [PubMed] [Google Scholar]
- 21.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 22.Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH American Society of Clinical O. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(31):4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D, Group EMGW. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2010;21(Suppl 5):v232–243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 24.National Comprehensive Cancer Network (NCCN) [Accessed 18 Feb 2015];Clinical Practice Guidelines in Oncology, Anti-emesis. Version 1.2012. [Internet] http://www.medicine.wisc.edu/~williams/anti-emesis.pdf.
- 25.Roila F, Hesketh PJ, Herrstedt J Antiemetic Subcommitte of the Multinational Association of Supportive Care in C. Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2006;17(1):20–28. doi: 10.1093/annonc/mdj078. [DOI] [PubMed] [Google Scholar]
- 26.Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(18):2932–2947. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- 27.Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21(8):1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 28.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 29.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 30.Smedley BD, Stith AY, Nelson AR. Institute of Medicine, Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal treatment: confronting racial and ethnic disparities in health care. 2003 [PubMed] [Google Scholar]
- 31.Le Cook B, McGuire TG, Lock K, Zaslavsky AM. Comparing methods of racial and ethnic disparities measurement across different settings of mental health care. Health services research. 2010;45(3):825–847. doi: 10.1111/j.1475-6773.2010.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuire TG, Alegria M, Cook BL, Wells KB, Zaslavsky AM. Implementing the Institute of Medicine definition of disparities: an application to mental health care. Health services research. 2006;41(5):1979–2005. doi: 10.1111/j.1475-6773.2006.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Archives of internal medicine. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 34.Mehta RS, Lenzner D, Argiris A. Race and health disparities in patient refusal of surgery for early-stage non-small cell lung cancer: a SEER cohort study. Annals of surgical oncology. 2012;19(3):722–727. doi: 10.1245/s10434-011-2087-3. [DOI] [PubMed] [Google Scholar]
- 35.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain medicine. 2003;4(3):277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 36.Yoon J, Malin JL, Tisnado DM, Tao ML, Adams JL, Timmer MJ, Ganz PA, Kahn KL. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast cancer research and treatment. 2008;108(1):69–77. doi: 10.1007/s10549-007-9580-1. [DOI] [PubMed] [Google Scholar]
- 37.Maly RC, Umezawa Y, Leake B, Silliman RA. Determinants of participation in treatment decision-making by older breast cancer patients. Breast cancer research and treatment. 2004;85(3):201–209. doi: 10.1023/B:BREA.0000025408.46234.66. [DOI] [PubMed] [Google Scholar]
- 38.Maly RC, Liu Y, Leake B, Thind A, Diamant AL. Treatment-related symptoms among underserved women with breast cancer: the impact of physician-patient communication. Breast cancer research and treatment. 2010;119(3):707–716. doi: 10.1007/s10549-009-0418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avis NE, Stellato R, Crawford S, Bromberger J, Ganz P, Cain V, Kagawa-Singer M. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Social science & medicine. 2001;52(3):345–356. doi: 10.1016/s0277-9536(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 40.Giedzinska AS, Meyerowitz BE, Ganz PA, Rowland JH. Health-related quality of life in a multiethnic sample of breast cancer survivors. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine. 2004;28(1):39–51. doi: 10.1207/s15324796abm2801_6. [DOI] [PubMed] [Google Scholar]
- 41.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Archives of internal medicine. 2005;165(11):1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 42.Jha AK, Orav EJ, Epstein AM. Low-quality, high-cost hospitals, mainly in South, care for sharply higher shares of elderly black, Hispanic, and medicaid patients. Health affairs. 2011;30(10):1904–1911. doi: 10.1377/hlthaff.2011.0027. [DOI] [PubMed] [Google Scholar]
- 43.Miller PJ, Balu S, Buchner D, Walker MS, Stepanski EJ, Schwartzberg LS. Willingness to pay to prevent chemotherapy induced nausea and vomiting among patients with breast, lung, or colorectal cancer. Journal of medical ecnomics. 2013;16(10):1179–1189. doi: 10.3111/13696998.2013.832257. [DOI] [PubMed] [Google Scholar]