Abstract
Interleukin-1β (IL-1β) is known to trigger beta cell dysfunction in vitro and could potentially play a role during the pathogenesis of type 1 diabetes and type 2 diabetes. However, several clinical trials attempting to block IL-1β function have had minimal success. We therefore reinvestigated local expression of IL-1β in human diabetic and non-diabetic pancreata.
We obtained pancreatic tissue sections from the Network for Pancreatic Organ Donors with Diabetes (nPOD) including non-diabetic control (n=9), non-diabetic auto-antibody positive (AAb+, n=5), type 1 diabetes (n=6), and type 2 diabetes (n=6) donors. Islets were systematically investigated for the presence of IL-1β mRNA by in situ hybridization and IL-1β protein by indirect immunofluorescence. We found that intra-islet IL-1β was produced at comparable levels in both non-diabetic and diabetic donors. Interestingly, the main source for IL-1β was alpha cells but not beta cells.
Our findings call into question the role of IL-1β in the diabetic pancreas as it has been proposed in previous literature. Additionally, our results regarding the localization of IL-1β should lead to further investigation into the role of IL-1β in the physiology of pancreatic alpha cells.
Keywords: Alpha Cells, Interleukin-1β, Type 1 Diabetes, Pancreas
1. Introduction
Type 1 diabetes (T1D) is an autoimmune disease which is characterized by the progressive loss of beta cell (β-cells) during an ongoing inflammatory process. According to the bi-hormonal hypothesis of diabetes pathogenesis [1], the loss of insulin secretion is often followed by impairment in the control of glucagon secretion. However, little is known about the fate of alpha cells (α-cells) within the inflamed islets.
During the last 30 years, intense efforts have been made to understand the role of cytokines in the pathogenesis of T1D with the hope that anti-cytokine therapies may eventually provide clinical benefits [2]. IL-1β is a pro-inflammatory cytokine, which, due to multiple biological effects on the inflammatory response and cell viability, has emerged as an interesting therapeutic target. Although the IL-1β gene is polymorphic and is reported to be associated with inflammation and Type 2 diabetes (T2D) [3], IL-1β maturation is mainly regulated at the posttranslational level. Previously, there have been discrepancies reported in the data obtained from mRNA and protein IL-1β in the islets from diabetes [4, 5]. We therefore examined the expression of pancreatic IL-1β and its role in the pathogenesis of T1D.
The modulation of innate and adaptive immune responses through IL-1β is believed to promote β-cell dysfunction and apoptosis in vitro [6–8]. However, bimodal effects have also been described for IL-1β [9]. In animal models, administration of a high concentration of IL-1β accelerated T1D development, whereas low dose IL-1β reduced the frequency of T1D [10]. Moreover, the effect of IL-1β blockade was found to be minor in several animal models of T1D including the RIP-LCMV mouse model [11] and the non-obese diabetic (NOD) mouse model (13). In recent trials, IL-1β blockade was not effective as a single immunomodulatory therapeutic approach in recent-onset T1D [12].
In T2D, based on the observation that insulin secretion and number of pancreatic β-cells is decreased, it has been suggested that IL-1β might contribute to the premature demise of these cells [13]. Larsen et al demonstrated that IL-1 blockade in patients with T2D slightly improved Hb1Ac and β-cell secretory function and reduced markers of systemic inflammation [14]. However, it only provided transient benefits since HBA1c reduction was not sustainable after one year [15].
In humans, IL-1β is typically produced by activated immune cells (mostly macrophages), ductal cells and vascular endothelial cells. Furthermore, rat islet studies have revealed that IL-1β can also be produced by β-cells [7]. Since clinical studies could not demonstrate any sustainable benefit following IL-1β blockade in patients with T1D and T2D, we decided to systematically re-evaluate the expression of IL-1β in human pancreata.
We analyzed pancreatic tissues from pre-diabetic, diabetic and non-diabetic donors obtained from the Network for Pancreatic Organ Donors with Diabetes (nPOD; www.jdrfnpod.org) in order to examine the expression of intra-islet IL-1β at the mRNA and the protein level, and to determine its cellular origin.
2. Materials and Methods
2.1. Subjects
Human pancreata were collected from cadaveric organ donors via nPOD. Six μm sections from formalin-fixed paraffin-embedded (FFPE) tissue samples were obtained from non-diabetic (n=9) non-diabetic auto-antibody positive (n=5), T1D (n=6), and T2D (n=6) donors which were age and BMI-matched. A total of 10 donors were non-obese and 16 donors were obese. The age of onset in T1D is heterogeneous, but this study is focused on donors with age of onset between 14 and 19 years old. Detailed demographic and histological information for each group is provided in Supplementary Table 1.
2.2. In situ hybridization
To detect IL-1β mRNA expression level, RNAscope® 2.0 High Definition BROWN Assay (ACD, Hayward, CA) was used according to the manufacturer’s instructions (see Supplementary material for more details).
2.3. Indirect immunofluorescence
Pancreas sections were subjected to a standard triple indirect immunofluorescence (IF) staining protocol to determine the expression of IL-1β at the protein level and its localization in α and β cells (see Supplementary Materials for more details).
2.4. Analysis
In situ hybridization (ISH)
Islets were quantified by Image-pro Premier software (Media Cybernetics, Rockville, MD). For each islet, the percentage of the positive area (or positive staining, defined by Image-pro Premier software) within the islet boundary was assessed and the differences between the groups and within the regions of each section were presented as the mean of analyzed islets (mean±SD).
Immunofluorescence (IF)
Image analysis was performed with Image Pro Premier software on randomly selected islets. For each islet, the difference of the mean intensity in the positive area (MIP), and the mean intensity in the negative area (MIN) (background) was measured (MIP-MIN). The differences between the groups and within the regions of each section were presented as the mean of (MIP-MIN) ± SD. All techniques, image acquisition and analysis have been validated by histology and microscopy specialists, at LJI which enabled us to rightfully quantify the IL-1β expression level.
2.5. Statistics
Data are presented as mean ± SD and were analyzed using either one-way analysis of variance (ANOVA) or an unpaired Student t test. All analyses were performed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA) and reviewed by a statistician. A value of P < 0.05 was considered significant.
2.6. Study approval
La Jolla Institute for Allergy and Immunology Institutional Review Board approved all experimental procedures (approved protocol number DI3-054-1112).
3. Results and Discussion
The number of islets in nPOD pancreas sections varies from ten to several hundreds. The definition of what appears ‘representative’ in human pancreatic pathology is therefore a subject of scrutiny, but, to our knowledge, has not been clearly defined. Hence, a first analysis was performed in order to estimate how many islets should be analyzed to reach a representative result (see Supplementary Materials). We found that a stable mean and confidence interval of either the percentage of positive area (ISH) or the mean intensity (Immunofluorescence; IF) was attained when at least 30 islets were included in the analysis (Supplementary Figure 1). We then investigated the heterogeneity of IL-1β mRNA and protein expression in 30 islets from 9 donors in three regions of the pancreas (tail, body and head). The results were highly variable between regions (Supplementary Figure 2A and 2B), yet the level of mRNA and protein expression was not statistically significantly different between the three regions. Additionally, a high amount of variation in IL-1β expression both at the mRNA and the protein level was observed within sections (the average coefficient of variation of IL-1β ranged from 5% to 80% for both ISH and IF measurements), although there was less heterogeneity in IF than ISH (30% vs. 80%). These results confirmed the need for a systematic analysis to take into account the diversity of pancreatic lobes. Consequently, we established a new method to investigate IL-1β expression in the pancreas, which allows high confidence in the reliability and the reproducibility of our findings. We systematically tested pancreas sections from 26 nPOD samples (a significant number of 780 islets in total) for the presence of IL-1β both at the mRNA level, by ISH, and protein level, by IF.
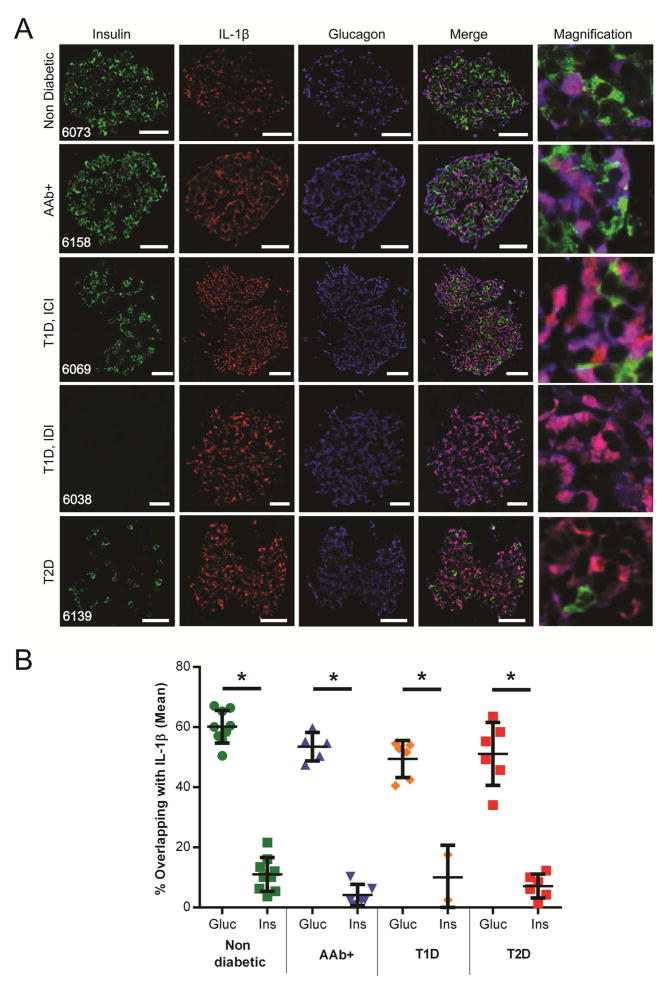
In order to determine the cellular source of IL-1β, we performed a triple immunostaining for IL-1β, insulin and glucagon and defined overlapping of IL-1β positive staining with insulin (β-cells) or glucagon (α-cells) positive staining (Figure 1A). We showed for the first time that more α-cells were producing IL-1β (40%–68%) than β-cells (1%–21%) independently of disease status (Figure 1B). In all T1D specimens, α-cells were found to be positive for IL-1β, with homogenous staining and no specific distribution pattern within the islets. In T1D donors, some of the remaining insulin positive β-cells were also found to co-express IL-1β but the majority of IL-1β positive cells were α-cells.
Figure 1. a-cells mainly expressed IL-1β, independently of the diabetic status.
A: Pancreatic tissue sections from non-diabetic, AAb+, T1D (ICIs and IDIs), and T2D donors were analyzed by indirect immunofluorescence. Representative images of islets from sections are shown. First column displays insulin (green), second column shows IL-1β (red), third column represents glucagon (blue), fourth column is the merge of the first three columns, and fifth column is a magnification of the fourth column. B: Percentage of overlapping IL-1β positive signal with either glucagon (Gluc) or insulin (Ins) positive signal was determined using Fiji. Each dot represents the mean of 10 randomly selected islets. Data are mean ± SD and were compared by t-test. A p-value less than 0.05 is considered as significant (* p<0.001). Scale bars represent 20 micrometers. T1D: Type 1 Diabetes; T2D: Type 2 Diabetes; AAb+: Auto-antibody positive; ICI: Insulin Containing Islets; IDI: Insulin Deficient Islets
There are conflicting reports regarding the effect of elevated glucose concentration on the production of IL-1β by human or rodent islets. Gene array analysis and real time PCR from laser-captured islets from patients with T2D have previously shown increased IL-1β expression when compared with non-diabetic control samples [16, 17]. This observation had also been reported in human and rodent islets exposed to high concentrations of glucose [4]. However, Welsh and colleagues showed that neither high glucose in vitro nor the diabetic state in vivo led to IL-1β production in human islets [18]. Collectively, these results suggest that locally produced IL-1β is not necessarily linked to the glycemic status in diabetes and might be heterogeneous.
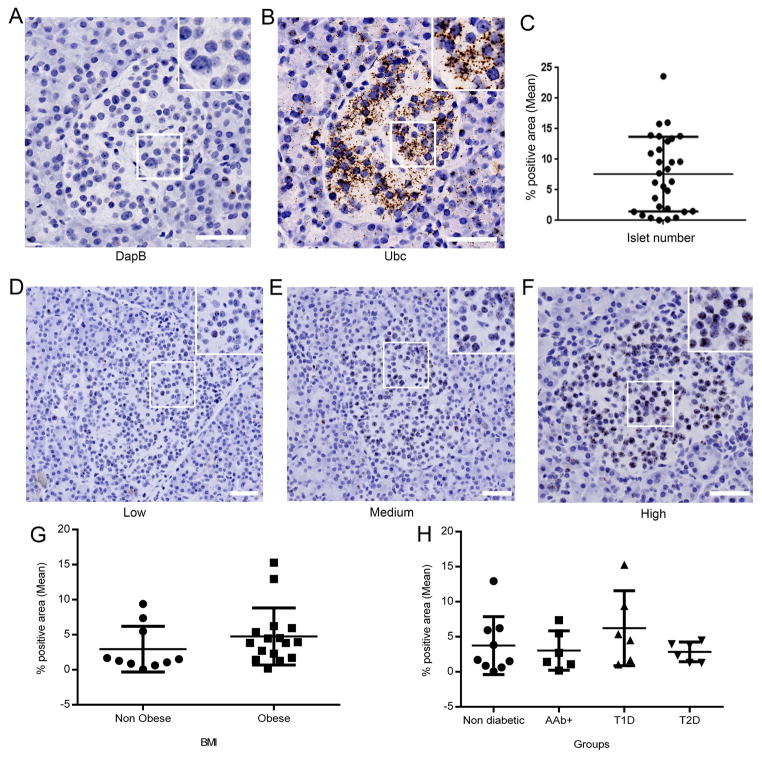
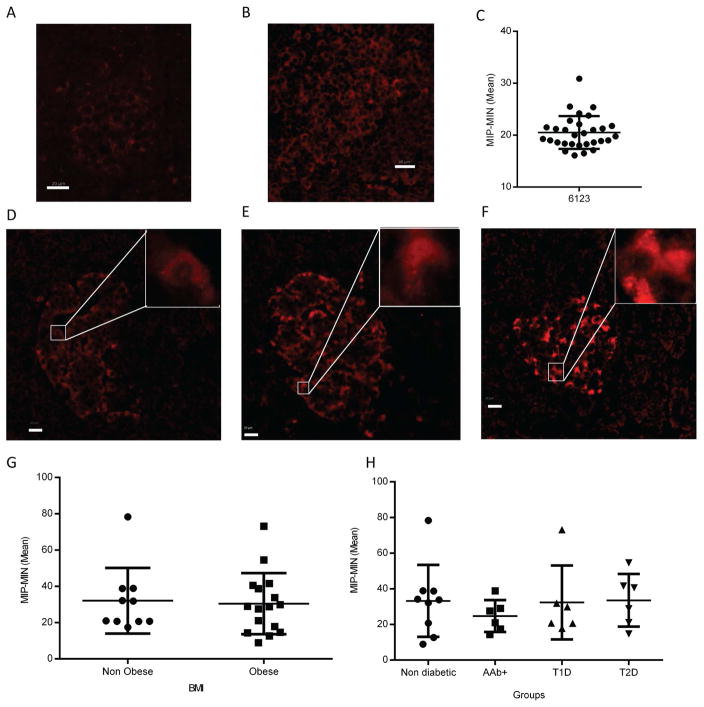
In ISH experiments, tonsil or pancreas sections served as positive and negative control using Ubc and DapB probes, respectively (Figure 2A and 2B). An important IL-1β variability between individuals and within islets of the same section was noticed (Figure 2C–F). There was no significant difference in IL-1β mRNA expression between groups based on BMI (Figure 2G) or disease status (Figure 2H). In IF experiments, the specificity of IL-1β antibody was validated in non-diabetic pancreata (negative control, Figure 3A), and human tonsil sections (positive control, Figure 3B). IL-1β within pancreatic islets was detected in most of the samples at various levels of expression (Figure 3C–F). In our cohort, the expression level of IL-1β protein did not differ between obese and non-obese donors (Figure 3G) or among individuals with different disease status (Figure 3H). Case 6152 exhibited high expression of IL-1β and was considered an outlier, while 5 cases (6038, 6039, 6069, 6087, 6198) were negative for IL-1β expression. We compared data with 54 variables available from the nPOD database (including demographics, histology evaluation, patient lifestyle and medical history, serology and HLA); however, we did not identify any positive correlation with expression of IL-1β (data not shown). These results clearly demonstrate that IL-1β can be detected in the islets of patients with T1D, T2D and also in islets from non-diabetic donors. Furthermore, we assessed the correlation of islet infiltration by myeloid cells (CD45 positive) with IL-1β expression but we could not find any association either inside or in the periphery of the islets (Supplementary Figure 3). Thus, we ruled out the possibility of the involvement of infiltrating cells in the IL-1β expression by α-cells. Additionally, we did not observe any association of IL-1β expression with macrophages (CD68 positive cells, data not shown), confirming that α-cells are the main source of IL-1β. However, we observed cells which were positive for both CD68 and IL-1β (Supplementary Figure 4), hence further validating our antibody directed against IL-1β.
Figure 2. IL-1β mRNA expression is independent of BMI and disease status.
A–F: Representative images from the case 6181. DapB (A) or Ubc (B) served as a negative and positive control, respectively. (C) Intra-section heterogeneity of IL-1β expression (30 islets from case 6181). Images of (D) low (0–2%), (E) medium (2–5%), and (F) high (> 5%) expression of IL-1β. (G, H) Quantification of intra-islet IL-1β expression in pancreas sections from non-diabetic (n=9), T1D (n=6), AAb+ (n=5), and T2D (n=6) donors. Data are obtained from 30 islets and are presented as the percentage of positive area within islets (mean ± SD). Scale bars represent 50μm. Inserts show a magnification of the area inside the box. Data are displayed as mean ± SD and were compared with (G) t-test or (H) one-way ANOVA. T1D: Type 1 Diabetes; T2D: Type 2 Diabetes; AAb+: Auto-antibody positive.
Figure 3. IL-1β protein expression is independent of BMI and disease status.
A–F: Non-diabetic pancreata stained with secondary antibody only (A) or human tonsil (B) served as negative and positive controls, respectively. Heterogeneity of IL-1β expression between islets from the pancreatic tail of case 6123 (C). Images of low (0–15) (D), medium (15–30) (E), and high (>30) (F) expression of IL-1β. (G, H) Quantification of intra-islet IL-1β expression in pancreas sections from non-diabetic (n=9), T1D (n=6), AAb+ (n=5), and T2D (n=6) donors. Data are obtained from 30 islets and are presented as the mean of the difference of MIP and MIN (mean ± SD). Scale bars represent 20μm. Inserts show a magnification of the area inside the box. Data are displayed as mean ± SD and were compared with (G) t-test or (H) one-way ANOVA. MIP: mean intensity in the positive area, MIN: mean intensity in the negative area T1D: Type 1 Diabetes; T2D: Type 2 Diabetes; AAb+: Auto-antibody positive.
Previous studies of transcriptomes of purified human islet cells from healthy donors showed 26 fold higher IL-1β gene expression in β-cells than in α-cells [5]. Our mRNA data does not allow a direct comparison with these results because we did not measure insulin or glucagon expression at the mRNA level. However, our protein data clearly reveal that α-cells are the main source of IL-1β. Our finding that α-cells predominantly express IL-1β and this expression is independent of diabetic status is novel and intriguing. Potentially, α-cell-derived IL-1β could exert paracrine effects on β-cells expressing IL-1 receptors. Indeed, Maedler and colleagues showed that the IL-1 receptor antagonist (IL-1Ra) is expressed by human pancreatic β-cells and down-regulated in patients with T2D [19]. The authors observed that leptin, which is mainly produced by the adipose tissue, decreased IL-1Ra expression in human islets at the mRNA and the protein level. Even though there was no difference in IL-1β expression, locally produced IL-1β could have a distinct effect if IL-1Ra was modulated by other factors. Expression of IL-1β was studied in pancreata sections from five T2D patients with poor glycemic control. In contrast with our findings, their results by double immunostaining of IL-1β and insulin showed higher levels of IL-1β in T2D donors compared with non-diabetic donors [4]. The high degree of heterogeneity in IL-1β expression that we observed in our systematic study may account for these discrepancies. We believe that we have addressed this issue in a systematic fashion by pre-defining how many islets and regions need to be analyzed to give reproducible results. We therefore demonstrate for the first time that the expression of IL-1β both at the mRNA and the protein level is independent of disease status and is mainly localized to α-cells.
Our results are novel and differ from previous reported studies but they do resonate with the marginal clinical effects observed in several IL-1β blockade trials [12, 15, 20]. Data used to build the rationale for IL-1β blockade were obtained either from animal models [21], isolated β-cells [4] or at the systemic level [7, 22] but not from human pancreatic tissues which may explain the discrepancy between expected results from the literature and our observations. The present study is the first quantification of IL-1β in human pancreas tissue and therefore may provide a more representative image of its role in human T1D pathophysiology. However, even if intra-islet IL-1β is not involved in pancreatic inflammation, IL-1β is known to be produced by other sources, such as macrophages from the adipose tissue [23, 24]. In T2D, this source of IL-1β might be involved in the physiopathology of the disease, which may explain the limited significant effect observed in clinical trials [14].
The presence of intra-islet IL-1β in α-cells independent of BMI or disease status is certainly puzzling. However, IL-1β has been shown to act directly on β-cells as a non-deleterious cytokine to potentiate insulin secretion [25]. In the islet, the presence of IL-1β may therefore be associated with glucose homeostasis rather than inflammation.
4. Conclusion
Based on our current data, the limited beneficial outcomes of IL-1β blockade and therefore, since side effects are expected, we believe that such therapy should be considered carefully. Furthermore, our observations should help to better understand the pathogenesis of T1D and T2D, and opens up new avenues of investigation pertaining to the potential physiological and pathogenic roles of IL-1β produced by α-cells.
Supplementary Material
Highlights.
Alpha cells, but not beta cells, are the main source for IL-1β in human pancreatic islets
Non-diabetic and diabetic donors produce intra-islet IL-1β at comparable levels independently from BMI or disease status
The presence of IL-1β in islets may be associated with glucose homeostasis rather than inflammation
Acknowledgments
The authors thank Zbigniew Mikulski, William Kiosses, Margaret Chadwell and Priscilla Colby of La Jolla Institute for Allergy and Immunology for help with image acquisition and analysis, histology core and administrative assistance respectively.
M.G.v.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors acknowledge the editorial assistance of Ellie Ling.
This research was performed with the support of nPOD, sponsored by the Juvenile Diabetes Research Foundation International. This study was also supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases grant #U01AI102370-08 and Charles Thivolet was supported by the Claude Bernard University, Lyon France.
Footnotes
Conflict of interest: The Authors have declared that no conflict of interest exist
Author contributions
F.A., S.S., C.T. performed, designed experiments and wrote the manuscript. F.A., S.S., C.T., T.R.-C., D.S. interpreted data. J.Z.-G. assisted with the statistical analysis. N.A. and E.C. performed experiments. M.G.v.H. designed experiments, interpreted data, and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1:14–6. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- 2.Nepom GT, Ehlers M, Mandrup-Poulsen T. Anti-cytokine therapies in T1D: Concepts and strategies. Clinical immunology. 2013;149:279–85. doi: 10.1016/j.clim.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee M, Saxena M. Genetic polymorphisms of cytokine genes in type 2 diabetes mellitus. World journal of diabetes. 2014;5:493–504. doi: 10.4239/wjd.v5.i4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. The Journal of clinical investigation. 2002;110:851–60. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorrell C, Schug J, Lin CF, Canaday PS, Fox AJ, Smirnova O, et al. Transcriptomes of the major human pancreatic cell types. Diabetologia. 2011;54:2832–44. doi: 10.1007/s00125-011-2283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150:5218–29. doi: 10.1210/en.2009-0543. [DOI] [PubMed] [Google Scholar]
- 7.Donath MY, Boni-Schnetzler M, Ellingsgaard H, Halban PA, Ehses JA. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends in endocrinology and metabolism: TEM. 2010;21:261–7. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Eizirik DL, Sammeth M, Bouckenooghe T, Bottu G, Sisino G, Igoillo-Esteve M, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS genetics. 2012;8:e1002552. doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spinas GA, Palmer JP, Mandrup-Poulsen T, Andersen H, Nielsen JH, Nerup J. The bimodal effect of interleukin 1 on rat pancreatic beta-cells--stimulation followed by inhibition--depends upon dose, duration of exposure, and ambient glucose concentration. Acta endocrinologica. 1988;119:307–11. doi: 10.1530/acta.0.1190307. [DOI] [PubMed] [Google Scholar]
- 10.Wilson CA, Jacobs C, Baker P, Baskin DG, Dower S, Lernmark A, et al. IL-1 beta modulation of spontaneous autoimmune diabetes and thyroiditis in the BB rat. Journal of immunology. 1990;144:3784–8. [PubMed] [Google Scholar]
- 11.Barral AM, Thomas HE, Ling EM, Darwiche R, Rodrigo E, Christen U, et al. SOCS-1 protects from virally-induced CD8 T cell mediated type 1 diabetes. Journal of autoimmunity. 2006;27:166–73. doi: 10.1016/j.jaut.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–15. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Current opinion in endocrinology, diabetes, and obesity. 2010;17:314–21. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 14.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. The New England journal of medicine. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 15.Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes care. 2009;32:1663–8. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marselli L, Thorne J, Dahiya S, Sgroi DC, Sharma A, Bonner-Weir S, et al. Gene expression profiles of Beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PloS one. 2010;5:e11499. doi: 10.1371/journal.pone.0011499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. The Journal of clinical endocrinology and metabolism. 2008;93:4065–74. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welsh N, Cnop M, Kharroubi I, Bugliani M, Lupi R, Marchetti P, et al. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes. 2005;54:3238–44. doi: 10.2337/diabetes.54.11.3238. [DOI] [PubMed] [Google Scholar]
- 19.Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, et al. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8138–43. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumpter KM, Adhikari S, Grishman EK, White PC. Preliminary studies related to anti-interleukin-1beta therapy in children with newly diagnosed type 1 diabetes. Pediatric diabetes. 2011;12:656–67. doi: 10.1111/j.1399-5448.2011.00761.x. [DOI] [PubMed] [Google Scholar]
- 21.Aharon-Hananel G, Jorns A, Lenzen S, Raz I, Weksler-Zangen S. Antidiabetic Effect of Interleukin-1beta Antibody Therapy Through beta-Cell Protection in the Cohen Diabetes-Sensitive Rat. Diabetes. 2015;64:1780–5. doi: 10.2337/db14-1018. [DOI] [PubMed] [Google Scholar]
- 22.Mandrup-Poulsen T, Pickersgill L, Donath MY. Blockade of interleukin 1 in type 1 diabetes mellitus. Nature reviews Endocrinology. 2010;6:158–66. doi: 10.1038/nrendo.2009.271. [DOI] [PubMed] [Google Scholar]
- 23.Wewers MD, Dare HA, Winnard AV, Parker JM, Miller DK. IL-1 beta-converting enzyme (ICE) is present and functional in human alveolar macrophages: macrophage IL-1 beta release limitation is ICE independent. Journal of immunology. 1997;159:5964–72. [PubMed] [Google Scholar]
- 24.Zhao R, Zhou H, Su SB. A critical role for interleukin-1beta in the progression of autoimmune diseases. International immunopharmacology. 2013;17:658–69. doi: 10.1016/j.intimp.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Yelich MR. In vivo endotoxin and IL-1 potentiate insulin secretion in pancreatic islets. The American journal of physiology. 1990;258:R1070–7. doi: 10.1152/ajpregu.1990.258.4.R1070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.