Cholangiocarcinoma (CCA) is a lethal malignancy originating from the biliary tree. Because CCA is usually detected in advanced stages, patients are infrequently eligible for surgery, which is the only curative intervention. Unfortunately, there are currently no other efficient therapeutic options, and these tumors are particularly resistant to chemotherapy.(1) Therefore, new strategies for the treatment of CCA are needed. In this issue of HEPATOLOGY, Li et al.(2) provide the basis for a potential new approach based on the use of extracellular vesicles (EVs). Their study addresses two important topics in CCA biology: the bidirectional intercellular communication between tumor and stromal cells by means of EVs and the potential therapeutic use of these vesicles for delivering molecules of interest such as microRNAs (miRNAs) into tumor cells.
EVs are bilayered membrane vesicles released from a variety of cell types, and they can be found in almost any biological fluid. In addition to apoptotic bodies, which are measured in micrometers, EVs can be divided in two groups based on their size, but more importantly on their origin—namely, microvesicles (also known as ectosomes or microparticles, 0.2–1 μm in diameter) and exosomes (0.04–0.1 μm in diameter). Microvesicles are originated by budding from the plasma membrane, whereas exosome biogenesis starts from the multivesicular bodies that are redirected to and fuse with the plasma membrane, delivering the exosomes by way of exocytosis into the extracellular media. In addition to lipids and proteins, EVs contain cytokines, hormones, transcription factors, and nucleic acids, including miRNAs.(3,4) In recent years, it has become evident that EVs may be integral components of the tumor developmental process, from the communication between tumor and stromal cells to the colonization of new niches during metastasis. For example, exosomes derived from CCA cells are capable of transferring oncogenic proteins to normal cholangiocytes and induce migration and invasion,(5) and also induce the differentiation of mesenchymal stem cells into fibroblasts, which may contribute to stromal generation.(6) Furthermore, the characterization of EVs in bile suggests their potential use as a diagnostic tool.(7)
MiRNA dysregulation is a common feature of tumor cells. These short noncoding RNAs act as critical regulators of gene expression by specifically binding to messenger RNAs and blocking translation and/or inducing RNA degradation. To date, there is much evidence to show that EVs are able to transport signals from a donor cell and communicate with adjacent or more distant cells by fusing with the plasma membrane and delivering their cargo inside the target cell. Moreover, transported miRNAs are capable of targeting messenger RNAs or activate receptors in recipient cells,(8) suggesting that the use of artificially overloaded vesicles with a particular therapeutic miRNA may be of utmost interest.
Li et al. report that the coculture of a hepatic stellate cell line (LX2, a human cell line extensively used for in vitro modeling of CCA stroma) with a CCA cell line (HuCCT-1) induced the down-regulation of several miRNAs, including miR-195, in stellate cells by unknown mechanisms. Interestingly, the experimental overexpression of this particular miRNA in different stromal cells—including LX2 and the portal fibroblast cell line RGF (another cell type that may contribute to the stroma cell population in CCA)—inhibits the tumorigenic phenotype of cocultured CCA cells, suggesting a mutual regulation wherein the tumor cells decrease miR-195 expression in the stromal cells and the stromal cells reduce CCA cells growth and invasion in an miR-195–dependent manner by a mechanism involving the transport of miR-195 in EVs. These results uncover a tumor cell survival strategy by inhibiting the production of the tumor suppressor miRNA-195 in stromal cells (Fig. 1).
FIG. 1.
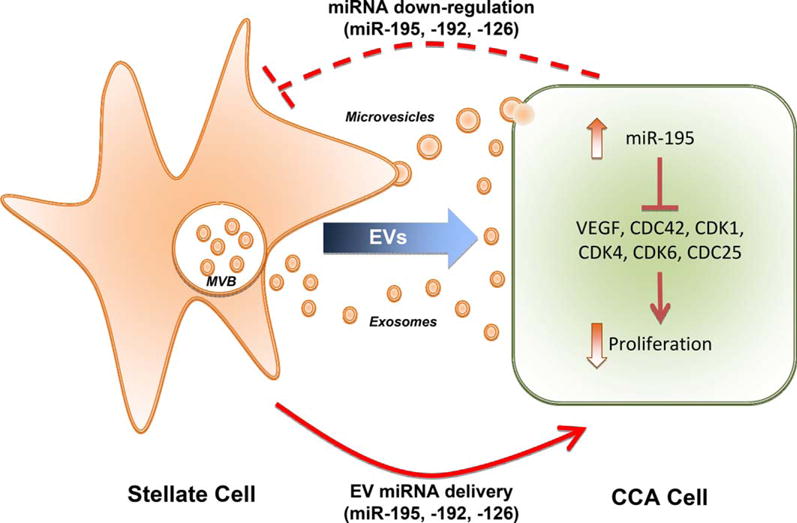
Mutual regulation between tumor and stromal cells. Coculture of CCA and stellate cell lines induces the down-regulation of specific miRNAs in the stromal cells, including miR-195. In turn, stellate cells secrete EVs (exosomes derived from the multivesicular bodies [MVB] and microvesicles originated by direct budding from the plasma membrane). Experimental overloading of EVs with miR-195 specifically targeted tumor cells and discharged their content inside the cytoplasm, regulating several growth factors and cell cycle–related proteins and resulting in inhibited proliferation.
The authors further found that LX2-derived EVs artificially loaded with miR-195 were able to reduce tumor growth and increase survival, both in vitro and in vivo, using a rat orthotopic model of CCA. Further analysis of rat tumors revealed that miR-195–loaded EVs suppressed cell proliferation and decreased the desmoplastic reaction. In addition, six described messenger RNA targets of miR-195 were reduced in the treated animals, including vascular endothelial growth factor and cdc25. To increase the relevance of their findings, the authors analyzed a set of 20 human CCA specimens with matching controls and found that miR-195 was down-regulated in 80% of cases.
Finally, the authors explored the potential application of EVs as a therapeutic approach. To this end, using labeled EVs with mCherry-TSG101 or CRE reporter tumor cells, the authors found that the systemic application of these vesicles accumulate preferentially in the tumors but not in the normal liver parenchyma, suggesting that the LX2-derived EVs are not only able to deliver miRNAs to the tumor cells, but that they do so in a specific manner.
As in any good study, Li et al. have generated several new questions that need to be addressed. For instance, it would be interesting to know which EV population is more important for tumor–stromal communication, and vice versa. Are exosomes or microvesicles the key players? Knowing the exact identity of the EVs responsible for the therapeutic effect may open new avenues to specifically modulate EV production in both tumor and stromal cells. Furthermore, as the authors noted, more studies are needed to identify the mechanisms that concede the specific tropism to these EVs toward tumor cells. The role of hepatic stellate cells and their diffusible secreted products has been addressed previously in both hepatocellular carcinoma and CCA, the latter characterized by abundant desmoplastic stroma. A reciprocal cross-talk between tumor and stromal cells has been described to contribute to tumor progression.(9,10) Therefore, the present studies deepen our knowledge of these intercellular communications and warrant further studies on the stromal cells derived EVs and their effects on tumor cells.
In conclusion, the study by Li et al. contributes to the growing body of evidence supporting the importance of miRNAs and EVs in tumor biology. In particular, it provides the foundation for a potential therapeutic approach for CCA that takes advantage of the tropism of EVs isolated from stromal cells toward CCA cells, suggesting that these vesicles may be useful for the targeted delivery of a variety of therapeutic agents.
Abbreviations
- CCA
cholangiocarcinoma
- EV
extracellular vesicle
- miRNA
microRNA
Footnotes
Potential conflict of interest: Nothing to report.
Author names in bold designate shared co-first authorship.
References
- 1.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Piontek K, Ishida M, Fausther M, Dranoff JA, Fu R, et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. HEPATOLOGY. 2017;65:501–514. doi: 10.1002/hep.28735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol. 2016;65:213–221. doi: 10.1016/j.jhep.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desrochers LM, Antonyak MA, Cerione RA. Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev Cell. 2016;37:301–309. doi: 10.1016/j.devcel.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta S, Reamtong O, Panvongsa W, Kitdumrongthum S, Janpipatkul K, Sangvanich P, et al. Proteomics profiling of cholangiocarcinoma exosomes: a potential role of oncogenic protein transferring in cancer progression. Biochim Biophys Acta. 2015;1852:1989–1999. doi: 10.1016/j.bbadis.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles. 2015;4:24900. doi: 10.3402/jev.v4.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Masica D, Ishida M, Tomuleasa C, Umegaki S, Kalloo AN, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. HEPATOLOGY. 2014;60:896–907. doi: 10.1002/hep.27050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 9.Clapéron A, Mergey M, Aoudjehane L, Ho-Bouldoires TH, Wendum D, Prignon A, et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. HEPATOLOGY. 2013;58:2001–2011. doi: 10.1002/hep.26585. [DOI] [PubMed] [Google Scholar]
- 10.Ji J, Eggert T, Budhu A, Forgues M, Takai A, Dang H, et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. HEPATOLOGY. 2015;62:481–495. doi: 10.1002/hep.27822. [DOI] [PMC free article] [PubMed] [Google Scholar]


