Abstract
Chemotherapeutic multidrug resistance (MDR) is a significant challenge to overcome in clinic practice. Several mechanisms contribute to MDR, one of which is the augmented drug efflux induced by the upregulation of ABCB1 in cancer cells. Regorafenib, a multikinase inhibitor targeting the RAS/RAF/MEK/ERK pathway, was approved by the FDA to treat metastatic colorectal cancer and gastrointestinal stromal tumors. We investigated whether and how regorafenib overcame MDR mediated by ABCB1. The results showed that regorafenib reversed the ABCB1-mediated MDR and increased the accumulation of [3H]-paclitaxel in ABCB1-overexpressing cells by suppressing efflux activity of ABCB1, but not altering expression level and localization of ABCB1. Regorafenib inhibited ATPase activity of ABCB1. In mice bearing resistant colorectal tumors, regorafenib raised the intratumoral concentration of paclitaxel and suppressed the growth of resistant colorectal tumors. But regorafenib did not induce cardiotoxicity/myelosuppression of paclitaxel in mice. Strategy to reposition one FDA-approved anticancer drug regorafenib to overcome the resistance of another FDA-approved, widely used chemotherapeutic paclitaxel, may be a promising direction for the field of adjuvant chemotherapy. This study provides clinical rationale for combination of conventional chemotherapy and targeted anticancer agents.
Keywords: multidrug resistance, ABCB1 transporter, combination chemotherapy, regorafenib, tumor xenograft mouse model
1. Introduction
Chemotherapeutic multidrug resistance (MDR) remains a major obstruction of conventional chemotherapy and targeted therapies. This prevailing and pernicious clinical conundrum frequently results in cancer recurrence and low survival rate. The mechanisms of MDR have been investigated thoroughly, but not all mechanisms that induce MDR have been clarified. One of the most common mechanisms that induce MDR is the overexpression of ATP-binding cassette (ABC) transporters on the membrane of cancer cells. ABC transporters are a family of ATP energy dependent, active transporter proteins that have diverse functions and exist in both prokaryotes and eukaryotes [1,2]. The primary functions of ABC transporter proteins include: (1) conserved mechanisms related to nutrition and pathogenesis in bacteria, (2) spore formation in fungi, and (3) signal transduction, protein secretion and antigen presentation in eukaryotes [3]. Many studies have reported that ABC transporters are responsible for extruding toxic endogenous molecules and xenobiotics out of the cell [4,5]. It has been proposed that an antagonist of ABC transporter may be used in combination with clinical chemotherapeutics to potentiate their antitumor activity. Until now, 48 human ABC transporters have been identified and classified into 7 subfamilies, from ABCA to ABCG [6]. Among them, ABCB1 is a major contributor to MDR. ABCB1 (P-gp/MDR1) is an apical membrane transporter that is ubiquitously expressed in kidney, intestine, placenta, liver, adrenal glands and blood-brain barrier (BBB) [7,8]. It can transport a wide range of anticancer drugs including taxanes (paclitaxel and docetaxel), epipodophyllotoxins (etoposide and teniposide), vinca alkaloids (vincristine and vinblastine), anthracyclines (doxorubicin and daunorubicin), and antibiotics (actinomycin D) [9–11]. Paclitaxel accumulates in the gastrointestinal tract and brain of Abcb1 knockout mice, suggesting that ABCB1 inhibits the excretion of paclitaxel into the bile and its crossing of the BBB [12]. The evaluation of ABCB1 expression in the National Cancer Institute (NCI) 60 cancer cell lines anticancer drug screening panel, using quantitative polymerase chain reaction, indicated a significant negative correlation coefficient (−0.896) of ABCB1 expression with the sensitivity profile of paclitaxel [13], proving that ABCB1 overexpression results in paclitaxel resistance. Overexpression of ABCB1 has been found in consequence of: (1) ABCB1 gene amplification [14]; (2) increased transcription of the ABCB1 gene by novel transcription factors such as RGP8.5 [15]; (3) changes in ABCB1 translational efficiency [14]; (4) mutations in the ABCB1 gene [16,17] and (5) chromosomal rearrangement in the ABCB1 gene that produce hybrid genes [18]. Overexpression of ABCB1 has been associated with various cancers, such as acute myeloid leukemia, childhood tumors, breast cancers, hematological malignancies and solid tumors [19–22]. A number of drugs that modulate MDR-ABCB1 transporter have been discovered or synthesized in the last decade. Some of them not only exhibited promising activity in vitro and in vivo, but also proved encouraging efficacy in clinical trials [23–26]. The clinical significance of ABCB1 transporter antagonism has been studied extensively as a potential therapeutic strategy.
Regorafenib is a multikinase inhibitor that interferes with the RAS/RAF/MEK/ERK pathway. It has been approved by FDA for the treatment of metastatic colorectal cancer (mCRC) and gastrointestinal stromal tumors [27,28]. The common side effects of regorafenib are fatigue, hand-foot skin reaction, diarrhea, anorexia, voice changes, hypertension, oral mucositis, rash, and weight loss [28]. Regorafenib is undergoing numerous clinical trials for the treatment of other malignancies, such as lung, liver and esophageal cancers. It inhibits some kinases that are often aberrantly activated in cancer cells, including c-Raf, B-Raf, vascular endothelial growth factor receptors (VEGFR), platelet-derived growth factor receptor (PDGFR), c-Kit, and Tie-2 [29]. The antitumor activity of regorafenib has been demonstrated in various preclinical studies, and is correlated with inhibition of tumor angiogenesis, suppression of cell proliferation, and induction of apoptosis [29]. For example, it was reported that regorafenib induced tumor shrinkage through the cell autonomous process of apoptosis induction, progressing from ERK inhibition, GSK3β activation, and p65 nuclear translocation, eventually triggering PUMA induction and onset of mitochondria-mediated apoptosis [30]. Hence MDR-ABCB1 inhibitor co-administered with clinically used chemotherapeutics would be a promising regimen for MDR cancer patients. In this study, we determined whether and how regorafenib overcome chemotherapeutic MDR mediated by ABCB1 transporter in CRC cells.
2. Materials and methods
2.1. Cells and reagents
SW620/Ad300 cells overexpressing ABCB1, were established by exposure of SW620, the parental human CRC cells, to 300 μg/L doxorubicin. HEK293/pcDNA3.1 and HEK/ABCB1 were generated by transfecting the HEK293 cells with empty and ABCB1 expressing vector [31]. [3H]-paclitaxel (23 Ci/mmol, MT552) was purchased from Moravek Biochemicals, Inc. (Brea, CA). Regorafenib was obtained from Bayer HealthCare Pharmaceuticals Inc. (Whippany, NJ). Paclitaxel (T7402), doxorubicin (D1515), vincristine (V8388), cisplatin (C2210000), verapamil (1711202), Triton (X-100), paraformaldehyde (P6148), DMSO (PHR1309), and MTT (M2128) were obtained from Sigma Chemical Co. (St. Louis, MO). The monoclonal antibody C219 (against ABCB1, sc-59591), sc-47778 (against β-Actin) and the secondary horseradish peroxidase-labeled rabbit anti-mouse IgG (sc-358914) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). The Alexa flour 488-conjugated goat anti-mouse IgG (A-11001) was purchased from Molecular Probes (Eugene, OR). WBC Diluting Fluid and Platelet Diluent (ES5401) were purchased from Eng Scientific Inc. (Clifton, NJ). High sensitivity mouse cardiac troponin-I ELISA KIT (CTNI-1-HSP) was purchased from Life Diagnostics, Inc. (West Chester, PA).
2.2. Cytotoxicity assay
The sensitivity of cells to anticancer drugs was measured as previously described [32] using the MTT colorimetric assay.
2.3. Immunoblotting analysis
60 μg protein cell lysates were loaded in each lane. ABCB1 was determined from C219, and β-Actin was used as internal loading control as previously mentioned in [33].
2.4. Immunofluorescence assay
Cells were seeded into 96-well plate and cultured with medium containing regorafenib (10 μM). The immunofluorescence assay was carried out as described previously [34].
2.5. Accumulation and efflux assay
The accumulation of [3H]-paclitaxel in SW620 and SW620/Ad300 cells was measured in the absence or presence of regorafenib or verapamil at 5 μM and 10 μM. The drug accumulation and efflux assay were performed as described previously [35].
2.6. ABCB1 ATPase assay
The vanadate-sensitive ATPase activity of ABCB1 in crude membranes of High-five insect cells, in the presence of concentrations of regorafenib ranging from 0 to 40 μM, was measured as previously described [36].
2.7. Induced-fit docking of regorafenib into human homology ABCB1
Human ABCB1 homology model based on refined mouse ABCB1 (PDB ID: 4M1M) was provided by S. Aller and the docking grid was refined as previously described [37,38]. The energy minimized structure of regorafenib was then subjected to Glide v6.6 XP (extra precision) docking. In this study, to allow for the conformational changes of ABCB1, the IFD (induced-fit docking) [39] protocol was carried out using Glide v6.6 (Schrödinger, USA, 2015). At the transmembrane domain of ABCB1, the best scored pose of regorafenib was used to generate the grid for IFD calculation. The default Glide IFD protocol was followed and the docking score (kcal/mol) was calculated. Top scoring docked pose of regorafenib-ABCB1 complex was subjected to interactions analysis and figure representation. All computations were carried out on a 6-core Intel Xeon Processor with a Mac OS.
2.8. Colorectal resistant tumor xenograft mouse model
The SW620 and SW620/Ad300 models were modified from the colorectal tumor xenograft model previously established by Chen’s laboratory [40,41]. There were four treatment groups. Group 1 animals received vehicle A (polypropylene glycol/PEG400/Pluronic F68/Saline, 34%/34%/12%/20%) orally every 3rd day, 1 hour prior to intraperitoneal administration of vehicle B (ethanol/Cremophor ELP/saline, 12.5%/12.5%/75%). Group 2 animals received 45 mg/kg regorafenib orally (prepared in vehicle A) administered every 3rd day, 1 hour prior to intraperitoneal administration of vehicle B. Group 3 animals received vehicle A orally every 3rd day, 1 hour prior to 15 mg/kg intraperitoneal paclitaxel administration. Group 4 animals received a combination of the regorafenib, administered every 3rd day orally, 1 hour prior to intraperitoneal paclitaxel administration. Eight mice were used for each group. The tumor sizes and body weights were measured as previously described [42,43]. Subsequently, all mice were euthanized using carbon dioxide, then tumor tissues were excised and stored at −80 °C. The IACUC at St. John’s University approved this project, and the research was conducted in compliance with the Animal Welfare Act and other federal statutes.
2.9. Tumor and plasma collection
Mice bearing SW620 and SW620/Ad300 tumors were divided into three groups: (i) regorafenib; (ii) paclitaxel; (iii) regorafenib + paclitaxel. After treatment, animals were anesthetized and blood was obtained using supraorbital puncture and placed in heparinized tubes, and plasma was harvested at 10, 30, 60, 120, or 240 min after paclitaxel administration in both groups.
2.10. Quantification of regorafenib and paclitaxel
The quantification of regorafenib in plasma and tumors was conducted using an isocratic Shimadzu LC-20AB HPLC (Shimadzu, OR). Chromatographic separation was performed using a reversed phase, Agilent, Eclipse XDB-C18 column. The mobile phase was 0.5% phosphate buffer PH 3.5- acetonitrile 30:70, v/v. Sample detection was carried at 260 nm. Injection volume was 20 microliter. The total run time was 6 minutes and the retention time was 3.6 minutes. Standard curves for regorafenib in plasma and tissue homogenates were prepared in the ranges of 10–10,000 ng/ml. The preparation and storage of samples and quantification of paclitaxel in tumor and plasma were performed as previously described [44].
2.11. Blood cell counting
The platelets and WBCs in mice were counted as described previously [40].
2.12. Mouse cardiac troponin-I ELISA assay
The plasma concentrations of cTnI in mice were measured as described previously [41].
2.13. Statistical analysis
All experiments were repeated at least three times, each done in triplicate. The statistical significance between two groups was determined with Student’s t-test, whereas the comparisons of multiple groups was carried out by one-way ANOVA, followed by Bonferroni’s post-test using Microsoft Excel software. A probability value of *P < 0.05 was considered to be significant.
3. Results
3.1. Regorafenib sensitizes ABCB1-overexpressing cell lines to ABCB1 substrate chemotherapeutics
In order to determine the concentration for MDR reversal study, different concentrations of regorafenib were used to treat the cell lines (data not shown). We used 5 μM and 10 μM because the viability of cell lines was above 85% when incubated with 10 μM regorafenib. To investigate if regorafenib was able to overcome ABCB1-mediated MDR, human CRC cell line SW620 and its doxorubicin-resistant counterpart SW620/Ad300 were utilized in MTT assay. Strikingly, 10 μM regorafenib potently diminished the resistance fold of paclitaxel, doxorubicin, vincristine (ABCB1 substrates) in SW620/Ad300 cells (Table 1). At 10 μM, the reversal effect of regorafenib was stronger than that of verapamil (positive control inhibitor of ABCB1). No significant alterations were found in IC50 values for SW620 and SW620/Ad300 when either regorafenib or verapamil was combined with cisplatin (which is not a substrate of ABCB1).
Table 1.
The reversal effect of regorafenib and verapamil on the cytotoxicity of paclitaxel, doxorubicin, vincristine and cisplatin to SW620 and SW620/Ad300, HEK293/pcDNA3.1 and HEK/ABCB1 cell lines.
| Treatment | SW620 | SW620/Ad300 | HEK293/pcDNA3.1 | HEK/ABCB1 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| IC50 ± SDa (μM) | FRb | IC50 ± SD(μM) | FR | IC50 ± SD(μM) | FR | IC50 ± SD(μM) | FR | |
| Paclitaxel | 0.007 ± 0.001 | [1.0] | 4.397 ± 0.194 | [628.1] | 0.049 ± 0.004 | [1.0] | 4.519 ± 0.357 | [92.2] |
| +Regorafenib (5 μM) | 0.007 ± 0.002 | [1.0] | 0.057 ± 0.006* | [8.1] | 0.048 ± 0.003 | [1.0] | 0.384 ± 0.038* | [7.8] |
| +Regorafenib (10 μM) | 0.007 ± 0.001 | [1.0] | 0.023 ± 0.004* | [3.3] | 0.047 ± 0.004 | [1.0] | 0.131 ± 0.014* | [2.7] |
| +Verapamil (10 μM) | 0.007 ± 0.001 | [1.0] | 0.027 ± 0.003* | [3.9] | 0.047 ± 0.004 | [1.0] | 0.156 ± 0.021* | [3.2] |
| Doxorubicin | 0.060 ± 0.005 | [1.0] | 16.343 ± 0.611 | [272.4] | 0.058 ± 0.004 | [1.0] | 4.155 ± 0.402 | [71.6] |
| + Regorafenib (5 μM) | 0.058 ± 0.004 | [1.0] | 0.592 ± 0.080* | [9.9] | 0.056 ± 0.004 | [1.0] | 0.484 ± 0.051* | [8.3] |
| + Regorafenib (10 μM) | 0.061 ± 0.005 | [1.0] | 0.378 ± 0.011* | [6.3] | 0.056 ± 0.003 | [1.0] | 0.334 ± 0.022* | [5.8] |
| +Verapamil (10 μM) | 0.060 ± 0.004 | [1.0] | 0.382 ± 0.021* | [6.4] | 0.058 ± 0.004 | [1.0] | 0.352 ± 0.047* | [6.1] |
| Vincristine | 0.025 ± 0.002 | [1.0] | 4.259 ± 0.338 | [170.4] | 0.028 ± 0.003 | [1.0] | 2.626 ± 0.312 | [93.8] |
| + Regorafenib (5 μM) | 0.025 ± 0.003 | [1.0] | 0.182 ± 0.019* | [7.3] | 0.027 ± 0.002 | [1.0] | 0.241 ± 0.036* | [8.6] |
| + Regorafenib (10 μM) | 0.024 ± 0.002 | [1.0] | 0.075 ± 0.007* | [3.0] | 0.027 ± 0.003 | [1.0] | 0.084 ± 0.014* | [3.0] |
| +Verapamil (10 μM) | 0.025 ± 0.003 | [1.0] | 0.077 ± 0.008* | [3.1] | 0.027 ± 0.002 | [1.0] | 0.094 ± 0.017* | [3.4] |
| Cisplatin | 1.254 ± 0.245 | [1.0] | 1.274 ± 0.224 | [1.0] | 1.372 ± 0.202 | [1.0] | 1.322 ± 0.252 | [1.0] |
| + Regorafenib (5 μM) | 1.316 ± 0.284 | [1.0] | 1.243 ± 0.147 | [1.0] | 1.315 ± 0.306 | [1.0] | 1.352 ± 0.238 | [1.0] |
| + Regorafenib (10 μM) | 1.272 ± 0.171 | [1.0] | 1.252 ± 0.142 | [1.0] | 1.385 ± 0.189 | [1.0] | 1.339 ± 0.134 | [1.0] |
| +Verapamil (10 μM) | 1.301 ± 0.172 | [1.0] | 1.277 ± 0.154 | [1.0] | 1.362 ± 0.226 | [1.0] | 1.379 ± 0.253 | [1.0] |
IC50: concentration that inhibited cell survival by 50% (means ± SD).
FR: fold-resistance represents IC50 value for paclitaxel, doxorubicin, vincristine, and cisplatin of SW620 and SW620/Ad300, HEK293/pcDNA3.1 and HEK/ABCB1 cells in the absence or presence of regorafenib and verapamil was divided by IC50 value for paclitaxel, doxorubicin, vincristine, and cisplatin in parental SW620 or HEK293/pcDNA3.1 cells. Values in table are determined from at least three independent experiments performed in triplicate. Verapamil was used as a positive control of ABCB1 inhibition.
Indicates significant difference from IC50 of SW620/Ad300 or HEK/ABCB1 without reversal drug (*P<0.05); one-way ANOVA with Bonferroni post-test.
Chemotherapeutic MDR results from assorted mechanisms [45]. Hence HEK293/pcDNA3.1 and ABCB1-transfected HEK/ABCB1 cells were used to limit the experiment to study the role of ABCB1 in MDR. As anticipated, regorafenib produced a concentration-dependent suppression in ABCB1-mediated resistance to paclitaxel, doxorubicin and vincristine in HEK/ABCB1 cells (Table 1). Overall, regorafenib potently resensitized both ABCB1-overexpressing drug selected and transfected cells to ABCB1 substrate anticancer agents.
3.2. Regorafenib does not affect the expression level and cellular localization of ABCB1
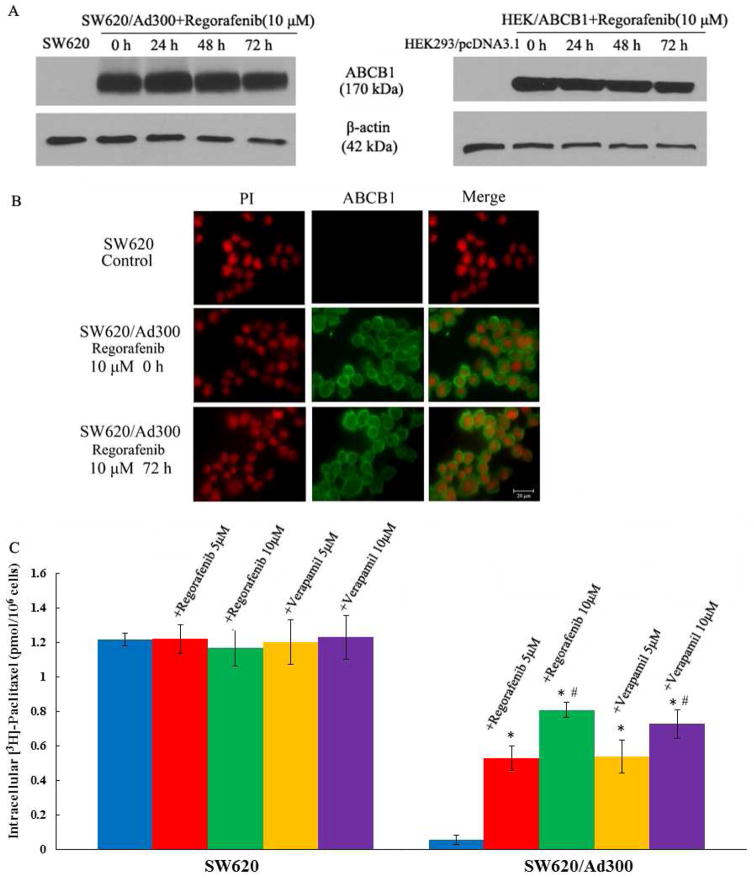
In order to demonstrate whether regorafenib circumvented ABCB1-mediated MDR via suppressing the protein expression level of ABCB1, cells were incubated with 10 μM regorafenib for 0, 24, 48 and 72 h. In both drug-selected SW620/Ad300 and transfected HEK/ABCB1 cells, we did not detect significant change in ABCB1 protein expression level (Fig. 1A). However, regorafenib might suppress the efficacy of ABCB1 by triggering its internalization from cell surface to cytosol. We performed the immunofluorescence assay to examine this possibility. The result suggested that regorafenib did not affect the localization of ABCB1 in SW620/Ad300 cells (Fig. 1B). The above findings inferred that regorafenib might functionally hinder ABCB1 transporter.
Figure 1.
(A) Western blot analysis showing the effect of regorafenib at 10 μM on the expression levels of ABCB1 in both SW620/Ad300 and HEK/ABCB1 cells for 0, 24, 48, and 72 h. Equal amounts (60 μg) of cell lysates were loaded into each well and subjected to Western blot analysis as described in the “Materials and Methods” section. Representative result is shown here and similar results were obtained in two other independent trials. (B) The immunofluorescence assays showing the effect of regorafenib at 10 μM on the subcellular localization of ABCB1 in SW620/Ad300 cells for 72 h. (C) The accumulation of [3H]-paclitaxel was measured after the cells were pre-incubated with or without regorafenib or verapamil for 2 h at 37 °C and then incubated with 0.01 mM [3H]-paclitaxel for another 2 h at 37 °C. Columns are the mean of triplicate determinations; error bars represent SD. *P < 0.05 versus control group (blue column, SW620/Ad300), #P < 0.05 versus group of 5 μM verapamil (yellow column, SW620/Ad300); one-way ANOVA with Bonferroni post-test.
3.3. Regorafenib raises the cellular accumulation of [3H]-paclitaxel
We performed accumulation assays to measure the amount of drug inside the cancer cells. Regorafenib significantly increased the intracellular accumulation of [3H]-paclitaxel in SW620/Ad300 as compared to SW620 cells (Fig. 1C). Consistent with MTT results, the intracellular accumulation of [3H]-paclitaxel in 10 μM regorafenib group was significantly higher than that in 10 μM verapamil group.
3.4. Regorafenib attenuates the efflux activity of ABCB1 transporter
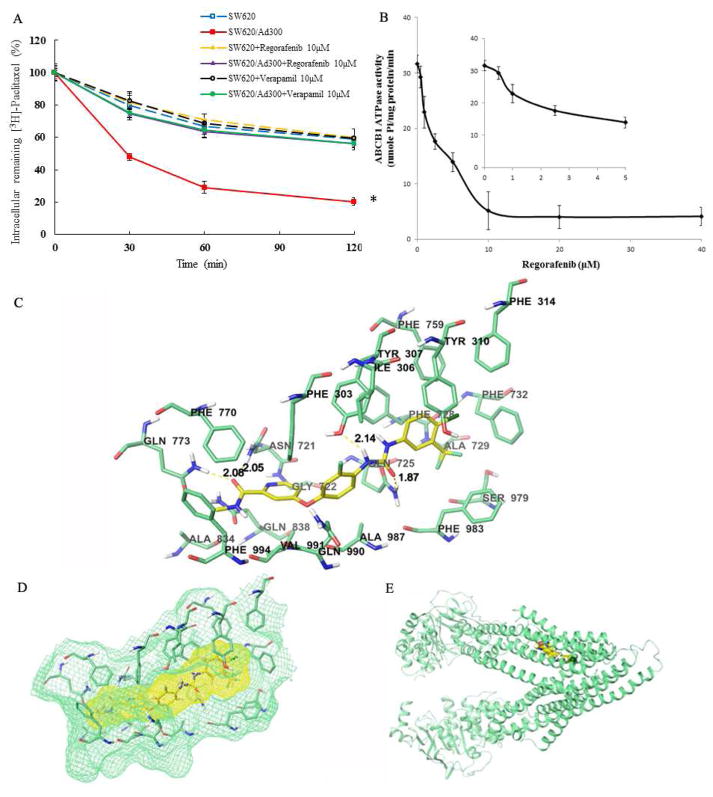
Drug efflux assay was utilized to determine whether the enhanced intracellular accumulation of [3H]-paclitaxel in resistant CRC cells was due to efflux inhibition of ABCB1 by regorafenib. Strikingly, ABCB1 transporters, which were overexpressed in SW620/Ad300 cells, actively pumped out a larger amount of [3H]-paclitaxel against the concentration gradient as compared to SW620 cells. However, regorafenib treatment efficiently attenuated the efflux of [3H]-paclitaxel through the inactivation of ABCB1 transporter. The remaining intracellular [3H]-paclitaxel level was raised from 20.6% to 56.2% after 10 μM regorafenib treatment for 120 min (Fig. 2A).
Figure 2.
(A) Effects of regorafenib or verapamil on the efflux of [3H]-paclitaxel from SW620 and SW620/Ad300 cells. Cells were pre-treated with or without regorafenib or verapamil at 10 μM for 2 h at 37 °C and further incubated with 0.01 mM [3H]-paclitaxel at 37 °C for 2 h. Cells were then incubated in fresh medium with or without the reversal agents for different time periods at 37 °C. Cells were then collected and the intracellular levels of [3H]-paclitaxel were determined by scintillation counting. A time course versus percentage of intracellular [3H]-paclitaxel remaining (%) was plotted (0, 30, 60, 120 min). Lines are the mean of triplicate determinations; error bars represent SD. *P < 0.05 versus control group (blue dash line); one-way ANOVA with Bonferroni post-test. Regorafenib is the test compound as an ABCB1 inhibitor. Verapamil is a positive control of ABCB1 inhibitor. (B) Crude membranes (100 μg protein/ml) from High-five cells expressing ABCB1 were incubated with increasing concentrations of regorafenib (0–40 μM), in the presence and absence of sodium orthovanadate (Vi) (0.3 mM), in ATPase assay buffer as described in the “Materials and Methods” section. Sodium orthovanadate is a selective inhibitor of ABCB1-transporter ATPase activity and is used to calculate the vanadate-sensitive activity by subtraction from the total ATPase activity. In this way, the ABCB1 transporter specific ATPase activity is obtained. The graph shows that ATPase activity was plotted with SD as a function of concentration of regorafenib. The inset shows stimulation of ATP hydrolysis at lower (0–5 μM) concentration of regorafenib. (C) Binding geometry of regorafenib into ABCB1 binding pocket by Glide docking algorithms. Interactions between regorafenib (carbon: yellow) and nearby residues (carbon: green) inside ABCB1. Dotted yellow line: hydrogen bonds. (D) Binding site surfaces of regorafenib-ABCB1 complex. (E) Overall view of regorafenib-ABCB1 complex.
3.5. Regorafenib interferes with ATPase activity of ABCB1
Since ABCB1 is an ATP energy-dependent, active transporter, we investigated whether and how regorafenib affected the ATP hydrolysis mediated by ABCB1. Interestingly, regorafenib inhibited the ATPase activity of ABCB1 in a concentration-dependent fashion, with a maximal inhibition of 88% of the basal activity. The inset in Fig. 2B demonstrates that the concentration of regorafenib required to obtain 50% inhibition is 3.73 ± 0.28 μM. The result disclosed that regorafenib suppressed the efflux capacity of ABCB1 transporters through the inhibition of ATPase activity of ABCB1, thereby overcoming MDR.
3.6. Interactions analysis of regorafenib-ABCB1 induced-fit docked complex
The best scored pose of regorafenib exhibited a high docking score of −12.963 kcal/mol inside the transmembrane domain of ABCB1. As shown in Figure 2C, the ligand regorafenib was well-fitted into a druggable cavity in TMD of human ABCB1. The 4-chloro-3-trifluoromethy phenyl ring was stabilized by nearby hydrophobic residues such as Tyr310, Phe314, Ala729, Phe728, Phe759, and Phe983. Residue Phe303 formed two π-π stacking interaction between 3-fluorophenyl ring and pyridine ring of regorafenib. Furthermore, four hydrogen bonding interactions were observed between regorafenib and ABCB1, suggesting high binding affinity of the regorafenib-ABCB1 complex. These hydrogen bonding interactions involve side chain hydroxyl group of Tyr307 (Tyr307-HO·· NH-, 2.14 Å), side chain amino group of Asn721 (Asn721-NH2·· O, 2.05 Å), side chain amino group of Gln725 (Gln725-NH2·· O, 1.87Å), and side chain amino group of Gln773 (Gln773-NH2·· O, 2.08 Å). Other nearby residues such as Ile306, Gly722, Phe770, Ala834, Gln838, Gln990, Val991, and Phe994 also contributed in forming the druggable cavity.
3.7. Synergistic antitumor effect of regorafenib and paclitaxel on resistant colorectal tumors
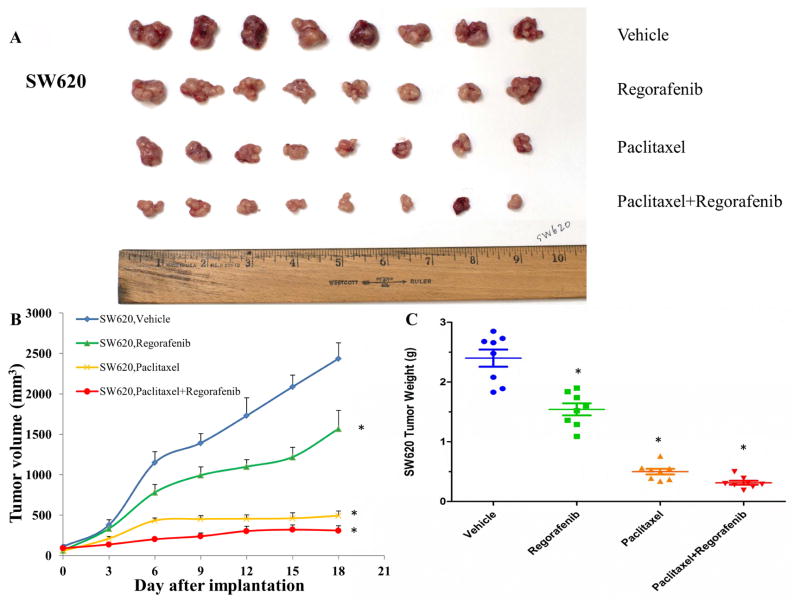
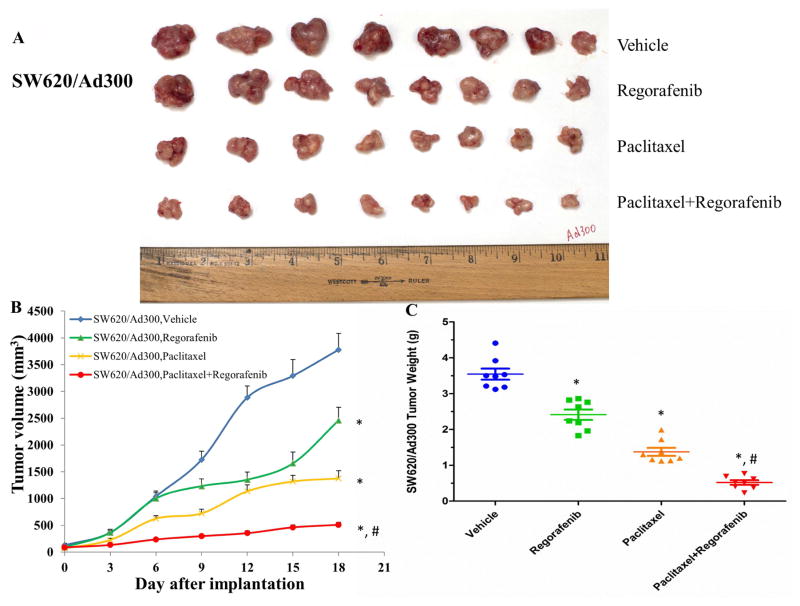
We translated the in vitro findings of regorafenib into the in vivo model. In parental colorectal tumor-bearing mice, combination of regorafenib and paclitaxel showed the additive antitumor effect on the growth of SW620 tumors (Fig. 3). Importantly, we observed synergistic antitumor effect of combination treatment on the growth of SW620/Ad300 tumors, which demonstrated that regorafenib overcame the resistance of paclitaxel to ABCB1-overexpressing tumors in mice (Fig. 4).
Figure 3. Effect of regorafenib and paclitaxel on the growth of SW620 tumors in nude athymic mice.
(A) The images of excised SW620 tumors implanted subcutaneously in athymic NCR nude mice (n = 8) that were treated with vehicle, regorafenib, paclitaxel, or the combination of regorafenib and paclitaxel, at the end of the 18-day treatment period. (B) The changes in tumor volume over time following the implantation. Data points represent the mean tumor volume for each treatment group (n = 8). Error bars, SEM. *P < 0.05 versus the vehicle group; #P < 0.05 versus the paclitaxel group; one-way ANOVA with Bonferroni post-test. (C) The mean weight (n = 8) of the excised SW620 tumors from the mice treated with vehicle, regorafenib, paclitaxel, or the combination of regorafenib and paclitaxel, at the end of the 18-day treatment period. Error bars, SEM. *P < 0.05 versus the vehicle group; #P < 0.05 versus the paclitaxel group; one-way ANOVA with Bonferroni post-test.
Figure 4. Effect of regorafenib and paclitaxel on the growth of SW620/Ad300 tumors in nude athymic mice.
(A) The images of excised SW620/Ad300 tumors implanted subcutaneously in athymic NCR nude mice (n = 8) that were treated with vehicle, regorafenib, paclitaxel, or the combination of regorafenib and paclitaxel, at the end of the 18-day treatment period. (B) The changes in tumor volume over time following the implantation. Data points represent the mean tumor volume for each treatment group (n = 8). Error bars, SEM. *P < 0.05 versus the vehicle group; #P < 0.05 versus the paclitaxel group; one-way ANOVA with Bonferroni post-test. (C) The mean weight (n = 8) of the excised SW620/Ad300 tumors from the mice treated with vehicle, regorafenib, paclitaxel, or the combination of regorafenib and paclitaxel, at the end of the 18-day treatment period. Error bars, SEM. *P < 0.05 versus the vehicle group; #P < 0.05 versus the paclitaxel group; one-way ANOVA with Bonferroni post-test.
3.8. Toxicity assessment of regorafenib in colorectal tumor-bearing mice
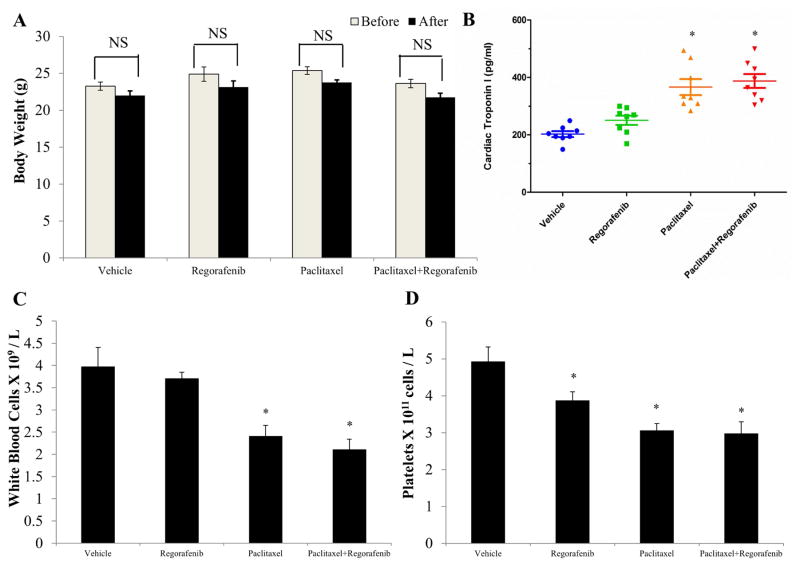
We demonstrated that regorafenib reversed chemotherapeutic MDR both in vitro and in vivo. Then toxicity of regorafenib was assessed in colorectal tumor-bearing mice. Administration of regorafenib only, paclitaxel only, and their combination did not significantly affect the body weight of colorectal tumor-bearing mice (Fig. 5A). Cardiomyopathy is reported to be a dangerous side effect of paclitaxel, resulting in congestive heart failure [46]. Cardiac troponin-I (cTnI) is a cardiac regulatory protein that controls the calcium mediated interaction between actin and myosin. Thus cTnI is considered to be a hallmark of heart muscle impairment [47]. Regorafenib did not significantly affect the cTnI concentrations as compared with the vehicle group. Mean cTnI concentration of the combination group was similar to that of paclitaxel group, suggesting that regorafenib alone was not cardiotoxic, and did not potentiate the side effect of paclitaxel in the combination treatment (Fig. 5B). Since myelosuppression (neutropenia and thrombocytopenia) are also common adverse effects of paclitaxel, we conducted a blood smear test to investigate the number of white blood cells (WBC) and platelets in mice. It has been reported that the normal range of WBC and platelets in mice are 1.32 x 109 ~ 8.38 x 109 WBC/L and 0.7 x 1011 ~ 12.0 x 1011 platelets/L [48]. The mean numbers of WBC and platelets in the four treatment groups were all in the normal range, suggesting that regorafenib alone and in combination with paclitaxel did not induce neutropenia or thrombocytopenia in mice (Fig. 5C and D).
Figure 5. Effect of regorafenib and paclitaxel on body weight, cardiac troponin I level, white blood cells and platelets in nude athymic mice.
(A) The changes in mean body weight of mice (n = 8) before and after the treatment. NS, not statistically significant (P > 0.05). (B) The changes in mean levels of cardiac troponin I in nude mice (n = 8) at the end of the 18-day treatment period. *P < 0.05 versus the vehicle group; #P < 0.05 versus the paclitaxel group; one-way ANOVA with Bonferroni post-test. (C) The changes in mean white blood cells in nude mice (n = 8) at the end of the 18-day treatment period. (D) The changes in mean platelets in nude mice (n = 8) at the end of the 18-day treatment period. *P < 0.05 versus the vehicle group; one-way ANOVA with Bonferroni post-test.
3.9. Regorafenib promotes accumulation of paclitaxel inside the resistant colorectal tumors
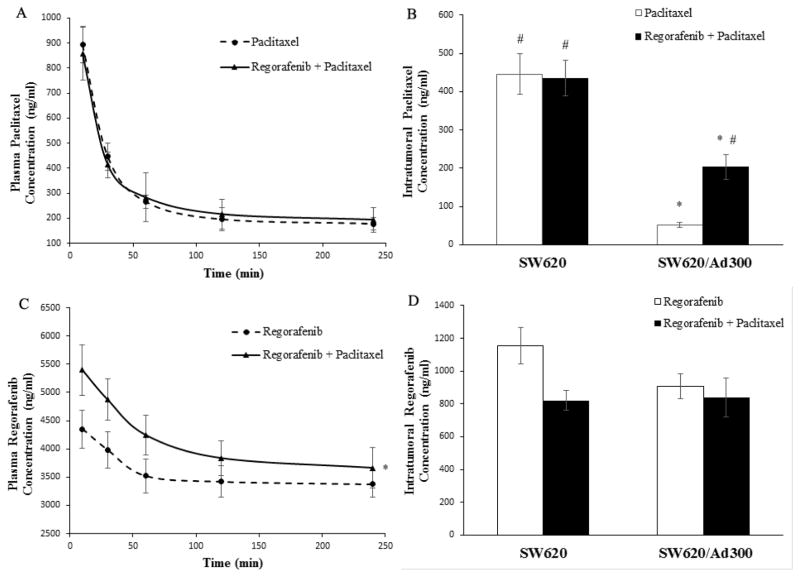
In order to understand the pharmacokinetic features of paclitaxel, plasma and intratumoral concentrations of paclitaxel were monitored in mice treated with paclitaxel only, and combination of paclitaxel and regorafenib. Regorafenib did not significantly alter the plasma concentrations of paclitaxel (Fig. 6A). Inside SW620 tumors, paclitaxel concentration of combination group was similar to that of paclitaxel alone group. Strikingly, regorafenib elevated the paclitaxel concentration within SW620/Ad300 resistant tumors as compared to paclitaxel only group (Fig. 6B). On the other hand, paclitaxel moderately increased the plasma concentration of regorafenib (Fig. 6C). Within SW620 tumors, regorafenib concentration of combination group was lower than that of regorafenib alone group. In SW620/Ad300 tumors, no significant difference of regorafenib concentration was observed between regorafenib group and combination group (Fig. 6D). Collectively, synergistic antitumor activity of regorafenib and paclitaxel on colorectal resistant tumors is primarily attributed to the inhibitory effect of regorafenib on ABCB1 efflux activity, thereby facilitating the paclitaxel accumulation within the resistant tumor microenvironment.
Figure 6. Pharmacokinetic characteristics of regorafenib and paclitaxel in nude athymic mice.
(A) Plasma paclitaxel concentrations in nude athymic mice at 10, 30, 60, 120, 240 min following administration of paclitaxel alone or a combination of regorafenib and paclitaxel (n=8). (B) Intratumoral paclitaxel concentrations in SW620 (n=8) and SW620/Ad300 tumors (n=8) after 240 min following administration of paclitaxel alone or a combination of regorafenib and paclitaxel (n=8). Columns and error bars represent mean ± SEM. *P < 0.05 versus the paclitaxel SW620 group; #P < 0.05 versus the paclitaxel SW620/Ad300 group; one-way ANOVA with Bonferroni post-test. (C) Plasma regorafenib concentrations in nude athymic mice at 10, 30, 60, 120, 240 min following administration of regorafenib alone or a combination of regorafenib and paclitaxel (n=8). *P < 0.05 versus the regorafenib group; Student’s t-test. (D) Intratumoral regorafenib concentrations in SW620 (n=8) and SW620/Ad300 tumors (n=8) after 240 min following administration of regorafenib alone or a combination of regorafenib and paclitaxel (n=8). Columns and error bars represent mean ± SEM. *P < 0.05 versus the regorafenib SW620 group; #P < 0.05 versus the regorafenib SW620/Ad300 group; one-way ANOVA with Bonferroni post-test.
4. Discussion
Acquired MDR represents a critical limitation of cancer chemotherapy. ABCB1 serves as a primary regulator in the distribution and elimination of chemotherapeutic agents, as well as intrinsic and acquired drug resistance of human cancers. Overexpression of ABCB1 in tumors is one of the most important mechanisms of MDR, which accelerates chemotherapeutics efflux out of tumor cells. Hence it’s stimulating more endeavor to develop modulators of ABCB1 that are capable of suppressing these transporters to elevate the amount of chemotherapeutics inside tumor cells. Nevertheless, it’s difficult to establish ABCB1 modulators as a therapeutic approach in clinical settings. For instance, the pharmacological inhibition of ABCB1 might change the pharmacokinetic features of chemotherapeutic agents (e.g., taxanes, anthracyclines and etoposide), resulting from nonspecific inhibition of hepatic and intestinal cytochrome P450 enzymes, along with other transporters [49,50]. Repositioning of commonly used tyrosine kinase inhibitor (TKI) as MDR-ABCB1 modulators, co-administered with conventional chemotherapy, might be a beneficial treatment for MDR cancer patients. The RAS/RAF/MEK/ERK pathway is one of the most deregulated pathways in human cancer, which is associated with increased cell proliferation, cell motility, angiogenesis, and resistance to apoptosis. Approximately 50% of CRC carries KRAS/BRAF mutations and the numbers are higher in more advanced tumors [51]. Regorafenib is the first small molecule inhibitor of this pathway that has been approved by the FDA for the treatment of mCRC. Although regorafenib extends overall survival rate of mCRC patients, its clinical efficacy is restricted. Combination chemotherapy becomes a promising strategy to circumvent MDR, potentiate antitumor efficacy and raise the disease-free/overall survival rate [52,53]. For instance, a combination of regorafenib and lapatinib suppressed the expression of anti-apoptotic protein Bcl-2, Mcl-1, XIAP, Survivin and increased the expression of pro-apoptotic proteins Bax, synergistically leading to apoptosis, human colorectal growth inhibition and cell cycle arrest [54]. A combination of regorafenib and irinotecan significantly delayed the growth of patient-derived CRC xenografts that were refractory to oxaliplatin and bevacizumab treatment [55]. Regorafenib exhibited antimetastatic activity in the murine MC38 CRC liver metastasis model through interference with the growth of formed liver metastases and establishment of new metastases in other organs [55]. It has been reported that regorafenib showed potent antiangiogenic, antitumorigenic and antimetastatic effects on aggressive colon carcinomas [56].
In the present study, MTT assays demonstrated that regorafenib significantly abrogated ABCB1-mediated MDR in both drug selected SW620/Ad300 cells and transfected HEK/ABCB1 cells. Furthermore, regorafenib increased the concentration of [3H]-paclitaxel in resistant SW620/Ad300 cells through inactivation of ABCB1 efflux, without altering the protein expression level and cellular localization of ABCB1. Regorafenib inhibited ABCB1 ATPase activity in a concentration-dependent manner. Docking study suggested that the ligand regorafenib was well-fitted into a druggable cavity in TMD of human ABCB1. By comparing the potency of anti-tumor effects between SW620 and SW620/Ad300 tumors, paclitaxel and combination treatment can shrink SW620 tumors by 4.8 times and 7.7 times as compared to vehicle control group. Furthermore, paclitaxel and combination treatment can inhibit the growth of SW620/Ad300 tumors by 2.6 times and 7.0 times. The only 2.6 times inhibition of paclitaxel on the SW620/Ad300 tumors is much smaller than the 4.8 times shrinkage of SW620 tumors by paclitaxel, revealing that the ABCB1-overexpressing SW620/Ad300 tumors are resistant to paclitaxel treatment. Interestingly, the 7.0 times inhibition of combination treatment is obviously stronger than the 2.6 times inhibition of paclitaxel on the SW620/Ad300 tumors. A combination of regorafenib and paclitaxel exerted synergistic inhibitory effect on the growth of resistant human CRC tumors in nude mice by markedly increasing the intratumoral concentration of paclitaxel. Moreover, regorafenib did not elicit the toxicity (cardiotoxicity/myelosuppression) of paclitaxel. Collectively, regorafenib is capable of blunting the efflux function of ABCB1 and enhance paclitaxel concentration within tumors, leading to a synergistic antitumor effect in combination with paclitaxel. This study establishes a clinical rationale for further research into the combination of conventional chemotherapy and targeted anticancer agents.
Highlights.
Regorafenib reverses the ABCB1-mediated multidrug resistance in vitro and in vivo.
Regorafenib inhibits the efflux activity of ABCB1 transporter.
Regorafenib and paclitaxel synergistically shrink resistant colorectal tumors.
Regorafenib does not induce cardiotoxicity/myelosuppression of paclitaxel in mice.
Acknowledgments
We thank Drs. Susan E. Bates and Robert W. Robey (NCI, NIH, Bethesda, MD) for providing SW620 and SW620/Ad300 cell lines, and Yangmin Chen (MediMedia Managed Markets, an ICON company, Yardley, PA) for editorial support.
Funding: This work was supported by funds from NIH (No. 1R15CA143701) and St. John’s University Research Seed Grant (No. 579-1110-7002) to Z.-S. Chen, the Chinese National Natural Science Foundation (No. 31271444 and 81201726), the Guangdong Natural Science Funds for Distinguished Young Scholar (No. 2014A030306001), the Guangdong Special Support Program for Young Talent (No. 2015TQ01R350), the Science and Technology Program of Guangdong (No. 2016A050502027) and the Foundation for Research Cultivation and Innovation of Jinan University (No. 21616119) to Z. Shi. S.S and S.V.A were supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors have declared no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shukla S, Wu C, Ambudkar SV. Development of inhibitors of ATP-binding cassette drug transporters–present status and challenges. Expert Opinion on Drug Metabolism & Toxicology. 2008;4:205–223. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Wu C, Hsieh C, Wu Y. The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Molecular Pharmaceutics. 2011;8:1996–2011. doi: 10.1021/mp200261n. [DOI] [PubMed] [Google Scholar]
- 3.Kathawala RJ, Gupta P, Ashby CR, Chen Z. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resistance Updates. 2015;18:1–17. doi: 10.1016/j.drup.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Holland IB. ABC transporters, mechanisms and biology: An overview. Essays Biochem. 2011;50:1–17. doi: 10.1042/bse0500001. [DOI] [PubMed] [Google Scholar]
- 5.Assaraf YG. Molecular basis of antifolate resistance. Cancer Metastasis Rev. 2007;26:153–181. doi: 10.1007/s10555-007-9049-z. [DOI] [PubMed] [Google Scholar]
- 6.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 8.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: Participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 9.Sodani K, Patel A, Kathawala RJ, Chen ZS. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31:58–72. doi: 10.5732/cjc.011.10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiwari AK, Sodani K, Dai C, Ashby CR, Chen Z. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:570–594. doi: 10.2174/138920111795164048. [DOI] [PubMed] [Google Scholar]
- 11.Sauna ZE, Smith MM, Müller M, Kerr KM, Ambudkar SV. The mechanism of action of multidrug-resistance-linked P-glycoprotein. J Bioenerg Biomembr. 2001;33:481–491. doi: 10.1023/a:1012875105006. [DOI] [PubMed] [Google Scholar]
- 12.Schinkel AH, Mayer U, Wagenaar E, et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A. 1997;94:4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez M, Paull K, Monks A, Hose C, Lee JS, Weinstein J, Grever M, Bates S, Fojo T. Generation of a drug resistance profile by quantitation of mdr-1/P-glycoprotein in the cell lines of the national cancer institute anticancer drug screen. J Clin Invest. 1995;95:2205–2214. doi: 10.1172/JCI117910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schondorf T, Kurbacher CM, Gohring UJ, Benz C, Becker M, Sartorius J, Kolhagen H, Mallman P, Neumann R. Induction of MDR1-gene expression by antineoplastic agents in ovarian cancer cell lines. Anticancer Res. 2002;22:2199–2203. [PubMed] [Google Scholar]
- 15.Xu X, Leo C, Jang Y, Chan E, Padilla D, Huang BC, Lin T, Gururaja T, Hitoshi Y, Lorens JB. Dominant effector genetics in mammalian cells. Nat Genet. 2001;27:23–29. doi: 10.1038/83717. [DOI] [PubMed] [Google Scholar]
- 16.Devine SE, Ling V, Melera PW. Amino acid substitutions in the sixth transmembrane domain of P-glycoprotein alter multidrug resistance. Proc Natl Acad Sci U S A. 1992;89:4564–4568. doi: 10.1073/pnas.89.10.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gros P, Dhir R, Croop J, Talbot F. A single amino acid substitution strongly modulates the activity and substrate specificity of the mouse mdr1 and mdr3 drug efflux pumps. Proc Natl Acad Sci U S A. 1991;88:7289–7293. doi: 10.1073/pnas.88.16.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mickley LA, Spengler BA, Knutsen TA, Biedler JL, Fojo T. Gene rearrangement: A novel mechanism for MDR-1 gene activation. J Clin Invest. 1997;99:1947–1957. doi: 10.1172/JCI119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leighton JC, Jr, Goldstein LJ. P-glycoprotein in adult solid tumors. expression and prognostic significance. Hematol Oncol Clin North Am. 1995;9:251–273. [PubMed] [Google Scholar]
- 20.Pérez-Sayáns M, Somoza-Martín JM, Barros-Angueira F, Diz PG, Rey JMG, García-García A. Multidrug resistance in oral squamous cell carcinoma: The role of vacuolar ATPases. Cancer Lett. 2010;295:135–143. doi: 10.1016/j.canlet.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Marie JP. P-glycoprotein in adult hematologic malignancies. Hematol Oncol Clin North Am. 1995;9:239–249. [PubMed] [Google Scholar]
- 22.Verrelle P, Meissonnier F, Fonck Y, Feillel V, Dionet C, Kwiatkowski F, Plagne R, Chassagne J. Clinical relevance of immunohistochemical detection of multidrug resistance P-glycoprotein in breast carcinoma. J Natl Cancer Inst. 1991;83:111–116. doi: 10.1093/jnci/83.2.111. [DOI] [PubMed] [Google Scholar]
- 23.Kelly RJ, Robey RW, Chen CC, Draper D, Luchenko V, Barnett D, Oldham RK, Caluag Z, Frye AR, Steinberg SM. A pharmacodynamic study of the P-glycoprotein antagonist CBT-1® in combination with paclitaxel in solid tumors. Oncologist. 2012 doi: 10.1634/theoncologist.2012-0080. theoncologist. 2012–0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunz PL, He AR, Colevas AD, Pishvaian MJ, Hwang JJ, Clemens PL, Messina M, Kaleta R, Abrahao F, Sikic BI. Phase I trial of ixabepilone administered as three oral doses each separated by 6 hours every 3 weeks in patients with advanced solid tumors. Invest New Drugs. 2012;30:2364–2370. doi: 10.1007/s10637-012-9800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien MM, Lacayo NJ, Lum BL, Kshirsagar S, Buck S, Ravindranath Y, Bernstein M, Weinstein H, Chang MN, Arceci RJ. Phase I study of valspodar (PSC-833) with mitoxantrone and etoposide in refractory and relapsed pediatric acute leukemia: A report from the children’s oncology group. Pediatric Blood & Cancer. 2010;54:694–702. doi: 10.1002/pbc.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Patel A, Ma S, Li XJ, Zhang Y, Yang P, Kathawala RJ, Wang Y, Anreddy N, Fu L. In vitro, in vivo and ex vivo characterization of ibrutinib: A potent inhibitor of the efflux function of the transporter MRP1. Br J Pharmacol. 2014;171:5845–5857. doi: 10.1111/bph.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demetri GD, Reichardt P, Kang Y, Blay J, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M, Joensuu H. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch K, Zopf D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. International Journal of Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 30.Chen D, Wei L, Yu J, Zhang L. Regorafenib inhibits colorectal tumor growth through PUMA-mediated apoptosis. Clin Cancer Res. 2014;20:3472–3484. doi: 10.1158/1078-0432.CCR-13-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Kathawala RJ, Zhang Y, Patel A, Kumar P, Shukla S, Fung KL, Ambudkar SV, Talele TT, Chen Z. Motesanib (AMG706), a potent multikinase inhibitor, antagonizes multidrug resistance by inhibiting the efflux activity of the ABCB1. Biochem Pharmacol. 2014;90:367–378. doi: 10.1016/j.bcp.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Kathawala RJ, Wang Y, Zhang Y, Patel A, Shukla S, Robey RW, Talele TT, Ashby CR, Ambudkar SV. Linsitinib (OSI-906) antagonizes ATP-binding cassette subfamily G member 2 and subfamily C member 10-mediated drug resistance. Int J Biochem Cell Biol. 2014;51:111–119. doi: 10.1016/j.biocel.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang Y, Wang Y, Vispute SG, Jain S, Chen Y, Li J, Youssef DT, Sayed KAE, Chen Z. Esters of the marine-derived triterpene sipholenol A reverse P-GP-mediated drug resistance. Marine Drugs. 2015;13:2267–2286. doi: 10.3390/md13042267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo HQ, Zhang GN, Wang YJ, Zhang YK, Sodani K, Talele TT, Ashby CR, Jr, Chen ZS. Beta-elemene, a compound derived from rhizoma zedoariae, reverses multidrug resistance mediated by the ABCB1 transporter. Oncol Rep. 2014;31:858–866. doi: 10.3892/or.2013.2870. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Wang Y, Zhang Y, Wang D, Kathawala RJ, Patel A, Talele TT, Chen Z, Fu L. AST1306, a potent EGFR inhibitor, antagonizes ATP-binding cassette subfamily G member 2-mediated multidrug resistance. Cancer Lett. 2014;350:61–68. doi: 10.1016/j.canlet.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–514. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- 37.Zhang YK, Zhang GN, Wang YJ, Patel BA, Talele TT, Yang DH, Chen ZS. Bafetinib (INNO-406) reverses multidrug resistance by inhibiting the efflux function of ABCB1 and ABCG2 transporters. Sci Rep. 2016;6:25694. doi: 10.1038/srep25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang YK, Zhang H, Zhang GN, Wang YJ, Kathawala RJ, Si R, Patel BA, Xu J, Chen ZS. Semi-synthetic ocotillol analogues as selective ABCB1-mediated drug resistance reversal agents. Oncotarget. 2015;6:24277–24290. doi: 10.18632/oncotarget.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherman W, Day T, Jacobson MP, Friesner RA, Farid R. Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem. 2006;49:534–553. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- 40.Wang YJ, Huang Y, Anreddy N, Zhang GN, Zhang YK, Xie M, Lin D, Yang DH, Zhang M, Chen ZS. Tea nanoparticle, a safe and biocompatible nanocarrier, greatly potentiates the anticancer activity of doxorubicin. Oncotarget. 2016;7:5877–5891. doi: 10.18632/oncotarget.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YJ, Patel BA, Anreddy N, et al. Thiazole-valine peptidomimetic (TTT-28) antagonizes multidrug resistance in vitro and in vivo by selectively inhibiting the efflux activity of ABCB1. Sci Rep. 2017;7:42106. doi: 10.1038/srep42106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Wang DS, Patel A, Shukla S, et al. Icotinib antagonizes ABCG2-mediated multidrug resistance, but not the pemetrexed resistance mediated by thymidylate synthase and ABCG2. Oncotarget. 2014;5:4529–4542. doi: 10.18632/oncotarget.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anreddy N, Patel A, Zhang YK, et al. A-803467, a tetrodotoxin-resistant sodium channel blocker, modulates ABCG2-mediated MDR in vitro and in vivo. Oncotarget. 2015;6:39276–39291. doi: 10.18632/oncotarget.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kathawala RJ, Wei L, Anreddy N, et al. The small molecule tyrosine kinase inhibitor NVP-BHG712 antagonizes ABCC10-mediated paclitaxel resistance: A preclinical and pharmacokinetic study. Oncotarget. 2015;6:510–521. doi: 10.18632/oncotarget.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calcagno AM, Ambudkar SV. Molecular mechanisms of drug resistance in single-step and multi-step drug-selected cancer cells. Methods Mol Biol. 2010;596:77–93. doi: 10.1007/978-1-60761-416-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Missov E, Calzolari C, Pau B. Circulating cardiac troponin I in severe congestive heart failure. Circulation. 1997;96:2953–2958. doi: 10.1161/01.cir.96.9.2953. [DOI] [PubMed] [Google Scholar]
- 47.Wang TJ. Significance of circulating troponins in heart failure: If these walls could talk. Circulation. 2007;116:1217–1220. doi: 10.1161/CIRCULATIONAHA.107.721845. [DOI] [PubMed] [Google Scholar]
- 48.Nemzek J, Bolgos G, Williams B, Remick D. Differences in normal values for murine white blood cell counts and other hematological parameters based on sampling site. Inflammation Res. 2001;50:523–527. doi: 10.1007/PL00000229. [DOI] [PubMed] [Google Scholar]
- 49.Chen KG, Sikic BI. Molecular pathways: Regulation and therapeutic implications of multidrug resistance. Clin Cancer Res. 2012;18:1863–1869. doi: 10.1158/1078-0432.CCR-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baer MR, George SL, Dodge RK, et al. Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and leukemia group B study 9720. Blood. 2002;100:1224–1232. [PubMed] [Google Scholar]
- 51.He K, Chen D, Ruan H, Li X, Tong J, Xu X, Zhang L, Yu J. BRAFV600E-dependent mcl-1 stabilization leads to everolimus resistance in colon cancer cells. Oncotarget. 2016;7:47699–47710. doi: 10.18632/oncotarget.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diyabalanage HV, Granda ML, Hooker JM. Combination therapy: Histone deacetylase inhibitors and platinum-based chemotherapeutics for cancer. Cancer Lett. 2013;329:1–8. doi: 10.1016/j.canlet.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang W, Li Y, Wei M, Chen Y, Qiu J, Jiang Q, Yang Y, Zheng D, Qin W, Huang J. Synergistic antitumor activity of regorafenib and lapatinib in preclinical models of human colorectal cancer. Cancer Lett. 2017;386:100–109. doi: 10.1016/j.canlet.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Schmieder R, Hoffmann J, Becker M, Bhargava A, Müller T, Kahmann N, Ellinghaus P, Adams R, Rosenthal A, Thierauch K. Regorafenib (BAY 73-4506): Antitumor and antimetastatic activities in preclinical models of colorectal cancer. International Journal of Cancer. 2014;135:1487–1496. doi: 10.1002/ijc.28669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, Lederle W. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12:1322–1331. doi: 10.1158/1535-7163.MCT-12-1162. [DOI] [PubMed] [Google Scholar]