Abstract
Background
Marked functional impairment has been reported by patients with Post- Treatment Lyme Disease Syndrome (PTLDS), but the clinical features which contribute most strongly to the impaired health status remain unknown.
Methods
Enrolled patients had a well-documented history of Lyme disease, prior treatment with at least 3 weeks with IV ceftriaxone, a positive IgG Western blot, and objective problems with memory. An index score to capture aggregate cognitive functioning, Short-Form 36 (SF-36) physical and mental component scores, and scores on other clinical and demographic measures were examined. Multiple linear regressions were performed to determine significant predictors of perceptions of impaired life functioning as delineated by the SF-36.
Results
Fatigue was the most important contributor to perceived impairments in overall physical functioning and fatigue and depression significantly predicted perceived impairments in overall mental functioning.
Conclusions
Because fatigue and depression contribute prominently to reports of impaired physical and mental functioning among patients with PTLDS, clinicians should assess carefully for these symptoms and consider targeting these symptoms in the selection of treatment interventions. Future controlled studies should examine the effectiveness of such agents for patients with PTLDS
Lyme borreliosis, caused by the tick-borne pathogen Borrelia burgdorferi, is a multi-systemic illness that can adversely affect the skin, joints, heart, eyes, and nervous system.1 In most cases, when Lyme borreliosis is recognized early, treatment is successful with a short course of antibiotics. However, treatment is less effective when infection is not recognized early, with a subset of patients reporting persistent and marked symptoms.2, 3 Some of these patients with persistent symptoms will have objective evidence of disease, such as those with chronic Lyme arthritis4 or those with post-treatment Lyme encephalopathy (PTLE).5 Others who do not have objectively measurable somatic markers of disease are said to have Post-Treatment Lyme Disease Syndrome (PTLDS);6 these patients typically report chronic subjective pain, fatigue, and/or cognitive impairment despite having received standard courses of antibiotic therapy.2, 5
The symptoms of PTLDS are not specific and have been observed in other illnesses such as chronic fatigue syndrome (CFS)7 and fibromyalgia.8 For example, moderate to severe fatigue is an essential component of CFS and is also often a key component of PTLDS. In all three disorders, these symptoms are quite debilitating – representing more than the normal aches and fatigue of daily life. Differences between these disorders however are worth noting. The pain in PTLDS may be more focused than in fibromyalgia, centering on certain joints or in the extremities due to a neuropathy.3 The cognitive deficits in PTLDS may be more prominent than in fibromyalgia9 and CFS,10 most often affecting memory, verbal fluency, and psychomotor speed, but also in some reports involving working memory and fine motor control.11, 12, 13, 14, 5 Finally, biological differences may be found between these syndromes; for example, a recent study demonstrated that the cerebrospinal fluid (CSF) proteomic profile of patients with post-treatment Lyme encephalopathy differs from the CSF profile of those with CFS with 692 unique proteins in PTLE and 738 unique proteins in CFS.15
Two principal views have emerged regarding the etiology of these persistent symptoms; one attributes symptoms to persistent infection,16 while the other posits a delayed immunologic response to prior infection or remnants of infection.17, 18 Because the etiology of post-treatment symptoms is uncertain and most likely heterogeneous with some patients having active infection and others a post-infectious process, for this article we will use the term “Post-Treatment Lyme Disease Syndrome (PTLDS)” if objective cognitive impairment is not present (or unmeasured) and “post-treatment Lyme encephalopathy (PTLE)” if objective cognitive impairment is present.
Research has shown that in addition to experiencing debilitating symptoms, patients with PTLDS often report subjectively impaired functioning in the mental and physical domains.19, 5, 2, 20 One study examined associations between clinical features that appear early in the illness and functional outcome in PTLDS patients, but did not examine the influence of prominent clinical variables that manifest after treatment.21 Knowing the extent to which commonly reported symptoms such as cognitive impairment, pain, fatigue, or psychopathology contribute to functional impairment would enable more targeted therapies for patients with persistent symptoms, while further elucidating their specific nature and impact in PTLDS.
The current study focuses on patients with objective cognitive impairment that persists after treatment for well-defined Lyme disease. This sample of patients with PTLE was used to determine the clinical correlates of perceived health status as measured by the SF-36. We explored the impact of two prominent physical symptoms (pain and fatigue), psychopathology (depression and anxiety), and a summary index of cognition. For these clinical variables, we chose commonly reported persistent symptoms that best captured the multisystemic nature of the illness.22 Due to the severity of symptoms of pain and fatigue among these patients,5 we hypothesized that these symptoms would have a strong negative impact on perceptions of physical functional status. Given the impaired functioning typically associated with depression and anxiety and the limitations imposed by cognitive deficits in PTLE, we hypothesized that these variables would have a negative association with self-reported mental functioning.
Methods
Patients
37 patients with a history of Lyme disease and cognitive impairment (“encephalopathy”) who had participated in a randomized placebo-controlled treatment trial of persistent Lyme encephalopathy5 were included in this study. Results of the treatment study have been described previously.5 Briefly, between 2000 and 2004, individuals between the ages of 18 to 65 years with a history of Lyme disease were recruited. All enrolled patients had a well-documented diagnosis of Lyme disease based on the Center for Disease Control (CDC) defined clinical and serologic surveillance criteria, a positive IgG Western blot for Lyme disease at the time of entry, a history of persistent cognitive impairment despite at least 3 weeks of intravenous (IV) ceftriaxone therapy, and subjective and objective evidence for memory impairment. Subjective memory impairment was based on self-report, with all patients complaining of problems with memory. Objective impairment was determined based on impaired performance of at least 1 SD below age-, gender-, and education-adjusted norms on core subtests of the Wechsler Memory Scale (WMS) III. This level of impairment can be considered moderate and was used to define probable Lyme encephalopathy prior to study entry. Patients were excluded if they had pre-existing medical, psychiatric, or neurologic conditions that could account for cognitive impairment or if they had an allergy to ceftriaxone. Within the context of the clinical trial patients received 10 weeks of randomized IV treatment with either ceftriaxone or placebo. See Table 1 for descriptive statistics on subject characteristics. The research protocol was approved by the New York State Psychiatric Institute and all patients signed informed consent prior to study participation.
Table 1.
Descriptive Statistics on 37 Patients with Post-treatment Lyme Encephalopathy
| Mean (+/− SD) | |
|---|---|
| Age | 45.47 (12.9) |
| Gender (% male) | 43.24 |
| Education Level | 14.69 (2.5) |
| Fatigue Score | 5.30 (1.3) |
| Beck Depression Score | 11.76 (8.0) |
| Zung Anxiety Score | 47.87 (10.7) |
| McGill Pain Score | 5.05 (3.3) |
| Cognitive Index score | −.41 (0.6) |
| SF-36 Physical Composite Score | 35.74 (8.4) |
| SF-36 Mental Composite Score | 41.09 (11.2) |
Assessments
All self-report assessments and cognitive testing were collected prior to the initiation of treatment in the clinical trial. While the screening battery for enrollment employed the Wechsler Memory Scale to identify objective memory impairment, the cognitive tests for the treatment study used a neurocognitive battery that has been shown to be sensitive to change and which focused on six cognitive domains: motor function (tests: finger tapping, simple reaction time, choice reaction time), psychomotor function (tests: Trail Making A and B, digit symbol), attention (tests: continuous performance test, Stroop task), memory (Bushke Selective Reminding Test, Benton Visual Retention Test), working memory (A not B logical reasoning test, N-Back test), and verbal fluency (Controlled Oral Word Association Test, Category Fluency Test). Information on these tests can be found in several outside sources.23, 24 Z-scores were obtained for patients’ performance on each of these tests using age-, gender-, and education-adjusted norms.5 Domain-specific scores were then calculated by averaging the z-scores for the tests within each respective cognitive domain. Cognitive index scores were obtained by averaging the domain scores for each patient; these scores were used to capture neurocognitive functioning within the context of this study. For psychopathology, the Beck Depression Inventory II25 and the Zung Anxiety Index26 were used. For energy, the Fatigue Severity Scale27 was used. For pain, the visual analog scale (VAS) for current pain of the McGill Pain Questionnaire28 was used. To capture baseline perceived health status and functioning, the two SF-36 summary indices were used: the Physical Component Summary (PCS) and the Mental Component Summary (MCS).29, 30 See Table 1 for the descriptive statistics on these measures.
Data Analysis
Statistical analysis was conducted using the software SPSS version 20 (Statistical Package for Social Sciences Inc., Chicago, IL, USA). To determine whether a significant relationship existed between demographic and clinical variables and functional status as determined by scores on the SF-36, stepwise multiple linear regression analyses were conducted. Each regression examined the relative contribution to the SF-36 of the key variables: neurocognitive functioning as summarized by the cognitive index, psychopathology (Beck Depression Score, Zung Anxiety Index Score), physical symptomology (McGill Pain Score, Fatigue Severity Score), age and gender. The outcome dependent variables were the two summary indices of the SF-36 subscales: Physical Composite Score (PCS) and Mental Composite Score (MCS).
Results
The 37 patients in this study were on average middle aged with 2 years of college, slightly more women than men. Their physical symptom self-report scales revealed moderate to severe levels of fatigue and pain. Psychopathology was evident but not prominent, with minimal to mild levels of depression and anxiety. Although these patients were recruited for memory impairment, their cognitive index score revealed only a mild level of global cognitive impairment. Functional impairment however was severe, worse in the physical realm than the mental realm.
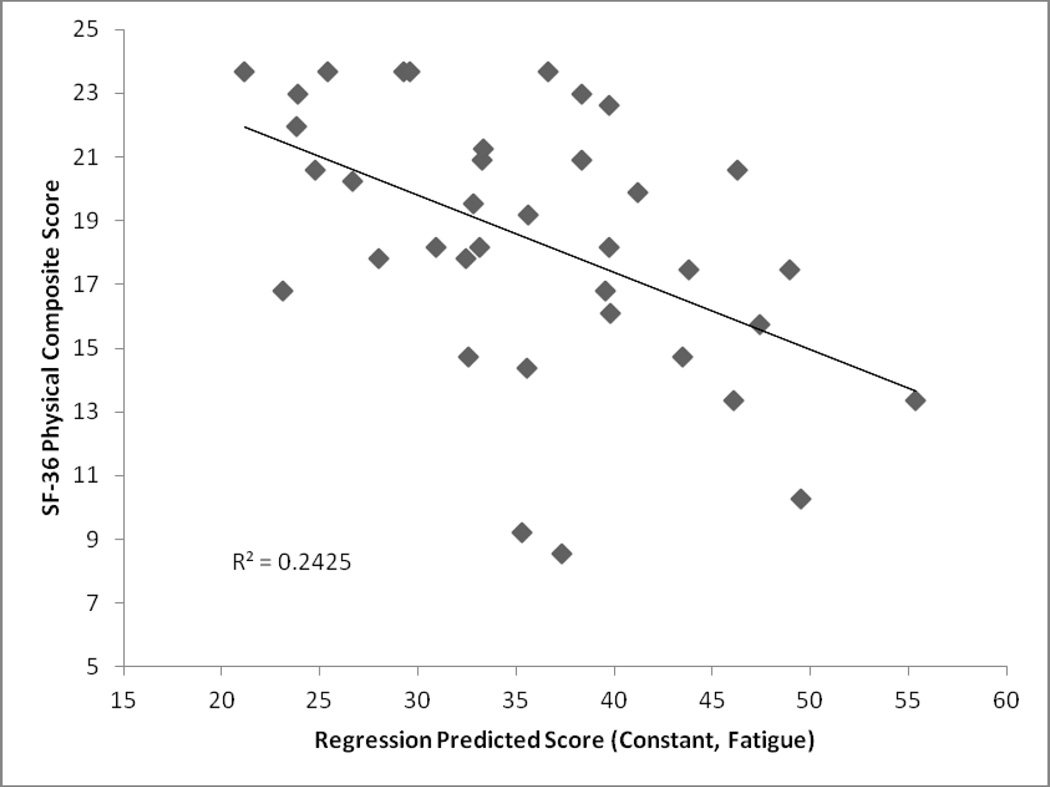
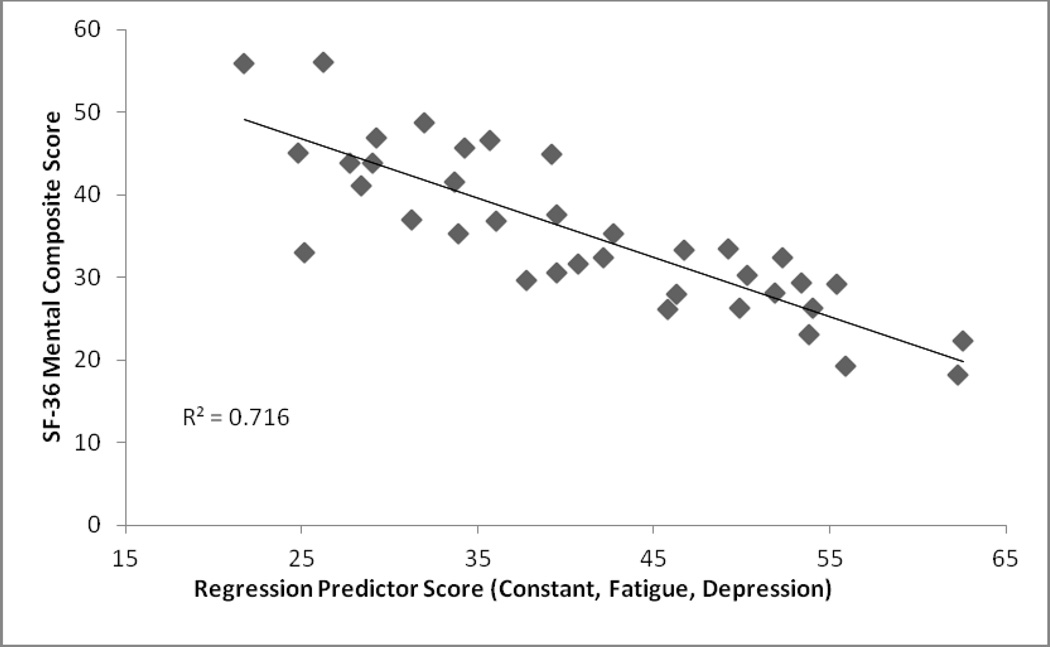
The stepwise regression revealed that only the Fatigue Severity Score was significantly associated with the Physical Composite Score. Results from this regression can be found in Table 2 and a graphical representation can be found in Figure 1. It was also found that the Beck Depression Score and the Fatigue Severity Score were significantly associated with the Mental Composite Score. Results from this regression can be found in Table 3 and a graphical representation can be found in Figure 2.
Table 2.
Stepwise Regression Results for Primary Outcome Variables (PCS, & MCS)
| Model 1 (DV: PCS) |
B | Standard Error |
β | t | p value |
|---|---|---|---|---|---|
| Constant | 52.124 | 5.044 | 10.333 | .000 | |
| Fatigue Severity Score | −3.092 | .924 | −.492 | −3.347 | .002 |
R2 of this model is .24(F (1, 35) = 11.20, p<.05)
VIF = 1; Durbin-Watson Test Statistic = 2.14
Figure 1.
Stepwise Regression Results: Physical Composite Score (Dependent Variable; Regression Score was Scaled to Reflect Direction of Component Scales)
Table 3.
Stepwise Regression Results for Primary Outcome Variables (PCS, & MCS)
| Model 2 (DV: MCS) |
B | Standard Error |
β | t | p value |
|---|---|---|---|---|---|
| Constant | 63.638 | 4.249 | 14.976 | .000 | |
| Beck Depression Score | −.969 | .148 | −.692 | −6.541 | .000 |
| Fatigue Severity Score | −2.106 | .891 | −.250 | −2.364 | .024 |
R2 of this model is .72 (F (2, 34) = 42.86, p<.05)
VIF = 1.34; Durbin-Watson Test Statistic = 1.6
Figure 2.
Stepwise Regression Results: Mental Composite Score (Dependent Variable; Regression Score was Scaled to Reflect Direction of Component Scales)
It was inferred from relatively low variance inflation factors (VIFs) that multicollinearity was not a significant issue.31 In addition, Durban-Watson test statistics were consistently above one, indicating that autocorrelation of errors wasn’t a problem.32 These values can be found in Tables 2 and 3.
Discussion
The current study sought to determine the correlates of perceptions of impaired life functioning, as quantified by SF-36 Physical and Mental Component Scales, in a sample of patients with post-treatment Lyme encephalopathy.5 Results demonstrate that fatigue is the most important contributor to the physical component scale (PCS), accounting for 24.2% of the variance, and that both symptoms of fatigue and depression were significant contributors to the mental component scale (MCS), accounting for 71.6% of the variance. However, no significant associations were found between cognitive functioning, symptoms of anxiety, or symptoms of pain and perceptions of health status. While it is clear that the measures of fatigue and depression together account for most of the variance in the mental functioning score, it is also clear that most of the variance in physical functioning was not accounted for by the variables we entered into the regression equation. This may be because the selected clinical variables were not the right ones, or this may have occurred because the measures were not sensitive or inclusive enough with respect to the full range of symptomatology in PTLE or PTLDS. For example, pain was assessed using a visual analog scale. While the VAS has been well-validated in pain research, there are other more detailed self-report measures of pain as well as experimental assays of pain tolerance (e.g., pressure pain or thermal pain thresholds) that may have been preferable for inclusion.
These results underscore the prominence of fatigue as a disabling symptom among patients with PTLDS. Persistent physical exhaustion and overwhelming tiredness contribute to impaired physical and mental health. In addition to vitality, the composite summary scales for mental and physical functioning of the SF-36 include items that assess social functioning, one’s ability to fulfill necessary roles due to affective and bodily issues, persistent psychological distress, and one’s inability to care for oneself.33 The magnitude of fatigue in PTLDS is high – similar to that seen among patients with Multiple Sclerosis (MS)27 and comparable to Systematic Lupus Erythematosus (SLE).34 However, the fatigue in PTLDS is not as circumstance-dependant as in MS.34 Recent research indicates that patients with PTLDS may have an ongoing hyper-activated immune response with levels of anti-neuronal antibodies comparable to what has been reported among patients with SLE,35 as well as elevated markers of interferon-α activity;36 it is reasonable therefore to hypothesize that the fatigue among patients with PTLDS may be mediated partially by this immune-activated state.
The results of this study should lead clinicians to focus on treatment interventions designed to target persistent symptoms of fatigue. Current literature suggests that cognitive-behavioral strategies37 and pharmacologic strategies38 can be effective among some patients with persistently fatigued states. Whether additional antibiotic therapy among PTLDS patients with moderate to marked fatigue is indicated is an area of controversy in the medical literature. While the current guidelines of the IDSA39 and the American Academy of Neurology40 do not recommend repeated antibiotic therapy – partly due to the adverse risks associated with intravenous administration, these guidelines have been criticized for failing to encourage clinicians to discuss the actual study results with patients so that an informed risk-benefit decision could be made. A patient with marked to severe fatigue that impairs functioning may conclude that the possibility of a sustained reduction of fatigue as a result of repeated antibiotic therapy, as was demonstrated in one controlled study41 and supported by a second controlled study5, may outweigh the risk of a serious adverse event from an indwelling catheter.42, 43
The observation that symptoms of depression were a major contributor to deteriorated perceptions of mental health status is of clinical importance. While a high prevalence of depression has been documented among patients with PTLDS,22, 44 the impact of depressive symptoms on perceived health and functioning in PTLDS has not been well-studied. The items covering mental health functioning on the SF-36 that might be impacted by these symptoms include carelessness and reduced time in activities, accomplishing less than expected, and a reduction in the extent and time in interpersonal events.33 These study findings highlight the need to include appropriate psychopharmacological and psychological interventions for depression in treating symptoms of PTLDS. Clinicians should carefully assess depression among patients with PTLDS, perhaps with the assistance of self-report scales such as the Beck Depression Inventory25 or the Patient Health Questionnaire-9.45 Although antibiotic treatment may result in an improvement in depressive symptoms among patients with active infection with Borrelia burgdorferi, it should not be assumed that antibiotic treatment alone will be adequate to treat depression among patients with PTLDS. Research studies indicate that some patients who develop depression after acquiring Lyme disease may have depressive symptoms that persist despite having received considerable prior antibiotic treatment.19, 44 Because there are effective treatments for depression, patients with PTLDS may find that their lives are markedly improved as a result of such treatment, enabling them to cope with remaining somatic symptoms in a more effective way.
The clinical variables of interest in this study may interact. For example, fatigue can lead to poor motivation or effort on cognitive testing. Fatigue has also previously been correlated with memory impairment in patients with PTLDS,12 although the current study did not find a significant correlation between fatigue and global cognitive impairment (r=0.10). Depression may lead to decreased vitality, as was demonstrated by a significant correlation between depressive symptoms and fatigue (r=.50; p<.01). Depression may also lead to poor cognitive performance, but the correlation was not significant in our dataset (r=−.01). Finally, depression and pain are often related, as was shown by a significant correlation (r=.46, p<.01). The degree of variance however accounted for by these correlations is low, supporting their use as independent variables in the primary regression analysis. The relationship between depression, fatigue and pain do suggest however that interventions targeting depressive symptoms in patients with PTLDS may, to some degree, also attenuate their fatigue and reduce pain.
Of note in this study is that the neurocognitive index summary score did not contribute significantly to the level of perceived mental or physical functional impairment. One possible explanation for this finding is that the cognitive deficits among these patients were only mild in severity. In addition, the sample size for this study was too small to be able to detect the contribution to impaired functioning of variables with lesser impact. Another possible explanation is that the SF-36 is not an adequate or sensitive measure of functional impairment in the cognitive realm. The SF-36 is a measure of health-related functional status that focuses in particular on physical and emotional health; as such, it may not have been constructed to detect the deficits in functioning seen among patients with mild to moderate cognitive impairment.
While the results of this report are compelling, they should be interpreted with caution. The patients enrolled in this study, while diagnosed using highly rigorous criteria for the definition of post-treatment Lyme encephalopathy, may be too narrowly defined to reflect the full population of patients with PTLDS. In addition, because the cohort for this report came from a prior treatment trial with explicit enrollment criteria, patients were excluded for many reasons, including negative serologic tests at screening, pre-existing psychiatric and neurologic conditions that might cause memory impairment, and current memory impairment that was not at least moderate in severity. These exclusionary criteria further limit the generalizability of our results to the larger community of patients with PTLDS. Finally, the sample size was small for the statistical analysis used in this study, and other variables that affect perceptions of health status may not have been recognized because their contributions to mental and health perceptions are more modest. Nonetheless, this is a rigorously defined sample of patients representing a disorder about which there is a great deal of controversy. Our goal was to identify those factors that had the largest and most significant effects on perceptions of health status, in order to assist clinicians who treat these patients.
In conclusion, the current study demonstrates that fatigue is the largest contributor to impaired health-related functional status in both the physical and mental realm in patients with PTLDS and that depression plays a significant role in reducing perceived mental functioning. Future studies should be undertaken to test these findings in larger patient samples, with a particular focus on developing treatment strategies for these disabling symptoms.
Sample Questions from the SF-3646.
Compared to one year ago, how much would you rate your health in general now?
- The following items are about activities you might do during a typical day. Does your health now limit you in these activities? If so, how much?
- Vigorous activities, such as running, lifting heavy objects, participating in strenuous sports
- Moderate activities, such as moving a table, pushing a vacuum cleaner, bowling, or playing golf
- Lifting or carrying groceries
- Walking more than a mile
- Bathing or dressing yourself
- During the past 4 weeks, have you had any of the following problems with your work or other regular daily activities as a result of your physical health?
- Cut down the amount of time you spent on work or other activities
- Accomplished less than you would like
- Were limited in the kind of work or other activities
During the past 4 weeks, to what extent has your physical health or emotional problems interfered with your normal social activities with family, friends, neighbors, or groups?
- How TRUE or FALSE is each of the following statements for you?
- I seem to get sick a little easier than other people
- I am as healthy as anybody I know
- I expect my health to get worse
- My health is excellent
Acknowledgments
Disclosure Statement
This study was conducted through the support of NINDS R01 NS38636 to Dr. Fallon and with the support of the Lyme and Tick-borne Diseases Research Center at Columbia University Medical Center established by the Lyme Disease Association, Inc and Lyme Research Alliance, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345(2):115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Shadick NA, Phillips CB, Sangha O, Logigian EL, Kaplan RF, Wright EA, et al. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Ann Intern Med. 1999;131(12):919–926. doi: 10.7326/0003-4819-131-12-199912210-00003. [DOI] [PubMed] [Google Scholar]
- 3.Cairns V, Godwin J. Post-Lyme borreliosis syndrome: a meta-analysis of reported symptoms. Int J Epidemiol. 2005;34(6):1340–1345. doi: 10.1093/ije/dyi129. [DOI] [PubMed] [Google Scholar]
- 4.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4(2):143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 5.Fallon BA, Keilp JG, Corbera KM, Petkova E, Britton CB, Dwyer E, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70(13):992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Post-Treatment Lyme Disease Syndrome. 2013 Retrieved from http://www.cdc.gov/lyme/postLDS/index.html.
- 7.Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741–746. [PubMed] [Google Scholar]
- 8.Helfenstein M, Jr, Goldenfum MA, Siena CA. Fibromyalgia: Clinical and occupational aspects. Rev Assoc Med Bras. 2012;58(3):358–365. [PubMed] [Google Scholar]
- 9.Kaplan RF, Meadows ME, Vincent LC, Logigian EL, Steere AC. Memory impairment and depression in patients with Lyme encephalopathy: comparison with fibromyalgia and nonpsychotically depressed patients. Neurology. 1992;42:1263. doi: 10.1212/wnl.42.7.1263. [DOI] [PubMed] [Google Scholar]
- 10.Gaudino EA, Coyle PK, Krupp LB. Post-Lyme syndrome and chronic fatigue syndrome. Neuropsychiatric similarities and differences. Arch Neurol. 1997;54(11):1372–1376. doi: 10.1001/archneur.1997.00550230045015. [DOI] [PubMed] [Google Scholar]
- 11.Keilp JG, Corbera KM, Slavov I, Taylor MJ, Sackeim HA, Fallon BA. WAIS-III and WMS-III performance in chronic Lyme disease. J Int Neuropsychol Soc. 2006;12(1):119–129. doi: 10.1017/S1355617706060231. [DOI] [PubMed] [Google Scholar]
- 12.Krupp LB, Masur D, Schwartz J, Coyle PK, Langenbach LJ, Fernquist SK, et al. Cognitive functioning in late Lyme borreliosis. Arch Neurol. 1991;48(11):1125–1129. doi: 10.1001/archneur.1991.00530230033017. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan RF, Trevino RP, Johnson GM, Levy L, Dornbush R, Hu LT, et al. Cognitive function in posttreatment Lyme disease: Do additional antibiotics help? Neurology. 2003;60(12):916–1922. doi: 10.1212/01.wnl.0000068030.26992.25. [DOI] [PubMed] [Google Scholar]
- 14.Shadick NA, Phillips CV, Logigian EL, Steere AC, Kaplan RF, Berardi VP, et al. The long-term clinical outcomes of Lyme disease: A population-based retrospective cohort study. Ann Intern Med. 1994;121(8):560–567. doi: 10.7326/0003-4819-121-8-199410150-00002. [DOI] [PubMed] [Google Scholar]
- 15.Schutzer SE, Angel TE, Liu T, Schepmoes AA, Clauss TR, Adkins JN, et al. Distinct cerebrospinal fluid proteomes differentiate post-treatment lyme disease from chronic fatigue syndrome. PLoS One. 2011;6(2):e17287. doi: 10.1371/journal.pone.0017287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stricker RB. Counterpoint: long-term antibiotic therapy improves persistent symptoms associated with lyme disease. Clin Infect Dis. 2007;45(2):149–157. doi: 10.1086/518853. [DOI] [PubMed] [Google Scholar]
- 17.Rupprecht TA, Koedel U, Fingerle V, Pfister HW. The pathogenesis of Lyme Neuroborreliosis: From infection to inflammation. Mol Med. 2008;14(3–4):205–212. doi: 10.2119/2007-00091.Rupprecht. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest. 2012;122(7):2652–2660. doi: 10.1172/JCI58813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345(2):85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
- 20.Aucott JN, Rebman AW, Crowder LA, Kortte KB. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: is there something here? Qual Life Res. 2013;22(1):75–78. doi: 10.1007/s11136-012-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eikeland R, Mygland A, Herlofson K, Ljostad U. Risk factors for a non-favorable outcome after treated European neuroborreliosis. Acta Neurol Scand. 2013;127(3):154–160. doi: 10.1111/j.1600-0404.2012.01690.x. [DOI] [PubMed] [Google Scholar]
- 22.Fallon BA, Nields JA. Lyme disease: A neuropsychiatric illness. Am J Psychiatry. 1994;151(11):1571–1580. doi: 10.1176/ajp.151.11.1571. [DOI] [PubMed] [Google Scholar]
- 23.Sackeim HA, Keilp JG, Rush AJ, George MS, Marangell LB, Dormer JS, et al. The effects of vagus nerve stimulation on cognitive performance in patients with treatment resistant depression. Neuropsychiatry Behav Neurol. 2001;14:53–56. [PubMed] [Google Scholar]
- 24.Keilp JG, Sackheim H, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Res. 2005;135:191–201. doi: 10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Beck A, Steer RA. Beck Depression Inventory Manual. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- 26.Zung W. A rating scale for anxiety disorders. Psychosomatics. 1971;12:371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 27.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 28.Melzack E. The short-form McGill Pain Questionnaires. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston: The Health Institute; 1994. [Google Scholar]
- 30.Ware J, Kosinski M. SF-36, Manual for Users, Version 1. Boston: The Health Institute; 1994. [Google Scholar]
- 31.Bowerman BL, O’Connell RT. Linear statistical models: An applied approach (2nd ed.) Belmont, CA: Druxbury; 1990. [Google Scholar]
- 32.Durbin J, Watson GS. Testing for serial correlation in least squares regression, II. Biometrika. 1951;30(1–2):159–178. [PubMed] [Google Scholar]
- 33.Ware JE. SF-36 Health Survey Update. Spine. 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz JE, Jandorf L, Krupp LB. The measurement of fatigue: A new instrument. J Psychosom Res. 1993;37(7):753–762. doi: 10.1016/0022-3999(93)90104-n. [DOI] [PubMed] [Google Scholar]
- 35.Chandra A, Wormser GP, Klempner MS, Trevino RP, Crow MK, Latov N, et al. Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms. Brain Behav Immun. 2010;24(6):1018–1024. doi: 10.1016/j.bbi.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacek E, Fallon BA, Chandra A, Crow MK, Wormser GP, Alaedini A. Increased IFNα activity and differential antibody response in patients with a history of Lyme disease and persistent cognitive deficits. Neuroimmunol. 2013;255(1–2):85–91. doi: 10.1016/j.jneuroim.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet. 2011;377(9768):823–836. doi: 10.1016/S0140-6736(11)60096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabkin JG, McElhiney MC, Rabkin R. Treatment of HIV-related fatigue with armodafinil: a placebo-controlled randomized trial. Psychosomatics. 2011;52(4):328–336. doi: 10.1016/j.psym.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 40.Halperin JJ, Shapiro ED, Logigian E, Belman AL, Dotevall L, Wormser GP, et al. Practice parameter: treatment of nervous system Lyme disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2007;69(1):91–102. doi: 10.1212/01.wnl.0000265517.66976.28. [DOI] [PubMed] [Google Scholar]
- 41.Krupp LB, Hyman LG, Grimson R, Coyle PK, Melville P, Ahnn S, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003;60(12):1923–1930. doi: 10.1212/01.wnl.0000071227.23769.9e. [DOI] [PubMed] [Google Scholar]
- 42.Fallon BA, Petkova E, Keilp JG, Britton CB. A reappraisal of the u.s. Clinical trials of post-treatment lyme disease syndrome. Open Neurol J. 2012;6:79–87. doi: 10.2174/1874205X01206010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stricker RB, Green CL, Savely VR, Chamallas SN, Johnson L. Safety of intravenous antibiotic therapy in patients referred for treatment of neurologic Lyme disease. Minerva Med. 2010;101(1):1–7. [PubMed] [Google Scholar]
- 44.Hassett AL, Radvanski DC, Buyske S, Savage SV, Gara M, Escobar JI, et al. Role of psychiatric comorbidity in chronic Lyme disease. Arthritis Rheum. 2008;59(12):1742–1749. doi: 10.1002/art.24314. [DOI] [PubMed] [Google Scholar]
- 45.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hays RD. The Medical Outcomes Study (MOS) Measures of Patient Adherence. 1994 Retrieved from http://www.rand.org/health/surveys/MOS.adherence.measures.pdf. [Google Scholar]