Abstract
Importance
Extramammary Paget disease (EMPD) is commonly refractory to surgical and non-surgical therapies. Identifying recurrent/persistent disease is challenging as the disease is multifocal and multiple blind scouting biopsies are usually performed in this setting. Handheld reflectance confocal microscopy (HRCM) has been used to diagnose and map primary EMPD, and therefore may be used to identify EMPD recurrences.
Objectives
To evaluate the diagnostic accuracy of HRCM in the setting of recurrent/persistent EMPD and to evaluate its potential diagnostic pitfalls.
Design
Prospective study including patients between 2014–2016 with biopsy-proven EMPD in whom HRCM was used to monitor treatment response.
Setting
Dermatology Service, Memorial Sloan Kettering Cancer Center, New York
Participants
Five patients were included, and 22 sites clinically concerning for recurrent/persistent disease were evaluated using HRCM and histology. In 2 patients, videomosaics were created to evaluate large areas.
Main Outcomes and Measures
We calculated the sensitivity and specificity of HRCM in identifying recurrent/persistent EMPD. We also reviewed the causes for false negatives according to its location, histopathologic findings and previous treatments.
Results
HRCM had a sensitivity of 75% and a specificity of 100% in identifying recurrent/persistent EMPD. The false negatives occurred at the margins of EMPD close to previous biopsies. Videomosaicking seemed to improve the detection of EMPD.
Conclusions and Relevance
HRCM is a useful auxiliary tool to diagnose EMPD recurrences and can be used to guide scouting biopsies, thus reducing the number of biopsies needed to render a correct diagnosis.
Keywords: reflectance confocal microscopy, extramammary Paget disease, surgery, radiation therapy, imiquimod
MANUSCRIPT
Extramammary Paget disease (EMPD) is a rare cutaneous adenocarcinoma that is difficult to visually assess due to its nonspecific clinical appearance and multifocal growth.1 Surgery is the mainstay of treatment for EMPD as nonsurgical therapy responses are variable and difficult to monitor. However, disease recurrence is common both after surgical and non-surgical approaches.1 As such, mapping skin biopsies are often performed to assess therapeutic response.2
Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that allows visualization of the epidermis and papillary dermis with cellular-level resolution.3 RCM has been utilized as a diagnostic adjunct for mammary Paget disease and EMPD.2,4–10 Traditional wide-probe RCM (Vivascope 1500, Caliber ID, Rochester, NY) requires attaching a metal ring onto the skin which is challenging on the genitalia. Conversely, the handheld RCM (HRCM) Vivascope 3000 (Caliber ID, Rochester, NY) allows free-form translation on the skin along any spontaneously user-chosen unconstrained path, permitting its use on curved areas such as the genitalia. Recently, HRCM has been reported to guide surgical management of EMPD.4 RCM has also been used to monitor other types of skin cancer such as lentigo maligna after therapies such as imiquimod.11 However, no studies have evaluated HRCM in monitoring EMPD after treatment. The aim of this study is to evaluate the diagnostic accuracy of HRCM in the setting of recurrent EMPD and its potential pitfalls.
Methods
After approval from the institutional review board from Memorial Sloan Kettering Cancer Center, we prospectively included patients referred to our service between 2014–2016 with biopsy-proven EMPD in whom HRCM was used to monitor treatment response. HRCM was used to evaluate areas clinically concerning for active disease. In 3 cases, adhesive paper rings (product number 1529; 3M, Flemington, NJ) were placed at the clinically-defined areas to facilitate imaging localization.12 Stacks of images were taken at clinically-suspicious areas. In the last 2 cases, videos were taken in the en-face planes at different depths and converted into videomosaics. To obtain the videomosaics, we extracted the video frames and using a novel algorithm based on our previous studies13 we stitched them together to compose an overall mosaic of the imaged area.
As per previous studies,5,6,8 we considered the site to be RCM-positive if we identified Paget cells (PC) (dark holes 1–2 times the size of keratinocytes, or target structures with bright center and surrounding dark halo) in the epidermis or forming nests at the dermoepidermal junction. Epidermal disarray or increased dermal vessels were considered supportive features but not specific for the diagnosis of EMPD.
Biopsies were taken either at areas that were positive after HRCM evaluation or at areas that were highly suspicious for recurrence, such as eroded areas or intensely erythematous areas. Histopathological results were later correlated with the HRCM findings.
Results
We included five patients (4 men, 1 woman) with a median age of 70 years (range 56–77 years). One patient had an associated internal organ malignancy; four patients received previous treatments including surgery, radiotherapy, imiquimod and a HER2 tyrosine kinase inhibitor (HER2-TKI) (Table 1).
Table 1.
Summary of the demographic and clinical findings and its confocal and histological diagnosis.
| Case no. | Gender | Ethnicity | Concomitant malignancy | Date of RCM imaging | Treatments for EMPD prior to RCM | Clinical description and location | Margin or center of lesion | RCM diagnosis | Histopathology diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | Caucasian | None | April, 2016 | Surgery (2014) | Erythematous macule, anterior perineum | Margin | Negative | Negative |
| Erythematous macule, right perineum | Margin | Negative | Negative | ||||||
| Erythematous macule, posterior perineum | Margin | EMPD | EMPD | ||||||
| 2 | Female | Caucasian | None | June, 2014 | Surgery (2014) | Erythematous macule, mons pubis | Margin | EMPD | EMPD |
| November, 2014 | Recurrent EMPD treated with imiquimod (2014) | Approximate area of previous biopsy, mons pubis | Margin | Negative | EMPD | ||||
| Erythematous patch, labia minora | Margin | EMPD | EMPD | ||||||
| 3 | Male | Caucasian | None | June, 2015 | Radiotherapy (2013) | Erythematous patch, left anterior scrotum | Margin | Negative | Negative |
| Erythematous patch, left inferior scrotum | Center | EMPD | EMPD | ||||||
| Erythematous patch, left mid inguinal crease | Margin | Negative | Negative | ||||||
| Erythematous patch, anterior perineum | Margin | Negative | Negative | ||||||
| Erythematous patch, posterior perineum | Margin | Negative | Negative | ||||||
| Erythematous patch, right lateral scrotum | Margin | Negative | Negative | ||||||
| January, 2016 | Recurrent EMPD treated with oral HER2-TKI (2015) | Erythematous patch, left inferior scrotum next to biopsy scar | Margin | Negative | EMPD | ||||
| Erythaematous patch, left inferior scrotum below biopsy scar | Margin | Negative | EMPD | ||||||
| 4 | Male | Caucasian | Papillary renal cell carcinoma | January, 2016 | Surgery (2011), residual EMPD treated with RT (2011) | Erythematous patch, penile shaft | Margin | EMPD | EMPD |
| Erythematous patch, left scrotum | Center | EMPD | EMPD | ||||||
| 5 | Male | Caucasian | None | April, 2016 | WLE (January, 2015); Mohs surgery (October, 2015) | Erythematous patch, right anterior scrotum | Margin | EMPD | EMPD |
| Erythematous patch, left anterior scrotal margin | Margin | EMPD | EMPD | ||||||
| Erythematous patch, center of scrotum | Center | EMPD | EMPD | ||||||
| Eroded erythematous patch, right posterior scrotum | Center | Negative | Negative | ||||||
| Erythematous macule, outside right border of the inguinal crease | Margin | Negative | Negative | ||||||
| Erythematous macule, margin of previous graft in the right ventral penile shaft | Margin | Negative | Negative |
Abbreviations: EMPD, extramammary Paget disease; HER2-TKI, HER2 tyrosine kinase inhibitor; RT, radiotherapy; WLE: wide local excision, RCM, reflectance confocal microscopy
In total, 22 clinically suspicious sites (4 in the center and 18 at the margins) were interrogated with HRCM and subsequently biopsied. Of those, 9 sites were positive on HRCM and histologically confirmed (Figure 1E, F). Conversely, 13 sites were negative on HRCM; of these, 3 were positive for EMPD on histological examination (Table 1). Overall, the sensitivity of HRCM in identifying recurrent/persistent disease was 75% and the specificity was 100%. The false negatives (FN) were found in two patients who had received topical imiquimod 5% (case 2) and radiotherapy followed by oral HER2-TKI 240 mg daily (case 3) prior to assessment.
Figure 1.
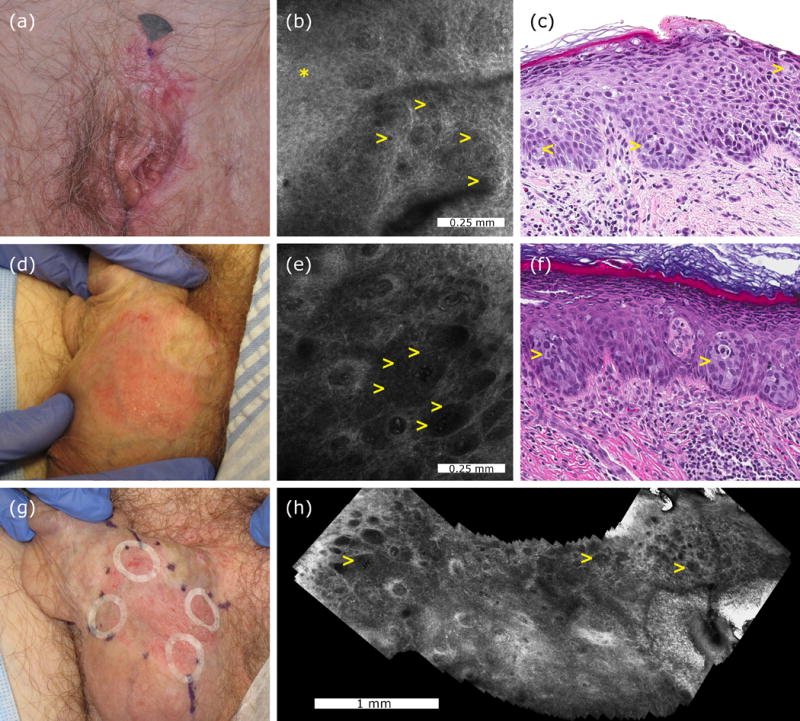
Representative cases of a confocal false-negative extramammary Paget disease, and a confocal true-positive extramammary Paget disease. Clinical appearance of a vulvar recurrent extramammary Paget disease (case 2, panel a). Confocal examination of the mons pubis was interpreted as negative although after reevaluation focal dark holes (yellow arrowheads) were identified in the stratum spinosum (asterisk) (b). Histologically, case 2 showed scattered Paget cells (yellow arrowheads) in the inflamed and spongiotic epidermis (H&E, 20× magnification, panel c). Clinical appearance of a scrotal extramammary Paget disease prior to treatment (case 4, panel d). Confocal examination revealed multiple target cells (yellow arrowheads) with bright centre and peripheral dark halo forming nests at the dermoepidermal junction (e). Histological examination showed large cells with pale cytoplasm forming nests (yellow arrows) in the lower epidermis confirming the diagnosis of extramammary Paget disease (H&E, 20× magnification, panel f). Adhesive paper rings were placed at 12, 3, 6 and 9 o’clock to improve confocal navigation, and later moved to adjacent areas to cover the entire margins and to reduce sampling bias (g). Confocal videomosaic taken at the dermoepidermal junction captured inside one paper ring to facilitate identification of large nests of Paget cells (yellow arrows) over a larger field of view (h).
When assessing the location of the biopsies, all the lesions biopsied in the center were correctly identified with HRCM, whereas the 3 FN occurred in the margins. The FN occurred in areas located close to previous biopsy sites (Table 1). To identify any additional cause for misdiagnosis, RCM images and histology slides from the FN were reviewed. The patient treated with imiquimod (case 2; Figure 1A) had focal dark holes on RCM in the stratum spinosum (Figure 1B) that corresponded to scattered individual PC within the epidermis on histopathology (Figure 1C). Conversely, the patient treated with HER2-TKI had unequivocal EMPD on histology that was not identified on RCM.
Discussion
Our results suggest that HRCM is useful to identify recurrent/persistent EMPD and to guide scouting biopsies due to its high specificity. This is particularly useful as it may reduce the number of biopsies needed to render a correct diagnosis in such sensitive location. However, negative HRCM findings should be interpreted with caution when monitoring treatment response as FN may occur.
In case 2, we identified lower PC density within the epidermis, making visualization of PC difficult with HRCM. We hypothesize that this may result from the effect of skin-directed therapies on the epidermis, and the fact that the area evaluated was located at the lesion margin where lower density of PC is expected compared to the center. However, this was not true for the false positives in case 3, also located on the margins. In addition, the three false negatives were close to recent biopsy sites, suggesting that scarring may obscure the identification of PC on HRCM. However, after histological review of the FN, no significant fibrosis was present. Hence, the most plausible explanation for FN seems sampling bias as PC are hyporeflective on RCM and can be easily missed when sparse.
To overcome sampling bias, in the last cases we have used an innovative approach combining adhesive paper rings (Figure 1G) and videomosaicking (Figure 1H). A common challenge with HRCM is locating on the skin the findings identified on the screen. This is especially relevant in EMPD as PC are distributed multifocally over a broad and curved area. Recently, Marino et al have used adhesive paper rings to locate the boundaries of a given lesion during HRCM.12 After this publication, we have been using adhesive paper rings also to delineate the margins of large lesions and facilitate HRCM navigation. In cases 4 and 5, we placed adhesive paper rings at areas of clinical interest, and we obtained stacks and videos within the paper rings. The videos were later converted into videomosaics and reviewed in order to identify PC.
Videomosaicking allows the visualization of large areas (centimeters) making the identification of focal features easier than when evaluating the native HRCM small field of view (1×1 mm to 0.75×0.75 mm depending on the device generation). Therefore, videomosaicking combined with adhesive paper rings may be very valuable to identify and locate scattered PC. Interestingly, no FN occurred when this approach was used. However, we acknowledge that our sample is very small and further studies are needed to assess the impact on reducing sampling bias by using this approach. In addition, newer videomosaicking algorithms including real-time mosaic composition and integration of the method into the RCM software are needed to achieve diagnostic assessment at the bedside.
Conclusion
HRCM can be a very useful auxiliary tool to diagnose EMPD recurrences but may fail to identify foci of scattered PC. Reassuringly, we have not had false positive cases, suggesting that HRCM can be used as an alternative to biopsy in EMPD cases with positive confocal findings, potentially reducing the need for confirmatory skin biopsies.
KEY POINTS.
-
-
Question: Is handheld reflectance confocal microscopy (HRCM) useful to identify recurrent/persistent extramammary Paget disease (EMPD)?
-
-
Findings: In this prospective study we included 5 patients with previously treated EMPD and evaluated 22 clinically suspicious sites with HRCM and histology. HRCM had a sensitivity of 75% and a specificity of 100% in identifying recurrent/persistent EMPD.
-
-
Meaning: HRCM is a useful auxiliary tool to diagnose EMPD recurrences and can be used to guide scouting biopsies, thus reducing the number of biopsies needed to render a correct diagnosis in such sensitive location.
Acknowledgments
Funding/Support: This research was funded by the NIH/NCI grant R01CA199673, and partially by the NIH/NCI Cancer Center Support Grant P30 CA008748 and the Beca Excelencia Fundación Piel Sana.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
| Funding/Sponsor was involved? | ||
| Design and conduct of the study | Yes__ | No X |
| Collection, management, analysis and interpretation of data | Yes__ | No X |
| Preparation, review, or approval of the manuscript | Yes__ | No X |
| Decision to submit the manuscript for publication | Yes__ | No X |
Footnotes
Author Contributions: Drs Yélamos and Jain had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Yélamos, Jain, Marchetti, Cordova, Rossi.
Acquisition, analysis, and interpretation of data: Cordova, Jain, Hibler, Rossi, Yélamos, Hollmann, Pulitzer. Drafting of the manuscript: Yélamos, Jain, Marchetti, Hibler, Cordova, Hollmann, Myskowski, Rossi, Kose, Rajadhyaksha. Critical revision of the manuscript for important intellectual content: Jain, Marchetti, Cordova, Rossi, Kose, Hollmann, Rajadhyaksha. Statistical analysis: Yélamos. Obtained funding: Yélamos, Rajadhyaksha. Administrative, technical, or material support: Pulitzer, Hollmann, Cordova. Study supervision: Jain, Marchetti.
Financial Disclosure: Milind Rajadhyaksha is a former employee of and owns equity in Caliber Imaging and Diagnostics (formerly Lucid Inc.), the company that manufactures and sells the Vivascope confocal microscope. The Vivascope is the commercial version of an original laboratory prototype that he had developed at Massachusetts General Hospital, Harvard Medical School. The other authors have no disclosures or conflicts of interest to report.
References
- 1.Lopes Filho LL, Lopes IM, Lopes LR, Enokihara MM, Michalany AO, Matsunaga N. Mammary and extramammary Paget’s disease. An Bras Dermatol. 2015;90(2):225–231. doi: 10.1590/abd1806-4841.20153189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim BJ, Park SK, Chang H. The Effectiveness of Mapping Biopsy in Patients with Extramammary Paget’s Disease. Arch Plast Surg. 2014;41(6):753–758. doi: 10.5999/aps.2014.41.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajadhyaksha M, Gonzalez S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113(3):293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 4.Terrier JE, Tiffet O, Raynaud N, Cinotti E. In Vivo Reflectance Confocal Microscopy Combined With the “Spaghetti” Technique: A New Procedure for Defining Surgical Margins of Genital Paget Disease. Dermatol Surg. 2015;41(7):862–864. doi: 10.1097/DSS.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 5.Longo C, Fantini F, Cesinaro AM, Bassoli S, Seidenari S, Pellacani G. Pigmented mammary Paget disease: dermoscopic, in vivo reflectance-mode confocal microscopic, and immunohistochemical study of a case. Arch Dermatol. 2007;143(6):752–754. doi: 10.1001/archderm.143.6.752. [DOI] [PubMed] [Google Scholar]
- 6.Guitera P, Scolyer RA, Gill M, et al. Reflectance confocal microscopy for diagnosis of mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2013;27(1):e24–29. doi: 10.1111/j.1468-3083.2011.04423.x. [DOI] [PubMed] [Google Scholar]
- 7.Debarbieux S, Dalle S, Depaepe L, Jeanniot PY, Poulalhon N, Thomas L. Extramammary Paget’s disease of the scalp: examination by in vivo and ex vivo reflectance confocal microscopy. Skin Res Technol. 2014;20(1):124–126. doi: 10.1111/srt.12087. [DOI] [PubMed] [Google Scholar]
- 8.Pan ZY, Liang J, Zhang QA, Lin JR, Zheng ZZ. In vivo reflectance confocal microscopy of extramammary Paget disease: diagnostic evaluation and surgical management. J Am Acad Dermatol. 2012;66(2):e47–53. doi: 10.1016/j.jaad.2010.09.722. [DOI] [PubMed] [Google Scholar]
- 9.Cinotti E, Perrot JL, Labeille B, Cambazard F, Groupe imagerie cutanee non invasive de la Societe francaise de d The contribution of reflectance confocal microscopy in the diagnosis of Paget’s disease of the breast. Ann Dermatol Venereol. 2013;140(12):829–832. doi: 10.1016/j.annder.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Suppa M, Marneffe A, Miyamoto M, Rorive S, Boone M, Del Marmol V. Contribution of reflectance confocal microscopy in the diagnosis of extra-mammary Paget’s disease. Ann Dermatol Venereol. 2015;142(1):70–73. doi: 10.1016/j.annder.2014.09.608. [DOI] [PubMed] [Google Scholar]
- 11.Alarcon I, Carrera C, Alos L, Palou J, Malvehy J, Puig S. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71(1):49–55. doi: 10.1016/j.jaad.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 12.Marino ML, Rogers T, Sierra Gil H, Rajadhyaksha M, Cordova MA, Marghoob AA. Improving lesion localization when imaging with handheld reflectance confocal microscope. Skin Res Technol. 2016 doi: 10.1111/srt.12280. [DOI] [PubMed] [Google Scholar]
- 13.Kose K, Cordova M, Duffy M, Flores ES, Brooks DH, Rajadhyaksha M. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171(5):1239–1241. doi: 10.1111/bjd.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]


