Abstract
Akt is a downstream target of B cell receptor signaling and is a central regulator of CLL cell survival. We aim to investigate the safety and efficacy of the Akt inhibitor MK-2206 in combination with bendamustine and rituximab (BR) in relapsed and/or refractory CLL in a phase I/II study. A standard phase I design was used with cohorts of three plus three patients to determine the maximum tolerated dose (MTD) of MK-2206 in combination with BR in relapsed CLL. Single-agent MK-2206 (weekly dosed) was administered one-week in advance before BR on cycle 1 and subsequently was given with BR at the same time for cycle 2–6. Phase II employed the MTD of MK-2206 with BR to evaluate safety and efficacy of this study combination. Thirteen relapsed/refractory CLL were treated for maximal 6-cycle of therapy. The maximum tolerated dose of MK-2206 was 90 mg by mouth once weekly. The most common grade 3/4 adverse events were neutropenia (46%), febrile neutropenia (23%), rash (15%), diarrhea (15%), and thrombocytopenia (15%). Overall response rate was 92% with a median progression free survival and treatment free survival of 16 and 24 months, respectively. Five patients (38%) achieved complete remission or complete remission with incomplete count recovery, two of whom were MRD negative. The efficacy and tolerability of this combination indicates that Akt inhibition combined with chemoimmunotherapy is a promising novel treatment combination in CLL and deserves further prospective clinical trial.
Keywords: AKT, Bendamustine, Rituximab, CLL
Introduction
An improved understanding of the fundamental role of B-cell receptor (BCR) activation in the biology of CLL has led to major breakthroughs of new therapies including the Bruton Tyrosine Kinase (BTK) inhibitor ibrutinib[1] and Phosphoinositide-3 Kinase (PI3K) δ inhibitor idelalisib.[2] The protein serine/threonine kinase Akt is a downstream target of BCR, PI3K, and growth factor receptor signaling pathways and is a central regulator of apoptosis, proliferation, and metabolism. In addition Akt activation results in phosphorylation of targets capable of promoting cell-cycle, stimulating cell metabolism, and antagonism of pro-apoptotic proteins. Importantly constitutive Akt activity is observed in CLL/SLL[3] and is known to be triggered through BCR ligation and cytokine stimulation[4, 5] and can also occur via bi-directional interactions between the CLL cells and the microenvironment leading to a promotion of cell survival and growth.[6, 7] In addition Increased Akt activation through PI3K pathway signaling as well as PI3K-independent pathways such as PKCβ[8] enhances CLL cell viability and chemotherapy resistance. Notably, direct Akt inhibition has been shown to result in death of CLL cells even within protective niches including CLL B cell clones designated as high risk with unmutated IgVH genes, del (17p) and high CD38 expression.[9] This latter finding adds to the attractive possibility that targeting Akt in CLL B cells will be of benefit in therapy of this disease.
Initial evaluation of in vitro Akt inhibition in CLL cells demonstrated induction of apoptosis in a dose dependent manner and was associated with a decrease in MCL-1 expression.[10] We have investigated the effect of Akt in vitro inhibition using MK-2206, a specific allosteric inhibitor of Akt on both apoptosis and signal activation in CLL. MK-2206 induced dose-dependent apoptosis of CLL and pre-treatment of CLL cells with MK-2206 selectively blocked the BCR ligation-mediated increase of CCL3, CCL4, CCL2, and IL-2Rα[11]. In addition, Akt activation appears to be essential in DNA damage repair mediated cell survival through PI3 kinase-like kinases (PIKKs) ATM, ATR, and DNA-PK[12]. Based on the above, bendamustine as an alkylator and DNA-damaging agent was combined with MK-2206 in our in vitro studies and an additive or synergistic effect was observed in 11 of the 12 CLL patient samples tested. It is of interest that bendamustine and rituximab (BR) as a salvage therapy for relapsed CLL has been demonstrated to be tolerable and achieves ~9% complete remission (CR) with ~60% overall response rate (ORR), but with much room to improve for efficacy[13].
A phase 1 dose-escalation trial in advanced solid tumors using MK-2206 on an every other day schedule demonstrated dose-limiting toxicities of skin rash and stomatitis at 60 mg.[14] Median terminal half-life ranged from 60–80 hours and subsequently the use of higher dose levels on a weekly schedule in order to maximize peak target inhibition were found to alleviate rash toxicities. Given our preclinical data and the established safety data of MK-226 in solid tumors, as well as the early clinical trial data for BR, we hypothesized that combination therapy of Akt inhibitor MK-2206 with bendamustine and rituximab would result in synergistic CLL cell death and abrogation of microenvironmental mediated protection. Here we report a phase I/II study in relapsed and/or refractory CLL patients that evaluated the safety and clinical efficacy of once weekly MK-2206 in combination with BR chemoimmunotherapy (protocol N1087, NCT01369849).
Methods
The phase I/II study was open for accrual in November 2011. CLL/SLL patients with relapsed and/or refractory symptomatic disease with ECOG performance status of two or less and adequate end organ function (total bilirubin ≤1.5, creatinine <1.5) were eligible to participate in the open-label, dose escalation phase I study (see supplemental data for complete protocol N1087, NCT01369849). Exclusion criteria were primary refractory disease as defined by progression while receiving or within 6 months of completion of a chemoimmunotherapy regimen such as fludarabine, cyclophosphamide and rituximab (FCR) or pentostatin, cyclophosphamide and rituximab (PCR), del (17p), four or more prior lines of therapy, or significant medical comorbidities that would impede their participation. These eligibility criteria were designed to match the ones published in German CLL group BR study for relapsed CLL[13] to facilitate the historical comparison of the two trials. The protocol was approved by the Institutional Review Board and an independent ethics committee. Informed consent was provided by each study participant in accordance with the Declaration of Helsinki.
A standard 3 + 3 dose escalation design was used in the phase 1 trial in order to define the maximum tolerated dose (MTD) and safety of MK-2206 in combination with BR. MK-2206 doses of 90 mg, 135 mg or 200 mg weekly was to be tested in combination with bendamustine (70 mg/m2 IV daily two days per cycle) and rituximab (cycle 1: 375 mg/m2, cycle 2–6: 500 mg/m2) for a maximum of 6 cycles. MTD was defined as the dose level below the lowest dose that induced dose-limiting toxicity (DLT) in at least one-third of patients during cycle 1. The defined MTD was then used for an expansion cohort (designated as the phase II portion of this trial). Primary aim in the phase II was to assess the rate of complete response (CR and complete remission with incomplete marrow recovery [CRi]) of MK-2206 in combination with BR. Response was evaluated two months after the last cycle of therapy per IWCLL 2008 criteria.[15] ORR (CR, CRi, complete clinical remission [CCR], nodular partial remission [nPR], and partial remission [PR]), progression free survival (PFS), treatment free survival (TFS), overall survival (OS), and minimal residual disease (MRD) were also evaluated based on IWCLL 2008 criteria. MK-2206 monotherapy for seven days prior to BR on cycle 1 was designed in order to conduct correlative analysis such as multiplex plasma cytokine analysis (Invitrogen, Life Technologies, Grand Island, NY) at baseline and after one week of MK-2206 therapy. The distributions of all time-to-event endpoints were estimated using the method of Kaplan-Meier. Progression free survival (PFS) was defined as the time interval from registration to the first date of disease progression or death or last follow-up. Treatment free survival (TFS) was defined to be the time from registration to the date of initiation of subsequent therapy or death or last follow-up. Overall survival (OS) time was defined as the time interval from registration to death from any cause or last follow-up. Minimal residual disease (MRD) was assessed by using 8-color flow cytometric analysis (analysis of CD5, CD19, CD20, CD23, CD38, CD45, and kappa and lambda light chains) of percentage of CLL B cells detected in bone marrow nucleated cells. MRD was defined as negative when less than one CLL cell in 10,000 leukocytes in bone marrow. Adverse events were evaluated using NCI Common Terminology Criteria for Adverse Events v4.0 except for anemia and thrombocytopenia, which were graded according to the grading scale for hematologic toxicity in CLL studies based on IWCLL2008 criteria.
Results
Patients
Fifteen patients were accrued between November 2011 and December 2013. Two patients were determined to be ineligible due to co-existing myelodysplastic syndrome and newly found del (17p). The thirteen evaluable patients with a median age of 68 years (range: 44–75) were treated with the combination of MK-2206 and BR. Baseline characteristics are listed in Table 1. Median follow-up was 28.7 months (range: 19.1–37.1 months). The majority of patients had at least one adverse prognostic factor including unmutated IGHV in 10 of 11 tested patients, del(11q) in four patients, ZAP-70 positive status in 8 of 11 tested patients, and CD38 positive in 8 of 13 tested patients. Patients had received a median of 1 prior therapies (range: 1–2), where nine patients (69%) were previously treated with purine nucleoside analogue, alkylator and anti-CD20 based chemoimmunotherapy. Five patients had aggressive disease characterized by progression within ~24 months of completing chemoimmunotherapy. As detailed in a preliminary report of 8 patients[11], lymphocyte mobilization after one-dose of MK-2206 resulted in a mean increase in absolute lymphocyte counts of 37% (range, 0 to 226%) after one week in the majority of tested patients (n=11).
Table 1.
Patient baseline characteristics (n=13).
| Patient characteristics at initiation of study treatment | N (%) |
|---|---|
|
| |
| Male sex | 11 (85%) |
|
| |
| Median age, years (range) | 68 (44–75) |
|
| |
| Prior chemoimmunotherapy | 8 (62%) |
|
| |
| Median prior treatments (range) | 1 (1–2) |
| • Prior purine analogue | 9 (69%) |
| • Prior rituximab | 10 (77%) |
| • Prior alemtuzumab | 5 (38%) |
| • Chlorambucil | 1 (7.7%) |
|
| |
| Rai stage, median (range) | 3 (1–4) |
| • Rai 1–2 | 5 (38%) |
| • Rai 3–4 | 8 (61%) |
|
| |
| CLL FISH | |
| • 11q- | 4 (31%) |
| • Trisomy 12 | 2 (15%) |
| • 13q- | 3 (23%) |
| • Normal | 2 (15%) |
| • Other | 2 (15%) |
|
| |
| Median β-2 microglobulin (range) | 7.11 (3.4–9.9) |
|
| |
| IgHV status | |
| • Unmutated | 10 (77%) |
| • Mutated | 1 (7.7%) |
| • Not done | 2 (15%) |
|
| |
| ZAP-70 expression | |
| • Positive | 8 (62%) |
| • Negative | 3 (23%) |
| • Not done | 2 (15%) |
|
| |
| CD38 expression | |
| • Positive | 8 (46%) |
| • Negative | 5 (23%) |
|
| |
| Median ALC (×103/uL) (range) | 43.9 (1.5 – 306.3) |
Safety
Patients received a median of 6 cycles (range: 1–6). A total of 9 patients were treated in the phase 1 portion of the trial. Six patients were treated at dose level 1 of 90 mg weekly (MK-2206) and a single DLT of febrile neutropenia and hemolytic anemia was observed. Two DLTs occurred in the three patients treated at dose level 2 (135 mg weekly) consisting of a grade 3 acneiform rash in one patient and febrile neutropenia with dehydration in the second patient. The 90 mg weekly was defined as the MTD in combination with BR for cohort expansion during phase II testing. An additional 4 patients were subsequently treated at this dose for phase II portion of this trial. Of 13 treated patients, nine (69%) completed six cycles of therapy. Three discontinued treatment early due to adverse events: grade 4 neutropenia and grade 3 hemolytic anemia considered as DLT in one patient, febrile neutropenia and grade 3 diarrhea considered as DLT in the second patient and grade 1 acute allergic reaction of facial edema and grade 3 rash in the third patient. One patient discontinued after one cycle due to progressive disease.
In all 13 evaluable patients, the most frequent grade 3 or 4 treatment-related adverse events observed in >1 patient were neutropenia (46%), febrile neutropenia (23%), rash (15%), diarrhea (15%), and thrombocytopenia (15%). A grade 3/4 treatment-related adverse event of nausea and vomiting, dehydration, sinusitis, hemolysis, or tumor lysis syndrome was observed in one patient each. The most frequent non-hematologic treatment-related adverse event of any grade (reported in ≥10%) were: nausea (54%), diarrhea (39%), rash (39%), vomiting (31%), hyperglycemia (31%, all grade 1), oral mucositis (23%), fatigue (15%), fever (15%) and sinus bradycardia (15%) as shown in table 2.
Table 2.
Common drug-related adverse events (≥ 10%) observed in N1087 trial.
| Grade 1–2 | Grade 3–4 | |||
|---|---|---|---|---|
| hematological toxicity | N | % | N | % |
| Neutropenia | 3 | 23.1 | 6 | 46.2 |
| Platelet count decrease | 6 | 46.2 | 2 | 15.4 |
| Anemia | 7 | 53.8 | ||
| Febrile neutropenia | 3 | 23.1 | ||
|
| ||||
| Grade 1–2 | Grade 3–4 | |||
|
| ||||
| non-hematological toxicity | N | % | N | % |
| Nausea | 6 | 46.2 | 1 | 7.7 |
| Diarrhea | 3 | 23.1 | 2 | 15.4 |
| Rash | 3 | 23.1 | 2 | 15.4 |
| Vomiting | 3 | 23.1 | 1 | 7.7 |
| Hyperglycemia | 4 | 30.8 | ||
| Mucositis | 3 | 23.1 | ||
| Fever | 2 | 15.4 | ||
| Fatigue | 2 | 15.4 | ||
| Sinus Bradycardia | 2 | 15.4 | ||
Efficacy
Considering all 13 patients in the study, 12 (92%; 95% CI: 64% – 99.8%) experienced a response including 5 (38%, 95% CI: 14%–68%) who achieved CR/CRi at the time of response evaluation (table 3). One patient with CRi later had recovery of counts in 3 months consistent with conversion to CR. Two of five (40%) patients who achieved CR or CRi had bone marrow MRD negative status at the final response evaluation that was 3 months after completion of therapy. One additional patient achieved CCR after two cycles of therapy and came off therapy due to a grade 3 rash. He was treated with BR alone off study and achieved a CR as documented after 6 cycles of BR. One patient achieved a nodular partial remission (nPR, 8%), and five patients had a partial response (PR, 38%). One patient had progressive disease and discontinued treatment after one cycle due to progressive pneumonitis proven to be CLL infiltrates in biopsy of lung tissue. Six of the 12 responders have since progressed. Eight patients in the whole cohort have received subsequent treatment. The median duration of response has not been reached (95% CI: 3 – NA months). Median progression free survival (PFS) was 16 months (95% CI: 10.4 – NA) and median treatment free survival was 24.5 months (95% CI: 11.3 – NA) (figure 1). Two deaths occurred during the study period. One patient developed Richter transformation 11 months after registration and died at 15 months post registration. The second death occurred in a patient who had achieved PR, but who had to come off trial after one cycle due to autoimmune hemolytic anemia, and died from fungal infection at 27 months post registration. The median overall survival has not been reached with last analysis performed in May 2016.
Table 3.
Clinical response1
| Efficacy | n, (%) |
|---|---|
| ORR | 12 (92%) |
| CR/CRi | 5 (38%) |
| CCR | 1 (7.7%) |
| nPR | 1 (7.7%) |
| PR | 5 (38%) |
| PD | 1 (7.7%) |
Response was evaluated two months after the last cycle of therapy per IWCLL 2008 criteria.[15]
Figure 1.
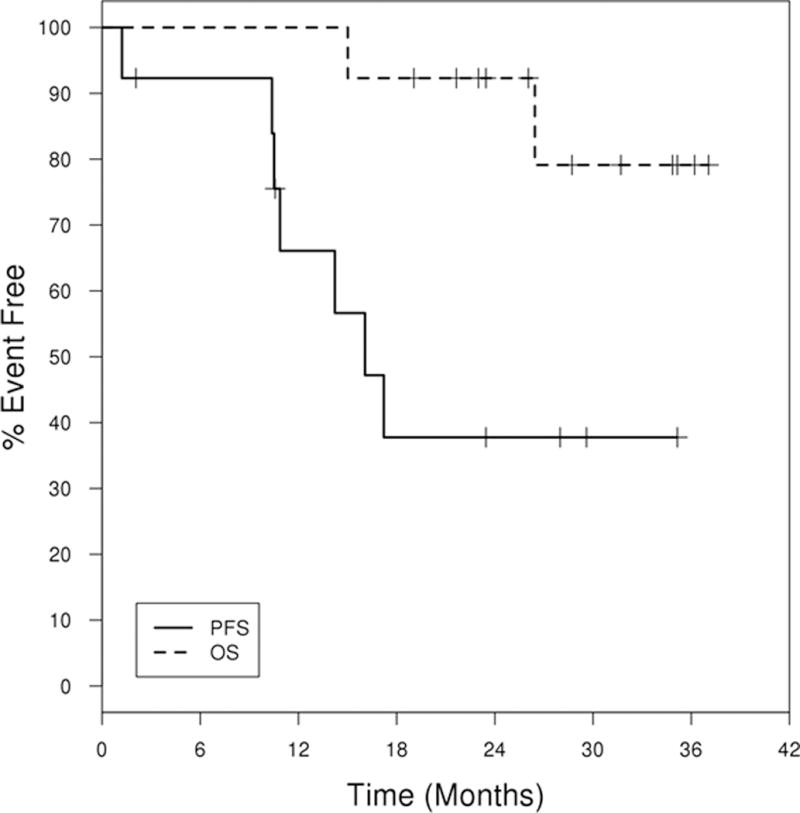
Progression free survival (PFS) and Overall survival (OS) curve. PFS is defined to be the time from registration to the date of progression or death or last follow-up. OS is defined to be the time from registration to the date of death or last follow-up.
Discussion
In this early phase study, we demonstrate that Akt inhibition with MK-2206 in combination with BR in relapsed and/or refractory CLL patients is tolerable and active. This above combination resulted in an CR/CRi rate 38%, PFS and TFS being 16 and ~25 months respectively. These data at least compare similarly to, if not better than, the previous reported experience with BR in relapsed CLL [13]. In this previous study, an ORR of 60%, a CR rate of 9% and an event free survival being 15 months were observed. In an effort to improve the efficacy of BR-based therapy, combination of targeted therapy using ibrutinib or idelalisib with BR were tested. The CR rate in trials evaluating the combination of ibrutinib with BR[16–18] and idelalisib with BR[19] were 20% and 10% before the single-agent extension phase, respectively. It is notable that these trials also included maintenance ibrutinib/idelalisib as single–agent extension after BR combination whereas in our study MK2206 was stopped after 6 cycles of BR combination. The differences in the duration of therapy in our trial were likely the causes for the shorter PFS observed in this study compared to the longer PFS seen with the trials used BR and ibrutinib combination. [16–18] This current study was initially designed to assess the synergy of Akt inhibition with BR chemotherapy and thus was not designed to have a maintenance therapy of Akt inhibitor to assess the effect of Akt inhibition on the PFS of this combination.
Novel targeted therapy has changed the landscape of CLL therapy. International phase 3 studies to compare the efficacy of front-line chemoimmunotherapy with ibrutinib-based therapy have been ongoing and results are eagerly waited. Despite the excellent efficacy of novel targeted therapy in relapsed CLL patients, a significant portion of CLL patients continue to progress or stop therapy due to intolerability[20, 21]. How to manage these patients is an active area of CLL research. It is to be determined whether progressive CLL patients after prior targeted therapies can still respond to chemoimmunotherapy or combination of targeted therapy with chemotherapy. Our early phase trial presented in this study provided an initial proof-of-concept of the combination therapy using Akt inhibitor with BR though none of the patients in this study had prior targeted therapy. The primary limitation of our study is the small sample size as patient accrual was terminated early due to the drug-sponsor’s decision to discontinue further MK-2206 development. Nonetheless with the favorable efficacy results and tolerability of Akt inhibition in combination with BR in the relapsed or refractory CLL cohort in this trial, further prospective testing of Akt inhibitors in CLL appears warranted.
Supplementary Material
Acknowledgments
The work was supported by funding from NCI grant K23CA160345 (WD), NCI grant CA95241 (NEK) and Alliance for Clinical Trials in Oncology (CA0252224 and CA33601).
Footnotes
Authorship contributions
JTL extracted data, performed statistical analysis, interpreted the results, and wrote the paper. TDS and NEK designed research, analyzed and interpreted the results, and wrote the paper. JFL, TGC, CZ, CE, TH, CR, DN, DB, and MC recruited patient, provided input, and reviewed the manuscript. BL, AP, CL, and DJ designed research, interpreted results, and performed statistical analysis. JB, CS, CL, and RT performed the experiments and interpreted the results. WD designed the study, contributed patients, performed statistical analysis, performed experiments, and wrote the paper.
Disclosure of Conflicts of Interest
TDS has received research support from Celgene, Hospira, Cephalon, Genentech, Glaxo-Smith-kline and Polyphenon E International. CE was on the advisory board of Merck over 1 year ago. NEK has received research support from Celgene and Gilead. WD received research support from Merck. All other authors declare no competing financial interests.
References
- 1.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringshausen I, Schneller F, Bogner C, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100:3741–3748. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 4.Petlickovski A, Laurenti L, Li X, et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105:4820–4827. doi: 10.1182/blood-2004-07-2669. [DOI] [PubMed] [Google Scholar]
- 5.Longo PG, Laurenti L, Gobessi S, et al. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–855. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 6.Ding W, Nowakowski GS, Knox TR, et al. Bi-directional activation between mesenchymal stem cells and CLL B-cells: implication for CLL disease progression. Br J Haematol. 2009;147:471–483. doi: 10.1111/j.1365-2141.2009.07868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holler C, Pinon JD, Denk U, et al. PKCbeta is essential for the development of chronic lymphocytic leukemia in the TCL1 transgenic mouse model: validation of PKCbeta as a therapeutic target in chronic lymphocytic leukemia. Blood. 2009;113:2791–2794. doi: 10.1182/blood-2008-06-160713. [DOI] [PubMed] [Google Scholar]
- 9.Hofbauer SW, Pinon JD, Brachtl G, et al. Modifying akt signaling in B-cell chronic lymphocytic leukemia cells. Cancer Res. 2010;70:7336–7344. doi: 10.1158/0008-5472.CAN-09-4411. [DOI] [PubMed] [Google Scholar]
- 10.Zhuang J, Hawkins SF, Glenn MA, et al. Akt is activated in chronic lymphocytic leukemia cells and delivers a pro-survival signal: the therapeutic potential of Akt inhibition. Haematologica. 2010;95:110–118. doi: 10.3324/haematol.2009.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding W, Shanafelt TD, Lesnick CE, et al. Akt inhibitor MK2206 selectively targets CLL B-cell receptor induced cytokines, mobilizes lymphocytes and synergizes with bendamustine to induce CLL apoptosis. Br J Haematol. 2014;164:146–150. doi: 10.1111/bjh.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Turner KM, Alfred Yung WK, et al. Role of AKT signaling in DNA repair and clinical response to cancer therapy. Neuro Oncol. 2014;16:1313–1323. doi: 10.1093/neuonc/nou058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 14.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 15.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. The Lancet Oncology. 2016;17:200–211. doi: 10.1016/S1470-2045(15)00465-9. [DOI] [PubMed] [Google Scholar]
- 17.Barrientos JC, Barr PM, Flinn I, et al. Ibrutinib In Combination With Bendamustine and Rituximab Is Active and Tolerable In Patients With Relapsed/Refractory CLL/SLL. Final Results Of a Phase 1b Study. 2013:525–525. [Google Scholar]
- 18.Brown JR, Barrientos JC, Barr PM, et al. The Bruton tyrosine kinase inhibitor ibrutinib with chemoimmunotherapy in patients with chronic lymphocytic leukemia. Blood. 2015;125:2915–2922. doi: 10.1182/blood-2014-09-585869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner-Johnston ND, De Vos S, Coutre SE, et al. Chemo-Immunotherapy Combination Of Idelalisib With Bendamustine/Rituximab Or Chlorambucil/Rituximab In Patients With Relapsed/Refractory CLL Demonstrates Efficacy and Tolerability. 2013:4176–4176. [Google Scholar]
- 20.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA Oncol. 2015;1:80–87. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125:2062–2067. doi: 10.1182/blood-2014-09-603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


