Abstract
Individuals with psychosis have been reported to show either reduced or augmented brain responses under seemingly similar conditions. It is likely that inconsistent baseline-adjustment methods are partly responsible for this discrepancy. Using steady-state stimuli during a pro-/anti-saccade task, this study addressed the relationship between nonspecific and stimulus-related neural activity, and how these activities are modulated as a function of cognitive demands. In ninety-eight psychosis probands (schizophrenia, schizoaffective disorder, and bipolar disorder with psychosis), neural activity was assessed during baseline and during a 5sec period in preparation for the pro-/anti-saccade task. To maximize the ability to identify meaningful differences between psychosis subtypes, analyses were conducted as a function of subgrouping probands by standard clinical diagnoses and neurobiological features. These psychosis “Biotypes” were created using brain-based biomarkers, independent of symptomatology (Clementz et al., 2016).
Psychosis probands as a whole showed poor antisaccade performance and diminished baseline oscillatory phase synchrony. Psychosis Biotypes differed on both behavioral and brain measures, in ways predicted from Clementz et al. (2016). Two Biotype groups showed similarly deficient behavior and baseline synchrony, despite diametrically opposed neural activity amplitudes. Another Biotype subgroup was more similar to healthy on behavioral and brain measures, despite the presence of psychosis. This study provides evidence that (i) consideration of baseline levels of activation and synchrony will be essential for a comprehensive understanding of neural response differences in psychosis and (ii) distinct psychosis subgroups exhibit reduced versus augmented intrinsic neural activity, despite cognitive performance and clinical similarities.
Keywords: psychosis, EEG, biomarkers, diagnosis, schizophrenia, bipolar disorder, steady-state
Introduction
There is an intriguing discrepancy in the schizophrenia (SZ) electroencephalography (EEG)/ event-related potentials (ERP) literature. Under many circumstances, SZ show reduced amplitude brain responses to rapidly repeating stimuli across a range of stimulation frequencies (Brenner et al., 2009; Ferrarelli et al., 2008; Hamm, Gilmore, Picchetti, Sponheim, & Clementz, 2011; Teale et al., 2008). It is often assumed that such deficient response amplitudes are a defining feature of SZ, with theories of neuropathology built around this supposition. Other reports, however, indicate SZ have augmented amplitude brain responses during what seem to be similar stimulation conditions (Clementz, Wang, & Keil, 2008; Ethridge, Moratti, Gao, Keil, & Clementz, 2011; Farzan et al., 2010; Flynn et al., 2008; Hamm, Gilmore, & Clementz, 2012; Riečanský, Kašpárek, Řehulová, Katina, & Přikryl, 2010; Spencer et al., 2004).
This study has two main goals, and one methodological issue of note, designed to address various aspects of this discrepancy in the existing literature. First, perhaps inconsistent baseline adjustment methods are partly responsible for the disparity in brain responses among psychosis subjects. The paradigm used here was designed to examine the relationship between baseline stimulus-related neural activity. Second, the task used here allowed for assessment of cortical modulation of baseline activity and sensory processing as a function of cognitive demands (Clementz et al., 2010). This addresses a fundamentally important question in the clinical neuroscience of psychosis: how modulation of sensory systems facilitates context-appropriate behavior. Finally, it is likely that the outcomes of sensory responsiveness studies have been influenced by the extensive neurobiological variability found in psychosis. The ability to identify neurobiological distinctiveness of psychosis subtypes will be limited if these subtypes are not defined by neurobiology, and there is evidence that such variability in psychosis is not adequately captured by DSM-type clinical diagnoses (Clementz et al., 2016). In this study, sensory responsiveness and neural modulation abilities will be evaluated as a function of subgrouping cases by clinical versus neurobiological features.
Although the possibility that SZ have augmented brain responses seems contrary to decades of psychophysiological research (see Winterer & McCarley, 2011), there are multiple indications such is the case, including high levels of nonspecific, or intrinsic, neural activity (Clementz & Blumenfeld, 2001; Clementz, Sponheim, Iacono, & Beiser, 1994; Krishnan et al., 2005; Rolls, Loh, Deco, & Winterer, 2008; Winterer et al., 2000, 2004, 2006; Winterer & Weinberger, 2004). Intrinsic neural activity is not directly linked to stimulus processing and has been operationalized in a number of ways, including a ratio of single trials to average trial power (Winterer et al., 2000, 2004), activity at frequencies other than those of interest (Butler et al., 2001; Kim, Wylie, Pasternak, Butler, & Javitt, 2006; Kim, Zemon, Saperstein, Butler, & Javitt, 2005; Krishnan et al., 2005; Wang, Brown, Dobkins, McDowell, & Clementz, 2010), baseline activity (Clementz, Keil, & Kissler, 2004), or activity in relation to a stimulus that has not undergone baseline correction (Ethridge et al., 2011). Increased intrinsic activity is correlated with augmented sensory cortical responses (e.g., Clementz et al., 2008; Spencer et al., 2004; Wang et al., 2010). It has been theorized that either abnormally high or low intrinsic activity may lead to low signal-to-noise ratios, reducing the ability to parse stimulus relevance and accurately code perceptual events (Rolls et al., 2008). Low signal-to-noise ratios have been reported in SZ and may be particularly problematic for identifying stimulus salience (Clementz et al., 2008; Ethridge et al., 2011; Wang et al., 2010). Higher intrinsic activity in SZ is also associated with prolonged neural activation following stimulus termination (Clementz et al., 2004; Ethridge et al., 2011); this sluggishness of neural systems could be a basis for behavioral inflexibility in the face of changing task demands.
The present study provides additional evaluation of psychosis subjects’ neural regulation in response to rapidly repeating “steady-state” visual stimuli. The visual steady state response (vSSR) develops in primary visual cortex in relation to stimuli flickering at a specific rate (Clementz et al., 2008; Di Russo, Taddei, Apnile, & Spinelli, 2006; Pastor, Artieda, Arbizu, Valencia, & Masdeu, 2003). The resultant oscillations resonate at the stimulus flicker rate and vary in amplitude with the task relevance of the stimulus (Wang, Clementz, & Keil, 2007). The vSSR provides an unambiguous ability to evaluate preparation for and response to stimuli as a function of their importance because the input frequency (vSSR oscillation rate) is known and neural activity at that frequency yields a specific probe for hypothesis testing (Clementz et al., 2004, 2008).
The vSSR paradigm used here was designed to examine baseline differences in neural activity among psychosis subjects, and the relationship between baseline and stimulus-related activities. In a less cognitively demanding vSSR paradigm, Ethridge et al. (2011) observed augmented baseline activity among SZ compared to healthy persons that was associated with stimulus-related activations (i.e., high prestimulus activity determined high stimulus-related activity). Many vSSR studies in psychosis subtract prestimulus from stimulus-related activity and report only the difference scores, a procedure that yields apparently lower neural activations among SZ in response to steady-state stimulation (Ethridge et al., 2011). Although baseline subtraction provides for an examination of stimulus-specific neural responses, it yields an incomplete picture of overall neural activation among psychosis cases. Analyses of vSSRs here will be more comprehensive. Specifically, we will examine baseline, stimulus-related (i.e., not baseline-adjusted), and stimulus-specific (i.e., baseline-adjusted) activity, the former two being considered variants of intrinsic activity. This will allow for a determination of which of these types of activations are more important for indexing neuronal response differences among psychosis cases.
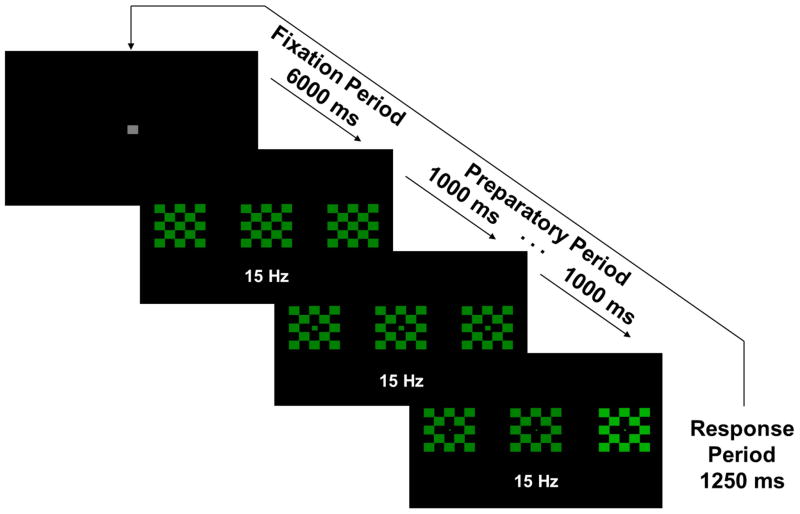
The task used here also allowed for assessment of cortical modulation of intrinsic activity and sensory processing as a function of task demands (Clementz et al., 2010). The paradigm elicits the vSSR in preparation for pro- versus anti-saccade responses, with quantification of neural activity occurring before presentation of the behavioral cue (see Figure 1). Unlike prosaccade tasks, where a glance is made towards a peripheral cue, antisaccade tasks require suppressing a glance to a peripheral cue and instead looking to that cue’s mirror image location (Hallett, 1978). In a previous vSSR investigation of neural regulation preceding correct pro- versus anti-saccade responses, Clementz et al. (2010) found that healthy persons decreased visual cortical neural investment to peripheral anti- relative to pro-saccade stimuli. Such a down-regulation of neural activity should be adaptive because it could reduce the probability of generating an unwanted pro-response on anti-trials (Munoz & Everling, 2004). This effect should be reversed with respect to the central fixation stimulus, with increased investment during anti- relative to pro-saccade trials, with preparatory modulation of sensoricortical activity theoretically generated via top-down control by prefrontal cortex (Johnston & Everling, 2006). While abnormal preparatory modulation of neural activity in psychosis is suggested by their high antisaccade error rates (Reilly et al., 2014), it is not yet established in direct neural system observation.
Figure 1.
Example prosaccade trial. Central checkerboard flickered at 15 Hz. For the anti-task, stimuli would be red rather than green. At each ¼ of the fixation period (1500 ms), the central square decreased in size by ¼ in order to give a “countdown” to the next trial. Similarly, at 1sec intervals during the Preparatory Period, the center square of the central checkerboard decreased in size by ¼ in order to give the participants information about the impending response requirement.
Finally, while measures relating to these primary goals are likely to differ among individuals with psychosis, there is growing evidence that DSM-type clinical psychosis diagnoses are not neurobiologically distinct; within- and between-disorder heterogeneity characterizes purported biomarker distributions (see Clementz et al., 2016). The gold standard for evaluating the validity of biomarkers for psychosis subgroups, however, has been DSM psychosis definitions. If such definitions are not defined by neurobiology, the ability to identify neurobiological distinctiveness of psychosis subtypes may be limited. This has led to a growing interest in biological markers for psychiatric disorders (Insel et al., 2010). Clementz et al. (2016) created neurobiologically distinct psychosis subgroups using brain-based biomarkers, independent of reliance on clinical characteristics. Their three psychosis Biotypes were not concordant with clinical diagnoses, but had greater neurobiological distinctiveness than clinical diagnoses given group differences on multiple biomarkers and external validators (measures not part of Biotype formulation but used to test their validity). DSM diagnoses were spread across Biotypes, suggesting that heterogeneity on vSSR results across studies from different laboratories could be accounted for by differences in case sampling. For instance, Biotype-1 cases, the most neurobiologically compromised with low sensory responsiveness, might be more likely recruited from chronic care settings. Biotype-2s, who were moderately less cognitively compromised but hyper-responsive to stimuli, might be more common in settings treating higher functioning patients. To maximize the ability to identify meaningful differences between psychosis subtypes, all measures will be evaluated as a function of subgrouping cases by clinical versus neurobiological features. For comparisons based on clinical features, probands will be subdivided based on their scores on the Schizo-Bipolar Scale (SBS), an ordinal scale ranking individuals on a continuum of affective to non-affective psychosis (Keshavan et al., 2011). This will allow for comparison of probands based on a spectrum of psychosis.
It is hypothesized that: (i) clinical subgroups will show significantly decreased behavioral performance compared to healthy subjects, as well as marginally reduced intrinsic and stimulus-specific brain activity and signal-to-noise; (ii) both behavioral performance and brain activity measures will differentiate Biotype subgroups; (iii) Biotype-1 will have the worst antisaccade performance and the most deficient intrinsic and stimulus-specific neural activations, Biotype-2 will show slightly less deviant antisaccade performance but accentuated intrinsic and stimulus-specific neural activations, and Biotype-3 will be most similar to healthy subjects on both performance and brain responses; (iv) despite differing levels of intrinsic neural activity, neural signal-to-noise will be low in both Biotypes-1/2; and (v) brain activity measures will predict antisaccade performance (Hamm, Dyckman, McDowell, & Clementz, 2012).
Method
Participants
As part of a larger, multisite data collection project, the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP), subjects were recruited, interviewed, and tested at the University of Illinois-Chicago. Ninety-eight clinically stable psychosis probands (schizophrenia, N = 33; schizoaffective disorder, N = 20; bipolar disorder with psychosis, N = 45) and 58 healthy comparison subjects were recruited via community advertisements, linked community facilities and programs, and local National Alliance on Mental Health type organizations (see Table 1 for clinical and demographic information). Clinical diagnosis was determined through administration of the Structured Clinical Interview for DSM-IV Diagnosis (First, Spitzer, Gibbon, & Williams, 1997); see Tamminga et al. (2013) for complete clinical evaluation details. Healthy subjects had no personal history of psychotic, bipolar, or recurrent major depressive disorder, or a family history of schizophrenia-bipolar spectrum disorders in first- and second-degree (Tamminga et al., 2013). All subjects provided written consent before participating, and the study was approved by University of Illinois-Chicago and University of Georgia Institutional Review Boards.
Table 1.
Demographic and Clinical Characteristics of Probands and Healthy Comparison Subjects
| Healthy (N=58) | SZ (N=33) | BDP (N=45) | SZA (N=20) | Significance Test | |||||
|---|---|---|---|---|---|---|---|---|---|
| 48 | 69 | 36 | 45 | χ2(3)=12.69, p=.005 | |||||
|
| |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
|
| |||||||||
| Age | 39.1 | 12.2 | 32.8 | 12.3 | 32.3 | 13.8 | 35.5 | 12.9 | F(3,152)=2.67, p=.049 |
| Trials accepted | |||||||||
| Prosaccades | 42.6 | 6.9 | 37.7 | 9.9 | 38.5 | 7.4 | 36.3 | 10.6 | F(3,153)=0.442, p=.72 |
| Antisaccades | 46.4 | 4.0 | 35.2 | 9.6 | 34.4 | 8.3 | 32.8 | 9.7 | F(3,153)=2.38, p=.07 |
| Percent Correct | |||||||||
| Prosaccades | 98.7 | 1.8 | 97.7 | 5.1 | 98.4 | 2.3 | 98.7 | 1.8 | F(3,153)=.847, p=.47 |
| Antisaccades | 90.3 | 8.0 | 80.3 | 19.2 | 83.0 | 12.6 | 79.8 | 16.5 | F(3,153)=5.73, p=.001 |
| Response Latency (ms)a | |||||||||
| Correct Prosaccades | 361.8 | 57.7 | 336.6 | 70.5 | 376.8 | 89.4 | 347.6 | 73.2 | F(3,153)=2.14, p=.10 |
| Correct Antisaccades | 399.5 | 78.0 | 390.2 | 89.6 | 428.0 | 78.7 | 392.2 | 74.2 | F(3,153)=1.86, p=.14 |
| Incorrect Antisaccades | 348.8 | 81.3 | 350.0 | 95.0 | 340.1 | 68.2 | 326.0 | 74.2 | F(3,153)=0.49, p=.69 |
| GAFb | 84.8 | 5.3 | 43.7 | 7.6 | 58.5 | 12.6 | 42.1 | 5.6 | F(2,95)=29.59, p<.001 |
| BACS | −0.09 | 1.09 | −1.50 | 1.31 | −0.72 | 1.17 | −1.55 | 1.21 | F(2,93)=13.28, p=9.8E-8 |
| PANSS-positive | – | – | 19.6 | 5.5 | 13 | 3.8 | 18.2 | 4.7 | F(2,94)=19.32, p<.001 |
| PANSS-negative | – | – | 20.1 | 6.3 | 13.9 | 4.2 | 16.9 | 7.2 | F(2,94)=10.43, p<.001 |
| PANSS-general | – | – | 37.3 | 7.1 | 32.3 | 7.8 | 36.1 | 8.3 | F(2,94)=3.33, p=.04 |
| MADRS | – | – | 10.2 | 8.1 | 12.9 | 8 | 17 | 7.8 | F(2,94)=4.44, p=.14 |
| Young Mania Scale | – | – | 8.3 | 6 | 5.9 | 5.5 | 8.7 | 5.3 | F(2,94)=1.72, p=.18 |
| Medication Class | |||||||||
| Antipsychotic first generation | 0% | 18.2% | 2.2% | 10.0% | χ2(2)=5.83, p=.05 | ||||
| Antipsychotic second generation | 0% | 66.7% | 64.4% | 65.0% | χ2(2)=0.04, p=.98 | ||||
| Mood stabilizer | 0% | 21.2% | 64.4% | 50.0% | χ2(2)=14.38, p=.001 | ||||
| Lithium | 0% | 0% | 22.2% | 10.0% | χ2(2)=8.87, p=.01 | ||||
| Antidepressant | 1.7% | 36.4% | 42.2% | 55.0% | χ2(2)=1.78, p=.41 | ||||
| Sedative/anxiolytic | 1.7% | 24.2% | 26.7% | 35.0% | χ2(2)=0.76, p=.69 | ||||
| Stimulant | 0% | 9.1% | 13.3% | 10.0% | χ2(2)=0.38, p=.83 | ||||
| Anticholinergic | 0% | 9.1% | 8.9% | 5.0% | χ2(2)=0.34, p=.85 | ||||
GAF, Global Assessment of Functioning; PANSS, Positive and Negative Symptom Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; BACS, Brief Assessment of Cognition in Schizophrenia.
Only 40% of participants made any incorrect responses during prosaccade trials.
Healthy subjects were excluded from comparisons of clinical characteristics and medication status
For comparisons based on clinical features, probands were subdivided based on Schizo-Bipolar Scale (SBS) ratings into three groups: those scoring 0–2 (SBS-BP; N = 45), 3–6 (SBS-MID; N = 26), and 7–9 (SBS-SZ; N = 27) (Keshavan et al., 2011). The SBS is an ordinal scale ranking individuals on a continuum of affective to non-affective psychosis, with lower scores representing cases more prototypical of bipolar disorder with psychosis and higher scores representing cases more prototypical of schizophrenia. All analyses make use of these SBS-Groups, rather than DSM diagnoses. Biotype membership (Biotype-1, N = 18; Biotype-2, N = 27; Biotype-3, N = 53) was determined by biomarker data as described in Clementz et al. (2016), and all participants in the present study were included in Clementz et al. (2016). Biotype-1 was most reminiscent of poor outcome, chronic, and neurobiologically impaired psychosis cases, Biotype-2 was characterized by hyper-responsiveness to sensorimotor events, while Biotype-3 were closer to healthy subjects than to the other Biotype subgroups across biomarkers (despite presence of psychosis). The distinctiveness of the Biotypes was also supported by clinical and neuroanatomical data from probands, as well as clinical and biomarker data from first-degree relatives, none of which were used in biotypes creation (Clementz et al., 2016). Saccade data used for developing Biotypes was independent from visual steady-state data that are the subject of this investigation (see Discussion below).
Stimuli
Stimuli were presented on a 19-inch flat-surface computer monitor with a refresh rate of 60Hz. Stimuli consisted of three 5x5cm checkerboards (alternating colored and black squares, each 1deg square) located at central fixation and 8 deg to the left and right of central fixation (Fig 1). Checkerboards were either green (during the prosaccade block) or red (during the antisaccade block). Both colors had equal luminance (5 cd/m2). The middle checkerboard was luminance modulated (100% modulation depth) at 15Hz, with the peripheral checkerboards not flickering. Blocks consisted of 50 trials of either pro- or anti-saccades, for a total of 100 trials.
Each trial was preceded by an inter-trial interval of 6 sec, in which a gray central fixation square (1 deg square) was presented. At each ¼ of this fixation period (1.5 sec), the central square decreased in size by ¼ in order to give a “countdown” to the next trial. Trials began with the central checkerboard flickering for 5 sec. Participants were instructed to maintain fixation on the central checkerboard throughout this preparatory period. Similar to the fixation period, at 1sec intervals during the Preparatory Period the center square of the central checkerboard decreased in size by ¼ in order to give the participants information about the impending response requirement. After the 5 sec preparatory interval, one of the peripheral checkerboards (pseudo-randomly determined) increased in luminance to 20 cd/m2 for 1.25 sec (the saccadic response period), while the central checkerboard continued flickering. On prosaccade trials, participants were to look as quickly and accurately as possible toward this target; on antisaccade trials, participants were instructed to look as quickly and accurately as possible to the mirror image location (opposite checkerboard).
Electrophysiological Recording and Data Preprocessing
EEG recording
EEG data was collected from 64 Ag/AgCl sensors (impedance was kept below 5kΩ; Quik-Cap, Compumedics Neuroscan, El Paso, TX) positioned according to the 10–10 EEG system (with the inclusion of mastoids and CP1/2 locations to provide for greater signal sampling below the cantho-meatal line), with a forehead ground and nose reference. EEG recordings were amplified (12,500×) and digitized (1000 Hz) using Neuroscan Acquire and Synamp2 (Compumedics, Charlotte, NC). Additional sensors located above and below the eyes, as well as at the outer canthi of each eye, recorded blinks and eye movements.
Data preprocessing
Visually identified bad sensors were interpolated (no more than 3 sensors per participant) using a spherical spline interpolation method (BESA 5.3; MEGIS Software, Grafelfing, Germany). Trials containing activity greater than 100 μV at any sensor during baseline and steady-state stimulation were eliminated. Data were then transformed to average reference and digitally filtered .5–50 Hz (zero phase filter; rolloff: 6 and 48 dB/octave, respectively). Artifacts related to eye blinks and heart rate were removed using the Independent Component Analysis (ICA) toolbox in EEGLAB 6.0 for Matlab (Version 7.0, MathWorks, Natick, MA) (Delorme & Makeig, 2004). Data were subsampled to 300Hz. Eye movements were manually scored for latency and correctness of the initial saccade using established protocols (Dyckman & McDowell, 2005).
Data Analyses
Two approaches were used to quantify brain activity. First, evoked potentials were used to evaluate the amplitude and spatial distribution of brain activity at the onset of visual stimulation (at the beginning of the Preparatory Period, before stabilization of the steady-state response). Second, as in Clementz et al. (Clementz et al., 2010), spectral measures were used to assess the power, phase stability, and spatial distribution of the vSSR over time before and during the Preparatory Period (−500ms:5000ms).
Visual Evoked Potentials to Onset of the Preparatory Period
The voltage data were used to assess for differences in brain activations, at the beginning of visual processing, between the pro- and anti-saccade conditions and between groups (Clementz et al., 2010; Clementz, Brahmbhatt, McDowell, Brown, & Sweeney, 2007; McDowell et al., 2005). As the overwhelming majority of frequency composition for early VEPs is below 10 Hz (Moratti, Clementz, Gao, Ortiz, & Keil, 2007), the EEG data were digitally low-pass filtered at 10 Hz (12 dB/octave rolloff), which also served to reduce possible confusion between the early VEPs and the initiation of the vSSR (at 15 Hz). For each participant, EEG data from 250ms before to 500 ms after the onset of the Preparatory Period were averaged across trials and baseline-subtracted using the average of −250:0 ms. Global field power (GFP; the root mean square of voltage over all sensors at each time point) was calculated for each participant and task.
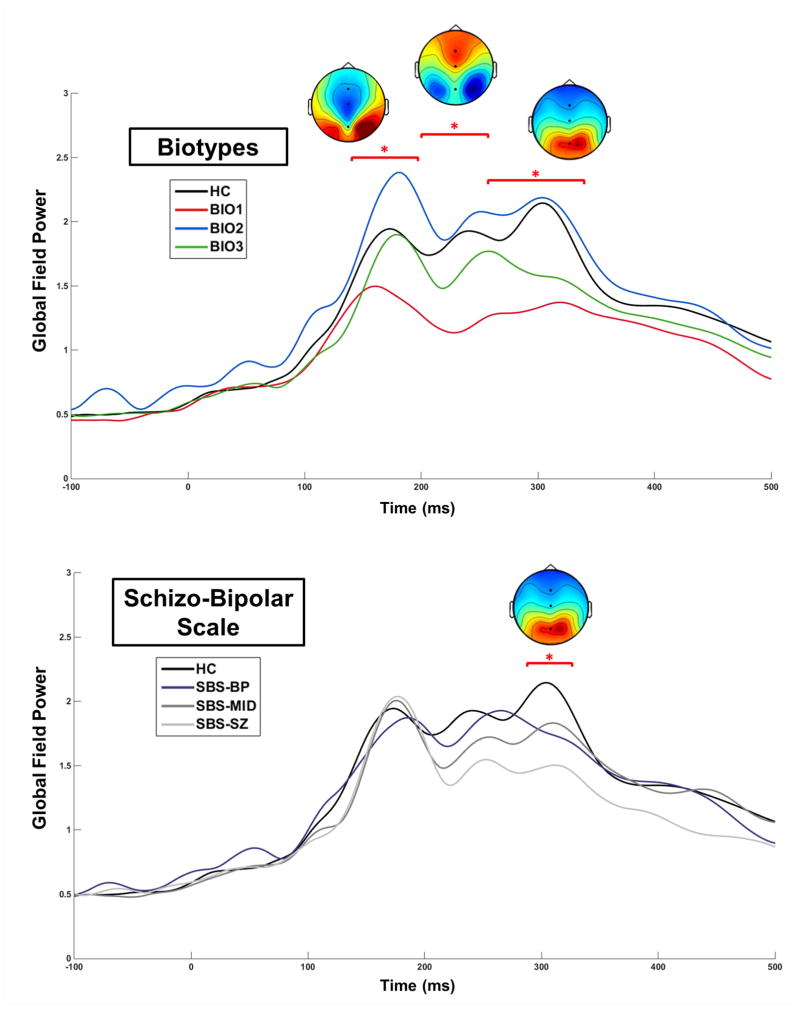
GFP waveforms were averaged into 10 ms bins and analyzed using a 2-way ANOVA (group x saccade type) at each time bin. To control for increased family-wise error rate due to multiple comparisons, a clustering method (Forman et al., 1995) was used to take account of the non-independence of data from adjacent time bins (for examples, see Hamm et al. 2012; Hudgens-Haney et al., 2013; and Monte Carlo simulations calculated using AlphaSim, Cox, 1996). Time bin “clusters” were considered significant at overall family-wise α < .01 if at least three adjacent time bins were significant at p < .05. Surviving time bin clusters were subjected to planned posthoc t-tests. Voltage topographies averaged over significant time bin clusters were used to visualize the spatial distributions of brain activations at those times (Figures 2 and 3).
Figure 2.
Group comparisons for Global Field Power waveforms. The colored top-down topographies are voltage maps (μV) from the three time bin clusters at which a significant Main Effect of Group was found for the Biotypes (top) and the one time bin cluster found for SBS groups (bottom). *p < .05.
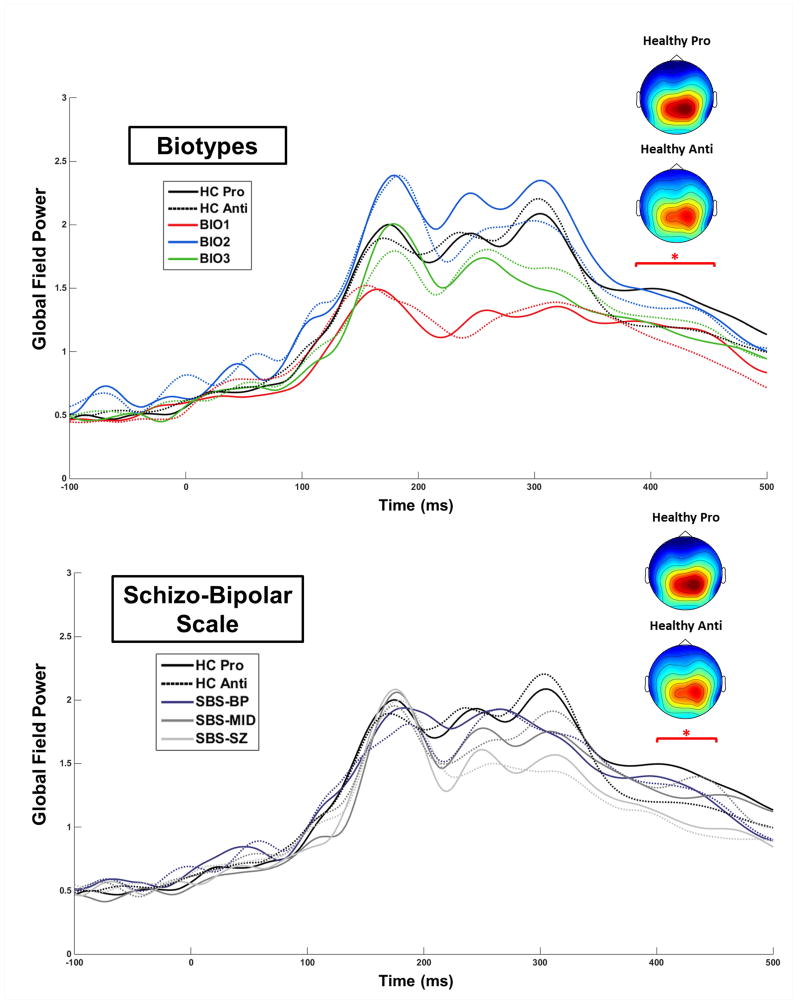
Figure 3.
Group by Task comparisons for Global Field Power waveforms. The colored top-down topographies are voltage maps (μV) from the time bin cluster at which a significant Group by Task Interaction was found for the Biotypes (top) and for SBS groups (bottom). *p < .05.
Spectral measures
Previous work has demonstrated that under typical circumstances the vSSR is determined primarily by increased intertrial phase coherence (ITC; increased across-trial phase similarity of the EEG signals in relation to frequency of visual flickering stimuli) without substantial changes in single-trial power (Ding, Sperling, & Srinivasan, 2006; Moratti et al., 2007). In the present study, however, subjects were required to impose sustained top-down control as a function of pro- versus anti-saccade task demands. As the effects of this top-down control have been found to manifest through changes in single-trial power (STP) using a similar task (Clementz et al., 2010), the vSSR response was quantified here using both ITC and STP.
Intertrial phase coherence and STP of the steady-state response across time were estimated at the flicker rate (15 Hz) of the checkerboards for each subject and task condition (Regan & Regan, 2003). Data for each useable trial were multiplied by a sliding 300-point Hanning window before fast Fourier transformation (FFT). ITC, the normalized phase vector, and STP, the absolute square of the complex result of the FFT, were each averaged across trials individually for each sensor. Due to the sensitivity of ITC to the number of trials contributed (i.e., ITC is positively biased with fewer trials or spectral estimates), we calculated ITC expected by chance given the number of trials:
where Rchance is chance ITC, n is number of trials, and c is 0.5 (Hipp, Engel, & Siegel, 2011; Moratti et al., 2007). Each participant’s chance ITC was subtracted from the observed ITC.
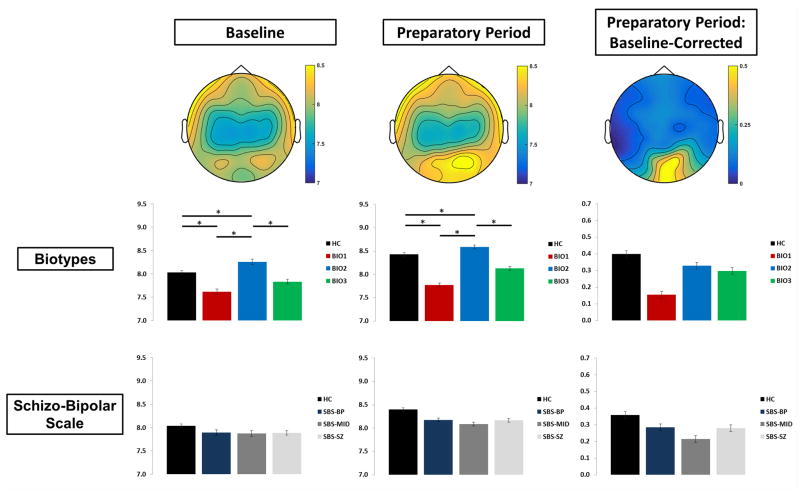
Power and ITC values were then averaged over 9 posterior cortex sensors that captured the peak vSSR activity (Figure 4; Clementz et al., 2010, 2008; Ethridge et al., 2011; Wang et al., 2007). Averaged spectral values for the 500ms baseline period were subjected to a 2-way ANOVA (group x saccade type). Averaged values for each of the five 1 sec Preparatory Period “countdowns” were analyzed both before and after baseline subtraction using mixed effects 3-way MANOVAs (group x saccade type x time bin). Power during the baseline period and non-baseline-corrected power during the Preparatory Period were considered to be measures of intrinsic activity. Power was also analyzed using percentage increase over baseline, providing a measure of the signal-to-noise ratio.
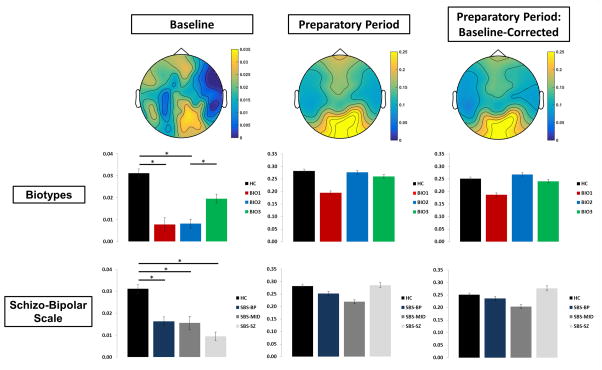
Figure 4.
Single-Trial Oscillatory Power. Top-down topographies of oscillatory power at the steady-state driving frequency (15Hz; top) for the baseline period, the Preparatory Period, and baseline-corrected entrainment. Bar plots for Biotypes (middle) and Schizo-Bipolar Scale (bottom) represent power over visual cortex. Error bars indicate standard error of the mean. *p < .05.
Results
Behavioral Data
Error rate was greater for anti- than pro-saccades, F(1,153) = 187.17, p = 2.46E-28. Latency of correct responses was greater for anti- than pro-saccades, F(1,153) = 109.9, p = 1.03E-19, but did not differ on incorrect responses. No effects involving group were found in response latency for either correct or incorrect trials (Tables 1 and 2).
Table 2.
Demographic and Clinical Characteristics of Healthy Comparison Subjects and Probands as a Function of Biotype
| Healthy (N=58) | Biotype-1 (N=18) | Biotype-2 (N=27) | Biotype-3 (N=53) | Significance Test | |||||
|---|---|---|---|---|---|---|---|---|---|
| 48 | 22 | 48 | 58 | χ2(3)=7.09, p=.07 | |||||
|
| |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
|
| |||||||||
| Age | 39.1 | 12.2 | 31.7 | 14.1 | 29.7 | 10.9 | 35.8 | 13.4 | F(3,152)=4.00, p=.009 |
| Trials accepted | |||||||||
| Prosaccades | 42.6 | 6.9 | 35.3 | 9.9 | 43.3 | 7.6 | 41.9 | 7.5 | F(3,153)=5.00, p=.002 |
| Antisaccades | 46.4 | 4.0 | 44.6 | 5.2 | 45.6 | 5.6 | 46.1 | 5.5 | F(3,153)=0.71, p=.55 |
| Percent Correct | |||||||||
| Prosaccades | 98.7 | 1.8 | 99.0 | 2.0 | 98.1 | 2.6 | 98.0 | 4.2 | F(3,153)=0.73, p=.54 |
| Antisaccades | 90.3 | 8.0 | 75.8 | 17.6 | 79.1 | 19.5 | 84.5 | 12.1 | F(3,153)=7.91, p<.001 |
| Response Latency (ms)a | |||||||||
| Correct Prosaccades | 361.8 | 57.7 | 347.1 | 84.4 | 344.7 | 72.0 | 367.2 | 85.4 | F(3,153)=0.75, p=.53 |
| Correct Antisaccades | 399.5 | 78.0 | 419.5 | 90.2 | 394.2 | 93.9 | 411.1 | 75.0 | F(3,153)=0.54, p=.66 |
| Incorrect Antisaccades | 348.8 | 81.3 | 301.3 | 61.0 | 335.6 | 81.2 | 355.4 | 79.5 | F(3,153)=2.09, p=.11 |
| GAFb | 84.8 | 5.3 | 44.1 | 8.2 | 48.6 | 11.6 | 53.4 | 13.7 | F(2,95)=4.20, p=.02 |
| BACS | −0.09 | 1.09 | −2.57 | 0.8 | −1.88 | 0.95 | −0.28 | 0.79 | F(2,93)=63.5, p=4.1E-18 |
| PANSS-positive | – | – | 17.9 | 7.5 | 15.8 | 4.8 | 15.9 | 5.2 | F(2,94)=0.90, p=.41 |
| PANSS-negative | – | – | 20.0 | 7.3 | 15.7 | 4.9 | 15.6 | 6.0 | F(2,94)=6.98, p=.01 |
| PANSS-general | – | – | 37.4 | 9.8 | 33.7 | 7.8 | 34.1 | 7.4 | F(2,94)=1.30, p=.28 |
| MADRS | – | – | 14.6 | 9.1 | 11.0 | 9.2 | 10.1 | 8.2 | F(2,94)=1.76, p=.18 |
| Young Mania Scale | – | – | 5.9 | 6.7 | 6.6 | 5.5 | 6.6 | 6.4 | F(2,94)=0.08, p=.93 |
| Medication Class | |||||||||
| Antipsychotic first generation | 0% | 11.1% | 11.1% | 7.6% | χ2(2)=0.37, p=.83 | ||||
| Antipsychotic second generation | 0% | 77.8% | 74.1% | 56.6% | χ2(2)=3.92, p=.14 | ||||
| Mood stabilizer | 0% | 55.6% | 44.4% | 45.3% | χ2(2)=0.66, p=.72 | ||||
| Lithium | 0% | 5.6% | 14.8% | 13.2% | χ2(2)=0.96, p=.62 | ||||
| Antidepressant | 1.7% | 38.9% | 37.0% | 47.2% | χ2(2)=0.89, p=.64 | ||||
| Sedative/anxiolytic | 1.7% | 22.2% | 25.9% | 30.2% | χ2(2)=0.48, p=.79 | ||||
| Stimulant | 0% | 5.6% | 7.4% | 15.1% | χ2(2)=1.77, p=.41 | ||||
| Anticholinergic | 0% | 11.1% | 7.4% | 7.6% | χ2(2)=0.26, p=.88 | ||||
GAF, Global Assessment of Functioning; PANSS, Positive and Negative Symptom Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; BACS, Brief Assessment of Cognition in Schizophrenia.
Only 40% of participants made any incorrect responses during prosaccade trials.
Healthy subjects were excluded from comparisons of clinical characteristics and medication status.
Schizo-Bipolar Scale Groups
SBS-Group differences, F(3,153) = 6.16, p = .001, and SBS-Group x Task interactions, F(3,153) = 5.86, p = .001, were driven by healthy subjects showing a lower antisaccade error rate than all psychosis groups, while psychosis groups did not differ from one another.
Biotype Groups
Biotype-Group differences, F(3,153) = 7.27, p = .00014, and Biotype-Group x Task interactions, F(3,153) = 7.91, p = .00006,were driven by healthy subjects showing a lower antisaccade error rate than all psychosis groups, as well as Biotype-1 showing a higher antisaccade error rate than Biotype-3. This is consistent with the findings of Clementz et al. (2016) using a different testing paradigm (Reilly et al., 2014).
Evoked Potentials to Onset of the Preparatory Period
At the onset of the Preparatory Period, there were four clearly visually identifiable peaks in GFP (Figures 2 and 3). The latencies of these peaks (130 ms, 175 ms, 235 ms, and 300 ms) are consistent with studies using similar stimuli (Clementz et al., 2010; Ethridge et al., 2011). Preceding the earliest peak (100–130 ms), GFP was greater for anti- than prosaccade trials, F(1,153) = 4.79, p = .031, consistent with Clementz et al. (2010).
Schizo-Bipolar Scale Groups
An SBS-Group difference at 290–320 ms, F(3,153) = 2.88, p = .039, were driven by healthy showing greater GFP than all SBS-Groups (Figure 2 bottom). At no time did any SBS-Group differ from any other. An SBS-Group x Task interaction effect was found at 400–450 ms, F(3,153) = 3.07, p = .030, which was driven by healthy showing greater GFP during pro- than anti-saccade trials (Figure 3 bottom).
Biotype Groups
Biotype-Group differences in GFP were found at 160–250 ms, F(3,153) = 4.26, p = .009, and from 260–330 ms, F(3,153) = 4.71, p = .006 (Figure 2 top). From 160–200 ms, encompassing the GFP peak at 175 ms, these differences were driven by Biotype-1 showing lower GFP than all other groups. Group differences from 200–250 ms, encompassing the GFP peak at 235 ms, were driven by healthy and Biotype-2 showing greater GFP than Biotype-1 and Biotype-3. From 260–330 ms, encompassing the GFP peak at 300 ms, differences were driven by Biotype-1 showing lower GFP than all other groups as well as Biotype-3 showing lower GFP than healthy and Biotype-2. A Biotype-Group x Task interaction effect was found at 390–470 ms, F(3,153) = 3.53, p = .019, which was driven by healthy showing greater GFP during pro- than anti-saccade trials (the same effect as for SBS; Figure 3 top).
Single-Trial Oscillatory Power
Baseline STP did not differ between pro- and antisaccade trials. During the preparatory period, a main effect of Time Bin both with and without baseline adjustment as well as percentage increase over baseline, F(3,153) = 48.198, p < .0001, showed increases from the first countdown to the second, as well as incremental decreases throughout the remainder of the preparatory period (Figure 4). In addition, significant Task x Time Bin interactions were found for both with and without baseline adjustment as well as percentage increase over baseline, F(3,153) = 11.72, p < .0001, with antisaccade trials showing greater magnitude than prosaccade trials only during the first two time bins.
Schizo-Bipolar Scale Groups
No effects involving SBS-Group were found.
Biotype Groups
Biotype-Group differences in baseline power, F(3,153) = 4.09, p = .008, as well as power during entrainment, F(3,153) = 5.25, p = .002, were driven by Biotype-2 having greater power than other groups, and by Biotype-1 showing lower power than healthy subjects. This pattern of effects, with Biotype-1 showing hypoactivity, Biotype-2 showing hyperactivity, and Biotype-3 the closest to the healthy group, is consistent with expectations based on Clementz et al. (2016).
Inter-Trial Phase Coherence
Baseline ITC did not differ between pro- and anti-saccade trials. During the preparatory period, ITC both with and without baseline adjustment showed main effects of Task, F(1,153) = 5.82, p = .017, with antisaccade trials greater than prosaccade, and main effects of Time Bin, F(3,459) = 23.96, p < .0001, with the first countdown lower than the others (Figure 5). In addition, both ITC and percentage change from baseline showed significant Task x Time Bin interactions, F(3,153) = 5.13, p = .002, with values increasing from the second to third countdown for pro- but not antisaccade trials, resulting in antisaccade trials being greater than prosaccades only during the first and second countdown time bins.
Figure 5.
Inter-Trial Phase Coherence. Top-down topographies of inter-trial phase coherence at the steady-state driving frequency (15Hz; top) for the baseline period, the Preparatory Period, and baseline-corrected entrainment. Bar plots for Biotypes (middle) and Schizo-Bipolar Scale (bottom) represent power over visual cortex. Error bars indicate standard error of the mean. *p < .05.
Schizo-Bipolar Scale Groups
SBS-Group differences in baseline ITC, F(3,153) = 3.62, p = .015, were driven by healthy individuals showing greater ITC than all SBS subgroups. SBS-Groups did not differ from one another.
Biotype Groups
Biotype-Group differences in baseline ITC, F(3,153) = 4.38, p = .005, were driven by healthy and Biotype-3 showing greater ITC than Biotype-2, as well as healthy individuals showing greater ITC than Biotype-1.
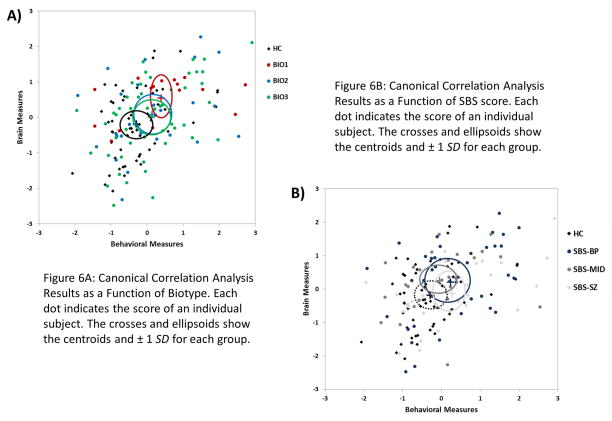
Canonical Correlation Analysis
To describe the relationship between behavior and neural activity, a canonical correlation was conducted with behavioral measures (error rate and correct/incorrect latencies for pro- and anti-saccade conditions) as the “criteria” and brain measures (STP and ITC for each task during baseline and the Preparatory Period) as the “predictors”. Brain measure values were averaged over the Preparatory Period due to the high level of collinearity between individual time bins. The first canonical variate was significant, canonical correlation = .436, Wilks lambda = .637, F(40,599) = 1.63, p = .009. Table 3 provides the standardized coefficients for each variable. ITC during the Preparatory Period for both pro- and anti-saccade trials was strongly negatively correlated with antisaccade error rate as well as correct latency on pro- and anti-saccades. In addition, baseline STP and error latency for antisaccade trials and prosaccade error rate were weakly positively correlated. This canonical variate suggests that decreased phase synchrony (i.e., less consistent neural responses) during the Preparatory Period and, to a lesser extent, atypical levels of baseline activity are associated with deviations in error rate and saccade latencies. Figure 6 shows scatterplots of individual subjects’ canonical variate scores as a function of SBS-Groups (Fig. 6a) and of Biotype-Groups (Fig. 6b), with centroids and ellipses of ±1 SD for each group. The SBS-Groups show little separation from one another, as evidenced by their ellipses encompassing all other centroids. The relationship between brain activity and behavioral performance represented by this canonical variate, however, discriminates between Biotype-Groups, as evidenced by a visible separation of Biotype-1 from healthy subjects, and to a lesser extent Biotype-2 and -3.
Table 3. Canonical Correlation Analysis Results.
Correlations between Canonical Variate and Constituent Variables
| Behavioral Variable
| |
| Error Rate – Pro | 0.240 |
| Error Rate – Anti | 0.752 |
| Correct Latency – Pro | 0.612 |
| Correct Latency – Anti | 0.749 |
| Error Latency – Anti | 0.283 |
| EEG Variable | |
|
| |
| STP Baseline – Pro | 0.178 |
| STP Baseline – Anti | 0.288 |
| STP Preparatory – Pro | −0.076 |
| STP Preparatory – Anti | −0.014 |
| ITC Baseline – Pro | −0.043 |
| ITC Baseline – Anti | −0.199 |
| ITC Preparatory – Pro | −0.895 |
| ITC Preparatory – Anti | −0.800 |
Pro, prosaccade; Anti, antisaccade; STP, single-trial power; ITC, inter-trial phase coherence
Figure 6.
Canonical Correlation Analysis Results as a Function of Biotype and of SBS score.
Discussion
There were two primary goals of this study of persons with psychosis: 1) examine differences in baseline, stimulus-related (i.e., not baseline-adjusted), and stimulus-specific (i.e., baseline-adjusted) neural activity, with baseline and stimulus-related being considered variants of intrinsic activity, and 2) determine how baseline, stimulus-related, and stimulus-specific neural activity may be modulated as a function of cognitive demands. Neural and behavioral measures were assessed using two psychosis subgrouping schemes, the Schizo-Bipolar Scale (Keshavan et al., 2011) and biologically-based Biotypes (Clementz et al., 2016). There were five primary outcomes of the present project. First, Biotype-Groups exhibited baseline and stimulus-related neural activity differences in opposite directions, with activity being diminished in Biotype-1 and accentuated in Biotype-2. Despite this, examination of stimulus-specific neural responses revealed no differences between Biotypes. Second, baseline ITC, a measure of the neural phase consistency across trials, was diminished in all SBS-Groups, while Biotype-1 and Biotype-2 were lower than both healthy and Biotype-3. Third, the Biotypes showed a number of differences in evoked potentials at stimulus onset from both healthy subjects and one another, including Biotype-Group x Task interactions, while SBS-Groups differed from healthy only once and never from one another. Fourth, psychosis subjects made the most antisaccade errors, but only Biotypes showed behavioral differences between psychosis subgroups, with Biotype-1 having the worst and Biotype-3 having the best antisaccade performance. Fifth, these behavioral and brain measures were related in a fashion that meaningfully separated Biotypes, but not SBS-Groups. The significance of these findings for understanding the neurobiology of psychosis and its relation to behavioral outcomes will be discussed below.
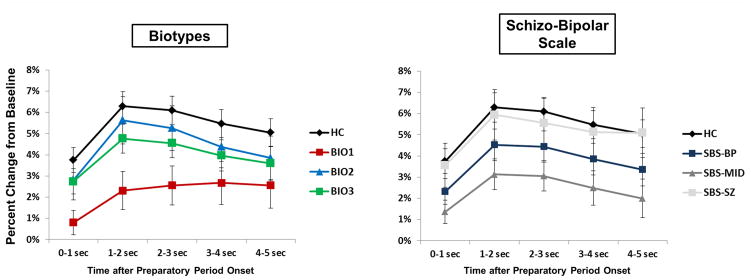
Ethridge et al. (2011) observed augmented stimulus-related responses in SZ, but these were determined by augmented baseline activity. Subtracting this baseline activity yielded lower stimulus-specific neural activations for SZ compared to healthy persons. Most studies of vSSR in psychosis report only this difference score, providing an informative yet incomplete picture of overall neural activation among psychosis cases. Similarly in the current study, a subgroup of individuals with psychosis, Biotype-2, had greater STP amplitude during the baseline and steady-state stimulation periods compared to both healthy persons and other Biotype-Groups. Baseline subtraction, however, revealed no differences between Biotype-Groups in stimulus-specific neural responses. High levels of intrinsic neural activity in SZ can masquerade as accentuated sensoricortical responsiveness (Clementz et al., 2008; Spencer et al., 2004; Wang et al., 2010) and can ultimately result in decreased signal-to-noise ratios. Indeed, Ethridge et al. (2011) found signal-to-noise in SZ to be increasingly deviant over time. The current examination revealed a trend for diminished SNR among individuals with psychosis (see Figure 7), most dramatically in Biotype-1. Biotype-1 had diminished activations during the baseline and Preparatory periods, although again this did not result in deviant levels of stimulus-specific, i.e., baseline subtracted, neural activity. It may be that both of these Biotype-Groups have “attractor state” (one of multiple, more-or-less stable firing patterns to which a given neural network can be attracted by internal or external cues) problems, although for different reasons (Rolls et al., 2008). For example, Biotype-2 have neuronal hyperexcitation, which given the stochastic nature of oscillatory neural activity would decrease the stability of cortical networks selected to support current behavioral requirements. Competing cortical networks could periodically attain higher signal strength, leading to behavioral indications of “distraction.” Biotype-1, characterized by hypoexcitation, would have difficulty initiating switches between cortical network states, which could also lead to many of the cognitive symptoms including reduced behavioral flexibility found in individuals with psychosis (Rolls et al., 2008). In this case, the problem of Biotype-2 would be easier to overcome probabilistically, consistent with Biotype-2 exhibiting slightly better cognitive performance than Biotype-1. This would mean the relatively similar behavioral performance of these Biotype-Groups resulted from distinct neurobiological deficits, likely requiring distinct treatments.
Figure 7.
Single-Trial Oscillatory Power: Percentage Change from Baseline. Line plots for Biotypes (left) and Schizo-Bipolar Scale (right) represent the percentage change from baseline of STP at the steady-state driving frequency (15 Hz). Error bars indicate standard error of the mean.
These findings are consistent with pharmaco-patho-physiological models of psychosis that invoke NMDA-receptor hypofunction (Javitt, 2007; Rujescu et al., 2006). Administration of NMDA antagonists, such as ketamine, produces clinical and neurophysiological deficits consistent with psychosis. Recently, Sivarao et al. demonstrated that ketamine can either reduce or augment auditory steady-state response amplitudes in mice, depending on dosage and time (2016). It is noteworthy that both Biotype-1 and Biotype-2 had diminished baseline ITC, as abnormally high or low NMDA current flow could decrease the stability of cortical networks (Rolls et al., 2008).
A similar pattern was found for evoked potentials at the onset of the Preparatory Period, with Biotype-1 exhibiting overall hypoexcitation compared to both healthy and other Biotype-Groups (see Figure 2) and Biotype-2 exhibiting hyperexcitation compared to other Biotype-Groups (see Figure 2). Together these findings constructively replicate those of Clementz et al. (2016), who found Biotype-1 to be the most behaviorally and neurobiologically impaired, characterized by diminished neural activity, decreased responsiveness to even simple stimuli, and poor cognitive performance. These results also integrate with previous findings that individuals with chronic SZ exhibit a slower buildup of steady-state response to steady-state stimuli (Clementz et al., 2004), providing further support for the idea that Biotype-1 may capture the essence of a particularly neurobiologically severe psychosis subform (Keshavan et al., 2011; Keshavan, Clementz, Pearlson, Sweeney, & Tamminga, 2013; Rosen et al., 2012; Tamminga et al., 2013). That Biotypes-1/2 had such diametrically opposed aberrations in intrinsic power, and that Biotype-2 exhibited greater VEPs than other Biotype-, and that, makes the finding that cognitive performance in Biotype-2 was only marginally better than in Biotype-1 all the more interesting. Further, although both Biotype-Groups had diminished baseline ITC, this decreased neural consistency correlated with cognitive performance to a much greater extent in Biotype-1 (see Figure 6). Further investigation is needed to understand how these two very different underlying neurobiological paths result in similar cognitive and clinical profiles.
Potential limitations include that, common to all studies of chronic psychosis, medication effects may be present and are not be easily controlled. Dosing and compliance information was based on physician choice and self-report, so firm conclusions on medication effects cannot be assessed. It is unlikely that this feature played a substantial role in the observed differences between the Biotype-Groups, however, as the Biotype-Groups were similarly medicated (see Table 2). Likewise, it is improbable that medication effects account for between-study differences in steady-state responses, as samples that were completely, or nearly completely, medicated with second-generation antipsychotics have shown reduced (Ferrarelli et al., 2008; Krishnan et al., 2009; Spencer et al., 2004; Teale et al., 2008) versus augmented responses (Ethridge et al., 2011; Hamm, Gilmore, et al., 2012). Unfortunately, while the current study had a modest sample size, certain psychosis subgroups (e.g., Biotype-1, N = 18) prevent reliable comparison of medicated versus non-medicated subsamples. Ideally, future studies will include sample sufficiently large for subsampling based on medication status. Second, it should be noted that the Biotype-Group sizes differ dramatically. This could not be rectified in the current study as all data was collected prior to the Biotype construction. Finally, it is important to address the potential limitation that pro-/anti-saccade performance measures were used in the Biotype construction. There are demonstrable task differences between those used for Biotype creation and those used in the current study, as indicated by antisaccade error rates being considerably lower here than is typical for psychosis studies and in the current sample with a standard laboratory task (Reilly et al., 2014), with only a moderate correlation (r = .54, p < .001) between error rate on the two antisaccade tasks. For comparison, this is much lower than typical test-retest correlations for antisaccade tasks found in studies of psychosis, even after many months (e.g., Calkins, Iacono, & Curtis, 2003). More importantly, no brain data related to saccades were included in the creation of the Biotypes. Further, all neurophysiological data in the current study were recorded during baseline and an extended visual steady-state period prior to the onset of the behavioral cue.
Although only one difference was found between SBS-Groups, it is noteworthy. Probands with scores in the middle of the Schizophrenia-Bipolar Scale showed a diminished percentage increase over baseline STP (Figure 7). Clinically these individuals are usually diagnosed with schizoaffective disorder and in research are often considered to be in between schizophrenia and bipolar disorder on a psychosis spectrum (Keshavan et al., 2011). In the literature, however, there are indications from a number of measures that schizoaffective disorder may not fit well as a midpoint on a psychosis spectrum but rather should be considered as a unique entity (Ethridge et al., 2014; Tamminga et al., 2014). Future studies of psychosis should include persons diagnosed with schizoaffective disorder in order to map the pattern of similarities and differences between this and other psychosis subgroups. In addition, this highlights the need for continued research into the neurobiological heterogeneity among persons with psychosis and the potential utility of neuroscience-based subgroups for both research and treatment for these debilitating diseases.
Acknowledgments
Funding for this study was provided by National Institutes of Health Grants MH077862 and MH085485.
Footnotes
Financial Disclosures
Author JAS has served as a consultant for Takeda, Inc. The remaining authors report no biomedical financial interests or potential conflicts of interest.
References
- Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, O’Donnell BF. Steady state responses: Electrophysiological assessment of sensory function in schizophrenia. Schizophrenia Bulletin. 2009;35(6):1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, … Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. American Journal of Psychiatry. 2001;158(7):1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Iacono WG, Curtis CE. Smooth pursuit and antisaccade performance evidence trait stability in schizophrenia patients and their relatives. International Journal of Psychophysiology. 2003;49(2):139–146. doi: 10.1016/S0167-8760(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld L. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Experimental Brain Research. 2001;139(4):377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Brahmbhatt SB, McDowell JE, Brown R, Sweeney Ja. When does the brain inform the eyes whether and where to move? An EEG study in humans. Cerebral Cortex. 2007;17(11):2634–43. doi: 10.1093/cercor/bhl171. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Gao Y, McDowell JE, Moratti S, Keedy SK, Sweeney JA. Top-down control of visual sensory processing during an ocular motor response inhibition task. Psychophysiology. 2010;47(6):1011–8. doi: 10.1111/j.1469-8986.2010.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Keil A, Kissler J. Aberrant brain dynamics in schizophrenia: delayed buildup and prolonged decay of the visual steady-state response. Cognitive Brain Research. 2004;18(2):121–129. doi: 10.1016/j.cogbrainres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sponheim SR, Iacono WG, Beiser M. Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology. 1994;31(5):486–94. doi: 10.1111/j.1469-8986.1994.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, … Tamminga CA. Identification of distinct psychosis biotypes using brain-based biomarkers. American Journal of Psychiatry. 2016 doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Wang J, Keil A. Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. Journal of Neuroscience. 2008;28(50):13411. doi: 10.1523/JNEUROSCI.4095-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Taddei F, Apnile T, Spinelli D. Neural correlates of fast stimulus discrimination and response selection in top-level fencers. Neuroscience Letters. 2006;408(2):113–8. doi: 10.1016/j.neulet.2006.08.085. [DOI] [PubMed] [Google Scholar]
- Ding J, Sperling G, Srinivasan R. Attentional modulation of SSVEP power depends on the network tagged by the flicker frequency. Cerebral Cortex (New York, NY: 1991) 2006;16(7):1016–29. doi: 10.1093/cercor/bhj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyckman KA, McDowell JE. Behavioral plasticity of antisaccade performance following daily practice. Experimental Brain Research. 2005;162(1):63–69. doi: 10.1007/s00221-004-2105-9. [DOI] [PubMed] [Google Scholar]
- Ethridge LE, Moratti S, Gao Y, Keil A, Clementz Ba. Sustained versus transient brain responses in schizophrenia: the role of intrinsic neural activity. Schizophrenia Research. 2011;133(1–3):106–11. doi: 10.1016/j.schres.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, Soilleux M, Nakonezny PA, Reilly JL, Kristian Hill S, Keefe RSE, … Sweeney JA. Behavioral response inhibition in psychotic disorders: Diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophrenia Research. 2014;159(2):491–498. doi: 10.1016/j.schres.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, Daskalakis ZJ. Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain. 2010;133(5):1505–1514. doi: 10.1093/brain/awq046. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, … Tononi G. Reduced Evoked Gamma Oscillations in the Frontal Cortex in Schizophrenia Patients: A TMS/EEG Study. American Journal of Psychiatry. 2008;165(8):996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. Arlington, VA, VA: American Psychiatric Publishing; 1997. [Google Scholar]
- Flynn G, Alexander D, Harris A, Whitford T, Wong W, Galletly C, … Williams LM. Increased absolute magnitude of gamma synchrony in first-episode psychosis. Schizophrenia Research. 2008;105(1):262–271. doi: 10.1016/j.schres.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved Assessment of Significant Activation in Functional Magnetic Resonance Imaging (fMRI): Use of a Cluster-Size Threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18(10):1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hamm JP, Dyckman Ka, McDowell JE, Clementz Ba. Pre-cue fronto-occipital alpha phase and distributed cortical oscillations predict failures of cognitive control. The Journal of Neuroscience. 2012;32(20):7034–41. doi: 10.1523/JNEUROSCI.5198-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Clementz Ba. Augmented gamma band auditory steady-state responses: Support for NMDA hypofunction in schizophrenia. Schizophrenia Research. 2012;138(1):1–7. doi: 10.1016/j.schres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Picchetti NAM, Sponheim SR, Clementz BA. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biological Psychiatry. 2011;69(10):989–96. doi: 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp JF, Engel AK, Siegel M. Oscillatory Synchronization in Large-Scale Cortical Networks Predicts Perception. Neuron. 2011;69(2):387–396. doi: 10.1016/j.neuron.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Hudgens-Haney ME, Hamm JP, Goodie AS, Krusemark EA, McDowell JE, Clementz BA. Neural correlates of the impact of control on decision making in pathological gambling. Biological Psychology. 2013;92(2):365–72. doi: 10.1016/j.biopsycho.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorder. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and Schizophrenia: Phencyclidine, N-Methyl-d-Aspartate Receptors, and Dopamine–Glutamate Interactions. International Review of Neurobiology. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Neural Activity in Monkey Prefrontal Cortex Is Modulated by Task Context and Behavioral Instruction during Delayed-match-to-sample and Conditional Prosaccade—Antisaccade Tasks. Journal of Cognitive Neuroscience. 2006;18(5):749–765. doi: 10.1162/jocn.2006.18.5.749. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Clementz Ba, Pearlson GD, Sweeney Ja, Tamminga Ca. Reimagining psychoses: An agnostic approach to diagnosis. Schizophrenia Research. 2013;146(1–3):10–6. doi: 10.1016/j.schres.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Morris DW, Sweeney JA, Pearlson GD, Thaker G, Seidman LJ, … Tamminga CA. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: The Schizo-Bipolar Scale. Schizophrenia Research. 2011;133(1–3):250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophrenia Research. 2006;82(1):1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Zemon V, Saperstein A, Butler PD, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia: harmonic analysis. Schizophrenia Research. 2005;76(1):55–65. doi: 10.1016/j.schres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O’Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. NeuroImage. 2009;47(4):1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O’Donnell BF. Steady state visual evoked potential abnormalities in schizophrenia. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2005;116(3):614–24. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Kissler JM, Berg P, Dyckman KA, Gao Y, Rockstroh B, Clementz BA. Electroencephalography/magnetoencephalography study of cortical activities preceding prosaccades and antisaccades. NeuroReport. 2005;16(7):663–668. doi: 10.1097/00001756-200505120-00002. [DOI] [PubMed] [Google Scholar]
- Moratti S, Clementz BA, Gao Y, Ortiz T, Keil A. Neural mechanisms of evoked oscillations: stability and interaction with transient events. Human Brain Mapping. 2007;28(12):1318–33. doi: 10.1002/hbm.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Reviews. Neuroscience. 2004;5(3):218–28. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Pastor MA, Artieda J, Arbizu J, Valencia M, Masdeu JC. Human Cerebral Activation during Steady-State Visual-Evoked Responses. J Neurosci. 2003;23(37):11621–11627. doi: 10.1523/JNEUROSCI.23-37-11621.2003. Retrieved from http://www.jneurosci.org/content/23/37/11621.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MP, Regan D. Magnetic source imaging of the human brain. Mahwah, NJ, NJ: Lawrence Erlbaum Associates; 2003. Techniques for investigating and exploiting nonlinearities in brain processes by recording responses evoked by sensory stimuli; pp. 135–157. [Google Scholar]
- Reilly JL, Frankovich K, Hill S, Gershon ES, Keefe RSE, Keshavan MS, … Sweeney JA. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophrenia Bulletin. 2014;40(5):1011–21. doi: 10.1093/schbul/sbt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riečanský I, Kašpárek T, Řehulová J, Katina S, Přikryl R. Aberrant EEG responses to gamma-frequency visual stimulation in schizophrenia. Schizophrenia Research. 2010;124(1):101–109. doi: 10.1016/j.schres.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nature Reviews. Neuroscience. 2008;9(9):696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- Rosen C, Marvin R, Reilly JL, DeLeon O, Harris MSH, Keedy SK, … Sweeney JA. Phenomenology of First-Episode Psychosis in Schizophrenia, Bipolar Disorder, and Unipolar Depression. Clinical Schizophrenia & Related Psychoses. 2012;6(July):145–151A. doi: 10.3371/CSRP.6.3.6. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, … Grunze H. A Pharmacological Model for Psychosis Based on N-methyl-D-aspartate Receptor Hypofunction: Molecular, Cellular, Functional and Behavioral Abnormalities. Biological Psychiatry. 2006;59(8):721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Chen P, Senapati A, Yang Y, Fernandes A, Benitex Y, … Ahlijanian MK. 40 Hz Auditory Steady-State Response Is a Pharmacodynamic Biomarker for Cortical NMDA Receptors. Neuropsychopharmacology. 2016;(August 2015):1–9. doi: 10.1038/npp.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz Ma, Klump MC, Frumin M, … McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(49):17288–93. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, … Sweeney JA. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) The American Journal of Psychiatry. 2013;170(11):1263–74. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, Clementz BA, Thaker GK. Bipolar and schizophrenia network for intermediate phenotypes: Outcomes across the psychosis continuum. Schizophrenia Bulletin. 2014;40(SUPPL 2):131–137. doi: 10.1093/schbul/sbt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. NeuroImage. 2008;42(4):1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Brown R, Dobkins KR, McDowell JE, Clementz BA. Diminished Parietal Cortex Activity Associated with Poor Motion Direction Discrimination Performance in Schizophrenia. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Clementz BA, Keil A. The neural correlates of feature-based selective attention when viewing spatially and temporally overlapping images. Neuropsychologia. 2007;45(7):1393–1399. doi: 10.1016/j.neuropsychologia.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR. Prefrontal Broadband Noise, Working Memory, and Genetic Risk for Schizophrenia. American Journal of Psychiatry. 2004;161(3):490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- Winterer G, McCarley RW. Schizophrenia. Oxford, UK: Wiley-Blackwell; 2011. Electrophysiology of Schizophrenia; pp. 311–333. [DOI] [Google Scholar]
- Winterer G, Musso F, Beckmann C, Mattay V, Egan MF, Jones DW, … Weinberger DR. Instability of prefrontal signal processing in schizophrenia. American Journal of Psychiatry. 2006;163(11):1960–1968. doi: 10.1176/appi.ajp.163.11.1960. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends in Neurosciences. 2004;27(11):683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, … Coppola R. Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clinical Neurophysiology. 2000;111(5):837–849. doi: 10.1016/S1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]