Abstract
This study examines whether frailty is associated with mortality independently of physiological dysregulation (PD) and, if so, which is the more accurate predictor of survival. Data come from the Social Environment and Biomarkers of Aging Study. We use Cox proportional hazard models to test the associations between PD, frailty and 4–5 year survival. We use Harrell’s C-index to compare predictive accuracy of the models. Both PD and frailty are significantly, positively, and independently correlated with mortality: worse PD scores and being frail are associated with a higher risk of dying. The overall PD score is a more accurate predictor of survival than frailty, although model prediction improves when both measures are included. Physiological dysregulation and frailty independently predict mortality, suggesting that the two measures may be capturing different aspects of the same construct, and that both may be important for identifying individuals at risk for adverse health outcomes.
Keywords: physiological dysregulation, frailty, mortality, older adults
INTRODUCTION
The purpose of this research is to explore and further elucidate the relationships among physiological dysregulation (PD), frailty, and poor health outcomes, specifically mortality, among older adults. Some studies have examined the associations between PD and frailty (Fried et al 2009; Lang, Michel, & Zekry, 2009 as reviewed in Walston, Hadly, Ferrucci, & Guralnik, 2006; Milot et al. 2014; Zaslavsky, Cochrane, Thompson, Woods & LaCroix, 2013), between frailty and mortality (Bandeen-Roche et al., 2006; Buchman, Wilson, Bienias, & Bennett, 2009), and between PD and mortality (Goldman, Turra, Glei, Seplaki, Lin & Weinstein, 2006; Milot et al. 2014). Few studies, however, have examined whether frailty and physiological dysregulation predict poor health independently of one another and, if so, whether one is a better predictor of poor health than the other.
Elucidating the relationships between dimensions of health has implications for both research and clinical practice. Fried and colleagues (2004) demonstrate that, although the literature once used the concepts of frailty, disability, and comorbidity interchangeably, the concepts are actually distinct but overlapping health concerns; “not all frail patients are disabled, not all disabled patients are frail, and comorbidities may or may not be present with these” (Fried et al., 2004; p.259). Distinguishing among them, though, is important for determining appropriate interventions as each carries with it differing needs and protocols for care (Fried et al., 2004).
Where does physiological dysregulation fit in to this picture? Physiological dysregulation or decline is often considered to underlie or be a catalyst of the morbidity process (Crimmins, Jung, & Vasunilashorn, 2010) or of health decline (Zaslavsky et al., 2013), of which frailty is a component. PD, however, has also been theorized to be a component of frailty (Lang et al., 2009) that needs to be considered not only in individuals’ care protocols but also in determining the prevalence of frailty at the population level. While Fried et al. (2004) demonstrate that not all individuals who have a disability are frail and that not all individuals who are frail have a disability, Figure 1 similarly shows that although there is substantial overlap between PD and frailty, not all frail individuals show high levels of physiological dysregulation and not all individuals with high PD are frail. We are, therefore, interested in further understanding the interrelationship between PD and frailty and their individual and joint relationships with mortality.
Figure 1. Overlap between Frailtya and Physiological Dysregulation (PD)b: Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan, 2006.
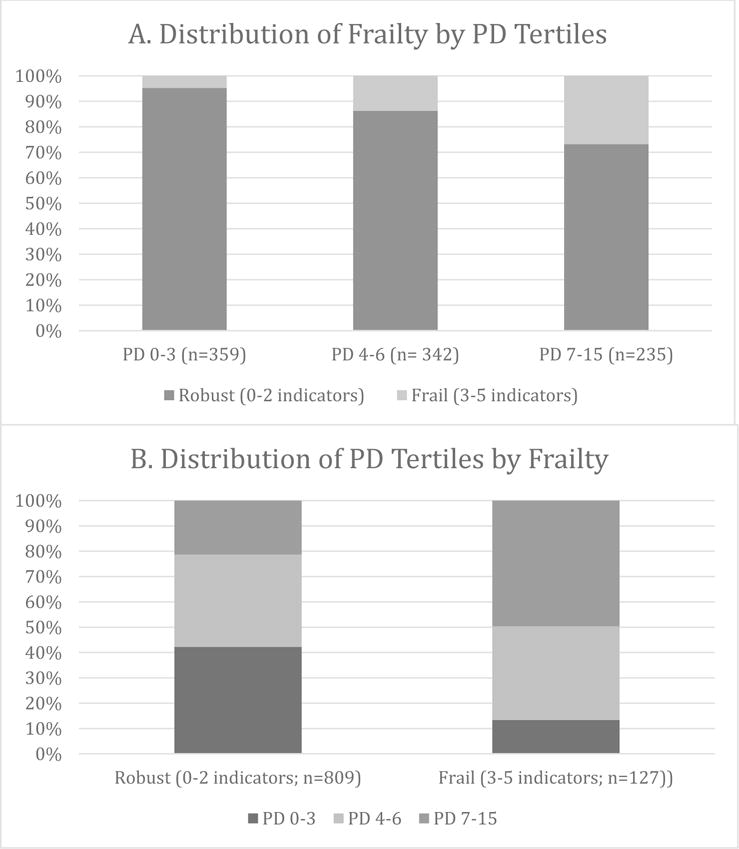
aFrailty indicators include weight loss or underweight, exhaustion, slowness, weakness and low physical activity (Fried et al., 2001). See Table 1 for more details.
bThe PD score is a count of the number of 24 biological markers (9 related to cardiovascular/metabolic risk, seven related to inflammation, four related to hypothalamic-pituitary-adrenal axis and sympathetic nervous system function, and another four associated with a variety of functions and systems) on which respondents scored in the high risk range (Glei, Goldman, Lin & Weinstein, 2012). The observed range of the PD score is 0–15.
Frailty and Physiological Dysregulation
While it is generally agreed that frailty is an indicator of health that identifies older adults who are vulnerable to, or at increased risk for, adverse health outcomes, there is some debate in the literature as to what constitutes frailty. One perspective suggests that frailty is a clinical or geriatric syndrome that develops progressively over time (Bergman et al., 2007; Bouillion et al., 2013; Gobbens, Luijkx, Wijnen-Sponselee, & Schols, 2010; Lang et al., 2009; Walston et al., 2006), is the result of the concurrent loss of reserves or dysregulation across numerous physiological systems (Fried et al., 2004), and whose elements are associated with a common, underlying “biological pathway” (Bergman et al., 2007). Although no single definition of the frailty syndrome exists, it is generally agreed that frailty involves a combination of clinical indicators including weakness, fatigue or exhaustion, unintentional loss of weight or lean body mass, poor balance, poor walking ability, low endurance, and low physical activity (Fried et al, 2001; Gruenewald, Seeman, Karlamangla, & Sarkisian, 2009; Walston et al., 2006). Some argue that definitions of frailty should also include psychological and social factors such as cognitive impairment and social withdrawal (Gruenewald et al., 2009; Walston et al., 2006; Zaslavsky et al., 2013). Because individuals can experience any one of these characteristics without others, a diagnosis or classification of frailty occurs only when individuals simultaneously experience several of these traits (Fried et al., 2001; Gruenewald et al., 2009; Lang et al, 2009; Walston et al., 2006). For instance, based on the frailty phenotype developed by Fried and colleagues (Fried et al., 2001), only individuals experiencing three or more symptoms (unintentional weight loss, weakness, poor endurance or low energy, slowness or physical inactivity) would be classified as frail.
In line with this conceptualization of frailty, a number of studies have examined the relationship between biological indicators of health and frailty in an effort to delineate the biological pathway that leads to frailty. Results show positive associations between frailty and biomarkers of inflammation, immune function, sympathetic nervous system function, endocrine system function, and cardiovascular and metabolic function (Fried et al., 2009; Lang et al., 2009 as reviewed in Walston et al., 2006; Zaslavsky et al., 2013). Research using both cross-sectional and cohort data also shows that physiological dysregulation, specifically allostatic load (Seeman, Singer, Rose, Horwitz, & McEwen, 1997), is significantly associated with frailty. One study, for example, reports that higher allostatic load scores (based on 10 biomarkers of cardiovascular and metabolic risk and immune function) are associated with higher levels of frailty (non-frail, pre-frail, or frail; Szanton, Allen, Seplaki, Bandeen-Roche, & Fried, 2009). Similarly, Gruenewald and colleagues (2009) find that worse allostatic load scores (based on 13 biomarkers of cardiovascular, endocrine, immune, and metabolic function) are associated with an increased likelihood of frailty three years later. Physiological dysregulation, therefore, may represent a preclinical indicator of frailty (Szanton et al., 2009; Zaslavsky et al., 2013).
A second perspective suggests that frailty is a multidimensional construct that potentially encompasses physical, psychological, social and biological functioning (Bergman et al., 2007; Bouillion e al., 2013; Fulop et al. 2010; Gobbens et al., 2010; Lang, et al., 2009; Rockwood & Mitnitski 2007) and may be viewed as a continuum or gradient (Bortz, 2002; Hogan, 2007). In this vein, frailty is seen as the accretion of health-related deficits across multiple physiological systems that occur with aging (Bouillion et al., 2013; Mitnitski, Mogilner, & Rockwood, 2001). Although most often operationalized as the number of or the proportion of the number of signs, symptoms and impairments that are present in an individual (Bouillion et al., 2013; Goggins, Woo, Sham, & Ho, 2005; Mitnitski et al., 2001), the literature suggests that there are also select biological markers that could be included (Lang et al., 2009). For example, studies have shown that biological indicators of inflammation, insulin resistance, and endocrine system function are worse in frail than non-frail populations (as reviewed in Lang et al., 2009). Frailty, therefore, may consist of multiple phenotypes or domains (Fried et al., 2004; Walston et al., 2006), including a biological one.
Frailty, Physiological Dysregulation, and Poor Health
Both frailty and physiological dysregulation have been hypothesized to be early indicators of the risk for poor health and mortality (Bandeen-Roche et al., 2006; Borrell, Dallo, & Nguyen, 2010; Buchman et al., 2009; Esplanoza, Jung, & Hazuda, 2012; Fried et al., 2001; Goldman et al., 2006; Hu, Wagle, Goldman, Weinstein, & Seeman, 2007; Karlamangla, Singer, McEwen, & Seeman, 2002; Macklai, Spagnoli, Junod, & Santos-Eggimann, 2013; Mattei, Demissie, Falcon, Ordovas & Tucker, 2010; Milot et al., 2014; Seeman et al., 1997). Separate studies have examined the association between measures of frailty or measures of physiological dysregulaton and the risk for poor health. Bandeen-Roche and colleagues (2006), for instance, show that frail women are more likely than non-frail women to develop difficulty with activities and instrumental activities of daily living and are more likely to die within a three year follow-up period. Many other studies have shown that higher physiological dysregulation scores are positively associated with having or developing chronic conditions or a disability, and with mortality (Borrell et al., 2010; Goldman et al., 2006; Hu et al., 2007; Karlamangla et al., 2002; Mattei et al., 2010; Seeman et al., 1997).
We know of only one study that has examined frailty and physiological dysregulation simultaneously in analyses predicting poor health. A recent analysis of data from a cohort of adults aged 85 and older (Mitnitski et al., 2015) examines an index of biological frailty (based on 40 biomarkers) and an index of clinical frailty (a measure of cumulative deficits based on 40 clinical markers that included items such as help with activities or instrumental activities of daily living, low energy, walking ability, and presence of chronic disease) and shows that both measures are significantly associated with mortality and improve the accuracy of predicting survival. While this study is an important step in furthering our understanding of the interrelationships between PD, frailty and negative health outcomes in late life, additional research is needed to determine whether these relationships hold for older adults more generally, e.g. those younger than age 85.
One theoretical model of the connections between PD, frailty and poor health proposes that physiological dysregulation works through frailty to lead to adverse health outcomes and ultimately death (Zaslavsky et al., 2013). As individuals progress from a non-frail to a frail state, their bodies experience changes at the cellular and physiological system (e.g. endocrine) levels, which are shaped by social, environmental and life style factors. After reaching a frail state, defined along the lines of a syndrome, intermediate adverse health outcomes develop (e.g. morbidity, disability), followed by death. This model, therefore, suggests that once frailty is accounted for, PD may no longer have an independent effect on mortality.
An alternative possibility is that PD and frailty have independent effects on the risk of dying. For instance, some previous work suggests that frailty is a multidimensional construct that encompasses both physical and biological domains (Bergman et al., 2007; Gobbens et al., 2010; Hogan, 2007; Sternberg, Schwartz, Karunananthan, Bergman & Clarfield, 2011), suggesting that measures such as physiological dysregulation and the Fried frailty phenotype (Fried et al., 2001) would both be independently associated with mortality. Independent effects could also emerge if PD is not a domain of the frailty construct, but rather, PD and frailty are simply independent factors in the morbidity process. This perspective suggests that in a single model PD and the Fried frailty phenotype will both be significantly associated with survival.
In this study, therefore, we analyze a population-based sample of adults aged 60 and older to assess whether frailty is associated with mortality independently of PD and, if so, whether frailty or PD is the more accurate predictor of mortality. We focus on the independence and significance of the predictors to provide insights into the interrelationships of PD and frailty and their relationship to the risk of dying. We also examine discriminatory power of the measures because statistical significance of a coefficient relating a predictor to an outcome does not necessarily indicate better model prediction (Pencina, D’Agostino, D’Agostino & Vasan, 2008). Both significance and discrimination are important in evaluating the clinical and theoretical importance of a variable (Pencina et al., 2008).
MATERIALS AND METHODS
Data
Data came from the Social Environment and Biomarkers of Aging Study (SEBAS), an extension of the Taiwan Longitudinal Study of Aging (TLSA) that was first conducted in 1989 (n=4049) with follow-ups approximately every three years and refreshed younger samples in 1996 and 2003. In 2000, a nationally representative sub-sample of Taiwanese aged 54 and older was selected from the 1999 TLSA to participate in the first wave of SEBAS; the study included a home interview and, several weeks later, a hospital-based physical exam. In addition to a 12-hour (7pm–7am) urine sample from the night before the exam and a fasting blood sample during the exam, height, weight, and waist-hip circumference were also collected. Additional study details have been described elsewhere (Chang et al., 2012).
A second wave of SEBAS, conducted in 2006, included the surviving SEBAS I exam participants and a subsample of respondents first interviewed in the 2003 TLSA. SEBAS II respondents were interviewed in their homes and participated in an examination similar to that in SEBAS I. The Social Environment and Biomarkers of Aging Study (SEBAS 2000 and 2006) was approved for human subjects concerns by the institutional review boards at Princeton University, RAND, Georgetown University, and the Bureau of Health Promotion, Taiwan.
The analyses presented here focused on the 639 respondents who participated in both the SEBAS I and SEBAS II clinical exams (84% of respondents eligible to participate in the SEBAS II exam [alive, completed the home interview, and living in a location with access to hospitals selected to perform the exam]), 51 of whom were excluded for having missing data on study covariates.
Measures
Frailty
The frailty measure used here was based on the measure first developed by Fried and colleagues (Fried et al., 2001) and further tested by others (Gruenewald et al., 2009; Szanton et al., 2009). It represents a count of the number of five criteria that were met at the time of the 2006 interview: weight loss/underweight, exhaustion, slowness, weakness, and low participation in activities. The weight loss/underweight criterion was met if respondents lost 5 % or more of their 2003 body weight between the 2003 survey (self-reported) and the 2006 exam (measured at exam) or if respondents’ body mass index (BMI) was less than 18.5 at the 2006 exam. Because this measurement of weight loss does not correspond precisely with that originally outlined by Fried and colleagues (Fried et al., 2001), we tested two additional measurements that used somewhat stricter thresholds for meeting the weight loss criteria: a) a weight loss of 5% or more of 2003 body weight among those with a 2003 BMI less than 25 or a 2006 BMI less than 18.5; and b) a weight loss of 10% or more of 2003 body weight or a 2006 BMI less than 18.5. Neither of these stricter specifications of weight loss affected the frailty-related results (not shown) so we utilized our original specification, which was the closest to that outlined in the Fried frailty phenotype (Fried et al., 2001) and similar to measures used in the literature (e.g. Gruenewald, 2009).
Exhaustion was determined by whether respondents reported feeling either of two symptoms two or more days in the past week: 1) doing anything was exhausting; and 2) could not gather the energy to do things. Respondents were considered slow if the time it took them to walk three meters (best of two trials) fell in the slowest 20%, with percentiles determined by height (above or below the median) and sex (Fried et al., 2001). Weakness was indicated by grip strength (kg) - the best result from six trials (3 on each hand) measured using a dynamometer - that was in the lowest 20%, with percentiles determined by BMI quartiles and sex (Fried et al., 2001). Respondents unable to perform the walking or grip strength test were considered to satisfy the slowness or weakness criterion, respectively.
Low energy expenditure has most often been measured using instruments that provide information for the calculation of kilocalories expended per week in leisure and other types of activities (for example, the Minnesota Leisure Time Activity Questionnaire (Szanton et al., 2009)). Although SEBAS II did not use such an instrument, the survey asked how many days a week (in ranges) a respondent did at least one of 10 activities (see Table 1) or exercised regularly doing something other than the listed activities. Our measure, low physical activity, was assessed as doing physical activity less than once per week. Because this measure was not the one typically used in analyses of frailty, we examined its association with a number of sociodemographic and health variables. Results (not shown) revealed that respondents doing physical activity less than once per week were older (85+), had worse self-rated health, more functional limitations, more difficulty with activities and instrumental activities of daily living, and more depressive symptoms than respondents who were more active.
Table 1.
Components of Frailty Measured in 2006 (n=639)a
| Component | Criteria met if… | Prevalence (%) (% missing) |
|
|---|---|---|---|
| Weight loss/under weight | Lost >=5% of 2003 weight based on difference between weight in 2003 (self-reported) and weight in 2006 (measured during exam) or BMI at 2006 exam < 18.5. | 19.6 (1.7) |
|
| Exhaustion | Felt during two or more days last week that doing anything was exhausting or that could not gather energy to do things. | 23.6 (3.0) |
|
| Slownessb | Fastest time of two attempts to walk three meters was in the slowest 20% by height (above or below median) and sex: | 28.0 (5.0) |
|
| height (cm) | Slowest 20% (seconds) | ||
| Males | <=164.0 | >=4.64 | |
| >164.0 | >=4.12 | ||
| Females | <=152.5 | >=6.00 | |
| >152.5 | >=4.78 | ||
| Weaknessb | Grip strength (kg) was in the weakest 20% by BMI quartiles and sex: | 30.4 (4.7) |
|
| BMI | Weakest 20% (kg) | ||
| Males | <22.33 | <=24 | |
| 22.33–24.47 | <=28 | ||
| 24.48–26.87 | <=26.4 | ||
| >26.87 | <=30 | ||
| Females | <22.45 | <=14 | |
| 22.46–24.76 | <=16 | ||
| 24.77–27.16 | <=16 | ||
| >=27.21 | <=14 | ||
| Low physical activity | Participated in physical activity less than 1 time per week. Physical activities included Chi Kung, Tai Chi, yoga, other activities similar to Chi Kung, gymnastics/stretching, walking, dancing, gardening, biking/swimming/playing ball, mountaineering, regular exercise other than activities previously mentioned. | 42.1 (0.0) |
|
Respondents who participated in both 2000 and 2006 exam.
Cutoffs established using the full 2006 biomarker sample.
For each frailty criterion that was met, respondents scored one point on the frailty indicator. Similar to previous operationalizations (Fried et al., 2001; Gruenewald et al., 2009; Lang et al., 2009; Walston et al., 2006), frailty was measured as a dichotomy that compared those who met 3 or more criteria with those who met 2 or fewer. Table 1 shows the prevalence of each frailty component.
Physiological Dysregulation
The theoretical model discussed above suggests that physiological dysregulation may precede the development of frailty (Gruenwald et al., 2009; Zaslavsky 2013). We, therefore, used biological markers from the 2000 hospital exam to derive a PD score. Based on a previous study (Glei, Goldman, Lin & Weinstein, 2012), the score in this analysis was a count of the number of 24 biological markers on which respondents scored in the high risk range. The biomarkers included 9 markers related to cardiovascular/metabolic [CV/metabolic] risk, seven related to inflammation, four related to hypothalamic-pituitary-adrenal axis and sympathetic nervous system [HPA/SNS] function, and another four associated with a variety of functions and systems). Biomarkers greater than or equal to the 80th percentile indicated high risk for systolic blood pressure, triglycerides, glycosylated hemoglobin, waist-hip ratio, resting pulse rate, urinary cortisol, norepinephrine, interleukin-6, high sensitivity C-reactive protein, soluble intercellular adhesion molecule-1, sE-selectin, soluble IL-6 receptor, white blood cell count, neutrophils, and homocysteine. For high-density lipoprotein, dehydroepiandrosterone-sulfate, albumin, insulin like growth factor-1, and creatinine clearance, high risk was determined by having a biomarker value less than or equal to the 20th percentile. For diastolic blood pressure, total cholesterol, fasting glucose, and epinephrine, an indication of high risk was having a biomarker value in the top or bottom decile of the distribution (Glei et al., 2012).
We also tested the relationship between mortality and four PD subscores: CV/metabolic risk, inflammation, HPA/SNS function, and a group of four other markers (OTHER). Specific biomarkers included in each subscore are shown in Supplemental Table 1. Each subscore was a count of the number of included biomarkers that were high risk.
Although researchers have developed various PD specifications based on different biomarkers and different definitions of risk, results are generally robust to the formulation of the physiological dysregulation score (Seplaki, Goldman, Glei, & Weinstein, 2005; Glei, Goldman, Wu, & Weinstein, 2013). Nonetheless, we tested the sensitivity of our findings using three alternative measurements of the PD score: transforming the individual biomarker variables into z-scores and averaging those scores; using high-risk quartiles rather than quintiles; and using the original 10-item allostatic load measure (Seeman et al., 1997). Although our theoretical model suggests that PD precedes the development of frailty, we also test a measure of PD based on the 2006 measurement of the 24 previously described biological markers because the six year lag between the measurements of PD and frailty could lead to weaker relationships and less discriminatory power between PD and mortality. These sensitivity analyses showed that the substantive findings were consistent regardless of the type of or date of measurement of PD (see Supplemental Table 3), so we presented here results using the PD measures described above.
Control variables
We controlled for socio-demographic and health variables, measured in 2006, that previously have been shown to be associated with the risk of dying: age, sex, education (completed years), urban residence, marital status (currently married vs. not), number of self-reported chronic/health conditions (high blood pressure, diabetes, heart disease, stroke, cancer/malignant tumor, lower respiratory tract disease, arthritis/rheumatism, gastric ulcer/stomach ailment, liver/gall bladder disease, hip fracture, cataracts, kidney disease, gout or spinal/vertebrae spur), cognitive function (number of cognitive function tests executed correctly out of a possible 28), and smoking (ever vs. never).
Mortality
Survival status was determined through linkage with the death certificate file from the Department of Health and with the household registration database from the Ministry of the Interior. By December 31, 2011, there were 104 deaths among the 639 respondents who completed the 2000 and 2006 physical exams. However, after excluding respondents who were missing data on any of the analysis predictors, the number of deaths was 86 out of 588 respondents (14.6%).
We tested the sensitivity of results to the exclusion of respondents missing on any analysis variable by re-estimating the models using multiple imputation (the ‘ice’ and ‘mim’ procedures in Stata 11.2; [StataCorp, 2012]). Because substantive findings using the imputed data (not shown) were the same as results using the sample of respondents with no missing data, we show only results for respondents with complete data.
Analysis
We estimated four Cox proportional hazard regression models to test the associations of frailty and physiological dysregulation with survival through the end of the 4.5 to 5 year period (2006 exam date through December 31, 2011). We first estimated a baseline model including only the control variables to act as a standard against which other models were evaluated (see Supplemental Table 2). We next estimated a model that included the control variables and the physiological dysregulation score from 2000 followed by a model that included controls and frailty measured in 2006. The final model included controls and both the 2000 PD score and 2006 frailty indicator. We repeated this series of models, substituting the PD subscores for the total PD score.
Since hazard ratios do not reflect the accuracy of mortality prediction (Pepe, Janes, Longton, Leisenring, & Newcomb, 2004), we estimated Harrell’s concordance index (C-index; [Newson, 2010]). The statistic measured the proportion of time that the model correctly ordered survival times (longer vs. shorter) for pairs of respondents among all usable pairs of respondents (i.e., pairs for which predictions were concordant with outcomes and not identical) in the dataset (Newson, 2010). Higher values of Harrell’s C-index indicated better predictive power. Although there are no set standards for evaluating the C-index, Yourman, Lee, Schonberg, Widera, & Smith (2012) employed the following criteria for assessing the predictive accuracy of a model: values below 0.60 = poor, 0.60–0.69 = moderate, 0.70–0.79 = good, 0.80–0.89 = very good, and 0.90 or higher = excellent.
Similarly, there are no established benchmarks for evaluating improvements in the C-index across models. However, because the C-index calculated from proportional hazard models is an extension of the area under the receiver-operating characteristic curve (AUC, Pencina & D’Agostino, 2004), we evaluated improvements in the C-index using criteria proposed for examining improvements in the AUC. Specifically, Pencina et al. (2008) suggest that an increase in the AUC of 0.01 is a meaningful improvement in the predictive ability of a model.
RESULTS
Table 2 shows the descriptive statistics for all predictors in the analysis. Approximately 21% of respondents are classified as frail in 2006. The PD score ranges from 0–13 (out of 24) and has a mean of 4.6. The mean CV/metabolic risk score is 1.8 and ranges from 0–7 (out of 9). The inflammation score, ranging from 0–6 (out of 7), has a mean of 1.2. The mean HPA/SNS score is less than 1.0 and ranges from 0–4. The subscore consisting of the other 4 biomarkers also has a mean that is less than 1.0.
Table 2.
Descriptive Statistics for Analysis Predictors (n=639 a)
| Mean (SD); % (n=639*) |
|
|---|---|
| Primary predictors | |
| Frail 2006 % | 21.1 |
| %missing | 3.3 |
| Physiological dysregulation (PD) 2000 | |
| Overall score (0–13 out of 24) | 4.6 (2.6) |
| % missing | 3.8 |
| Cardiovascular (CV)/metabolic risk (0–7 out of 9) | 1.8 (1.4) |
| % missing | 1.3 |
| Inflammation (0–6 out of 7) | 1.2 (1.3) |
| % missing | 2.2 |
| HPA/SNSb function (0–4 out of 4) | 0.8 (0.9) |
| % missing | 0.3 |
| OTHERc (0–4 out of 4) | 0.7 (0.8) |
| % missing | 1.1 |
| Controls (2006) | |
| Age at the time of the survey (60–97) | 72.5 (7.7) |
| Female, % | 43.5 |
| Urban resident, % | 54.3 |
| Years of completed education (0–17) | 5.5 (4.6) |
| Currently married, % | 69.8 |
| Number of chronic conditions (0–10 out of 14) | 2.6 (1.9) |
| Cognitive functioning (3–27 out of 28) | 19.6 (3.9) |
| % missing | 2.8 |
| Ever smoked | 42.3 |
Respondents who participated in both 2000 and 2006 exam.
HPA/SNS = hypothalamic-pituitary-adrenal axis and sympathetic nervous system function
Includes: insulin-like growth factor 1, creatinine clearance, albumin, and homocysteine
The mean age of respondents at the time of the 2006 survey is 72.5 years. Approximately 44% of respondents are female, 54% live in urban areas, 70% are currently married, and the average years of completed education is 5.5. On average, respondents report having nearly three chronic conditions (out of 14) and provide correct responses to approximately 20 (out of 28) of the questions used to assess cognitive functioning. Less than half of respondents (42%) have ever smoked.
Results from Cox proportional hazard models showing the associations of 2000 physiological dysregulation and 2006 frailty with survival are given in Table 3 (full models shown in Supplemental Tables 2). In Model 1, higher overall PD scores are significantly associated with a higher risk of dying during follow-up (hazard ratio [HR]=1.28, z=6.04, p<0.01). Being frail (vs. not frail) is also significantly associated with a higher risk of dying (Model 2; HR=3.08, z=4.38, p<0.01). Note that some of the control variables in the model (e.g. urban residence, marital status, chronic conditions, cognitive function, and smoking behavior) partially mediate the relationship between frailty and survival. In a model controlling only for age, sex, and education, the HR for is 3.57 (z=5.26, p<0.01; see footnotes in Table 3 and Supplemental Table 2). Finally, when both PD and frailty are included in Model 3, both measures significantly predict mortality, with relatively little attenuation in the size of the coefficients, particularly for the PD score (Model 3: PD Score HR=1.25, z=5.31, p<0.01; Frailty HR=2.43, z=3.39, p<0.01).
Table 3.
Hazard Ratios (standard errors) from Cox Proportional Hazard Models of Mortality (n=588 a)
| Modelse
|
|||||
|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | |
| PD Score 2000 (0–13) | 1.28**g (0.05) |
1.25** (0.05) |
|||
| Frail 2006 (vs. not frail) | 3.08**h (0.79) |
2.43** (0.64) |
2.52** (0.64) |
||
| PD subscores 2000 | |||||
| CVb/metabolic risk (0–7) | 1.34** (0.10) |
1.30** (0.10) |
|||
| HPA/SNSc (0–4) | 1.22 (0.15) |
1.17 (0.15) |
|||
| Inflammation (0–6) | 1.33** (0.11) |
1.31** (0.11) |
|||
| OTHERd (0–4) | 1.10 (0.14) |
1.03 (0.14) |
|||
| Harrell’s C-index f | 0.7670 | 0.7483 | 0.7848 | 0.7669 | 0.7833 |
Sample of respondents with complete data
CV=cardiovascular
HPA/SNS=Hypothalamic-pituitary-adrenal axis and sympathetic nervous system
OTHER biomarkers include: Insulin like growth factor-1 (IGF-1), creatinine clearance, albumin, homocysteine.
Model controls for age, sex, years of education, urban residence, marital status, number of chronic conditions, cognitive function, and smoking behavior, all measured in 2006.
Baseline model (includes only control variables): Harrell’s C-index = 0.7089.
In a model controlling only for age, sex and education the coefficient (standard error) for PD Score 2000 is 1.30 (0.05). See also Supplemental Table 2.
In a model controlling only for age, sex and education the coefficient (standard error) for Frail 2006 is 3.57 (0.86). See also Supplemental Table 2.
p<0.01
p<0.05
Harrell’s C-index values appear at the bottom of Table 3. Adding the overall PD score to the baseline model (control variables only; shown in Supplemental Table 2) increases the accuracy with which mortality is predicted (C-index=0.7670 vs. 0.7089 [see table notes]). Including frailty (Model 2) also improves predictive power over the baseline model (C-index=0.7483 vs.0.7089), but by a smaller margin than adding PD. However, even after including the PD score, the addition of frailty yields an improvement in the C-index (0.7848 for Model 3 vs. 0.7670 for Model 1).
The results for models incorporating the PD subscores (Models 4 and 5) show that two of the four subscales are significantly associated with mortality: worse scores for CV/metabolic risk (HR=1.34, z=3.75, p<0.01) and inflammation (HR=1.33, z=3.40, p<0.01) are both associated with a higher risk of dying over the period (Model 4). The same PD subscores significantly predict mortality even when frailty status, which is also significant (HR=2.52, z=3.53, p<0.01), is included in Model 5. Indeed, the addition of frailty to the model has virtually no effect on the HRs of the PD subscores.
DISCUSSION
The purpose of this research has been to examine among a population of adults aged 60 and over whether frailty is associated with mortality independently of physiological dysregulation and, if so, whether frailty or PD is a more accurate predictor of survival. Our results show that PD, whether a single score or several subscores, and frailty are independently associated with mortality. According to the Harrell’s C-index, the PD score is a more accurate predictor of mortality than frailty. In addition, when PD, either overall or as subscores, and frailty are included in a single model, the association of the PD scores (overall or subscores) with mortality is not attenuated. Taken together, these results suggest that physiological dysregulation may not necessarily work through frailty to affect mortality and that PD and frailty may be capturing different aspects of the process that results in mortality.
Although these findings are not consistent with the specific model proposed by Zaslavksy and colleagues (2013), they are consistent with recent reviews of the conceptual definition of frailty that suggest that frailty is multidimensional and includes physical, biological, social, and cognitive domains (Bergman et al., 2007; Gobbens et al., 2010; Hogan, 2007; Sternberg et al., 2011). Our results are also consistent with those reported by Mitnitski et al. (2015) that show that indicators of both biological and clinical frailty are associated with mortality and improve the accuracy of models predicting mortality among very old individuals. The finding that our indicators of physiological dysregulation and frailty independently predict mortality may signify that these indicators are measuring different aspects of the same construct. Indeed, some researchers have questioned whether frailty can be distinguished from the aging process, a process that has been defined as the accumulation of multiple deficits across physiological systems that make individuals more susceptible to unfavorable health outcomes (Bergman et al., 2007). The measures used in this study may have simply documented the accretion of deficits in different domains.
Our results further suggest that both biological and physical functioning domains are important for analyzing health risks. While our data show that there is overlap in the population of people who are frail and who have substantial physiological dysregulation, a sizeable proportion of those with high levels of physiological dysregulation are not frail and frail individuals do not necessarily have high PD. Furthermore, since both PD and frailty are independently and significantly associated with mortality and both improve the prediction of mortality, both are important in classifying individuals likely to experience adverse health outcomes and in determining appropriate interventions to reduce risk. At the same time, in developing measures of risk, we need to be cognizant of both respondent and patient burden and of time constraints in both the clinical and population survey settings; there needs to be a balance between thoroughness, precision, efficiency, and applicability to the provision of interventions (Fried et al., 2001; Gobbens et al., 2010; Rockwood, Andrew & Mitnitski, 2007).
This study has several limitations. First, we did not examine the dynamic nature of PD or frailty. Improvements or declines in physical and physiological capacity may be important for understanding a body’s reserves and capacity to handle stressors (Bergman et al., 2007; Buchman et al., 2009; Hardy, Perera, Roumani, Chandler, & Studenski, 2007; Karlamangla, Singer, & Seeman, 2006; Puts, Lips, & Deeg, 2005). For example, studies show that improvement in usual gait speed is associated with better survival (Hardy et al., 2007); worsening frailty results in higher mortality risk (Buchman et al., 2009); and declines (increases) in allostatic load scores are associated with lower (higher) risk of dying (Karlamangla et al., 2006), although controlling for baseline level of frailty may reduce the statistical effects of frailty decline on future health (Puts et al., 2005). Another study, of younger adults, suggests that understanding the pace of change in biological markers of aging also may be important for understanding future health (Belsky et al. 2015). It is possible that our estimates of the interrelationship between PD, frailty and mortality could have differed had we examined frailty and PD dynamically.
Second, the only adverse outcome that we examined was mortality. It would be interesting to see whether the same conclusions would be drawn if these relationships were tested with other outcomes such as disability, institutionalization or incidence of falls, all of which have been shown to be associated with frailty and PD in separate studies (see the discussion in the Introduction).
A third limitation is measurement. It is possible that the physiological measures available in SEBAS may not be the biological markers that are at the root of frailty. Testing these relationships with other measures of physiological function, such as neurological impairment (Walston et al., 2006), or with a more comprehensive measure of dysregulation, may result in a stronger relationship between PD and mortality or more accurate prediction. Also, dichotomizing the continuous biomarkers into high risk categories to create the PD score could introduce measurement error and reduce variability. However, we showed that our findings are robust to four alternative measures of PD (see Supplemental Table 3). Other studies have also shown that results are generally robust to the formulation of the physiological dysregulation score (Seplaki et al 2005; Glei et al., 2013) or of biological frailty (Mitnitski et al. 2015). Finally, our measure of frailty largely captures only the physical dimension of frailty. Although we control for cognitive function, results may have differed had other dimensions of frailty, such as psychological or social factors or sensory impairments, been included in our measure. Indeed, a study comparing three measures of frailty derived from different theoretical perspectives finds that each frailty measure identifies distinct, although overlapping, groups of older adults with the groups differing in their socidemographic and disease profiles (Cigolle, Ofstedal, Tian, & Blaum, 2009).
CONCLUSIONS
Theoretical models of the connections between physiological dysregulation, frailty, health and mortality are evolving as more and better data become available to test hypothesized relationships. Based on 24 biological markers of health and the Fried frailty phenotype, results from this study align more closely with a model that suggests that frailty may be a multidimensional construct that includes physical, psychosocial, and biological domains (Gobbens et al., 2010; Hogan, 2007; Sternberg, 2011) rather than a model that purports that PD operates primarily as an antecedent to frailty (Zaslavsky et al., 2013). Clearly more testing is needed, particularly with analyses that examine the dynamic nature of these relationships. Nonetheless, this study is one of the few to formally examine the independent effects of physiological dysregulation and physical frailty on mortality.
Supplementary Material
Acknowledgments
The Bureau of Health Promotion (BHP, Department of Health, Taiwan) provided additional financial support for SEBAS 2000. We acknowledge the hard work and dedication of the staff at the Center for Population and Health Survey Research (BHP), who were instrumental in the design and implementation of the SEBAS and supervised all aspects of the fieldwork and data processing.
Funding
This work was supported by and the SEBAS was funded by the National Institute on Aging (grant numbers R01AG16790, R01AG16661). This work was further supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number P2CHD047879). These funding agencies did not play a role in the study design, collection or analysis of the data, drafting of the manuscript or decision on where to submit the research for publication. Initial funding for the TLSA came from the Taiwan Department of Health, the Taiwan National Health Research Institute [grant number DD01-86IX-GR601S] and the Taiwan Provincial Government.
Biographies
Dr. Jennifer Cornman is a Research Consultant. Her work has focused on the relationships between social support, socioeconomic status and health and well-being among older adults in the United States and Taiwan.
Dr. Dana Glei is a Senior Research Investigator at Georgetown University. She is also a Research Consultant. Her work has investigated the relationships among the social environment, biological markers, and health outcomes in elderly populations as well as other social, demographic and health related issues.
Dr. Noreen Goldman is a Professor of Demography and Public Affairs at Princeton University’s Woodrow Wilson School and a Faculty Associate at the Office of Population Research. Her research examines the impact of social and economic factors on adult health and the physiological pathways through which these factors operate.
Dr. Maxine Weinstein is Distinguished Professor of Population and Health in the Graduate School of Arts and Sciences at Georgetown University. Her research focuses on biobehavioral aspects of reproduction and aging, with her most recent work in the emerging area of biodemography.
References
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: Characterization in the Women’s Health and Aging Studies. Journals of Gerontology: MEDICAL SCIENCES. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, et al. Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences. 2015;112:E4104–E4110. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: An emerging research and clinical paradigm—Issues and controversies. Journals of Gerontology: Biological Sciences, Medical Sciences. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Dallo FJ, Nguyen N. Racial/ethnic disparities in all-cause mortality in U.S. adults: The effect of allostatic load. Public Health Reports. 2010;125:810–816. doi: 10.1177/003335491012500608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz WM. A conceptual framework of frailty: A Review. Journals of Gerontology MEDICAL SCIENCES. 2002;57A:M283–M288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- Bouillion K, Kivimaki M, Hamer M, Sabia S, Fransson EI, Singh-Manoux A, Gale CR, Batty GD. Measures of frailty in population-based studies: an overview. BMC Geriatrics. 2013;13:64–74. doi: 10.1186/1471-2318-13-64. http://www.biomedcentral.com/1471-2318/13/64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Bienias JL, Bennett DA. Change in frailty and risk of death in older persons. Experimental Aging Research. 2009;35:61–82. doi: 10.1080/03610730802545051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Lin H, Chuang Y, Goldman N, Peterson CE, Glei DA, Weinstein M, Hurng B, Lin Y, Lin S, Liu I, Liu H, Lin S, Wu C, Hsiao M, Wu S. Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan, 2000 and 2006: main documentation for SEBAS longitudinal public use data (released 2012), Inter-university Consortium for Political and Social Research [distributor] Ann Arbor, MI: 2012. [Google Scholar]
- Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: The Health and Retirement Study. Journal of the American Geriatrics Society. 2009;57:830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- Crimmins E, Jung KK, Vasunilashorn S. Biodemography: New approaches to understanding trends and difficulties in population health and mortality. Demography. 2010;47:S41–S64. doi: 10.1353/dem.2010.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza SE, Jung I, Hazuda H. Frailty transitions in the San Antonio longitudinal study of aging. Journal of the American Geriatrics Society. 2012;60:652–660. doi: 10.1111/j.1532-5415.2011.03882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, Pawelec G. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. Journals of Gerontology: MEDICAL SCIENCES. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. Journals of Gerontology: MEDICAL SCIENCES. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- Fried LP, Xue Q, Cappoloa AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, Blaum CS, Bandeen-Roche K. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. Journals of Gerontology A Biological Sciences Medical Sciences. 2009;10:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Lin Y, Weinstein M. Relaxation practice and physiological regulation in a national sample of older Taiwanese. Journal of Alternative and Complementary Medicine. 2012;18:653–661. doi: 10.1089/acm.2010.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Wu C, Weinstein M. Does exposure to stressors predict changes in physiological dysregulation? Annals of Behavioral Medicine. 2013;46:121–126. doi: 10.1007/s12160-013-9485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Toward a conceptual definition of frail community dwelling older people. Nursing Outlook. 2010;58:76–86. doi: 10.1016/j.outlook.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. Journals of Gerontology A Biological Sciences Medical Sciences. 2005;60A:1046–1051. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- Goldman N, Turra CM, Glei DA, Seplaki CL, Lin Y, Weinstein M. Predicting mortality from clinical and nonclinical biomarkers. Journals of Gerontology: MEDICAL SCIENCES. 2006;61A:1070–1074. doi: 10.1093/gerona/61.10.1070. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic load and frailty in older adults. Journal of the American Geriatrics Society. 2009;57:1525–1531. doi: 10.1111/j.1532-5415.2009.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy SE, Perera SP, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. Journal of the American Geriatrics Society. 2007;55:1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- Hogan DB. Current understanding of the concept of frailty in later life. Aging Health. 2007;3:767–776. [Google Scholar]
- Hu P, Wagle N, Goldman N, Weinstein M, Seeman TE. The associations between socioeconomic status, allostatic load and measures of health in older Taiwanese persons: Taiwan Social Environment and Biomarkers of Aging Study. Journal of Biosocial Science. 2007;39:545–556. doi: 10.1017/S0021932006001556. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. Journal of Clinical Epidemiology. 2002;55:696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur Studies of Successful Aging. Psychosomatic Medicine. 2006;68:500–507. doi: 10.1097/01.psy.0000221270.93985.82. [DOI] [PubMed] [Google Scholar]
- Lang P, Michel J, Zekry D. Frailty syndrome: A transitional state in a dynamic process. Gerontology. 2009;55:539–549. doi: 10.1159/000211949. [DOI] [PubMed] [Google Scholar]
- Macklai NS, Spagnoli J, Junod J, Santos-Eggimann B. Prospective association of the SHARE-operationalized frailty phenotype with adverse health outcomes: evidence from 60+ community-dwelling Europeans living in 11 countries. BMC Geriatrics. 2013;13:3. doi: 10.1186/1471-2318-13-3. Accessed on line at http://www.biomedcentral.com/1471-2318/13/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei J, Demissie S, Falcon LM, Ordovas JM, Tucker KL. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Social Science & Medicine. 2010;70:1988–1996. doi: 10.1016/j.socscimed.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milot E, Morissette-Thomas V, Li Q, Fried LP, Ferrucci L, Cohen AA. Trajectories of physiological dysregulation predicts mortality and health outcomes in a consistent manner across three populations. Mechanisms of Ageing and Development. 2014:141–142. doi: 10.1016/j.mad.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy for measuring aging. The Scientific World. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski A, Collerton J, Martin-Ruiz C, Jagger C, von Zglinicki T, Rockwood K, Kirkwood TBL. Age-related frailty and its association with biological markers of ageing. BMC Medicine. 2015 doi: 10.1186/s12916-015-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newson RB. Comparing the predictive powers of survival models using Harrell’s C or Somer’s D. The Stata Journal. 2010;10:339–358. Accessed on line January 27, 2014 at http://www.stata-journal.com/article.html?article=st0198. [Google Scholar]
- Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Statistics in Medicine. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in Medicine. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. American Journal of Epidemiology. 2004;159:882–90. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- Puts MTE, Lips P, Deeg DJH. Static and dynamic measures of frailty predicted decline in performance-based and self-reported physical functioning. Journal of Clinical Epidemiology. 2005;58:1188–1198. doi: 10.1016/j.jclinepi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. Journals of Gerontology: MEDICAL SCIENCES. 2007;62A:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. Journals of Gerontology: MEDICAL SCIENCES. 2007;62A:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation-allostatic load and its health consequences. MacArthur Studies of Successful Aging. Archives of Internal Medicine. 1997;157:2259–68. [PubMed] [Google Scholar]
- Seplaki CL, Goldman N, Glei D, Weinstein M. A comparative analysis of measurement approaches for physiological dysregulation in an older population. Experimental Gerontology. 2005;40:438–449. doi: 10.1016/j.exger.2005.03.002. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 11.2. College Station, TX: StataCorp LP; 2012. [Google Scholar]
- Sternberg SA, Schwartz AW, Karunananthan S, Bergman H, Clarfield AM. The identification of frailty: A systematic review. Journal of the American Geriatrics Society. 2011;59:2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- Szanton SL, Allen JK, Seplaki CL, Bandeen-Roche K, Fried LP. Allostaic load and frailty in the Women’s Health and Aging Studies. Biological Research for Nursing. 2009;10:248–256. doi: 10.1177/1099800408323452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty and Older Adults. Journal of the American Geriatrics Society. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. Journal of the American Medical Association. 2012;307:182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslavsky O, Cochrane BB, Thompson HJ, Woods NF, LaCroix A. Frailty: A Review of the First Decade of Research. Biological Research for Nursing. 2013;15:422–432. doi: 10.1177/1099800412462866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


