Abstract
Purpose
Exposure of the general population to ionizing radiation has increased in the past decades, primarily due to long distance travel and medical procedures. On the other hand, accidental exposures, nuclear accidents, and elevated threats of terrorism with the potential detonation of a radiological dispersal device or improvised nuclear device in a major city, all have led to increased needs for rapid biodosimetry and assessment of exposure to different radiation qualities and scenarios. Metabolomics, the qualitative and quantitative assessment of small molecules in a given biological specimen, has emerged as a promising technology to allow for rapid determination of an individual's exposure level and metabolic phenotype. Advancements in mass spectrometry techniques have led to untargeted (discovery phase, global assessment) and targeted (quantitative phase) methods not only to identify biomarkers of radiation exposure, but also to assess general perturbations of metabolism with potential long-term consequences, such as cancer, cardiovascular, and pulmonary disease.
Conclusions
Metabolomics of radiation exposure has provided a highly informative snapshot of metabolic dysregulation. Biomarkers in easily accessible biofluids and biospecimens (urine, blood, saliva, sebum, fecal material) from mouse, rat, and minipig models, to non-human primates and humans have provided the basis for determination of a radiation signature to assess the need for medical intervention. Here we provide a comprehensive description of the current status of radiation metabolomic studies for the purpose of rapid high-throughput radiation biodosimetry in easily accessible biofluids and discuss future directions of radiation metabolomics research.
Keywords: Ionizing radiation, metabolomics, lipidomics, biofluids, rapid biodosimetry, radiation signatures
Introduction
Accidental or deliberate radiological events have been at the forefront of emergency response planning. Nuclear reactor accidents, such as Chernobyl and Fukushima-Daiichi, have highlighted that human error or natural disasters can lead to lasting effects in human and animal populations, surrounding environment, and socioeconomics. Improper disposal of medical equipment or improper use of industrial tools containing radioactive materials can lead to overexposure of individuals and contamination of a population, as in Goiânia in Brasil (Melo et al., 1994, Coeytaux et al., 2015). The atomic bombings of Hiroshima and Nagasaki demonstrated the power of nuclear weapons to cause maximum harm through death or injury and brought to attention the complications and long term effects arising from radiation exposure. Currently, the threat of terrorism or military action has escalated the possible use of radioactive materials or nuclear weapons in major cities and against various nations. Detonation of a radioactive dispersal device (RDD) or an improvised nuclear device (IND) will require an immediate and thorough assessment of individuals for radiation exposure. While an RDD will expose individuals to relatively low levels of radiation in the immediate vicinity, an IND will lead to significant deaths in a large radius around the epicenter and thousands of other individuals exposed due to ground shine or radioactive fallout. Various planning scenarios have been drafted for the immediate action after such an event (DiCarlo et al., 2011, Coleman et al., 2012, Sullivan et al., 2013, Homer et al., 2016), with evacuation and assessment of levels of radiation exposure of each individual followed by medical triage as the top priorities.
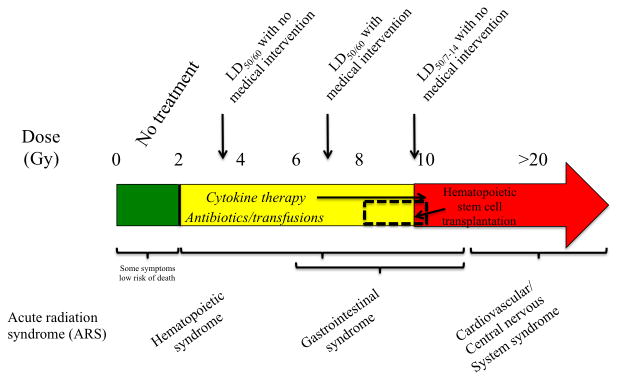
For this reason, significant efforts have been made to develop sensitive methods for radiation biodosimetry and medical countermeasures for mitigation of radiation effects. In the case of radiation biodosimetry, an estimated 50,000 individuals will need to be evaluated within 2 to 6 days after a 10 kt bomb, according to military scenarios for planning (Flood et al., 2016a), although in a city such as New York, NY or Washington, DC the number may well be much higher. Complete blood count (CBC) differential for lymphocyte depletion kinetics, premature chromosome condensation (PCC), dicentric measurement, gene expression, γ-H2AX, cytokinesis block micronucleus assay (CBMN), protein biomarkers, metabolic biomarkers, and electron paramagnetic resonance (EPR) or optically stimulated luminescence (OSL) of teeth have been proposed as some of the methods for radiation biodosimetry (Amundson and Fornace, 2003, Brengues et al., 2010, Coy et al., 2011, Gruel et al., 2013, Lamadrid Boada et al., 2013, Sharma and Moulder, 2013, Sullivan et al., 2013, Xu et al., 2013, Hu et al., 2015, Flood et al., 2016b, Garty et al., 2016, Sproull and Camphausen, 2016). However, some of these assays require a significant length of time for processing and highly trained personnel to perform the assays and interpret the results. According to the Biomedical Advanced Research and Development Authority (BARDA), results should ideally be available in less than 15 min and cover a dose range of 0.5-10 Gy (Homer et al., 2016). Radiation biomarkers [in general classified as diagnostic, prognostic, predictive, or pharmacodynamic (Singh et al., 2016)] should be able to accurately classify individuals at a point of care (POC) setting as having ≥ 2 Gy of exposure (Sullivan et al., 2013). A dose of ≥ 2 Gy would lead to acute radiation syndrome (ARS) that can manifest with emesis and diarrhea and can lead to death without appropriate medical intervention primarily through hematopoietic and gastrointestinal (GI) complications. A dose of < 2 Gy and particularly in the range of 0.75-1 Gy can still require treatment, however, attention of those individuals may be of less immediate priority (Sullivan et al., 2013). Figure 1 describes the current limits for ARS and medical interventions available.
Figure 1.
Acute radiation syndrome (ARS) in the human population is primarily divided in 4 different categories. Below 2 Gy, some individuals may experience some symptoms, such as emesis, however these individuals will not require immediate medical intervention for survival. Over doses of 2 Gy, individuals will experience and expire from myelosuppression (hematopoietic syndrome). The LD50/60 without medical intervention is ∼3.5 Gy, however with medical intervention it shifts upwards to ∼7 Gy. Only two medications have been FDA approved for the hematopoietic syndrome, Neupogen® and Neulasta®. Individuals exposed with a dose of 10 Gy and above will expire from gastrointestinal (GI) syndrome within 7-14 days post irradiation, although some individuals can have symptoms of GI syndrome with as low as 6 Gy. Individuals exposed to doses >20 Gy, although some can show symptoms as low as 10 Gy, will expire within days from cardiovascular and/or central nervous system syndromes. Since not all individuals can undergo an invasive procedure such as hematopoietic stem cell transplantation, only a small percentage falling between a dose range that cannot adequately benefit from cytokine therapy and antibiotic treatments, will be candidates for such treatments. Sources for the information include (DiCarlo et al., 2011, Sullivan et al., 2013, CDC, 2015).
To date, only three agents have been approved by the Food and Drug Administration (FDA) as medical countermeasures. Neupogen® and Neulasta® [filgrastim and polyethylene glycol (PEG)-filgrastim, respectively] have been approved as mitigators to increase survival of individuals exposed to doses that can lead to myelosuppression (Farese and MacVittie, 2015, Homer et al., 2016, Singh et al., 2016) and were developed with support from the National Institute of Allergy and Infectious Diseases (NIAID). Protectors, however, require prior knowledge of an incident and therefore can only be practically administered to first responders and military personnel (Coleman et al., 2012, Moulder, 2014). In addition, the FDA approved radioprotector Amifostine® has a very narrow window for administration in order to protect normal tissue and is unfortunately associated with severe side effects (Singh et al., 2016). In the case of first responders and military personnel, personal dosimetry will provide an accurate estimation of the external exposure and banked biological samples will serve as a point of reference for biodosimetry (Flood et al., 2016a), which is not currently feasible for the general population.
In any case, during such an incident, evaluation of individuals will be complicated by the very nature of the disaster. A significant number will experience other injuries besides radiation exposure, such as trauma, burns, wound, and/or sepsis, that collectively are termed radiation combined injury (RCI) (DiCarlo et al., 2010) and can account for up to 65% of all injuries. This is important as RCI can substantially increase mortality and lead to false categorization of individuals based on their physical symptoms, e.g. emesis. Other situations to be taken into account when assessing an individual for radiation exposure for appropriate medical management include total body or partial body exposures, external or internal exposure, dose rate, a potential neutron component, inter-individual variability and radiosensitivity, age, sex, pre-existing conditions (DiCarlo et al., 2011), current medications/supplements, and prior radiation exposures. Although classical cytogenetic biodosimetry techniques are the gold standard for biodosimetry, they are laborious, time consuming, and lack the ability to take into account the above criteria. Additionally, although individuals may survive the initial radiation exposure, downstream effects linked to radiation such as pulmonary pneumonitis, cardiovascular disease, and cancer may impact longer-term survival. For this, rapid biodosimetric methods and biomarker discovery have become priorities and forefront of research funded by NIAID.
One such promising method is metabolomics, which is the rapid, qualitative and quantitative assessment of small molecules of <1 kDa in a given biofluid or tissue, allowing for the phenotypic assessment of a given physiological state. Metabolomics, together with transcriptomics, genomics, proteomics, epigenomics, and glycomics, constitutes the current scope of systems biology approaches. In addition, lipidomics, the full assessment of changes in lipids, can be considered a component of metabolomics analyses, and with the variability and number of possible lipids can be considered an -omics field itself. Global approaches allow for the full scanning of the metabolome and pattern identification according to pathway interactions, whereas targeted approaches can be more quantitative and concentrate on specific metabolites or perturbations along a metabolic pathway. For the purposes of biodosimetry, easily accessible biofluids (urine, blood, saliva) have been the primary focus that will require minimally invasive methods of acquisition on the field.
Multiple reviews have detailed the technological aspects of radiation biodosimetry (Patterson et al., 2010, Coy et al., 2011, Di Girolamo et al., 2013, Menon et al., 2016), while others have focused on the strengths and challenges of this newer –omics field (Johnson and Gonzalez, 2012, Patti et al., 2012). Briefly, nuclear magnetic resonance (1H-NMR or NMR) had been the platform of choice historically, with samples analyzed in a non-destructive manner and because it provided information on the structure of a given biomarker (Menon et al., 2016). However, the tendency of NMR to identify high abundance molecules due to low sensitivity, left many metabolites undiscovered that could serve as biomarkers of a given injury. Liquid chromatography coupled to mass spectrometry (LC-MS) on the other hand, has provided high selectivity, chromatographic separation, and identification of molecules with variable polarity. The introduction of very high performance LC coupled with time-of-flight MS (TOFMS) further increased chromatographic resolution and sensitivity, thus improving the ability to identify a high number of ions in a given biofluid, including ions present in low abundance that can be utilized as biomarkers. In comparison to NMR, which typically can only assess higher abundance metabolites (typically up to 50-100 metabolites), LC-TOFMS can assess thousands. Additionally, the high level of mass accuracy from TOFMS has increased the confidence in the identification and validation of a specific metabolite. Furthermore, the technological ability to fragment each ion in a simultaneous run, collect MS data (data-independent acquisition) and match the fragmentation patterns to those in online databases, has significantly decreased the amount of time required for positive biomarker identification. Finally, gas chromatography MS (GC-MS) is a platform primarily focused on identification of thermally stable metabolites (Coy et al., 2011). Although it is complementary to LC-MS due to its superiority in identifying sugars, amino acids, and aromatic amines among others, the labor intensive sample preparation due to the need for chemical derivatization to provide thermal stability to metabolites, severely limits its utility for rapid biodosimetry. Nevertheless, it has provided excellent information that could potentially be translated into a different platform with more targeted approaches. Although the initial global metabolomic profiling may seem complex, as highlighted in Figure 2, the ultimate targeted approaches that would be utilized in radiation biodosimetry are more simplified. These types of analyses have been described previously in detail by (Chen et al., 2007, Patterson et al., 2010, Coy et al., 2011). Although significant progress has been achieved in radiation metabolomics, caveats with respect to global metabolomics should be considered. Many of the challenges have been described in detail in more in depth reviews on metabolomics as an –omics technology (Coy et al., 2011, Johnson and Gonzalez, 2012, Patti et al., 2012). Briefly, important issues to be considered include biological sample acquisition, storage and processing, standardization of analytical techniques, identification of metabolites through tandem mass spectrometry and database matching, and sensitivity and specificity of instruments and metabolites to name a few. However, advancements in these areas and efforts in standardization have begun to address some key issues.
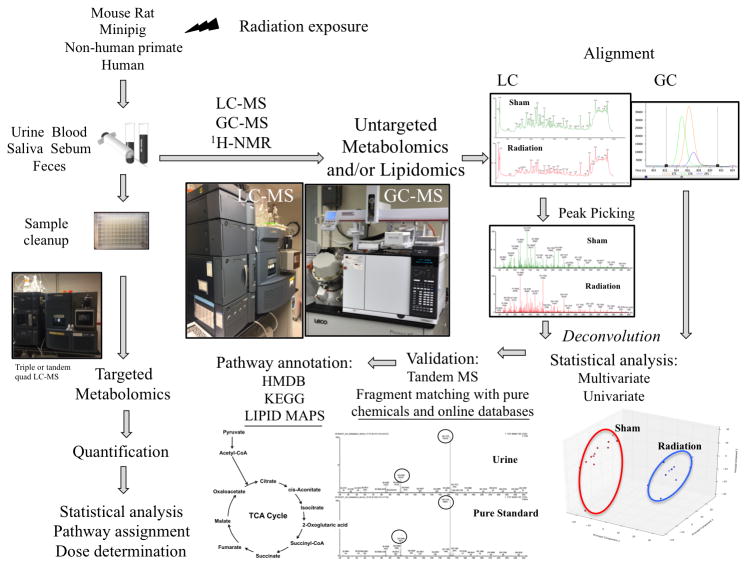
Figure 2.
A simplified illustration of the metabolomic processes, untargeted (global profiling) and targeted approaches. LC-MS, GC-MS, and 1H-NMR have all been used successfully to identify biomarkers of radiation exposure. Targeted approaches, primarily through LC-MS, have provided quantitative information on select metabolites and could be utilized for high-throughput analysis of samples in a real life scenario to accurately identify individuals who have been exposed to ionizing radiation.
In this review we discuss the current status of radiation metabolomic analysis as it pertains to biodosimetry and cross-species validation of biomarkers. We present the biomarkers identified from each study in animal models [mice, rats, minipigs, non-human primates (NHPs), humans] and biofluids (urine, blood, saliva, fecal material, sebum) with different radiation scenarios that have been explored. We finally offer some conclusions based on the information that is currently known and potential future directions and areas of research.
Mice
Urine
Initial in vivo experiments to determine the utility of metabolomics for radiation biodosimetry were conducted in mice. Murine models are ideal for studying the effects of radiation injury due to their relative genetic homogeneity, ability to tightly control the experimental conditions (such as age, sex, diet etc.), and use of appropriate experimental numbers for increased statistical power, among others. In addition, mice are generally more radioresistant than humans with a lethal dose (LD) 50/30 of 7-8 Gy for C57BL/6, while humans have an LD50/60 of ∼3.5 Gy. LD50/30 refers to 50% expected death within 30 days after exposure, while LD50/60 refers to 50% expected death within 60 days after exposure. For that purpose, Tyburski et al. published two papers (Tyburski et al., 2008, Tyburski et al., 2009) that explored the effects of total body external irradiation with a 137Cs source and the utility of urine as a rich matrix for biomarkers using LC-MS. In the first paper (Tyburski et al., 2008), urine from male mice was mined for biomarkers at 24 hours post-exposure and hence set the stage for radiation metabolomics. N-hexanoylglycine and β-thymidine were identified as markers for both 3 and 8 Gy exposures, while 3-hydroxy-2-methylbenzoic acid 3-O-sulfate was only identified for 3 Gy and taurine only for 8 Gy. Their study also pointed out the existence of dose responses in the global urinary metabolome (6, 7, 8, and 11 Gy). In their second paper (Tyburski et al., 2009) they explored lower doses (1, 2, and 3 Gy) and the kinetics of certain biomarkers up to 9 days post-exposure. At 2 and 3 Gy, several markers (thymidine, 2′-deoxyuridine, 2′-deoxyxanthosine, xanthine, xanthosine, and 2′-deoxycytidine) showed the highest alterations at 8 to 12 hours post-exposure, however their levels returned to normal by 36 hours, with no changes until the end of the study (9 days). Chen et al. also conducted a time course analysis with assessment of urine on the first week after irradiation with 8 Gy (Chen et al., 2011). Their results using a 1H-NMR platform revealed changes in citrate, taurine, 2-oxoglutarate, hippurate (a microbial metabolite), creatine, succinate, methylamine, N-methyl-nicotinamide, and choline. Laiakis et al. further identified uric acid, allantoin, taurine, and nicotinate as markers of 8 and 15 Gy (nicotinate was a marker for only 8 Gy), two doses that cover both the hematopoietic and GI syndromes that are of particular interest in a nuclear emergency (Laiakis et al., 2012). As metabolomic analysis provides a snapshot of the metabolic condition of an organism, urinary analysis therefore can reflect the current systemic effects.
These studies that initiated the radiation metabolomics field utilized a relatively high dose rate of 1Gy/min and mostly reflected changes associated with oxidative stress, DNA damage and apoptosis, and energy metabolism from products of the tricarboxylic acid (TCA) cycle. Low dose rate irradiations, encountered after contamination or ingestion of radioactive materials, were also proven to show distinct biomarkers from high dose rate. Goudarzi et al. identified perturbations at 2 days post-exposure for both 1.1 and 4.5 Gy when delivered with a high dose (1.03 Gy/min) or low dose rate (3 mGy/min) (Goudarzi et al., 2014a). While markers of energy metabolism were the primary classifiers, levels of some biomarkers differed between the two types of radiation exposure. In addition, phenylacetylglycine was a specific marker for high dose rate, while xanthurenic acid, decreased levels of acylcarnitines (hexanoylcarnitine, tiglylcarnitine, propenoylcarnitine), indole-3-carboxylic acid, and riboflavin were either specific for low dose rate or showed opposite fold change patterns from high dose rate. The results of this study highlight the potential to generate signatures in biofluids for different radiation exposure scenarios and further classify individuals in an effective manner for administration of appropriate medical triage.
As individuals may also be exposed internally from ingestion of radioactive food or water or inhalation and therefore receive a significant dose from low dose rate radionuclides, it is imperative to identify those individuals that will require different treatment and possible administration of chelators. Mice injected with 137CsCl2 show distinctly different metabolic profiles from control mice (Goudarzi et al., 2014b) at 2 days (1.95 Gy), 5 days (4.14 Gy), 20 days (9.46 Gy), and 30 days (9.91 Gy) post injection. While some markers were previously identified with either high or low dose rate external beam irradiation (xanthurenic acid, taurine, uric acid, citrate, alpha-ketoglutaric acid, hippuric acid, tiglylcarnitine, hexanoylcarnitine,) (Tyburski et al., 2008, Tyburski et al., 2009, Laiakis et al., 2012, Goudarzi et al., 2014a, Chen et al., 2016) and therefore do not reflect a specific internal emitter signature, others such as tiglylglycine, isoleucine/leucine, isovalerylglycine, and isethionic acid were specific for 137Cs. Urinary analysis from mice exposed to 85/90SrCl2, which is known to deliver most of the dose to the skeleton and bone marrow, also validated low dose rate markers (xanthurenic acid, riboflavin, tiglylcarnitine, hexanoylcarnitine, and retinoic acid) (Goudarzi et al., 2015c). Mice exposed to 85/90SrCl2 were assessed at 7 days (1.81 Gy), 9 days (2.12 Gy), 25 days (4.76 Gy), and 30 days (5.25 Gy) post injection. Other biomarkers from this 90Sr internal exposure study, such as 4-guanidinobutanoic acid, 3-hydroxybutanoate, 4-aminobutanoate, pantothenic acid, indolelactic acid, glutaconic acid, glutamate, quinolinic acid, and malate, were specific for this particular radionuclide. Taken together, patterns of radiation specific markers start to emerge, whether that is from differences in fold changes from control, presence or absence, or even metabolic pathway involvement, that can potentially allow for identification of the type of exposure that other biodosimetry assays, such as classical cytogenetics, may not fully allow.
In a real life situation however, the populations exposed will be far more genetically diverse and may also experience other types of injuries, such as infections, trauma, burns, that may complicate and alter the radiation signature and even survival. Some limited efforts have already explored these areas to further refine those effects. Mice were exposed to lipopolysaccharide (LPS), a bacterial toxin that can mimic the prodromal syndrome with emesis, diarrhea, and fever and can therefore falsely identify individuals as radiation victims. Laiakis et al. demonstrated that global metabolic differences were vastly different from 8 Gy of radiation in mice, a dose that will certainly induce the above symptoms (Laiakis et al., 2012). Furthermore, increases in excretion of cytosine, cortisol, adenine, O-propanoylcarnitine, and isethionic acid were identified as unique metabolites of LPS exposure at 24 hours post-exposure. Increases in isethionic acid were also reported by (Goudarzi et al., 2014b) in internal 137Cs exposure, indicating some possible overlap in metabolic responses. Additionally, various degrees of genetically based radiosensitivity in the human population should constitute such individuals recipients of careful and specialized treatments. As a model of radiosensitivity, Parp1-/- mice were exposed to an LD50/30 dose (Laiakis et al., 2016). The poly(ADP-ribose) polymerase 1 (PARP1) protein recognizes single strand breaks in DNA and facilitates their repair with recruitment of the base excision repair (BER) machinery, in addition to being a major regulator of metabolism (Kim et al., 2005, Patrono et al., 2014). Parp1-/- mice that are exposed to ionizing radiation exhibit high levels of DNA damage, G2 arrest, and mitotic catastrophe (Majuelos-Melguizo et al., 2015, Laiakis et al., 2016). Analysis of urine samples from Parp1-/- and wild type mice exposed to an LD50/30 dose showed time dependent changes in TCA cycle products (citric acid, cis-aconitic acid, alpha-ketoglutaric acid), together with amino acids and metabolites of general energy metabolism dysregulation. Interestingly, the profile of taurine was similar to previously published reports, as mentioned above, rendering this a robust radiation biomarker. Finally, Cook et al. utilized similar metabolomic analysis to predict radiation-induced cancer from urine collected over a 1-year period (Cook et al., 2016). Metabolomic profiles clearly stratified mice according to neoplasms (hematopoietic, solid, or benign), and also indicated that radiation leads to accelerated aging-like responses that are reflected in the urinary metabolome. The validated biomarkers from these studies are presented in Table 1 and pathway involvement in Supplementary Table 1.
Table 1. Validated urinary metabolites of radiation exposure through metabolomics.
| Organism | Strain/Species | Analytical Technique | Dose rate or initial activity | Exposure | Type | Doses in study | Validated metabolites of radiation exposure | Trend compared to control | Time points analysed after irradiation | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Mice | C57BL/6 | LC-MS | 2.57Gy/min | TBI | External 137 Cs | 3 and 8 Gy | 3-Hydroxy-2-methylbenzoic acid O-sulfate | ↑ | 24 hours | Tyburski et al., 2008 |
| Citric and/or isocitric acid | ↑ | |||||||||
| N-Hexanoyglycine | ↑ | |||||||||
| β-Thymidine | ↑ | |||||||||
| Taurine | ↑ | |||||||||
|
| ||||||||||
| Mice | C57BL/6N | LC-MS | 2.57Gy/min | TBI | External 137 Cs | 1, 2, and 3 Gy | 2′-Deoxyuridine | ↑ | 0- 9 days | Tyburski et al., 2009 |
| 2′-Deoxycytidine | ↓ (2 and 3 Gy) | |||||||||
| 2′-Deoxyxanthosine | ↑ | |||||||||
| Thymidine | ↑ | |||||||||
| Xanthine | ↑ | |||||||||
| N-Hexanoyglycine | ↑ | |||||||||
| Xanthosine | ↑ (3 Gy) | |||||||||
|
| ||||||||||
| Mice | C57BL/6 | 1H-NMR | 3.7Gy/min | TBI | 250 kVp X rays | 8 Gy | Creatine | ↑ | 0-7 days | Chen et al., 2011 |
| Succinate | ↓ | |||||||||
| Taurine | ↑ (D1) ↓ (D4) | |||||||||
| Methylamine | ↓ | |||||||||
| N-Methyl-nicotinamide | ↑ | |||||||||
| Choline | ↑ | |||||||||
| Citric acid (citrate) | ↓ | |||||||||
| 2-Oxoglutarate | ↓ | |||||||||
| Hippurate (Hippuric acid) | ↓ | |||||||||
|
| ||||||||||
| Mice | C57BL/6N | LC-MS | 0.309 cGy/min or 1.03 Gy/min | TBI | 250 kVp X rays | 1.1, 4.45 Gy | N1-methyl-2-pyridone-5-carboxamide | ↑ | 2-5 days | Goudarzi et al., 2014a |
| Pantothenic acid | ↑ | |||||||||
| Valine | ↑ | |||||||||
| Citrate | ↓ | |||||||||
| Hexanoylglycine | ↑ | |||||||||
| Tiglylglycine | ↑ | |||||||||
| Kynurenic acid | ↓ | |||||||||
| Hippuric acid | ↑ | |||||||||
| Tiglylcarnitine | ↓(LDR), ↑ (HDR) | |||||||||
| Phenylacetylglycine | ↓(LDR), ↑ (HDR) | |||||||||
| Hexanoylcarnitine | ↓(LDR), ↑ (HDR) | |||||||||
| Indole-3-carboxylic acid | ↓(LDR), ↓ (HDR) | |||||||||
| Propenoylcarnitine | ↓(LDR), ↑ (HDR) | |||||||||
| 5-Hydroxy-L-tryptophan | ↓(LDR), ↑ (HDR) | |||||||||
| Xanthurenic acid | ↓(LDR) | |||||||||
| Riboflavin | ↓(LDR) | |||||||||
|
| ||||||||||
| Mice | C57BL/6N | LC-MS | 1.67 Gy/min | TBI | External 137 Cs | 3, 8,15 Gy | Nicotinate | ↑ | 24 hours | Laiakis et al., 2012 |
| Taurine | ↑ | |||||||||
| Uric acid | ↑ | |||||||||
| Allantoin | ↑ | |||||||||
|
| ||||||||||
| Mice | 129S-Parp1tm1Zqw, 129S/J | LC-MS | 1.67 Gy/min | TBI | External 137 Cs | 6, 8.8 Gy | 2-Ketobutyric acid | ↓ (24hr) ↑ (D3) | 24 hours, 3 days | Laiakis et al., 2016a |
| 4-Pyridoxic acid | ↓ (24hr) | |||||||||
| Cortisol | ↓ (24hr) Parp1 | |||||||||
| Hexanoyglycine | ↑ (24h and D3) Parp1 | |||||||||
| Flavin mononucletotide | ↑WT↓ Parp1-/- | |||||||||
| Taurine | ↑ (24hr) | |||||||||
| Citric acid | ↑ (D3) Parp1 | |||||||||
| cis-Aconitic acid | ↑ (D3) Parp1 | |||||||||
| α-Ketoglutaric acid | ↓ (24hr) ↑ (D3) Parp1 | |||||||||
|
| ||||||||||
| Mice | C57BL/6 | LC-MS | Activity (injected) 8.0±0.3 MBq | Internal | 137CsCl | 1.95, 4.14, 9.49, 9.91 Gy | Xanthurenic acid | ↓ | 2-30 days | Goudarzi et al., 2014b |
| Tiglylcarnitine | ↓ | |||||||||
| Hexanoylcarnitine | ↓ | |||||||||
| Tiglylglycine | ↑ | |||||||||
| Isoleucine/Leucine | ↓ | |||||||||
| β-Hydroxyisovaleric acid | ↑ | |||||||||
| Hippuric acid | ↑ | |||||||||
| Isovaleryglycine | ↓ | |||||||||
| Citrate | ↓ | |||||||||
| Uric acid | ↑ | |||||||||
| Taurine | ↓ (D2, D5) ↑(D20, D30) | |||||||||
| Isethionic acid | ↑ | |||||||||
| α-Ketoglutaric acid | ↓ | |||||||||
|
| ||||||||||
| Mice | C57BL/6 | LC-MS | Activity (injected) 200±0.3 kBq | Internal | 85/90SrCl2 | 1.81, 2.12, 4.76, 5.25 Gy | Xanthurenic acid | ↓ | 7-30 days | Goudarzi et al., 2015c |
| Riboflavin | ↓ | |||||||||
| Retinoic acid | ↓ | |||||||||
| Tiglylcarnitine | ↓ | |||||||||
| Hexanoylcarnitine | ↓ | |||||||||
| Citrate | ↓ | |||||||||
| Hippuric acid | ↑ | |||||||||
| 4-Guanidinobutanoic acid | ↓ | |||||||||
| 3-Hydroxybutanoate | ↓ | |||||||||
| 4-Aminobutanoate | ↓ | |||||||||
| Pantothenic acid | ↓ | |||||||||
| Indoleacetic acid | ↓ | |||||||||
| Glutaconic acid | ↓ | |||||||||
| Glutamate | ↓ | |||||||||
| Quinolinic acid | ↓ | |||||||||
| Malate | ↓ | |||||||||
|
| ||||||||||
| Rats | Wistar | GC-MS | 1 Gy/min | TBI | External 137 Cs | 3 Gy | Citric acid | ↓ | 1-3 days | Lanz et al., 2009 |
| Phosphoric acid | ↑ | |||||||||
| Glyoxylic acid | ↑ | |||||||||
| Glycolic acid | ↑ | |||||||||
| Lactic acid | ↑ | |||||||||
| p-Cresol | ↑ | |||||||||
| Arabitol | ↓ | |||||||||
| Threonic acid | ↑ | |||||||||
| Ferulic acid | ↓ | |||||||||
| Azelaic acid | ↓ | |||||||||
| 2-Oxoglutaric acid | ↓ | |||||||||
| Pimelic acid | ↓ | |||||||||
| Uracil | ↑ | |||||||||
| Lactose | ↓ | |||||||||
| Maltose | ↓ | |||||||||
| Suberic acid | ↓ | |||||||||
| Gulonolactone | ↓ | |||||||||
| Fucose | ↑ | |||||||||
| Thymine | ↑ | |||||||||
| Adipic acid | ↓ | |||||||||
| Glycerol-3-phosphate | ↓ | |||||||||
|
| ||||||||||
| Rats | Wistar | LC-MS | 1 Gy/min | TBI | External 137 Cs | 3 Gy | N-Acetyltaurine | ↑ | 1-3 days | Johnson et al., 2011 |
| Isethionic acid (putative) | ↑ | |||||||||
| N-Acetyl-D-glucosamine/ galactosamine-6-sulfate | ↑ | |||||||||
| 2′-Deoxyuridine | ↑ | |||||||||
| 2′-Deoxyxanthosine | ↑ | |||||||||
| Thymidine | ↑ | |||||||||
| 1N -Acetylspermidine | ↑ | |||||||||
| Taurine | ↑ | |||||||||
| N-Hexanoyglycine | ↑ | |||||||||
| Azelaic acid | ↓ (related to starvation) | |||||||||
| Pimelic acid | ↓ (related to starvation) | |||||||||
| Dodecanedioic acid | ↓ (related to starvation) | |||||||||
| N-Isovalerylglycine | ↓ (related to starvation) | |||||||||
|
| ||||||||||
| Rats | Sprague-Dawley (Hsd:SD) |
LC-MS | 0.6Gy/min | TBI | External 60 Co | 0.5, 2.5, 5, 7.5,10 Gy | Threonate | ↑ | 6-72 hours | Mak et al., 2015b |
| Arabinonate | ↑ | Majority more prominent at doses >2.5 Gy | ||||||||
| Thymidine | ↑ | |||||||||
| Glucarate | ↑ | |||||||||
| Uric acid | ↑ | |||||||||
| Cytosine | ↑ | |||||||||
| Creatine | ↑ | |||||||||
| Carnitine | ↑ | |||||||||
| Alanine or sarcosine | ↑ | |||||||||
| Methylimidazoleacetic acid | ↑ | |||||||||
|
| ||||||||||
| Rats | Sprague Dawley | LC-MS | 2 Gy/min | TBI | 6 MV X rays | 2, 4, 6, 8 Gy | Targeted amino acid analysis | Dose and time dependent | 5-72 hours | Zhang et al., 2014a |
|
| ||||||||||
| Non-human primates | Macaca mulatta | LC-MS | 60 cGy/min | TBI | External 60 Co | 1, 3.5, 6.5, 8.5 Gy | Taurine | ↑ | 12-72 hours | Johnson et al., 2012 |
| N-acetyltaurine | ↑ | |||||||||
| Isethionic acid | ↑ | |||||||||
| Hypoxanthine | ↑ | |||||||||
| Uric acid | ↑ | |||||||||
| Xanthine | ↑ | |||||||||
| Adipic acid | ↑ | |||||||||
| N-acetylserotonin sulfate | ↑ | |||||||||
| 3-hydroxytyrosol sulfate | ↑ | |||||||||
| Tyramine sulfate | ↑ | |||||||||
| Tyrosol sulfate | ↑ | |||||||||
| Creatinine | ↑ | |||||||||
|
| ||||||||||
| Non-human primates | Macaca mulatta | LC-MS | 0.6Gy/min | TBI | External 60 Co | 2, 4, 6,7,10 Gy | Carnitine | ↑ | 7 days | Pannkuk et al., 2015 |
| L-Acetylcarnitine | ↑ | |||||||||
| Isobutyryl-L-carnitine/ butyryl carnitine | ↑ | |||||||||
| Taurine | ↑ | |||||||||
| Cortisone | ↑ | |||||||||
| Cortisol | ↑ | |||||||||
| Hypoxanthine | ↑ | |||||||||
| Xanthosine | ↑ | |||||||||
| Xanthine | ↓ | |||||||||
| Xanthurenic acid | ↑ | |||||||||
| Creatine | ↑ | |||||||||
| Creatinine | ↓ | |||||||||
| Kynurenic acid | ↑ | |||||||||
|
| ||||||||||
| Humans | Homo sapiens | LC-MS | ∼0.1 Gy/min | TBI | 15 MVX rays | 1.25 cGy | L-Octanoylcarnitine | ↓ | 6 hr | Laiakis et al., 2014a |
| Hypoxanthine | ↑ | |||||||||
| Trimethyl-L-Lysine | ↓ | |||||||||
| L-Acetylcarnitine | ↓ | |||||||||
| Decanoylcarnitine | ↑ | |||||||||
| Uric acid | ↑ | |||||||||
| Xanthine | ↑ | |||||||||
|
| ||||||||||
| Humans | Homo sapiens | GC-MS and LC-MS | Unknown | PBI | Unknown | Unknown | N-acetylphenylalanine | ↑ | Unknown | Tandle et al., 2013 |
| N-acetyltryptophan | ↑ | |||||||||
| N-acetyltyrosine | ↑ | |||||||||
| N-acetylproline | ↑ | |||||||||
| Citrate | ↑ | |||||||||
| Isocitrate | ↑ | |||||||||
| Alpha-ketoglutarate | ↑ | |||||||||
| Succinate | ↑ | |||||||||
| Fumarate | ↑ | |||||||||
| Malate | ↑ | |||||||||
| 2-Hydroxyglutarate | ↑ | |||||||||
Blood
Although urine has provided numerous biomarkers for radiation exposure, blood (serum or plasma) as another easily accessible biofluid has proven to be a rich matrix as well for radiation metabolomics. The metabolic phenotype, pro- or anti-inflammatory state, and even microbial contamination or markers associated with tissue injury have provided valuable information on how to predict radiation injury and allow for appropriate medical treatment. Khan et al. utilized 1H-NMR to investigate dose and time-dependent differences in strain A mice (3, 5, and 8 Gy investigated at 1, 3, and 5 days post exposure) (Khan et al., 2011). While limited changes in metabolites were observed at day 1 after exposure, lipids, branched chain amino acids, lactate, alanine, acetate, glutamine/glutamate, choline, phosphoethanol amine, and betaine were all elevated at the later time points to varying degrees. Glucose levels were decreased in the majority of experimental times and doses. The investigators concluded that the results reflected changes in radiation-induced oxidative stress and effects on energy metabolism that were also shown in urine as described above. A comprehensive analysis was undertaken combining both untargeted (metabolomics and lipidomics) and targeted approaches at 24 hours post-irradiation with a single dose of 8 Gy (Laiakis et al., 2014b). Targeted approaches, while allowing for identification of specific metabolites, also have the advantage of determining the absolute levels of metabolites in a sample, and do not require the extra step of validation as with untargeted LC-MS (Figure 2). Lipidomics showed differences in phospholipids, particularly phosphatidylcholines (PCs), indicating radiation-induced damage of cell membranes. One targeted approach utilized the Biocrates AbsoluteIDQ® p180 Kit, which allows for extraction, internal standard normalization, and achieving quantitative results on 180 compounds (amino acids, acylcarnitines, phospholipids, sphingomyelins, biogenic amines, and hexose) using a 96-well plate. This approach further showed changes in PCs and sphingomyelins (SMs), but also various amino acids and energy metabolism intermediates. Finally, a targeted approach to analyze omega-6 (pro-inflammatory) and omega-3 (anti-inflammatory) intermediates (i.e., prostaglandins, thromboxanes, leukotrienes) indicated a shift towards an inflammatory state in systemic circulation that is consistent with radiation-induced oxidative stress. This study utilizing both untargeted and targeted analysis was the first comprehensive study of metabolic dysregulation in blood with a dose (8 Gy) that leads to hematopoietic syndrome.
This study also identified decreased circulating citrulline that has been linked to radiation related GI and liver injury (Lutgens et al., 2003, Lutgens et al., 2004, Onal et al., 2011, Kurland et al., 2015). A targeted approach was developed in plasma of mice to identify and quantify citrulline (Jones et al., 2014a, Jones et al., 2014b, Jones et al., 2015, Bujold et al., 2016). In doses leading to GI syndrome, the citrulline decrease correlated with decreased numbers of crypts in the small intestine (Jones et al., 2014a, Jones et al., 2015). Citrulline is the first marker to show direct correlation to tissue injury and based on its levels can predict the level of injury and the specific total dose.
While citrulline was also identified by Kurland et al., these investigators proceeded to connect urea cycle defects to liver injury and branched chain amino acid (valine, leucine, and isoleucine) metabolic products to total body irradiation (TBI) with 10 Gy (Kurland et al., 2015). Most importantly, they further identified products of tryptophan metabolism that implicate the gut microbiome in the radiation response. This connection was also described by Ó Broin et al., who dissected the responses of this metabolic pathway in plasma of mice irradiated with 2 to 10.4 Gy at 24 hours post-irradiation (Ó Broin et al., 2015). As more research is being conducted on the microbiome effects (initial studies are described in a later section), how the host and microbiome interact after radiation exposure, and whether there is leakage of intestinal bacteria in the blood circulation, may be more significant in organ responses, such as liver, than previously thought.
Regarding internal emitters, as described above in the urine section, the cumulative dose on an organism will be based on the activity and type of isotope, and expected biological responses may also be due to dose rate, which will primarily be low. Significant perturbations were identified in blood (Goudarzi et al., 2015a, Goudarzi et al., 2015b), similarly to urine (Grison et al., 2012, Goudarzi et al., 2015c). In mice injected with 137CsCl2 (Goudarzi et al., 2015b), general increases were observed in lysophosphatidylcholines (LPCs), SMs, and phosphatidylethanolamines (PEs), while PCs were significantly decreased compared to control samples in all time points and doses, as described under the urine section since the analyses were conducted on the same set of experimental animals. General metabolic pathways that were also affected included amino acid metabolism, TCA cycle, and fatty acid metabolism, with most free fatty acids that were identified showing persistent decreases, with the exception of linoleic acid (carbon content 18: double bond number 2). In mice injected with 90SrCl2 on the other hand (Goudarzi et al., 2015a), increases were observed in triacylglycerides (TGs) and cholesteryl esters (ChoEs), while PCs and LPCs were significantly decreased. Interestingly, arachidonic acid (20:4) levels were also elevated, signifying the initiation of a pro-inflammatory response to these two radionuclides. Lipidomics is still an evolving -omics technology as current analyses usually indicate changes in lipid molecules by carbon:double bond number (e.g. TG 54:2), which can be composed of multiple different acyl chains. Further analysis, such as tandem MS (MS/MS) and/or ion-mobility spectrometry, is required to positively identify levels/positions of unsaturation in acyl side chains and their precise location on the parent lipid backbone. As an exception to this statement, free fatty acids are easier to positively identify due to their simpler structure (allowing for simpler confirmation by MS/MS).
Finally, while most data investigated typically utilize high doses that can lead to hematopoietic or GI syndromes, metabolomics can be used to discern changes of low doses as well, although not relevant to radiation biodosimetry. Lee et al. explored such effects in genetically diverse mice irradiated with 10 cGy (Lee et al., 2012). Their model was far more representative of a heterogeneous human population, as it was based on a cross between radiosensitive and radioresistant mouse strains that allowed to track genetic diversity. Two metabolites, thymine and 2-monostearin classified exposed from non-exposed mice. Overall metabolite abundances were primarily affected by dose, and not by underlying genetics. However, at higher doses that will be encountered in a radiological event, genetics may have a far more complex role in metabolism, as was shown above in the urine of Parp1-/- mice (Laiakis et al., 2016). The validated biomarkers from these studies are presented in Table 2 and pathway involvement in Supplementary Table 1.
Table 2. Validated blood (serum or plasma) metabolites of radiation exposure through metabolomics.
| Organism | Strain/Species | Analytical Technique | Dose rate or initial activity | Exposure | Type | Doses in study | Matrix | Validated metabolites of radiation exposure | Trend compared to control | Time points analysed after irradiation | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mice | C57BL/6 | LC-MS | 0.6 Gy/min | TBI | External 60Co | 13-17 Gy | Plasma | Citrulline | ↓(D<10)↑(D>10) | 1, 3.5, 5, 7, 10, 30 days | Bujold et al., 2016 |
|
| |||||||||||
| Mice | C57BL/6 | LC-MS | 79.5 cGy/min | TBI | 300 kV, 10 mA X ray | 8-15 Gy | Plasma | Citrulline | ↓ | 4, 6 days | Jones et al., 2014a |
|
| |||||||||||
| Mice | C57BL/6 | LC-MS | 81.2 cGy/min | TBI | 300 kV, 10 mA X ray | 6-15 Gy | Plasma | Citrulline | Variable by dose | 1-6 days | Jones et al., 2015 |
|
| |||||||||||
| Mice | C57BL/6 | GC-MS | 320 cGy/min | PBI (Liver) | 320 kVp, 10 mA X ray | 10 or 50 Gy | Plasma | Dimethylglycine | ↓ | 24 hours | Kurland et al., 2015 |
| FT-ICR MS | 236 cGy/min | TBI | External 137 Cs | Pipecolate | ↓ | ||||||
| LC-MS | Phenol sulfate | ↓ | |||||||||
| Phenyacetylglycine | ↓ | ||||||||||
| Isovalerylglycine | ↓ | ||||||||||
| 4-Guanidinobutanoic acid | ↓ | ||||||||||
| 3-Indoxyl sulfate | ↓ | ||||||||||
| Citrulline | ↓ | ||||||||||
| Urea | ↓ | ||||||||||
| N-acetyl-L-leucine | ↓ | ||||||||||
| Fructose | ↓ | ||||||||||
| Heme | ↓ | ||||||||||
| Trigonelline (N′-methylnicotinate) | ↓ | ||||||||||
| Niacinamide | ↓ | ||||||||||
| Fumaric acid | ↓ | ||||||||||
| Malic acid | ↑ | ||||||||||
| Riboflavin | ↑ | ||||||||||
| 2-Hydroxyglutarate | ↓ | ||||||||||
| Myristoleate (14:1n5) | ↑ | ||||||||||
| Palmitoleate (16:1n7) | ↑ | ||||||||||
| 1-Palmitoylglycerophosphoinositol | ↓ | ||||||||||
| Palmitoyl sphingomyelin | ↑ | ||||||||||
| Inositol | ↓ | ||||||||||
| Allantoin | ↓ | ||||||||||
| Cinnamoylglycine | ↓ | ||||||||||
| Equol sulfate | ↓ | ||||||||||
| Homostachydrine | ↓ | ||||||||||
| Stachydrine | ↓ | ||||||||||
| N-Acetylalanine | ↓ | ||||||||||
| Eicosapentanoate (EPA; 20:5n3) | ↓ | ||||||||||
| 1-Linoleoylglycerol (1-monolinolein) | ↓ | ||||||||||
| Docosahexaenoic acid | ↓ | ||||||||||
| Tryptophan | ↓ | ||||||||||
| Threonine | ↓ | ||||||||||
| Alanine | ↓ | ||||||||||
|
| |||||||||||
| Mice | C57BL/6 | LC-MS | 1.67 Gy/min | TBI | External 137Cs | 8 Gy | Serum | PC aa C32:0 | ↑ | 24 hours | Laiakis et al., 2014b |
| PC aa C30:0 | ↑ | ||||||||||
| PC aa C34:1 | ↓ | ||||||||||
| PC aa C34:2 | ↓ | ||||||||||
| PC aa C36:5 | ↓ | ||||||||||
| PC aa C38:0 | ↑ | ||||||||||
| PC aa C38:4 | ↑ | ||||||||||
| PC aa C38:5 | ↓ | ||||||||||
| PC aa C38:6 | ↓ | ||||||||||
| PC aa C34:4 | ↓ | ||||||||||
| PC aa C34:3 | ↓ | ||||||||||
| PC aa C36:6 | ↓ | ||||||||||
| PC aa C42:6 | ↓ | ||||||||||
| PC aa C40:4 | ↓ | ||||||||||
| PC ae C30:1 | ↑ | ||||||||||
| PC ae C36:4 | ↑ | ||||||||||
| PC ae C36:5 | ↑ | ||||||||||
| PC ae C38:0 | ↓ | ||||||||||
| PC ae C38:4 | ↑ | ||||||||||
| PC ae C38:5 | ↑ | ||||||||||
| PC ae C38:6 | ↑ | ||||||||||
| PC ae C32:1 | ↑ | ||||||||||
| PC ae C32:2 | ↓ | ||||||||||
| PC ae C34:2 | ↓ | ||||||||||
| SM C18:1 | ↑ | ||||||||||
| SM C18:0 | ↑ | ||||||||||
| SM C20:2 | ↓ | ||||||||||
| SM C16:0 | ↑ | ||||||||||
| SM (OH) C16:1 | ↑ | ||||||||||
| LysoPC a C18:0 | ↑ | ||||||||||
| LysoPC a C16:1 | ↓ | ||||||||||
| 12-HHTrE | ↑ | ||||||||||
| 11-HETE | ↑ | ||||||||||
| 14,15-DiHETrE | ↑ | ||||||||||
| 8-HETE | ↑ | ||||||||||
| 12-HETE | ↑ | ||||||||||
| 17,18-DiHETE | ↓ | ||||||||||
| 14,15-DiHETE | ↓ | ||||||||||
| 9-HOTrE | ↓ | ||||||||||
| Carnitine | ↑ | ||||||||||
| Serine | ↑ | ||||||||||
| Histidine | ↓ | ||||||||||
| Aspartic acid | ↑ | ||||||||||
| Acetylornithine | ↓ | ||||||||||
| 3-Hydroxyoleoylcarnitine C18:1-OH | ↑ | ||||||||||
| Palmitoylcarnitine | ↑ | ||||||||||
| Serotonin | ↓ | ||||||||||
| Citrulline | ↓ | ||||||||||
|
| |||||||||||
| Mice | C57BL/6 | LC-MS | Activity (injected) | Internal | 137CsCl | 1.95, 4.14, 9.49, 9.91 Gy | Serum | 4-Hydroxyphenylpyruvic acid | ↑ | 2-30 days | Goudarzi et al., 2015b |
| 8.0±0.3 MBq | Gentisic acid | ↑ | |||||||||
| Reduced Riboflavin | ↓(D 2)↑(D>3) | ||||||||||
| Hippuric acid | ↑ | ||||||||||
| Lactic acid | ↑ | ||||||||||
| Nicotinic acid | ↑ | ||||||||||
| Hydroquinone | ↑ | ||||||||||
| Glucose | ↑ | ||||||||||
| Dityrosine | ↑ | ||||||||||
| Inositol | ↑ | ||||||||||
| Dihydrolipoamide | ↑ | ||||||||||
| Uridine | ↓ | ||||||||||
| Taurine | ↓ | ||||||||||
| 2-Ketobutyric acid | ↑ | ||||||||||
| Malic acid | ↓(D 2)↑(D>5) | ||||||||||
| Uric acid | ↑ | ||||||||||
| LPC(16:0)/PC(36:4) | ↑ | ||||||||||
| Arachidonic acid | ↓(D 2)↑(D>3) | ||||||||||
| Leukotriene F4 | ↓(D 2)↑(D>3) | ||||||||||
| Palmitic acid | ↓ | ||||||||||
| Linoleic acid | ↓ | ||||||||||
| Oleic acid | ↓ | ||||||||||
| Carnitine/Acetylcarnitine | ↓ | ||||||||||
|
| |||||||||||
| Mice | C57BL/6 | LC-MS | Activity (injected) | Internal | 85/90SrCl2 | 1.2, 1.8, 2.1, 4.8, 5.2 Gy | Serum | Palmitic acid | ↓(D 4) | 7-30 days | Goudarzi et al., 2015c |
| 200±0.3 kBq | Linoleic acid | ↓(D 4) | |||||||||
| Arachidonic acid | ↑(D >4) | ||||||||||
| Azelaic acid | ↑(D >4) | ||||||||||
| Adrenic acid | ↑(D 4) | ||||||||||
| LPC(16:0) | ↓ | ||||||||||
| LPC(18:2) | ↓ | ||||||||||
| LPC(18:1) | ↓ | ||||||||||
| LPC(18:0) | ↓ | ||||||||||
| PC(32:2) | ↓ | ||||||||||
| PC(32:1) | ↓ | ||||||||||
| PC(34:2) | ↓ | ||||||||||
| PC(34:1) | ↓ | ||||||||||
| PC(36:3) | ↓ | ||||||||||
| PC(36:2) | ↓ | ||||||||||
| PC(36:1) | ↓ | ||||||||||
| PC(34:4) | ↓ | ||||||||||
| PC(33:4) | ↓ | ||||||||||
| SM(38:2) | ↓ | ||||||||||
| TG(56:1) | ↑ | ||||||||||
| TG(58:7) | ↑ | ||||||||||
| TG(51:6) | ↑ | ||||||||||
| TG(61:6) | ↑ | ||||||||||
| DG(43:3) | ↓ | ||||||||||
| DG(39:3) | ↓ | ||||||||||
| ChoE(20:4) | ↑ | ||||||||||
| ChoE(18:2) | ↑ | ||||||||||
|
| |||||||||||
| Mice | C57BL/6 | GC-MS | 236 cGy/min | TBI | External 137Cs | 2, 4, 8,10.4 Gy | Plasma | 5,6-Dihydrouracil | ↓ | 24 hours | Ó Broin et al., 2015 |
| beta-Alanine | ↓ | ||||||||||
| N-Acetyl-beta-alanine | ↑ | ||||||||||
| Thymidine | ↑ | ||||||||||
| 2′-Deoxycytidine | ↑ | ||||||||||
| 2′-Deoxyuridine | ↑ | ||||||||||
| Tryptophan | ↑ | ||||||||||
| Indoleacetate | ↓ | ||||||||||
| Indolelactate | ↓ | ||||||||||
| Indolepropionate | ↓ | ||||||||||
| Serotonin (5HT) | ↑ | ||||||||||
| 3-Indoxyl sulfate | ↓ | ||||||||||
| Indole-3-acetamide | ↓ | 48-96 hours | |||||||||
| Indolepropionate | ↓ | ||||||||||
| Indole lactate | ↓ | ||||||||||
| Indole-3-pyruvate | ↓ | ||||||||||
|
| |||||||||||
| Mice | A | 1H-NMR | 0.22 Gy/min | TBI | External 60Co | 3, 5, 8 Gy | Serum | Lipids (LDLs/VLDLs) | ↑ | 1, 3, 5 days | Khan et al., 2011 |
| Branched chain amino acids | ↑ | ||||||||||
| Lactate | ↑ | ||||||||||
| Alanine | ↑ | ||||||||||
| Glutamine/Glutamate | ↑ | ||||||||||
| Choline | ↑ | ||||||||||
| Phosphoethanol amine | ↑ | ||||||||||
| Betaine | ↑ | ||||||||||
| Glucose | ↓ | ||||||||||
|
| |||||||||||
| Mice | BALB/c x SPRET/EiJ | GC-MS | TBI | X rays | 10 cGy | Plasma | 2-Monostearin | ↑ | 36 hours | Lee et al., 2012 | |
| Genetically diverse population | Benzoic acid | ↑ | |||||||||
| Cysteine | ↑ | ||||||||||
| Cystine | ↑ | ||||||||||
| Glucuronic acid | ↑ | ||||||||||
| Glutamic acid | ↓ | ||||||||||
| Linoleic acid | ↑ | ||||||||||
| Lysine | ↑ | ||||||||||
| 1-Monopalmitin | ↑ | ||||||||||
| Pyrazine 2,5-dihydroxy NIST | ↑ | ||||||||||
| Saccharic acid | ↑ | ||||||||||
| Stearic acid | ↑ | ||||||||||
| Threonic acid | ↑ | ||||||||||
| Thymine | ↑ | ||||||||||
| Uracil | ↓ | ||||||||||
| Uric acid | ↑ | ||||||||||
|
| |||||||||||
| Rats | Sprague-Dawley | LC-MS | 2 Gy/min | TBI | 6 MV X rays | 2, 4, 6, 8 Gy | Plasma | Cystine | ↓ | 5, 24, 72 hours | Tanget al., 2013 |
| Phenylalanine | ↑ | Time and dose dependent | |||||||||
| Glutamine | ↑ | ||||||||||
| trans-4-hydroxy-L-proline | ↓ | ||||||||||
| Citrulline | ↓ | ||||||||||
| Serine | ↑ | ||||||||||
| Glycine | ↑ | ||||||||||
| Arginine | ↓ | ||||||||||
| Tyrosine | ↓ | ||||||||||
| Tryptophan | ↑ | ||||||||||
| Threonine | ↑ | ||||||||||
| Leucine | ↑ | ||||||||||
| Lysine | ↑ | ||||||||||
| Creatinine | ↑ | ||||||||||
| Cysteine | ↑ | ||||||||||
| Alanine | ↑ | ||||||||||
|
| |||||||||||
| Rats | GC-MS | 1.9 Gy/min | TBI | External 60Co | 0.75, 3, 8 Gy | Serum | Lysine | ↑ | 24 hours | Liu et al., 2013 | |
| Serine | ↑ | ||||||||||
| Inositol | ↑ | ||||||||||
| Gluconic acid | ↓ | ||||||||||
| Isocitrate | ↓ | ||||||||||
| Glycine | ↑ | ||||||||||
| Stearic acid | ↓ | ||||||||||
| Threonine | ↑ | ||||||||||
| Glycerol | ↑ | ||||||||||
|
| |||||||||||
| Rats | Sprague-Dawley | LC-MS | 1 Gy/min | TBI | External 60Co | 3.5 Gy | Plasma | Phospholipids | ↑ | 24 hours | Wanget al., 2009 |
| Phosphatidylethanolamines (PE) | ↑ | General lipid classes | |||||||||
| Phosphatidylinositols (PI) | ↓ | ||||||||||
| Phosphatidylserines (PS) | ↑ | ||||||||||
| Phosphatidylcholines (PC) | ↑ | ||||||||||
| Sphingomyelins (SM) | ↑ | ||||||||||
| Lysophosphatidylcholines (LysoPC) | ↑ | ||||||||||
|
| |||||||||||
| Rats | Sprague-Dawley | LC-MS | 170 Bq/rat/day | Internal | 137CsCl | 4 mGy | Urine, Plasma | 26 metabolites, all putative | 9 months continuous exposure | Grison et al., 2012 | |
|
| |||||||||||
| Non-human primates | Macaca mulatta | LC-MS | 0.6 Gy/min | TBI | External 60Co | 2, 4, 6, 7, 10 Gy | Serum | Carnitine | ↑ | 7 days | Pannkuk et al., 2016b |
| Acetylcarnitine | ↑ | ||||||||||
| Propionylcarnitine | ↓ (2Gy), ↑ | ||||||||||
| Butyrylcarnitine | ↑ | ||||||||||
| Valerylcarnitine | ↑ | ||||||||||
| Tetradecadienylcarnitine | ↓ | ||||||||||
| Octadecenoylcarnitine | ↓ | ||||||||||
| Octadecadienylcarnitine | ↓ | ||||||||||
| Glutamate | ↓ | ||||||||||
| Histidine | ↓ | ||||||||||
| Proline | ↓ | ||||||||||
| cis-OH Proline | ↓ | ||||||||||
| trans-OH Proline | ↓ | ||||||||||
| Alanine | ↓ | ||||||||||
| Arginine | ↓ | ||||||||||
| Citrulline | ↓ | ||||||||||
| Asparagine | ↓ | ||||||||||
| PC(34:2) | ↓ | ||||||||||
| PC(34:3) | ↓ | ||||||||||
| PC(36:1) | ↓ | ||||||||||
| PC(36:2) | ↓ | ||||||||||
| PC(36:5) | ↓ | ||||||||||
| PC(38:3) | ↓ | ||||||||||
| TG (50:5) | Variable by dose | ||||||||||
| TG (52:2) | ↓ | ||||||||||
| TG (52:3) | ↓ | ||||||||||
| TG (52:7) | ↓ | ||||||||||
| TG (54:6) | ↓ | ||||||||||
| TG (54:7) | ↓ | ||||||||||
| TG (56:2) | Variable by dose | ||||||||||
| TG (56:5) | ↓ | ||||||||||
| TG (56:6) | ↓ | ||||||||||
| TG (56:8) | Variable by dose | ||||||||||
| TG (56:9) | ↓ | ||||||||||
| TG (56:10) | Variable by dose | ||||||||||
| TG (58:3) | Variable by dose | ||||||||||
| TG (58:4) | Variable by dose | ||||||||||
| TG (58:5) | Variable by dose | ||||||||||
| TG (58:7) | Variable by dose | ||||||||||
| TG (60:4) | Variable by dose | ||||||||||
| TG (60:5) | Variable by dose | ||||||||||
| TG (60:6) | Variable by dose | ||||||||||
| TG (60:7) | Variable by dose | ||||||||||
| TG (62:9) | Variable by dose | ||||||||||
| TG (62:10) | Variable by dose | ||||||||||
| DG (34:1) | ↓ | ||||||||||
| DG (34:2) | ↓ | ||||||||||
| DG (36:1) | ↓ | ||||||||||
| DG (36:2) | ↓ | ||||||||||
| DG (36:3) | ↓ | ||||||||||
| DG (36:4) | ↓ | ||||||||||
| DG (44:12) | ↓ | ||||||||||
| LysoPC (22:6) | Variable by dose | ||||||||||
| LysoPC (22:4) | ↑ 10Gy | ||||||||||
| LysoPC (20:5) | ↑ 10Gy | ||||||||||
| LysoPC (20:4) | Variable by dose | ||||||||||
| LysoPC (20:3) | Variable by dose | ||||||||||
| LysoPC (20:1) | ↑ 10Gy | ||||||||||
| LysoPC (20:0) | Variable by dose | ||||||||||
| LysoPC (18:3) | ↓ | ||||||||||
| LysoPC (18:2) | ↑ 10Gy | ||||||||||
| LysoPC (18:1) | ↑ 10Gy | ||||||||||
| LysoPC (18:0) | ↑ 10Gy | ||||||||||
| LysoPC (16:1) | ↑ | ||||||||||
| LysoPC (16:0) | ↑ 10Gy | ||||||||||
| LysoPE (18:0) | ↑ 10Gy | ||||||||||
| LysoPE (18:2) | ↓ | ||||||||||
| LysoPE (20:1) | ↑ 10Gy | ||||||||||
| LysoPE (20:2) | Variable by dose | ||||||||||
| LysoPE (22:4) | ↑ 10Gy | ||||||||||
| LysoPE (22:6) | Variable by dose | ||||||||||
| PE (34:2) | ↓ | ||||||||||
| PE (36:1) | ↓ | ||||||||||
| PE (36:2) | ↓ | ||||||||||
| PE (36:3) | ↓ | ||||||||||
| PE (38:1) | ↓ | ||||||||||
| PE (38:2) | ↓ | ||||||||||
| PE (38:3) | ↓ | ||||||||||
| PE (38:4) | ↑ 10Gy | ||||||||||
| PE (40:2) | ↓ | ||||||||||
| PE (40:3) | ↓ | ||||||||||
| PE (40:4) | Variable by dose | ||||||||||
| ePE (34:3) | Variable by dose | ||||||||||
| ePE (36:2) | Variable by dose | ||||||||||
| ePE (36:3) | Variable by dose | ||||||||||
| ePE (36:4) | Variable by dose | ||||||||||
| ePE (38:5) | Variable by dose | ||||||||||
| ePE (38:6) | Variable by dose | ||||||||||
| ePE (38:7) | ↑ | ||||||||||
| ePE (40:5) | Variable by dose | ||||||||||
| ePE (40:7) | ↑ | ||||||||||
| ePE (40:8) | ↑ | ||||||||||
| PC (32:3) | Variable by dose | ||||||||||
| PC (36:4) | ↓ | ||||||||||
| PC (36:5) | Variable by dose | ||||||||||
| PC (36:6) | ↓ | ||||||||||
| PC (38:4) | ↑ 10Gy | ||||||||||
| PC (40:2) | Variable by dose | ||||||||||
| PC (40:5) | Variable by dose | ||||||||||
| PC (40:8) | Variable by dose | ||||||||||
| PC (41:2) | Variable by dose | ||||||||||
| PC (41:6) | Variable by dose | ||||||||||
| PC (42:10) | Variable by dose | ||||||||||
| PC (42:2) | Variable by dose | ||||||||||
| PC (42:5) | Variable by dose | ||||||||||
| PC (42:6) | ↓ | ||||||||||
| ePC (24:0) | Variable by dose | ||||||||||
| ePC (30:0) | ↓ | ||||||||||
| ePC (32:2) | Variable by dose | ||||||||||
| ePC (34:2) | Variable by dose | ||||||||||
| ePC (34:4) | Variable by dose | ||||||||||
| ePC (36:1) | ↑ 10Gy | ||||||||||
| ePC (36:2) | Variable by dose | ||||||||||
| ePC (36:5) | Variable by dose | ||||||||||
| ePC (40:6) | ↑ 10Gy | ||||||||||
| ePC (42:4) | ↑ | ||||||||||
|
| |||||||||||
| Non-human primates | Macaca mulatta | LC-MS | 0.6 Gy/min | TBI | External 60Co | 2, 4, 6, 7, 10 Gy | Serum | TG(52:4) | ↓ | 7 days | Pannkuk et al., 2016a |
| TG(54:2) | ↓ | ||||||||||
| TG(54:5) | ↓ | ||||||||||
| TG(60:10) | ↑ 10Gy | ||||||||||
| TG(60:11) | ↑ 10Gy | ||||||||||
| TG(62:14) | ↑ 10Gy | ||||||||||
| LysoPC(20:4) | ↑ 10Gy | ||||||||||
| Ly soPC(2 2:6) | ↑ 10Gy | ||||||||||
| LysoPE(22:6) | ↑ 10Gy | ||||||||||
| PC(38:2) | ↓ | ||||||||||
| PC(38:3) | ↓ | ||||||||||
| PC(38:6) | ↑ 10Gy | ||||||||||
| ChoE(18:1) | ↑ | ||||||||||
| ChoE(20:4) | ↑ | ||||||||||
| ChoE(22:6) | ↑ | ||||||||||
| Linoleic acid | ↓ | ||||||||||
| Arachidonic acid | ↓ | ||||||||||
| Docosahexaenoic acid | ↑ | ||||||||||
| Taurine | ↓ | ||||||||||
| Uridine | ↑ | ||||||||||
| Glucose | ↓ | ||||||||||
| Tyrosine | ↓ | ||||||||||
| Proline | ↓ | ||||||||||
| Hypoxanthine | ↓ | ||||||||||
| L-Carnitine | ↑ | ||||||||||
| Valine | ↓ | ||||||||||
|
| |||||||||||
| Non-human primates | Macaca mulatta | LC-MS | 0.8 Gy/min | TBI PBI 5% sparing | 6 MV LINAC | 13,10.5, 7.5 Gy 11 Gy | Plasma | Citrulline | ↓, ↑ overtime | Up to 180 days post IR | Jones et al., 2015 |
|
| |||||||||||
| Mice | C57BL/6 | LC-MS | 0.6 Gy/min | TBI, partial shielding | External 60Co | 13-17 Gy | Plasma | Citrulline | ↓ | 1-30 days | Bujold et al., 2016, Jones et al., 2014a, Jones et al., 2014b |
| Mini-pigs | Göttinger | LC-MS | 0.5 Gy/min | TBI, partial shielding | External Co | 8-16 Gy | Plasma | Citrulline | ↓ | 1-45 days | |
| Non-human primates | Rhesus macaques | LC-MS | 0.6-0.8 Gy/min | TBI | External 60Co | 6.7-13 Gy | Plasma | Citrulline | ↓ | 1-10 days | |
Rats
Urine
Following proof of principle demonstration of radiation metabolomics in mouse models and identification of metabolites that can serve as radiation markers, metabolomic analysis has also been performed in rat models. While as an experimental model rats are still genetically vastly different from humans, instigation of cross species validation of radiation biomarkers may provide further proof that animal-model-developed signatures can be applicable to human populations. The first reports on analysis of rat urine appeared in 2009. Utilizing GC-MS, Lanz et al. (Lanz et al., 2009) analyzed urine from male rats irradiated with 3 Gy. Analysis of urine samples for 3 days post-exposure revealed significant increases in glyoxylate, threonate, thymine, uracil, and p-cresol, most of them, however, decreasing after the first day. Several other metabolites (citrate, 2-oxoglutarate, adipate, pimelate, azelate) showed decreased levels, with citrate and 2-oxoglutarate showing some of the most prominent changes. The investigators concluded that oxidative stress and kidney function are most likely the largest contributors to the phenotype, while products of the pyrimidine pathway point to pathway similarities between mice and rats in response to radiation, without however excluding that food consumption may significantly affect the metabolomic responses (Lanz et al., 2009).
Building on samples from the same experiments, investigators from the same group (Johnson et al., 2011) utilized LC-MS to expand radiation-related metabolomic analysis. Their results identified increased acetylated metabolites (N-acetyltaurine, N1-acetylspermidine, N-acetylglucosamine/galactosamine-6-sulfate) and putative isethionic acid in the urine. Furthermore, increased thymidine, taurine, 2′-deoxyxanthosine, and 2′-deoxyuridine were previously identified as radiation markers of external exposure in mice (Tyburski et al., 2008, Tyburski et al., 2009, Laiakis et al., 2012), providing further evidence for cross-species validation of metabolomic responses to ionizing radiation. The investigators also provided further analysis on starvation related changes in markers, with azelaic acid, pimelic acid, dodecanedioic acid, and N-isovaleryglycine showing decreased levels correlating with radiation exposure.
The Johnson et al. study utilized a sublethal dose (3 Gy), however Mak et al. expanded on the dose range (0.5 to 10 Gy), while maintaining the same time range for analysis (Mak et al., 2015b). In order to effectively visualize the net changes in the metabolome, they also developed a novel approach termed Visual Analysis of Metabolomics Package (VAMP) that revealed progressive decreases in excreted ions with time and dose. Nevertheless, some metabolites were significantly increased in the urine post-exposure. Thymidine, uric acid, cytosine, creatine, and carnitine were positively confirmed through MS/MS, while threonate, arabinonate, glucarate, alanine or sarcosine, and methylimidazoleacetic acid were not validated through matching of fragmentation patterns to either pure chemicals or online databases. Creatine was the only biomarker increased at doses as low as 0.5 Gy with dose dependence up to 72 hours, whereas thymidine exhibited dose dependency at only ≤2.5 Gy up to 6 hours, which expanded for ≤10 Gy at 24 hours.
Although untargeted analysis has revealed significant information on the general metabolic status of a rat after irradiation, Zhang et al. conducted a targeted approach to specifically assess amino acid changes up to 72 hours post-exposure with doses from 2 to 8 Gy (Zhang et al., 2014a). Changes revealed that the amino acid signature can be utilized as an effective classifier at 72 hours post-exposure. Furthermore, the changes in the levels of certain amino acids, particularly with significant dose and time dependence, could have further implications in biological mechanisms such as in urea cycle, glycine, serine and threonine metabolism, and alanine, aspartate and glutamine metabolism. Taken together, global and targeted analysis of rat urine provides significant information on radiation metabolism and further enriches the radiation specific signature and provides evidence of cross-species effects. The validated biomarkers from these studies are presented in Table 1 and pathway involvement in Supplementary Table 1.
Blood
Unlike urine, only limited analysis has been conducted on blood (plasma and serum) radiation signatures in rats. The first report of radiation metabolomics in blood from rats was published by Tang et al. (Tang et al., 2013). Plasma analysis, with the same experimental conditions as in (Zhang et al., 2014a), also revealed time and dose dependent changes. Of particular importance is citrulline that has also been identified in mice as a biomarker of intestinal injury (Jones et al., 2014a, Jones et al., 2014b, Jones et al., 2015, Kurland et al., 2015, Bujold et al., 2016). This is further proof of cross-species validation and that biomarkers of intestinal injury and therefore GI syndrome can be utilized as predictive markers at early time points. Serum analysis (Liu et al., 2013) with GC-MS also identified elevated levels of serine, lysine, glycine, and threonine, in agreement with Tang et al. (Tang et al., 2013). Additionally, inositol and glycerol also increased, while isocitrate, gluconic acid, and stearic acid decreased after irradiation (at 24 hours-, 0.75, 3, or 8 Gy). Although 5 out of 9 markers showed statistical significance at the lower dose, the number increased to 7 out of 9 with the higher doses. Glycerol was identified as a marker for the 0.5 Gy dose, stearic acid for 3 Gy, threonine for 8 Gy, and glycine for both 3 and 8 Gy. The remaining markers were all altered significantly in all doses.
As the lipid content in blood is exceptionally high, Wang et al. concentrated on the analysis of phospholipid groups, the main components of membranes (Wang et al., 2009). Following exposure to 3.5 Gy at 24 hours post-exposure, total phospholipids were significantly increased in circulation in the irradiated group. When broken down by group, only PEs and phosphatidylserines (PSs) exhibited statistical significance. The rest of the groups, phosphatidylinositols (PIs), PCs, SMs, and lysophosphatidylcholines (LPCs), were elevated but not statistically significant.
While the above results refer to external exposure to photons with high dose rate, internal exposure to radionuclides such as 137Cs leading to a low dose rate exposure did show differences in mice (Goudarzi et al., 2014b, Goudarzi et al., 2015b). However, the first report to demonstrate that chronic internal exposure to 137Cs can lead to severe metabolic changes was published in 2012 by Grison et al. (Grison et al., 2012) in rats. Rats were exposed in utero to the radionuclide through their mothers and continued to receive it after birth in their water until 9 months of age, leading to a mean maximal whole-body absorbed dose of 4 mGy over this time period. Although standard clinical analysis of electrolytes and similar tests did not provide any information to differentiate the two groups, metabolomics was able to provide the predictive power through identification of 26 metabolites that initially determine the level of exposure of an individual in a real life scenario. Further analysis however, (Manens et al., 2016) showed that chronic exposure to 137Cs leads to increases in cholesterol, high-density lipoprotein (HDL) cholesterol, phospholipids B, phosphorus, and bilirubin (bilirubin increase was only observed in adults), through standard clinical testing. Finally, similar results of increased cholesterol were also observed in rats exposed to sublethal neutrons/gamma rays (Feurgard et al., 1998), indicating a potential metabolomic analysis may provide further information on the degree of damage and radiation quality. It remains to be determined whether cross species validation of markers exists for these different exposure scenarios. The validated biomarkers from these studies are presented in Table 2 and pathway involvement in Supplementary Table 1.
Minipigs
This animal model has recently been proposed as a more appropriate model for studying radiation injury compared to rats or mice (Shim et al., 2014), as symptoms that are indicators of ARS (emesis, fever, diarrhea) are not present or easily measured in rat and mouse models. In order to study GI injury through blood indicators, citrulline was measured by two groups (Shim et al., 2014, Bujold et al., 2016) (Table 3, Supplementary Table 1). Both groups identified decreased levels in plasma, correlating with small intestinal radiation-induced injury. This model has not been further utilized with radiation metabolomics studies, but may be a less expensive alternative and informative model as NHPs.
Table 3. Validated metabolites in saliva, fecal material, or sebum of radiation exposure through metabolomics.
| Organism | Strain/ Species | Analytical Technique | Dose rate or initial activity | Exposure | Type | Doses in study | Matrix | Validated metabolites of radiation exposure | Trend compared to control | Time points analysed after irradiation | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mice | C57BL/6 | LC-MS | 1.4 Gy/min | TBI | External137 Cs | 0.5, 3, 8 Gy | Saliva | 3-Oxo-octadecanoic acid | ↑(D2)↑(D7) | 24 hours (D1), D7 | Laiakis et al., 2016 |
| dAMP | ↓ | ||||||||||
| Dodecanedioic acid | ↑ | ||||||||||
| L-Histidine | ↑ | ||||||||||
| L-Phenylalanine | ↑(D2) variable (D7) | ||||||||||
| L-Proline | ↓, ↑(3Gy, D7) | ||||||||||
| Nicotinamide | ↓(D2)↓(8Gy, D7) | ||||||||||
| Nicotinic acid | ↑ | ||||||||||
|
| |||||||||||
| Mice | C57BL/6J | LC-MS | TBI | 20kVp, 12.5 mA X ra | 5, 12 Gy | Feces | Glyceric acid | ↓ | D3 | Goudarzi et al., 2016 | |
| Homogentisic acid | ↓ | ||||||||||
| Glutaconic acid | ↓ | ||||||||||
| Pipecolic acid | ↓ | ||||||||||
| Hippuric acid | ↑ | ||||||||||
| Taurine | ↑ | ||||||||||
| Urobilinogen | ↑ | ||||||||||
| Taurocholic acid | ↑ | ||||||||||
| 7-Sulfocholic acid | ↑ | ||||||||||
| 12-Ketodeoxycholic acid | ↓ | ||||||||||
| L-Alloisoleucine | ↓ | ||||||||||
| Tyrosine | ↓ | ||||||||||
| Ornithine | ↑ | ||||||||||
| Quinolinic acid | ↑ | ||||||||||
| Citric acid | ↑ | ||||||||||
| Chenodeoxycholic acid sulfate | ↓ | ||||||||||
| Serotonin | ↓ | 3, 14, 30 days | |||||||||
| Sebacic acid | ↓ | ||||||||||
| Hypoxanthine | ↑ | ||||||||||
| 2-Ketobutyric acid | ↑ | ||||||||||
|
| |||||||||||
| Rats | Fischer | GC-MS | 1 Gy/min | TBI | External137 Cs | 3 Gy | Sebum | Palmitic acid | Marginally ↑ | 24 hours | Lanz et al., 2011 |
| 2-Hydroxypalmitic acid | Marginally ↑ | ||||||||||
| Stearic acid | Marginally ↑ | ||||||||||
Non-human Primates (NHPs)
Urine
Biomarker discovery by MS on NHP models, primarily Rhesus macaques (Macaca mulatta), is sparse compared to murine models, possibly due to prohibitive expenses and ethical issues associated with use of NHPs in medical research. Two studies utilized an LC-MS platform for global metabolomics on NHP urine after exposure to γ-rays with 1, 3.5, 6.5, and 8.5 Gy (samples collected at 24, 48, and 72 hours) (Johnson et al., 2012) and 2, 4, 6, 7, and 10 Gy (samples collected at 7 days) (Pannkuk et al., 2015). Although early assessment of individuals is imperative, the later time point of 7 days remains a priority based not only on practical reasons for accessibility to POC centers, but also as the last time point of treatment initiation before the onset of possible GI syndrome leading to death. A total of 13 biomarkers increased in NHP urine collected within 72 hours, including markers novel from other species (tyrosol sulfate, 3-hydroxytyrosol sulfate, tyramine sulfate, creatinine), specific to NHPs and humans (hypoxanthine, see below), or previously identified in murine models (xanthine, taurine, N-acetyltaurine, isethionic acid, uric acid, creatine, adipic acid) (Johnson et al., 2012). A later study performed on urine collected 7 days post-irradiation corroborated these findings in that taurine, xanthine, hypoxanthine, and creatine are viable urinary biomarkers at later time points as well along with others (xanthurenic acid, xanthosine, cortisol, cortisone, carnitine, acetylcarnitine, kynurenic acid, and isobutyryl carnitine/butyrylcarnitine), some of which are sex specific (Pannkuk et al., 2015). Together, these studies suggest perturbations to metabolic processes including tryptophan metabolism, DNA damage, fatty acid β-oxidation, steroid metabolism, and taurine metabolism, and highlight the possibility of expanding urinary metabolomics to human biodosimetry. The validated biomarkers from these studies are presented in Table 1 and pathway involvement in Supplementary Table 1.
Blood
Recently, a number of studies have elucidated biomarkers in NHP serum and plasma, including global lipidomics/metabolomics on serum (Pannkuk et al., 2016a), targeted analysis of plasma citrulline as a biomarker of GI syndrome (Jones et al., 2014b, Jones et al., 2015, Wang et al., 2015), and targeted analysis of serum using the Biocrates AbsoluteIDQ® p180 Kit (Pannkuk et al., 2016b). Global lipidomics identified 604 putative lipid ions from multiple broad classes, many of which were perturbed at 7 days post-exposure (Pannkuk et al., 2016a) in doses 2, 4, 6, 7, and 10 Gy. Lipid levels can be altered in a variety of ways, including changes in diet (e.g., lower TGs), increased eicosanoid levels during inflammation, drug regimens, and many are prime targets for destruction by reactive oxygen species (ROS) due to the susceptibility of their polyunsaturated fatty acyl chains. Interestingly, despite the direct tissue damage caused from ionizing radiation and indirect damage through ROS generated from hydrolysis of water, increases in lipids containing arachidonic acid (20:4) and docosahexaenoic acid (22:6) as acyl chains were identified with 10 Gy at 7 days. Due to the importance of these molecules in inflammation, these compounds may be produced in radiation doses approaching levels inducing GI syndrome.
Another well-studied biomarker of GI syndrome is citrulline. NHPs exposed to 10.5 and 13.0 Gy TBI showed a ∼4-fold decrease in circulating citrulline levels at 7 days (Jones et al., 2014b, Jones et al., 2015); however, citrulline increased in concentration after 7 days at doses eliciting hematopoietic syndrome (i.e., 6.7 and 7.5 Gy) and or in animals exposed to partial body irradiation (Jones et al., 2015, Wang et al., 2015). A method for increasing positively identified metabolites (including citrulline) using higher sample throughput was attempted with the Biocrates AbsoluteIDQ® p180 Kit using NHP serum, its utility already demonstrated in mice (Laiakis et al., 2014b, Pannkuk et al., 2016b). A 96-well plate allows for higher number of samples to be processed as automated robotic instruments for sample prep are already commercially available, and plates can be inserted directly into a tandem quadrupole MS for analysis. This targeted analysis allowed for the quantitative identification of 7 novel acylcarnitines (acetylcarnitine, propionylcarnitine, butyrylcarnitine, valerylcarnitine, tetradecadienylcarnitine, octadecenoylcarnitine, octadecadienylcarnitine) including carnitine, 8 amino acids in addition to citrulline [glutamate, histidine, proline, cis-OH proline, trans-OH proline (hydroxyproline), alanine, arginine, asparagine], and 36 PCs and ether-linked PCs (ePCs) in NHP serum, some of which also exhibited sex specific differences. These studies provide evidence that NHPs will continue to play a pivotal role in determining suitable biomarker panels that can be utilized in human biodosimetry. The validated biomarkers from these studies are presented in Table 2 and pathway involvement in Supplementary Table 1.
Humans
Although animal models have provided a significant number of potential radiation biomarkers in both urine and blood with cross species validation of alterations in metabolites and dysregulation of metabolic pathways, developing biomarkers in the human population is of utmost importance. As already described, various scenarios should be anticipated and development of radiation biomarkers should not only address the specificity of the radiation signature, radiation quality (photons vs. neutrons), dose rate, or type of exposure (total body vs. partial body), but also age and sex that are contributing factors in alterations of the metabolome. However, tightly controlled experiments on human populations are obviously unethical and therefore samples may only be acquired from previously exposed individuals, such as cancer patients undergoing radiotherapy. To date, few studies have demonstrated the feasibility of metabolomics in the development of a human specific radiation signature (Tandle et al., 2013, Laiakis et al., 2014a). Urine from cancer patients undergoing TBI prior to hematopoietic stem cell transplantation was analyzed with LC-MS at ∼6 hours following exposure to one fraction of 1.25 Gy. Further time points were not assessed due to biological complications from fractionated dose and increased creatinine levels, indicative of kidney injury. Several biomarkers implicating fatty acid β-oxidation and mitochondrial involvement were identified (trimethyl-L-lysine, acetylcarnitine, decanoylcarnitine, octanoylcarnitine), together with metabolites of the purine catabolism pathway signifying increased oxidative stress and DNA damage (hypoxanthine, xanthine, uric acid) (Laiakis et al., 2014a), pathways and processes that have been observed in animal models as described in the previous sections. Examples of overlap include uric acid (Laiakis et al., 2012, Mak et al., 2015b) and xanthine (Tyburski et al., 2009, Johnson et al., 2012).
More importantly, evidence of sex differences in the excretion of these metabolites constitutes imperative to generate sex-specific biosignatures with regard to urinary metabolomics. Blood analysis (serum) is currently underway in our lab in patients of the same cohort, which also allows for further assessment of a pro-inflammatory state, as previously demonstrated in mice (Laiakis et al., 2014b). Liang et al. investigated basal metabolomic differences in control populations to putatively identify steroid metabolites as the primary discriminatory molecules (Liang et al., 2015), and therefore caution should be taken when utilizing such metabolites as radiation specific. Given the heterogeneity in the available human radiation cohorts due not only to genetic differences but also to underlying disease, it has been proposed that perhaps pairs of ions instead of single biomarkers and patterns of similar or dissimilar regulation may provide more power in distinguishing exposed from non-exposed individuals (Mak et al., 2015a). This study also further identified perturbations in lysine biosynthesis/degradation and folate biosynthesis.
A different study utilized patients with glioblastoma multiforme (GBM) undergoing treatment with radiation (Tandle et al., 2013). Since GBM is a brain tumor, irradiation is highly localized and therefore this study provides the basis for a partially irradiated model. However, total dose and time of urine sampling post-exposure were not provided, therefore deeming unusable the use of the identified biomarkers for radiation biodosimetry. Regardless, the investigators identified significant changes in N-acetylated metabolites (N-acetylphenylalanine, N-acetyltryptophan, N-acetyltyrosine, N-acetylproline) and TCA cycle intermediates (citrate, isocitrate, alpha-ketoglutarate, succinate, fumarate, malate, and 2-hydroxyglutarate) (Tandle et al., 2013). Other studies have investigated the stability of whole blood that can have implications for storage and transport in a real life incident. Patel et al. demonstrated that storage of >7 days may indeed accentuate the metabolic differences and therefore identification of exposed individuals (Patel et al., 2015). However, the dose of 25 Gy that was used would most likely lead to death of individuals due to GI and central nervous system syndromes with a TBI exposure. Nevertheless, this is the first study to demonstrate the stability of irradiated red blood cells and storage.
Although significant information has been obtained from these studies, the complicated nature of samples that are currently available for radiation metabolomic studies renders some caution in the development of a biosignature for the human population. Furthermore, validation of the results in samples from different institutions may provide significant power to radiation biodosimetry through metabolomics. The validated biomarkers from these studies are presented in Table 1 and pathway involvement in Supplementary Table 1.
Potential use of other easily accessible samples
Sebum
Sebum, an oily matrix secreted by sebaceous glands on the skin, was investigated for its potential for use in radiation biodosimetry (Lanz et al., 2011). Lipidomic analysis with GC-MS was conducted in sebum from γ-irradiated rats at 1 and 24 hours after exposure to 3 Gy. A comparative study with control human sebum was also conducted to investigate the potential translation of markers to humans. Results demonstrated the ineffectiveness of this matrix to differentiate between exposed and non-exposed groups, with only palmitic acid (16:0), 2-hydroxypalmitic acid, and stearic acid marginally increased in the 3 Gy group (Table 3, Supplementary Table 1). Nonetheless, the basal profiles between rat and human sebum were similar. Although this study did not identify any biomarkers of radiation exposure, it remains the first attempt to assess this substance's profile. However, the logistics of acquiring a clean sample in a possible IND scenario and the analytical method utilized in this study that is laborious and time consuming, limit its potential for rapid biodosimetry.
Saliva
Salivary glands and the oral mucosa are highly radiosensitive tissues (Moore et al., 2014), resulting in severe reduction to saliva production within the first week post-exposure that can lead to mucositis, dysphagia, and nutrient malabsorption (Grundmann et al., 2009, Pernot et al., 2014). Differences in electrolytes and enzymes such as amylase have already been reported to be directly associated with radiation exposure (Pernot et al., 2014, Soni et al., 2016), and salivary biomarkers have provided discriminatory power in various cancers and disease states (Tsuruoka et al., 2013, Mikkonen et al., 2014, Zhang et al., 2014b). Its availability and ease in acquisition therefore makes it an attractive biofluid for radiation biodosimetry. Its utility was recently demonstrated in a pilot study in mice (Laiakis et al., 2016). Male mice exposed to γ-radiation with low (0.5 Gy), sublethal (3 Gy), and 8 Gy (LD50/30 dose) were assessed at days 1 and 7 post-exposure. Metabolic profiles were able to distinguish each radiation group, even at day 7, from each other and controls. Markers such as 3-oxodecanoic acid, dAMP, histidine, proline, niacinamide, nicotinic acid, and dodecanedioic acid showed high sensitivity and specificity in distinguishing the 8 Gy from the sham-irradiated group. Markers identified in this study were suggestive of increased cell death, characteristic of known responses in irradiated salivary glands (Grundmann et al., 2009, Pernot et al., 2014) (Table 3, Supplementary Table 1). While this was a pilot study, it provided the first evidence of the ability to utilize this biofluid not only for assessment of radiation exposure, but also for biodosimetry through metabolomics. However, it remains to be determined what effect lifestyle, diet, and even oral microbiome will have on the metabolomic signature.
Fecal material
While time of emesis is a good indicator of the level of radiation exposure, GI injuries are generally characterized by severe diarrhea and pain. Although as previously described, reduced levels of circulating citrulline can correlate with GI injury (Jones et al., 2014a, Jones et al., 2014b, Jones et al., 2015, Jones et al., 2015, Wang et al., 2015, Bujold et al., 2016) and particularly small intestinal damage, they provide no direct assessment of the population of microbiota or their direct metabolites. Fecal material can be easily obtained and analyzed in a similar manner as urine, serum, saliva, and sebum. This was demonstrated in mice irradiated with 5 or 12 Gy and evaluated at 3 days post-irradiation (Goudarzi et al., 2016). Marked decreases in several species were observed after 16S rRNA sequencing, most notably in the Lactobacillaceae, Staphylococcaceae, Lachnospiraceae, Ruminococcaceae, and Clostridiaceae families. Although not definitive on the contribution of each family to the metabolic content, significant changes were observed in metabolites such as pipecolic acid, glutaconic acid, urobilinogen, taurocholic acid, and 12-ketodeoxycholic acid (Table 3, Supplementary Table 1). Furthermore, observed specific changes in bile acids may be associated with the bacteria R. gnacus. This was the first study to demonstrate the utility of feces as a rich material for radiation injury assessment through metabolomics. It has to be noted that when conducting such experiments, the housing conditions of mice (whether specific pathogen free, conventionally raised, conventionally derived, or germ-free) can have important implications in the metabolic profiles and radiosensitivity of mice (Crawford and Gordon, 2005, Wikoff et al., 2009), further complicated by possible administration of antibiotics that can restructure the gut microbiome (Ferrer et al., 2016). The effects of radiation on the microbiome in general are poorly understood and more research needs to be conducted in this area.
Conclusions and Future Directions
Radiation metabolomics has provided a myriad of potential markers of radiation exposure in easily accessible samples that will aid in constructing biodosimetric signatures for high-throughput analysis. The current state of research has led to identification of markers primarily for TBI, dose rate effects, internal vs. external exposure, genotypic effects, radiation quality, and even markers for specificity. Cross species validation of markers and metabolic pathways has allowed for the use of smaller and more versatile animal models in order to explore different scenarios that can affect the radiation biosignature. However, in a real life situation, burns and trauma combined with radiation exposure may significantly change the outcome of any response, whether the outcome includes metabolic changes, microRNA (miRNA), cytokine levels (Islam et al., 2015), and even survival (Ledney and Elliott, 2010). It is expected therefore that radiation metabolomic signatures, whether in urine or blood, will exhibit similar alterations.
Nevertheless, significant discoveries have been made in this aspect with the availability of new MS instrumentation. Radiation exposure leads to an early metabolic response, and general perturbations can persist for weeks and months. Through metabolomics it has become evident that radiation affects a plethora of metabolic pathways, some highly dependent on others. For example, acylcarnitine alterations are interconnected with fatty acid β-oxidation indicating a mitochondrial perturbation, an organelle known to be affected by ionizing radiation (Kam and Banati, 2013). Other pathways include purine and pyrimidine pathways, the products of which are key components of DNA and RNA and also serve as indicators of DNA damage when present in biofluids, the TCA cycle with implications for energy metabolism, pro-inflammatory pathways such as the omega-6 constituents and polyunsaturated fatty acids, and amino acids that can also indicate specific tissue injury responses. Metabolic pathways with the most significant perturbations are highlighted in Table 4.
Table 4. Metabolic pathways highly dysregulated with radiation biodosimetry implications.
| Fatty acid β-oxidation |
| Metabolism of amino acids |
| Omega-3 and Omega-6 pathways |
| Biosynthesis of unsaturated fatty acids |
| Lipid metabolism of various classes |
| Membrane integrity/ stability |
| Glycolysis/ Gluconeogenesis |
| Nicotinate and nicotinamide metabolism |
| Oxidative phosphorylation |
| Purine and pyrimidine metabolism |
| Riboflavin metabolism |
| Taurine and hypotaurine metabolism |
| TCA cycle |
However, the current assessment of radiation biodosimetry is conducted on a generally qualitative level, with few studies reporting a thorough quantitative approach. In order to construct a signature that will have the potential to determine dose or dose range exposures, it is required to include quantitative data, just like with any clinical test. It remains to be determined what concentration of biomarkers is detectable and quantifiable in each biospecimen and generate standards by which decisions will be made on treatment. Additionally, biomarkers of tissue specific injury will be informative to medical personnel not only for immediate treatment decisions, but also for future risk of delayed effects. To date, the only reliable tissue injury specific biomarker that has been identified through metabolomics is citrulline. However, radiation induced pulmonary and cardiovascular disease for example can appear months to many years after the initial radiation exposure and impact survival. Limited research on predictive biomarkers has been conducted, particularly with low doses (Hu et al., 2012, Lee et al., 2012), however metabolomics may be able to provide predictive markers of risk. Furthermore, tissue metabolomics in combination with other –omics analyses will provide a comprehensive systems biology approach that can lead to the design of more effective countermeasures.
Although significant progress has been made in radiation biomarker discovery, the reality remains that during an emergency thousands of samples from potentially radiation exposed individuals and first responders will need to be processed, something that may be achieved through compact deployable instrumentation. Alternatively, samples can be shipped to clinical laboratories around the country that have the capacity to process significant numbers of samples per day. While the second option still remains a possibility, with increased logistical difficulties compared to on site processing such as transportation of samples and dissemination of results, its implementation will require the coordination of multiple stakeholders from the federal and private sectors. Efforts in deployable devices have already identified technologies that can be sized down to a more compact instrumentation, unlike the ones currently utilized in the discovery and quantification phases. In particular, technologies like differential mobility spectrometry- mass spectrometry (DMS-MS) has shown encouraging results in the selectivity, speed, and accurate quantification of previously identified biomarkers (Coy et al., 2010, Coy et al., 2011, Coy et al., 2013, Chen et al., 2016), and will be explored more in detail in the future. Finally, it is encouraging that radiation metabolomics has not only provided answers on the molecular level, but it can be utilized successfully for assessment of radiation exposure, suggestion of appropriate medical triage, and potentially risk assessment.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health (National Institute of Allergy and Infectious Diseases) grant U19 AI067773 (P.I. David J. Brenner, performed as part of Columbia University Center for Medical Countermeasures against Radiation) and grant 1R01AI101798 (P.I. Albert J. Fornace, Jr). Dr. Pannkuk was supported by the training grant in the Tumor Biology Program 5T32CA9686-20. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would also like to thank Bill Morgan for his countless support and encouragement, and most importantly his friendship throughout the years.
Abbreviations
- 1H-NMR or NMR
nuclear magnetic resonance
- ARS
acute radiation syndrome
- BARDA
Biomedical Advanced Research and Development Authority
- BER
base excision repair
- CBC
complete blood count
- CBMN
cytokinesis block micronucleus assay
- ChoE
cholesteryl ester
- DG
diacylglycerol
- DMS
differential mobility spectrometry
- ePC
ether linked phosphatidylcholine
- EPR
electron paramagnetic resonance
- FDA
Food and Drug Administration
- γ-H2AX
gamma-H2AX or phosphorylated histone H2AX
- GC-MS
gas chromatography mass spectrometry
- GI
gastrointestinal
- HDL
high-density lipoprotein
- IND
improvised nuclear device
- LC-MS
liquid chromatography mass spectrometry
- LD50/30
lethal dose 50/30
- LPC or LysoPC
lysophosphatidylcholine
- LPS
lipopolysaccharide
- LysoPE
lysophosphatidylethanolamine
- miRNA
microRNA
- MS/MS
tandem mass spectrometry
- NHPs
non-human primates
- NIAID
National Institute of Allergy and Infectious Diseases
- OSL
optically stimulated luminescence
- PARP1
poly(ADP-ribose) polymerase 1
- PC
phosphatidylcholine
- PCC
premature chromosome condensation
- PE
phosphatidylethanolamine
- PEG-filgrastim
polyethylene glycol-filgrastim
- PI
phosphatidylinositol
- POC
point of care
- PS
phosphatidylserine
- RCI
radiation combined injury
- RDD
radioactive dispersal device
- ROS
reactive oxygen species
- SM
sphingomyelin
- TBI
total body irradiation
- TCA
tricarboxylic acid
- TG
triacylglyceride
- TOFMS
time-of-flight mass spectrometry
- VAMP
Visual Analysis of Metabolomics Package
Footnotes
Disclosure statement: The authors report no conflicts of interest.
References
- Amundson SA, Fornace AJ. Monitoring human radiation exposure by gene expression profiling: possibilities and pitfalls. Health Phys. 2003;85:36–42. doi: 10.1097/00004032-200307000-00009. [DOI] [PubMed] [Google Scholar]
- Brengues M, Paap B, Bittner M, Amundson S, Seligmann B, Korn R, Lenigk R, Zenhausern F. Biodosimetry on small blood volume using gene expression assay. Health Phys. 2010;98:179–185. doi: 10.1097/01.HP.0000346706.44253.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujold K, Hauer-Jensen M, Donini O, Rumage A, Hartman D, Hendrickson HP, Stamatopoulos J, Naraghi H, Pouliot M, Ascah A, Sebastian M, Pugsley MK, Wong K, Authier S. Citrulline as a Biomarker for Gastrointestinal-Acute Radiation Syndrome: Species Differences and Experimental Condition Effects. Radiat Res. 2016;186:71–78. doi: 10.1667/RR14305.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Acute Radiation Syndrome: A Fact Sheet for Clinicians 2015 [Google Scholar]
- Chen C, Brenner DJ, Brown TR. Identification of urinary biomarkers from X-irradiated mice using NMR spectroscopy. Radiat Res. 2011;175:622–630. doi: 10.1667/RR2388.1. [DOI] [PubMed] [Google Scholar]
- Chen C, Gonzalez FJ, Idle JR. LC-MS-based metabolomics in drug metabolism. Drug Metab Rev. 2007;39:581–597. doi: 10.1080/03602530701497804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Coy SL, Pannkuk EL, Laiakis EC, Hall AB, Fornace AJ, Vouros P. Rapid and High-Throughput Detection and Quantitation of Radiation Biomarkers in Human and Nonhuman Primates by Differential Mobility Spectrometry-Mass Spectrometry. J Am Soc Mass Spectrom. 2016;27:1626–1636. doi: 10.1007/s13361-016-1438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coeytaux K, Bey E, Christensen D, Glassman ES, Murdock B, Doucet C. Reported radiation overexposure accidents worldwide, 1980-2013: a systematic review. PLoS One. 2015;10:e0118709. doi: 10.1371/journal.pone.0118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CN, Adams S, Adrianopoli C, Ansari A, Bader JL, Buddemeier B, Caro JJ, Casagrande R, Case C, Caspary K, Chang AS, Chang HF, Chao N, Cliffer KD, Confer D, Deitchman S, Derenzo EG, Dobbs A, Dodgen D, Donnelly EH, Gorman S, Grace MB, Hatchett R, Hick JL, Hrdina C, Jones R, Kane E, Knebel A, Koerner JF, Laffan AM, Larson L, Livinski A, Mackinney J, Maidment BW, Manning R, Marinissen MJ, Martin C, Michael G, Murrain-Hill P, Nemhauser JB, Norwood AE, Nystrom S, Raheem M, Redlener I, Sheehan K, Simon SL, Taylor TP, Toner E, Wallace KS, Wieder J, Weinstock DM, Wiley AL, Yeskey K, Miller CW, Whitcomb RC. Medical planning and response for a nuclear detonation: a practical guide. Biosecur Bioterror. 2012;10:346–371. doi: 10.1089/bsp.2012.1025. [DOI] [PubMed] [Google Scholar]
- Cook JA, Chandramouli GV, Anver MR, Sowers AL, Thetford A, Krausz KW, Gonzalez FJ, Mitchell JB, Patterson AD. Mass Spectrometry-Based Metabolomics Identifies Longitudinal Urinary Metabolite Profiles Predictive of Radiation-Induced Cancer. Cancer Res. 2016;76:1569–1577. doi: 10.1158/0008-5472.CAN-15-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy SL, Cheema AK, Tyburski JB, Laiakis EC, Collins SP, Fornace A. Radiation metabolomics and its potential in biodosimetry. Int J Radiat Biol. 2011;87:802–823. doi: 10.3109/09553002.2011.556177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy SL, Krylov EV, Nazarov EG, Fornace AJ, Kidd RD. Differential mobility spectrometry with nanospray ion source as a compact detector for small organics and inorganics. Int J Ion Mobil Spectrom. 2013;16:217–227. doi: 10.1007/s12127-013-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy SL, Krylov EV, Schneider BB, Covey TR, Brenner DJ, Tyburski JB, Patterson AD, Krausz KW, Fornace AJ, Nazarov EG. Detection of Radiation-Exposure Biomarkers by Differential Mobility Prefiltered Mass Spectrometry (DMS-MS) Int J Mass Spectrom. 2010;291:108–117. doi: 10.1016/j.ijms.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A. 2005;102:13254–13259. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo F, Lante I, Muraca M, Putignani L. The Role of Mass Spectrometry in the “Omics” Era. Curr Org Chem. 2013;17:2891–2905. doi: 10.2174/1385272817888131118162725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, Bader JL, Coleman CN, Weinstock DM. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S32–44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo AL, Ramakrishnan N, Hatchett RJ. Radiation combined injury: overview of NIAID research. Health Phys. 2010;98:863–867. doi: 10.1097/HP.0b013e3181a6ee32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, MacVittie TJ. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today (Barc) 2015;51:537–548. doi: 10.1358/dot.2015.51.9.2386730. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Méndez-García C, Rojo D, Barbas C, Moya A. Antibiotic use and microbiome function. Biochem Pharmacol. 2016 doi: 10.1016/j.bcp.2016.09.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Feurgard C, Bayle D, Guézingar F, Sérougne C, Mazur A, Lutton C, Aigueperse J, Gourmelon P, Mathé D. Effects of ionizing radiation (neutrons/gamma rays) on plasma lipids and lipoproteins in rats. Radiat Res. 1998;150:43–51. [PubMed] [Google Scholar]
- Flood AB, Ali AN, Boyle HK, Du G, Satinsky VA, Swarts SG, Williams BB, Demidenko E, Schreiber W, Swartz HM. Evaluating the Special Needs of The Military for Radiation Biodosimetry for Tactical Warfare Against Deployed Troops: Comparing Military to Civilian Needs for Biodosimetry Methods. Health Phys. 2016a;111:169–182. doi: 10.1097/HP.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood AB, Williams BB, Schreiber W, Du G, Wood VA, Kmiec MM, Petryakov SV, Demidenko E, Swartz HM, EPR CTDPT. Advances in in vivo EPR Tooth BIOdosimetry: Meeting the targets for initial triage following a large-scale radiation event. Radiat Prot Dosimetry. 2016b doi: 10.1093/rpd/ncw165. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty G, Turner HC, Salerno A, Bertucci A, Zhang J, Chen Y, Dutta A, Sharma P, Bian D, Taveras M, Wang H, Bhatla A, Balajee A, Bigelow AW, Repin M, Lyulko OV, Simaan N, Yao YL, Brenner DJ. THE DECADE OF THE RABiT (2005-15) Radiat Prot Dosimetry. 2016 doi: 10.1093/rpd/ncw172. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi M, Mak TD, Chen C, Smilenov LB, Brenner DJ, Fornace AJ. The effect of low dose rate on metabolomic response to radiation in mice. Radiat Environ Biophys. 2014a;53:645–657. doi: 10.1007/s00411-014-0558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi M, Mak TD, Jacobs JP, Moon BH, Strawn SJ, Braun J, Brenner DJ, Fornace AJ, Li HH. An Integrated Multi-Omic Approach to Assess Radiation Injury on the Host-Microbiome Axis. Radiat Res. 2016;186:219–234. doi: 10.1667/RR14306.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi M, Weber W, Mak TD, Chung J, Doyle-Eisele M, Melo D, Brenner DJ, Guilmette RA, Fornace AJ. Development of urinary biomarkers for internal exposure by cesium-137 using a metabolomics approach in mice. Radiat Res. 2014b;181:54–64. doi: 10.1667/RR13479.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi M, Weber WM, Chung J, Doyle-Eisele M, Melo DR, Mak TD, Strawn SJ, Brenner DJ, Guilmette R, Fornace AJ. Serum Dyslipidemia Is Induced by Internal Exposure to Strontium-90 in Mice, Lipidomic Profiling Using a Data-Independent Liquid Chromatography-Mass Spectrometry Approach. J Proteome Res. 2015a;14:4039–4049. doi: 10.1021/acs.jproteome.5b00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi M, Weber WM, Mak TD, Chung J, Doyle-Eisele M, Melo DR, Brenner DJ, Guilmette RA, Fornace AJ. Metabolomic and lipidomic analysis of serum from mice exposed to an internal emitter, cesium-137, using a shotgun LC-MS(E) approach. J Proteome Res. 2015b;14:374–384. doi: 10.1021/pr500913n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi M, Weber WM, Mak TD, Chung J, Doyle-Eisele M, Melo DR, Strawn SJ, Brenner DJ, Guilmette RA, Fornace AJ. A Comprehensive Metabolomic Investigation in Urine of Mice Exposed to Strontium-90. Radiat Res. 2015c;183:665–674. doi: 10.1667/RR14011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grison S, Martin JC, Grandcolas L, Banzet N, Blanchardon E, Tourlonias E, Defoort C, Favé G, Bott R, Dublineau I, Gourmelon P, Souidi M. The metabolomic approach identifies a biological signature of low-dose chronic exposure to cesium 137. J Radiat Res. 2012;53:33–43. doi: 10.1269/jrr.11071. [DOI] [PubMed] [Google Scholar]
- Gruel G, Grégoire E, Lecas S, Martin C, Roch-Lefevre S, Vaurijoux A, Voisin P, Voisin P, Barquinero JF. Biological dosimetry by automated dicentric scoring in a simulated emergency. Radiat Res. 2013;179:557–569. doi: 10.1667/RR3196.1. [DOI] [PubMed] [Google Scholar]
- Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: from animal models to therapies. J Dent Res. 2009;88:894–903. doi: 10.1177/0022034509343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer MJ, Raulli R, DiCarlo-Cohen AL, Esker J, Hrdina C, Maidment BW, Moyer B, Rios C, Macchiarini F, Prasanna PG, Wathen L. UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES BIODOSIMETRY AND RADIOLOGICAL/NUCLEAR MEDICAL COUNTERMEASURE PROGRAMS. Radiat Prot Dosimetry. 2016;171:85–98. doi: 10.1093/rpd/ncw226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Blakely WF, Cucinotta FA. HEMODOSE: A Biodosimetry Tool Based on Multi-type Blood Cell Counts. Health Phys. 2015;109:54–68. doi: 10.1097/HP.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZP, Kim YM, Sowa MB, Robinson RJ, Gao X, Metz TO, Morgan WF, Zhang Q. Metabolomic response of human skin tissue to low dose ionizing radiation. Mol Biosyst. 2012;8:1979–1986. doi: 10.1039/c2mb25061f. [DOI] [PubMed] [Google Scholar]
- Islam A, Ghimbovschi S, Zhai M, Swift JM. An Exploration of Molecular Correlates Relevant to Radiation Combined Skin-Burn Trauma. PLoS One. 2015;10:e0134827. doi: 10.1371/journal.pone.0134827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Gonzalez FJ. Challenges and opportunities of metabolomics. J Cell Physiol. 2012;227:2975–2981. doi: 10.1002/jcp.24002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Patterson AD, Krausz KW, Kalinich JF, Tyburski JB, Kang DW, Luecke H, Gonzalez FJ, Blakely WF, Idle JR. Radiation metabolomics. 5. Identification of urinary biomarkers of ionizing radiation exposure in nonhuman primates by mass spectrometry-based metabolomics. Radiat Res. 2012;178:328–340. doi: 10.1667/rr2950.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Patterson AD, Krausz KW, Lanz C, Kang DW, Luecke H, Gonzalez FJ, Idle JR. Radiation metabolomics. 4. UPLC-ESI-QTOFMS-Based metabolomics for urinary biomarker discovery in gamma-irradiated rats. Radiat Res. 2011;175:473–484. doi: 10.1667/RR2437.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Bennett A, Carter CL, Tudor G, Hankey KG, Farese AM, Booth C, MacVittie TJ, Kane MA. Citrulline as a Biomarker in the Non-human Primate Total- and Partial-body Irradiation Models: Correlation of Circulating Citrulline to Acute and Prolonged Gastrointestinal Injury. Health Phys. 2015;109:440–451. doi: 10.1097/HP.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Scott AJ, Tudor G, Xu PT, Jackson IL, Vujaskovic Z, Booth C, MacVittie TJ, Ernst RK, Kane MA. Identification and quantitation of biomarkers for radiation-induced injury via mass spectrometry. Health Phys. 2014a;106:106–119. doi: 10.1097/HP.0b013e3182a4ed3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Tudor G, Bennett A, Farese AM, Moroni M, Booth C, MacVittie TJ, Kane MA. Development and validation of a LC-MS/MS assay for quantitation of plasma citrulline for application to animal models of the acute radiation syndrome across multiple species. Anal Bioanal Chem. 2014b;406:4663–4675. doi: 10.1007/s00216-014-7870-0. [DOI] [PubMed] [Google Scholar]
- Jones JW, Tudor G, Li F, Tong Y, Katz B, Farese AM, MacVittie TJ, Booth C, Kane MA. Citrulline as a Biomarker in the Murine Total-Body Irradiation Model: Correlation of Circulating and Tissue Citrulline to Small Intestine Epithelial Histopathology. Health Phys. 2015;109:452–465. doi: 10.1097/HP.0000000000000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam WW, Banati RB. Effects of ionizing radiation on mitochondria. Free Radic Biol Med. 2013;65:607–619. doi: 10.1016/j.freeradbiomed.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Khan AR, Rana P, Devi MM, Chaturvedi S, Javed S, Tripathi RP, Khushu S. Nuclear magnetic resonance spectroscopy-based metabonomic investigation of biochemical effects in serum of γ-irradiated mice. Int J Radiat Biol. 2011;87:91–97. doi: 10.3109/09553002.2010.518211. [DOI] [PubMed] [Google Scholar]
- Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- Kurland IJ, Broin PÓ, Golden A, Su G, Meng F, Liu L, Mohney R, Kulkarni S, Guha C. Integrative Metabolic Signatures for Hepatic Radiation Injury. PLoS One. 2015;10:e0124795. doi: 10.1371/journal.pone.0124795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Hyduke DR, Fornace AJ. Comparison of mouse urinary metabolic profiles after exposure to the inflammatory stressors γ radiation and lipopolysaccharide. Radiat Res. 2012;177:187–199. doi: 10.1667/rr2771.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Mak TD, Anizan S, Amundson SA, Barker CA, Wolden SL, Brenner DJ, Fornace AJ. Development of a metabolomic radiation signature in urine from patients undergoing total body irradiation. Radiat Res. 2014a;181:350–361. doi: 10.1667/RR13567.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Pannkuk EL, Diaz-Rubio ME, Wang YW, Mak TD, Simbulan-Rosenthal CM, Brenner DJ, Fornace AJ. Implications of genotypic differences in the generation of a urinary metabolomics radiation signature. Mutat Res. 2016;788:41–49. doi: 10.1016/j.mrfmmm.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Strassburg K, Bogumil R, Lai S, Vreeken RJ, Hankemeier T, Langridge J, Plumb RS, Fornace AJ, Astarita G. Metabolic phenotyping reveals a lipid mediator response to ionizing radiation. J Proteome Res. 2014b;13:4143–4154. doi: 10.1021/pr5005295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Strawn SJ, Brenner DJ, Fornace AJ. Assessment of Saliva as a Potential Biofluid for Biodosimetry: A Pilot Metabolomics Study in Mice. Radiat Res. 2016;186:92–97. doi: 10.1667/RR14433.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamadrid Boada AI, Romero Aguilera I, Terzoudi GI, González Mesa JE, Pantelias G, García O. Rapid assessment of high-dose radiation exposures through scoring of cell-fusion-induced premature chromosome condensation and ring chromosomes. Mutat Res. 2013;757:45–51. doi: 10.1016/j.mrgentox.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Lanz C, Ledermann M, Slavík J, Idle JR. The production and composition of rat sebum is unaffected by 3 Gy gamma radiation. Int J Radiat Biol. 2011;87:360–371. doi: 10.3109/09553002.2010.537432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz C, Patterson AD, Slavík J, Krausz KW, Ledermann M, Gonzalez FJ, Idle JR. Radiation metabolomics. 3. Biomarker discovery in the urine of gamma-irradiated rats using a simplified metabolomics protocol of gas chromatography-mass spectrometry combined with random forests machine learning algorithm. Radiat Res. 2009;172:198–212. doi: 10.1667/RR1796.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledney GD, Elliott TB. Combined injury: factors with potential to impact radiation dose assessments. Health Phys. 2010;98:145–152. doi: 10.1097/01.HP.0000348466.09978.77. [DOI] [PubMed] [Google Scholar]
- Lee DY, Bowen BP, Nguyen DH, Parsa S, Huang Y, Mao JH, Northen TR. Low-dose ionizing radiation-induced blood plasma metabolic response in a diverse genetic mouse population. Radiat Res. 2012;178:551–555. doi: 10.1667/RR2990.1. [DOI] [PubMed] [Google Scholar]
- Liang Q, Xu W, Hong Q, Xiao C, Yang L, Ma Z, Wang Y, Tan H, Tang X, Gao Y. Rapid comparison of metabolites in humans and rats of different sexes using untargeted UPLC-TOFMS and an in-house software platform. Eur J Mass Spectrom (Chichester) 2015;21:801–821. doi: 10.1255/ejms.1395. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang Z, Zhang X, Qiao Y, Wu S, Dong F, Chen Y. Selection of candidate radiation biomarkers in the serum of rats exposed to gamma-rays by GC/TOFMS-based metabolomics. Radiat Prot Dosimetry. 2013;154:9–17. doi: 10.1093/rpd/ncs138. [DOI] [PubMed] [Google Scholar]
- Lutgens LC, Deutz N, Granzier-Peeters M, Beets-Tan R, De Ruysscher D, Gueulette J, Cleutjens J, Berger M, Wouters B, von Meyenfeldt M, Lambin P. Plasma citrulline concentration: a surrogate end point for radiation-induced mucosal atrophy of the small bowel. A feasibility study in 23 patients. Int J Radiat Oncol Biol Phys. 2004;60:275–285. doi: 10.1016/j.ijrobp.2004.02.052. [DOI] [PubMed] [Google Scholar]
- Lutgens LC, Deutz NE, Gueulette J, Cleutjens JP, Berger MP, Wouters BG, von Meyenfeldt MF, Lambin P. Citrulline: a physiologic marker enabling quantitation and monitoring of epithelial radiation-induced small bowel damage. Int J Radiat Oncol Biol Phys. 2003;57:1067–1074. doi: 10.1016/s0360-3016(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Majuelos-Melguizo J, Rodríguez MI, López-Jiménez L, Rodríguez-Vargas JM, Martí Martín-Consuegra JM, Serrano-Sáenz S, Gavard J, de Almodóvar JM, Oliver FJ. PARP targeting counteracts gliomagenesis through induction of mitotic catastrophe and aggravation of deficiency in homologous recombination in PTEN-mutant glioma. Oncotarget. 2015;6:4790–4803. doi: 10.18632/oncotarget.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak TD, Laiakis EC, Goudarzi M, Fornace AJ. Selective paired ion contrast analysis: a novel algorithm for analyzing postprocessed LC-MS metabolomics data possessing high experimental noise. Anal Chem. 2015a;87:3177–3186. doi: 10.1021/ac504012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak TD, Tyburski JB, Krausz KW, Kalinich JF, Gonzalez FJ, Fornace AJ. Exposure to ionizing radiation reveals global dose- and time-dependent changes in the urinary metabolome of rat. Metabolomics. 2015b;11:1082–1094. doi: 10.1007/s11306-014-0765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manens L, Grison S, Bertho JM, Lestaevel P, Guéguen Y, Benderitter M, Aigueperse J, Souidi M. Chronic exposure of adult, postnatal and in utero rat models to low-dose 137Cesium: impact on circulating biomarkers. J Radiat Res. 2016:1–13. doi: 10.1093/jrr/rrw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo DR, Lipsztein JL, de Oliveira CA, Bertelli L. 137Cs internal contamination involving a Brazilian accident, and the efficacy of Prussian Blue treatment. Health Phys. 1994;66:245–252. doi: 10.1097/00004032-199403000-00002. [DOI] [PubMed] [Google Scholar]
- Menon SS, Uppal M, Randhawa S, Cheema MS, Aghdam N, Usala RL, Ghosh SP, Cheema AK, Dritschilo A. Radiation Metabolomics: Current Status and Future Directions. Front Oncol. 2016;6:20. doi: 10.3389/fonc.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkonen JJW, Herrala M, Soininen P, Lappalainen R, Tjäderhane L, Seitsalo H, Niemelä R, Salo T, Kullaa AM, Myllymaa S. Metabolic Profiling of Saliva in Patients with Primary Sj gren s syndrome. Metabolomics: Open Access. 2014;182:149–153. [Google Scholar]
- Moore HD, Ivey RG, Voytovich UJ, Lin C, Stirewalt DL, Pogosova-Agadjanyan EL, Paulovich AG. The human salivary proteome is radiation responsive. Radiat Res. 2014;181:521–530. doi: 10.1667/RR13586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE. 2013 Dade W. Moeller lecture: medical countermeasures against radiological terrorism. Health Phys. 2014;107:164–171. doi: 10.1097/HP.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ó Broin P, Vaitheesvaran B, Saha S, Hartil K, Chen EI, Goldman D, Fleming WH, Kurland IJ, Guha C, Golden A. Intestinal microbiota-derived metabolomic blood plasma markers for prior radiation injury. Int J Radiat Oncol Biol Phys. 2015;91:360–367. doi: 10.1016/j.ijrobp.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onal C, Kotek A, Unal B, Arslan G, Yavuz A, Topkan E, Yavuz M. Plasma citrulline levels predict intestinal toxicity in patients treated with pelvic radiotherapy. Acta Oncol. 2011;50:1167–1174. doi: 10.3109/0284186X.2011.584557. [DOI] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Authier S, Wong K, Fornace AJ. Global Metabolomic Identification of Long-Term Dose-Dependent Urinary Biomarkers in Nonhuman Primates Exposed to Ionizing Radiation. Radiat Res. 2015;184:121–133. doi: 10.1667/rr14091.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Mak TD, Astarita G, Authier S, Wong K, Fornace AJJ. A lipidomic and metabolomic serum signature from nonhuman primates exposed to ionizing radiation. Metabolomics. 2016a;12:80. doi: 10.1007/s11306-016-1010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Authier S, Wong K, Fornace AJ. Targeted metabolomics of nonhuman primate serum after exposure to ionizing radiation: potential tools for high-throughput biodosimetry. RSC Adv. 2016b;6:51192–51202. doi: 10.1039/C6RA07757A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RM, Roback JD, Uppal K, Yu T, Jones DP, Josephson CD. Metabolomics profile comparisons of irradiated and nonirradiated stored donor red blood cells. Transfusion. 2015;55:544–552. doi: 10.1111/trf.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrono C, Sterpone S, Testa A, Cozzi R. Polymorphisms in base excision repair genes: Breast cancer risk and individual radiosensitivity. World J Clin Oncol. 2014;5:874–882. doi: 10.5306/wjco.v5.i5.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Lanz C, Gonzalez FJ, Idle JR. The role of mass spectrometry-based metabolomics in medical countermeasures against radiation. Mass Spectrom Rev. 2010;29:503–521. doi: 10.1002/mas.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti GJ, Yanes O, Siuzdak G. Innovation: Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012;13:263–269. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernot E, Cardis E, Badie C. Usefulness of saliva samples for biomarker studies in radiation research. Cancer Epidemiol Biomarkers Prev. 2014;23:2673–2680. doi: 10.1158/1055-9965.EPI-14-0588. [DOI] [PubMed] [Google Scholar]
- Sharma M, Moulder JE. The urine proteome as a radiation biodosimeter. Adv Exp Med Biol. 2013;990:87–100. doi: 10.1007/978-94-007-5896-4_5. [DOI] [PubMed] [Google Scholar]
- Shim S, Jang WS, Lee SJ, Jin S, Kim J, Lee SS, Bang HY, Jeon BS, Park S. Development of a new minipig model to study radiation-induced gastrointestinal syndrome and its application in clinical research. Radiat Res. 2014;181:387–395. doi: 10.1667/RR13207.1. [DOI] [PubMed] [Google Scholar]
- Singh VK, Newman VL, Romaine PL, Hauer-Jensen M, Pollard HB. Use of biomarkers for assessing radiation injury and efficacy of countermeasures. Expert Rev Mol Diagn. 2016;16:65–81. doi: 10.1586/14737159.2016.1121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni S, Agrawal P, Kumar N, Mittal G, Nishad DK, Chaudhury NK, Bhatnagar A, Basu M, Chhillar N. Salivary biochemical markers as potential acute toxicity parameters for acute radiation injury: A study on small experimental animals. Hum Exp Toxicol. 2016;35:221–228. doi: 10.1177/0960327115579433. [DOI] [PubMed] [Google Scholar]
- Sproull M, Camphausen K. State-of-the-Art Advances in Radiation Biodosimetry for Mass Casualty Events Involving Radiation Exposure. Radiat Res. 2016 doi: 10.1667/RR14452.1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Prasanna PG, Grace MB, Wathen LK, Wallace RL, Koerner JF, Coleman CN. Assessment of biodosimetry methods for a mass-casualty radiological incident: medical response and management considerations. Health Phys. 2013;105:540–554. doi: 10.1097/HP.0b013e31829cf221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandle AT, Shankavaram U, Brown MV, Ho J, Graves C, Lita E, Pfohl J, Mohney R, Tofilon P, Camphausen K. Urinary metabolomic profiling of patients with glioblastoma multiforme. J Proteomics Bioinform. 2013;S6:003. doi: 10.4172/jpb.S6-003. [DOI] [Google Scholar]
- Tang X, Zheng M, Zhang Y, Fan S, Wang C. Estimation value of plasma amino acid target analysis to the acute radiation injury early triage in the rat model. Metabolomics. 2013;9:853–863. [Google Scholar]
- Tsuruoka M, Hara J, Hirayama A, Sugimoto M, Soga T, Shankle WR, Tomita M. Capillary electrophoresis mass spectrometry based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis. 2013;34:2865–2872. doi: 10.1002/elps.201300019. [DOI] [PubMed] [Google Scholar]
- Tyburski JB, Patterson AD, Krausz KW, Slavík J, Fornace AJ, Gonzalez FJ, Idle JR. Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiat Res. 2008;170:1–14. doi: 10.1667/RR1265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyburski JB, Patterson AD, Krausz KW, Slavík J, Fornace AJ, Gonzalez FJ, Idle JR. Radiation metabolomics. 2. Dose- and time-dependent urinary excretion of deaminated purines and pyrimidines after sublethal gamma-radiation exposure in mice. Radiat Res. 2009;172:42–57. doi: 10.1667/RR1703.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yang J, Nie J. Plasma phospholipid metabolic profiling and biomarkers of rats following radiation exposure based on liquid chromatography-mass spectrometry technique. Biomed Chromatogr. 2009;23:1079–1085. doi: 10.1002/bmc.1226. [DOI] [PubMed] [Google Scholar]
- Wang J, Shao L, Hendrickson HP, Liu L, Chang J, Luo Y, Seng J, Pouliot M, Authier S, Zhou D, Allaben W, Hauer-Jensen M. Total Body Irradiation in the “Hematopoietic” Dose Range Induces Substantial Intestinal Injury in Non-Human Primates. Radiat Res. 2015;184:545–553. doi: 10.1667/RR14191.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Turner HC, Garty G, Brenner D. A Rapid, Quantitative Method to Characterize The Human Lymphocyte Concentration for Automated High-Throughput Radiation Biodosimetry. Biomed Eng Res. 2013;2:16–19. doi: 10.5963/ber0201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou X, Li C, Wu J, Kuo JE, Wang C. Assessment of early triage for acute radiation injury in rat model based on urinary amino acid target analysis. Molecular BioSystems. 2014a;10:1441–1449. doi: 10.1039/c3mb70526a. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sun J, Lin CC, Abemayor E, Wang MB, Wong DTW. The emerging landscape of salivary diagnostics. Oral Health Dent Manag. 2014b;13:200–210. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.