Abstract
In diabetes, glucocorticoid secretion increases secondary to hyperglycemia and is associated with an extensive list of disease complications. Levels of cortisol in humans, or corticosterone in rodents, are usually measured as transitory biomarkers of stress in blood or saliva. Glucocorticoid concentrations accumulate in human or animal hair over weeks and could more accurately measure the cumulative stress burden of diseases like chronic diabetes. In this study, corticosterone levels were measured in hair in verified rodent models of diabetes mellitus. To induce type 1 diabetes, C57BL/6J mice were injected with streptozotocin and blood and hair samples were collected 28 days following induction. Leptin receptor deficient (db/db) mice were used as a spontaneous model of type 2 diabetes and blood and hair samples were collected at 8 weeks of age, after the development of hyperglycemia and obesity. Corticosterone levels from serum, new growth hair and total growth hair were analyzed using an enzyme immunoassay. Corticosterone levels in new growth hair and serum were significantly elevated in both models of diabetes compared to controls. In contrast, corticosterone levels in old hair growth did not differ significantly between diabetic and non-diabetic animals. Thus, hair removal and sampling of new hair growth was a more sensitive procedure for detecting changes in hair corticosterone levels induced by periods of hyperglycemia lasting for 4 weeks in mice. These results validate the use of hair to measure long-term changes in corticosterone induced by diabetes in rodent models. Further studies are now needed to validate the utility of hair cortisol as a tool for measuring the stress burden of individuals with diabetes and for following the effects of long-term medical treatments.
Keywords: diabetes, corticosterone, hair, streptozotocin, mouse
INTRODUCTION
The study of environmental, hormonal and nutritional influences to the development of metabolic disease was one of the greatest contributions of Dr. Randall Sakai [1–3], to whom this volume is dedicated. Diabetes mellitus is a chronic metabolic disorder characterized by relative or absolute lack of insulin resulting in prolonged hyperglycemia. Type 1 diabetes, previously known as juvenile diabetes, results from insufficient insulin production due to autoimmune destruction of insulin-producing beta cells in the pancreas; the primary cause is unknown [4]. Type 2 diabetes, previously known as adult-onset diabetes, accounts for the majority of diabetics and is characterized by insulin resistance, or ineffective use of insulin by the body. Diabetes affects more than 400 million people globally and significantly increases the risk for long-term health complications such as cardiovascular disease, kidney failure, blindness, and premature death [4]. Stress, in the form of hypothalamic-pituitary-adrenal (HPA) activity and glucocorticoid secretion, is increased in individuals with diabetes. Hypercortisolism is associated with poor metabolic control and subsequent development of diabetes [5]. In diabetic patients, degree of cortisol secretion has been correlated with the presence and number of chronic diabetic complications, including nephropathy, macroangiopathy, retinopathy and depression [6–9].
The primary glucocorticoid in humans and most mammals is cortisol, while the primary glucocorticoid in rodents is corticosterone. Although evaluation of HPA activity has relied on measurements of glucocorticoids in blood serum and saliva, this information is limited to the minutes to hours preceding collection [10–13]. Recently, measurement of cortisol in hair has been developed as a retrospective biomarker for long-term HPA activity due to environmental or physiologic stressors [14–16]. Compared to other biologic materials, hair can be used to evaluate chronic exposure to drugs, toxins, or hormones over much longer periods of weeks to years. Hair can also be collected non-invasively and remains stable for long periods of time – hundreds of years or more – prior to analysis [16–18]. Although the precise mechanism is not yet fully understood, compounds are thought to be incorporated during hair shaft formation by diffusion from blood into the growing hair follicle or after hair shaft formation by diffusion from sweat, sebum, and surrounding tissues [19, 20]. In humans, population surveys have associated scalp hair cortisol levels with environmental stressors, mental health disorders, and somatic health conditions [21–23]. Specifically, population studies reported that hair cortisol concentrations tended to be higher in a proportion of individuals that self-identified with diabetes mellitus [22, 24]. However, clinical variability and the involvement of factors other than hyperglycemia complicate studies of diabetic humans without a confirmed medical diagnosis. Nevertheless, if this were true, hair cortisol measurement could be a valuable new tool for screening and managing patients with diabetes.
In order to clarify the relationship between hyperglycemia and hair glucocorticoid levels in diabetes, we compared hair corticosterone levels in two different mouse models of diabetes mellitus. Type 1 diabetes is commonly induced in rodents by injection of streptozotocin (STZ), which results in chemical ablation of pancreatic beta cells [25]. Mice given a single high dose of STZ (100–200 mg/kg) develop hyperglycemia within 1 week of injection followed by clinical signs of diabetes (e.g., weight loss, polydypsia, polyuria) [26, 27]. Models of type 2 diabetes typically involve either genetic or diet-induced obesity. Leprdb/db mice have an autosomal recessive mutation in the leptin receptor; these animals are obese, hyperphagic, and begin developing hyperglycemia at approximately 4 weeks of age [25, 28]. We hypothesized that hair corticosterone would be elevated in diabetic mice compared to control mice 4 weeks following either diabetes induction or the onset of hyperglycemia. Furthermore, we compared corticosterone concentration between hair samples from previously shaved and unshaved areas of skin to determine whether previously unshaved hair would be useful in evaluating HPA activity in mice during this 4-week timeframe. Previous studies of hair corticosterone in rodents evaluated only new hair growth or old hair growth following the onset of experimentally altered HPA activity (e.g., chronic stress, parasitic infection) [29–33]. Given his career interest in rodent models of chronic social stress and metabolism [2], Dr. Sakai would likely have been interested in the development of measuring long-term corticosterone exposure in hair for his own rodent studies.
MATERIALS & METHODS
Animals
Mice were housed in an AAALAC-accredited facility on a 12:12-h light-dark cycle with four animals per cage in static polycarbonate microisolation cages (Max 75, Alternative Design, Siloam Springs, AR) on disposable bedding (0.12-in. Bed-O-Cobs, The Andersons, Maumee, OH). Mice were allowed 3 days to acclimate following arrival to the vivarium prior to experimental manipulation. Mice were fed standard pelleted laboratory rodent chow (5001, LabDiet, St. Louis, MO) and provided municipal water supplied by bottle. Sentinel mice were routinely tested for infectious diseases as part of the institutional health surveillance program. All procedures were approved by the University of Pennsylvania’s Institutional Animal Care and Use Committee.
Type 1 diabetes
To induce type 1 diabetes, 24 eight-week-old, male, C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were injected with STZ or vehicle (Veh). On day 0, mice were anesthetized for hair collection with isoflurane (Isothesia, Henry Schein Animal Health, Dublin, OH), fasted for 4 hours, and received a single intraperitoneal injection of Veh (n=12) or 150 mg/kg STZ (n=12) (Sigma-Aldrich, St. Louis, MO) prepared in 5M sodium citrate, pH 4.5. Mice were supplied with 10% sucrose water overnight following injection to avoid sudden hypoglycemia. Mice were weighed twice weekly on days 0, 2, 3, 7, 10, 14, 17, 21, 24, and 28. Blood glucose (BG) was measured on days 2, 4, 7, 14, 21, and 28; BG was measured more frequently during the first week following injection to monitor for onset of hyper- or hypoglycemia. On day 28 after induction of diabetes and hair collection, mice were euthanized by CO2 asphyxiation and hair and serum samples were collected for analysis.
Type 2 diabetes
As a model of type 2 diabetes, 16 five-week-old, male, BKS.Cg-Dock7m +/+ Leprdb/J mice were purchased from Jackson Laboratories (Bar Harbor, ME), including homozygous diabetic mice (db/db, n=8) and heterozygous non-diabetic controls (db/+, n=8). On day 0, mice were anesthetized for hair collection. Mice were weighed twice weekly on days 0, 2, 3, 7, 10, 14, 17, 21, 24, and 28. BG was measured on days 0, 7, 14, 21, and 28. On day 28, mice were euthanized by CO2 asphyxiation, and hair and serum samples were collected for analysis.
Monitoring of body weight and blood glucose
Animals were observed daily while on study to monitor for adverse effects of diabetes. As diabetic mice typically become polyuric, polydipsic, and/or polyphagic, cages were changed and additional food and water provided more frequently as needed. Additional food pellets were placed on the cage floor to ensure access for obese diabetic mice. During the 4 weeks on study, mice were individually weighed twice weekly. Non-fasted BG measurements were performed during the first 3 h of the light cycle to minimize effects of diurnal variation. Using a restraint device designed for tail access (Tailveiner Restrainer, Brainstree Scientific, Inc, Braintree, MA), blood was collected from the lateral tail vein. BG was measured using a handheld glucometer (AlphaTRAK 2 Blood Glucose Monitoring System, Abbott Animal Health, Zoetis, Florham Park, NJ) with a testing range of 20–750 mg/dL; several BG measurements for diabetic mice were above the testing range and were recorded as 750 mg/dL. Mice with blood glucose consistently >250 mg/dL were considered diabetic.
Collection of hair and serum samples
Hair samples were collected on days 0 and 28. After mice were anesthetized on day 0, hair was shaved from one side of each mouse using an electric razor. The shaved area extended from dorsal to ventral midline and from neck to tail base. On day 28, immediately following euthanasia by CO2 asphyxiation, blood was collected by cardiocentesis. Blood was centrifuged at 14,000 × g for 15 min at 4°C, and serum was separated and stored at −20°C until analysis. To assess differences in corticosterone levels between new and old hair growth, hair was shaved and collected separately from both previously shaved and unshaved sides of each mouse at the end of the experiment. Hair was stored in 10 mL polypropylene tubes at 4°C until steroid extraction and analysis.
Hair sample preparation
The method for hair corticosterone extraction and analysis was modified from protocols described in previous studies [30, 33–35]. Hair samples were washed with methanol to remove sebum, saliva, urine or external contaminants that might contain steroids. Hair was washed twice by adding 5 mL of high performance liquid chromatography (HPLC)-grade methanol (Fisher Scientific, Waltham, MA) to each sample, rotating for 3 min, followed by decanting excess methanol. After washing, hair samples were placed on aluminum foil and dried in a protected hood for 3 days. Dried hair samples were weighed and transferred to 2 mL polypropylene tubes containing stainless steel grinding beads (2.8 mm Stainless Steel Grinding Balls Pre-Filled Tubes, OPS Diagnostics, Lebanon, NJ). Tubes containing hair and grinding beads were placed in a bead beater (Mini-Beadbeater, BioSpec Products, Bartlesville, OK), and each sample was ground for 2 min to produce a powder. After grinding, 1.5 mL methanol was added to tubes containing powdered hair, and samples were incubated for 24 h on slow rotation to extract steroid. The tubes were centrifuged at 10,000 g for 4 min, and 0.6 mL of the steroid-containing methanol supernatant was transferred to a new 1.5 mL microcentrifuge tube. Samples were dried in a protected hood for 2–3 days to evaporate methanol. Dry extracts were reconstituted with 0.4 mL assay diluent provided in the corticosterone enzyme immunoassay kit.
Corticosterone assays
Corticosterone levels in hair and serum samples were quantified using a commercially available enzyme immunoassay kit (Corticosterone EIA, Immunodiagnostic Systems Inc., Gaithersburg, MD). As per the quality controls conducted by the manufacturer, the sensitivity of the corticosterone assay was 0.55 ng/mL, the intraassay CV for the low standard (4.6 ng/ml) was 4.9% and that for the high standard (45.7 ng/ml) was 7.7%. Cross-reactivity was as follows: 6.60% 11-dehydrocorticosterone; 5.93% 11-deoxycorticosterone; 1.39% progesterone; 0.85% cortisol; 0.60% prednisolone; 0.34% 21-deoxycortisol; 0.21% 5α-pregnan-3,20-dione; and <0.1% for all other analytes tested. Each sample was analyzed in duplicate according to the manufacturer’s instructions. Because the assay kit was designed to measure corticosterone in liquid samples, assay output values for hair samples were converted from ng/mL to pg/mg and adjusted according to the mass of the hair sample subjected to steroid extraction. All samples that were statistically compared were run in the same assay to avoid interassay variability.
Data analysis
Data were analyzed using Prism GraphPad (version 6.0h, La Jolla, CA). Serum corticosterone was analyzed using unpaired t-tests. Alterations in BG and body weight were analyzed using two-way repeated measures ANOVA with Sidak’s multiple comparisons. Two-way ANOVA was used to determine hair growth*treatment and hair growth*genotype interactions on hair corticosterone, Tukey multiple comparisons tests were used for post hoc analysis. For all tests, p<0.05 was considered statistically significant. Data are expressed as mean ± SEM.
RESULTS
Blood glucose and body weight
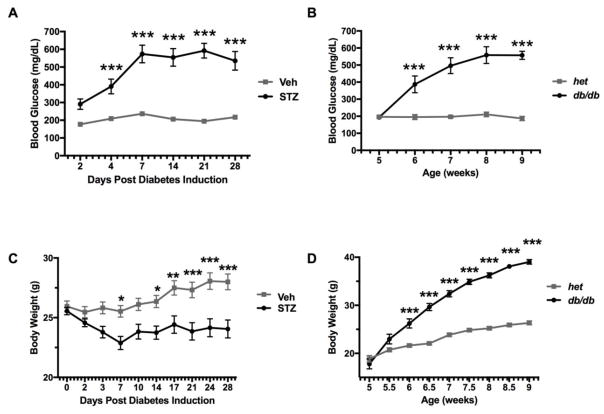
Figure 1 represents average BG and body weight over 28 days for all groups. In the type 1 diabetes model (Panels A and C), 11 of 12 mice treated with STZ developed diabetes, as defined by BG > 250 mg/dL. One mouse treated with STZ did not develop hyperglycemia during the 28 day study period and was excluded from further analysis. A significant time*treatment interaction was observed on BG levels (F5,105 = 19.87, p<0.001). Sidak’s multiple comparison tests revealed significantly higher levels of glucose in STZ-treated mice compared to controls on day 4 through day 28 (p<0.001). In addition, a significant time*treatment interaction on bodyweight was observed (F9,189 = 11.8, p<0.001). Over the 28 day study period, STZ mice showed a significant decrease in body weight compared to controls (day 7 and day 14 p<0.05, day 17 p<0.01, and day 21–28 p<0.001).
Figure 1.
Blood glucose (mg/dl) and body weight over time for diabetic and non-diabetic control mice. Panel A shows glucose levels over days following injection of A) Veh (n = 12) or STZ (n = 11). Panel B shows glucose levels in db/+ non-diabetic (n = 8) and db/db diabetic (n = 8) mice as a function of age. Body weights (mean ± SEM) are shown for the same mice injected with C) Veh (n = 12) or STZ (n = 11), and D) db/+ non-diabetic (n = 8) and db/db diabetic (n = 8) mice. Asterisks indicate significant difference between groups at each timepoint (***p < 0.001, ****p < 0.0001).
In the type 2 diabetes model (Panels B and D), a significant time*genotype interaction was observed on BG levels (F4,56 = 19.51, p<0.001). All 8 homozygous db/db mice developed diabetes (BG > 250 mg/dL) with posthoc tests identifying highly significant increases in BG from 6 weeks of age onwards compared to db/+ mice (p<0.001). Similarly, a significant time*genotype interaction was observed on bodyweight (F8,112 = 100.3, p<0.001). db/db mice were significantly heavier compared to their db/+ controls. This increase in bodyweight emerged at 6 weeks of age and remained statistically significant throughout the duration of study (p<0.001).
Serum corticosterone
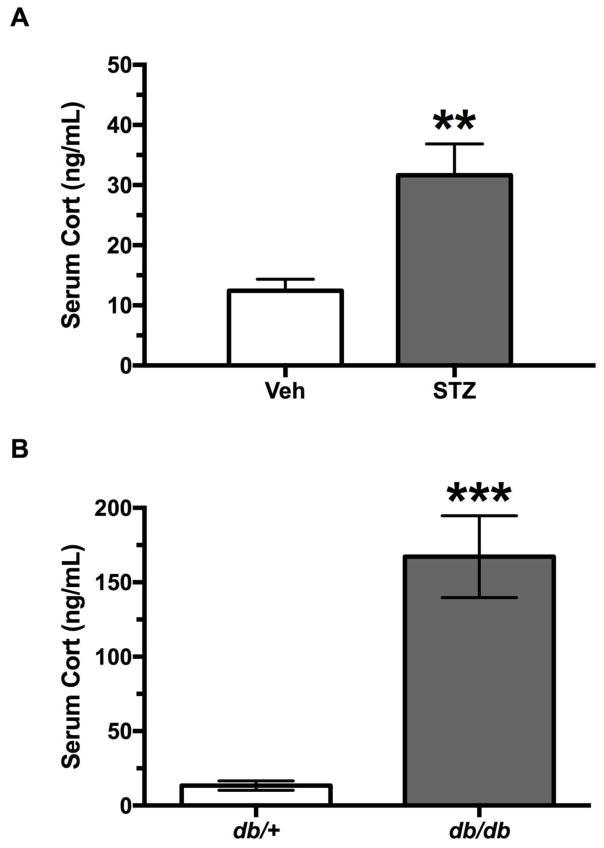
Figure 2 presents serum corticosterone levels on day 28 for all groups. STZ treated mice had significantly higher concentrations of serum corticosterone that was over twice as high as Veh-treated mice (t17 =3.62, p<0.003).
Figure 2.
Serum corticosterone levels (mean ± SEM) for diabetic and non-diabetic control mice. A) Mice injected with Veh (n = 10) or STZ (n = 9) at 28 days post-injection, and B) db/+ non-diabetic (n = 8) and db/db diabetic (n = 8) mice at 9 weeks of age. Asterisks indicate significant difference between groups (**p < 0.01, ***p < 0.001).
As there was a significant difference in the variances of serum corticosterone between groups in the type 2 diabetes model, an unpaired t-test with Welch’s correction was used to analyze the data. A statistically significant effect of genotype was observed on serum corticosterone concentrations (t14 =5.558, p<0.001), which were significantly elevated in db/db mice compared to their db/+ controls.
Hair samples
Mean hair sample mass subjected to steroid extraction was 22.2 ± 0.9 mg (range 1.2 – 61.2 mg). From the 118 total hair samples collected, 6 specimens weighing < 6 mg were subjected to steroid extraction and ELISA but did not show detectable levels of corticosterone. These hair samples were excluded from subsequent data analysis.
Hair corticosterone
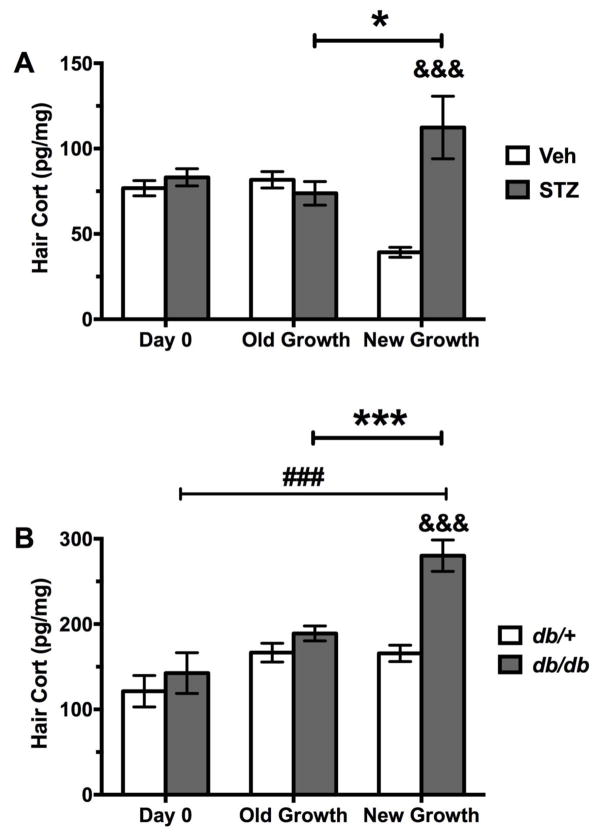
Figure 3 represents hair corticosterone concentrations (HCC) on days 0 and 28 for all groups, including new and old hair growth samples on day 28. As shown in Figure 3A, there was a significant hair growth*treatment interaction on HCC in the type 1 diabetes model (F2,54 =14.78, p<0.001). Posthoc tests revealed elevated HCC new growth from STZ-treated mice compared to new growth from vehicle treated mice on day 28 (p<0.001) and also when compared to old growth collected on day 28 from STZ-treated mice (p<0.05). In addition, there were significant differences between the vehicle groups, where HCC was lower in new growth obtained from vehicle treated mice on day 28 compared to the vehicle group on day 0 (p<0.01) and old growth from this group on day 28 (p<0.05).
Figure 3.
Hair corticosterone (mean ± SEM) for diabetic and non-diabetic control mice. A) Mice injected with Veh or STZ: Day 0 (Veh n = 11, STZ n = 11), Day 28 old hair growth (Veh n = 11, STZ n = 10), Day 28 new hair growth (Veh n = 9, STZ n = 8), and B) db/+ non-diabetic db/db diabetic mice: Day 0 (db/+ n = 7, db/db n = 8), Day 28 old hair growth (db/+ n = 7, db/db n = 7), Day 28 new hair growth (db/+ n = 8, db/db n = 7). Groups (STZ and Veh, db/+ and db/db) were statistically compared by two-way ANOVA. Ampersand indicates significant difference compared to corresponding control group (&&& p < 0.001). Number sign indicates significant difference between new and old hair growth at day 28 (### p < 0.001). Asterisks indicate significant difference between day 0 and day 28 new hair growth (*p < 0.05, ***p < 0.001).
For the type 2 diabetes model (Figure 3B), there was a significant hair growth*genotype interaction on HCC (F2,38 =5.449, p<0.009). Posthoc tests revealed that hair obtained from new growth of db/db mice on day 28 had greater HCC compared to that of new growth obtained from db/+ mice on day 28 (p<0.001). Moreover, new growth of db/db mice on day 28 had higher HCC compared to old growth obtained from db/db mice on day 28 (p<0.001) and on day 0, i.e., at 4 weeks of age and prior to the onset of diabetes (p<0.001).
DISCUSSION
The primary aim of this study was to determine whether hair corticosterone would be a useful measure of chronic HPA activation in mice that accompanies two mouse models of diabetes mellitus. The results of the study showed that hair corticosterone levels in both serum and hair samples were significantly elevated in mice treated with STZ and in genetically obese db/db mice compared to their respective non-diabetic controls.
The pathophysiologies of type 1 and type 2 diabetes mellitus are complex and not completely understood [36]. Diabetes and prolonged hyperglycemia have detrimental effects on all body systems, including endocrine functions and the HPA axis. Although growing evidence exists for a correlation between cortisol secretion and complications in diabetes, the causal association between these factors is not definitive and is likely cyclic in nature [37, 38]. Type 2 diabetes consistently produces elevation of cortisol or corticosterone values in both human patients and animal models [6–8, 38]. Type 1 diabetes has been associated with elevated serum and urine cortisol [9, 39–42], as well as blunted glucocorticoid responses to stress [43–45], attributable to dysregulation of the HPA axis. Adrenal cortex hypertrophy has been documented in rodent models of both type 1 and type 2 diabetes and likely reflects these perturbations in endocrine function and glucocorticoid secretion [38]. While previous studies have relied on acute measurements of cortisol or corticosterone from serum or urine samples, the analysis of increased glucocorticoids from hair samples may offer a better indication of the long-term impact of chronic glucocorticoid secretion and true disease burden.
In the current study, the magnitude of serum and hair corticosterone elevation compared to control animals was greater in type 2 diabetic mice compared to type 1 diabetic mice, despite similar elevations in blood glucose. In the Type 2 diabetes model, serum corticosterone levels were 12-fold higher in db/db mice compared to db/+ mice whereas the difference in hair corticosterone levels between db/db and db/+ mice was only 2-fold. In the Type 1 diabetes model, serum and hair corticosterone levels were both increased about 2-fold. We hypothesize that these differences in magnitude may be due to the fact that serum concentrations reflected circulating corticosterone at a single time point when db/db mice had developed full-fledged diabetes, while hair concentrations reflected average circulating corticosterone over the preceding hair growth period. As well, serum corticosterone levels can be further influenced by acute changes such as diurnal variation or stress due to handling. Specifically, dysregulation of the HPA axis and physiologic responses to stress have been demonstrated in obese and diabetic patients; the marked serum corticosterone elevations observed in db/db mice relative to db/+ mice may reflect both acute glucocorticoid variations and metabolic dysfunction [46].
Differences in the response of hair versus serum corticosterone levels in mice with type 1 or type 2 diabetes could also reflect the influence of corticosterone binding globulin (CBG), responsible for transport and release of the glucocorticoid. Total serum corticosterone levels reflects both the free and bound biologically active fraction accounted for by CBG. Hair corticosterone or cortisol levels have been assumed to reflect free rather than total glucocorticoids and differences between serum and hair samples may reflect variations in these separate pools, as shown previously for sex differences [30]. Given the association between insulin and CBG, it is reasonable to expect that the different hair:serum ratios for type 1 and type 2 diabetes reflect variations in CBG and free versus bound corticosterone levels that may arise from the insulin resistance selectively in type 2 diabetes. The response of free versus total corticosterone levels as biomarkers to physiological challenges reflected in different biological samples has widespread importance for clinical translation and could be studied experimentally using the methods described in this paper with CBG-deficient mice.
Collection and analysis of hair in mice can be challenging due to their small body size, short, fine hair coat, and irregular hair growth. In humans and some larger veterinary species like non-human primates, hair grows much longer and, in some cases, at a predictable rate such that analytes measured from different areas of the hair shaft can indicate exposure over specific time periods. Thus, hair samples collected at a single time point enable a degree of retrospective temporal evaluation of glucocorticoid exposure if they are long enough. In contrast, the hair shaft in C57BL/6 mice only reaches a maximum length of approximately 1 mm [47] and 5% of the samples in the present study could not be used due to insufficient new hair growth.
A secondary aim of this study was to compare corticosterone levels in old and new hair growth in rodents over the 4-week study period to determine whether old hair growth would provide a useful indicator of HPA activity. The ability to compare corticosterone levels in old hair growth would eliminate the need for hair removal prior to the onset of the experiment. Some studies that demonstrated significant differences in rodent hair corticosterone following up to 5 weeks of experimental manipulation analyzed only new hair growth [29, 30]. In contrast, other studies that examined hair corticosterone in rodents from old hair growth did not reveal significant differences between experimental groups [32, 48]. Because the complete hair growth cycle in mice is relatively short, approximately 3 weeks [49], we were able to determine whether prior hair removal and collection of new hair growth was necessary to detect changes in HPA activity over the 4 week study period. In contrast with corticosterone levels in new hair growth, we found that corticosterone in old hair growth did not differ significantly between diabetic and non-diabetic animals. These findings support the need for hair removal and measurement of new hair growth to detect changes in hair corticosterone levels over periods of 4 weeks or shorter in mice.
In summary, the results of the present study validated the elevation of hair corticosterone levels in two mouse models of diabetes mellitus. These findings are consistent with elevated plasma cortisol levels measured in human patients with untreated diabetes mellitus [3, 15–17]. Compared to other biologic samples, hair reflects the status of glucocorticoid levels over longer periods of time, is less affected by transient variables, can be collected less invasively, and samples can be stored for a long time prior to analysis. Future studies may aim at assessing the utility of hair corticosterone as a translational biomarker of risk for diabetic complications and to monitor the long-term efficacy of diabetic medications on glucocorticoid secretion in both experimental animal models and diabetic patients.
Highlights.
Mice with Type 1 diabetes after steptozotocin demonstrated higher levels of corticosterone in blood and hair samples.
Type 2 diabetes shown by leptin receptor deficient (db/db) mice showed higher levels of corticosterone in blood and hair samples.
Hair samples from recent growth were more sensitive to changes than old growth.
Hair samples can be used to measure long-term increases in corticosterone by diabetes in rodent models.
Acknowledgments
This research was supported by USPHS grant R01 MH092412. RE was supported in part by the Office of the Vice Provost for Research at the University of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, et al. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80:683–93. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol Behav. 2006;89:536–42. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Tamashiro KL, Sakai RR, Shively CA, Karatsoreos IN, Reagan LP. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14:468–74. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- 4.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005:28. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 5.Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. The Journal of clinical endocrinology and metabolism. 2003;88:5593–602. doi: 10.1210/jc.2003-030871. [DOI] [PubMed] [Google Scholar]
- 6.Chiodini I, Di Lembo S, Morelli V, Epaminonda P, Coletti F, Masserini B, et al. Hypothalamic-pituitary-adrenal activity in type 2 diabetes mellitus: role of autonomic imbalance. Metabolism: clinical and experimental. 2006;55:1135–40. doi: 10.1016/j.metabol.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, et al. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes care. 2007;30:83–8. doi: 10.2337/dc06-1267. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini I, Torlontano M, Scillitani A, Arosio M, Bacci S, Di Lembo S, et al. Association of subclinical hypercortisolism with type 2 diabetes mellitus: a case-control study in hospitalized patients. European journal of endocrinology/European Federation of Endocrine Societies. 2005;153:837–44. doi: 10.1530/eje.1.02045. [DOI] [PubMed] [Google Scholar]
- 9.Roy MS, Roy A, Brown S. Increased urinary-free cortisol outputs in diabetic patients. Journal of diabetes and its complications. 1998;12:24–7. doi: 10.1016/s1056-8727(97)00006-8. [DOI] [PubMed] [Google Scholar]
- 10.Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149:3244–53. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- 11.Gwinup G, Johnson B. Clinical testing of the hypothalamic-pituitary-adrenocortical system in states of hypo- and hypercortisolism. Metabolism: clinical and experimental. 1975;24:777–91. doi: 10.1016/0026-0495(75)90045-1. [DOI] [PubMed] [Google Scholar]
- 12.Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–6. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, et al. The significance of glucocorticoid pulsatility. European journal of pharmacology. 2008;583:255–62. doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 14.D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic–pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiology & behavior. 2011;104:348–53. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production—Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–7. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SHM. Hair Analysis Provides a Historical Record of Cortisol Levels in Cushing’s Syndrome. Exp Clin Endocrinol Diabetes. 2010;118:133–8. doi: 10.1055/s-0029-1220771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kintz P. Value of hair analysis in postmortem toxicology. Forensic science international. 2004;142:127–34. doi: 10.1016/j.forsciint.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Webb E, Thomson S, Nelson A, White C, Koren G, Rieder M, et al. Assessing individual systemic stress through cortisol analysis of archaeological hair. Journal of Archaeological Science. 2010;37:807–12. [Google Scholar]
- 19.Pragst F, Balikova MA. State of the art in hair analysis for detection of drug and alcohol abuse. Clinica chimica acta; international journal of clinical chemistry. 2006;370:17–49. doi: 10.1016/j.cca.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Raul JS, Cirimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical biochemistry. 2004;37:1105–11. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Dettenborn L, Muhtz C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, et al. Introducing a novel method to assess cumulative steroid concentrations: increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress. 2012;15:348–53. doi: 10.3109/10253890.2011.619239. [DOI] [PubMed] [Google Scholar]
- 22.Feller S, Vigl M, Bergmann MM, Boeing H, Kirschbaum C, Stalder T. Predictors of hair cortisol concentrations in older adults. Psychoneuroendocrinology. 2014;39:132–40. doi: 10.1016/j.psyneuen.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Staufenbiel SM, Penninx BW, de Rijke YB, van den Akker EL, van Rossum EF. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology. 2015;60:182–94. doi: 10.1016/j.psyneuen.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Manenschijn L, Schaap L, van Schoor NM, van der Pas S, Peeters GM, Lips P, et al. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. The Journal of clinical endocrinology and metabolism. 2013;98:2078–83. doi: 10.1210/jc.2012-3663. [DOI] [PubMed] [Google Scholar]
- 25.King AJF. The use of animal models in diabetes research. British journal of pharmacology. 2012;166:877–94. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dekel Y, Glucksam Y, Elron-Gross I, Margalit R. Insights into modeling streptozotocin-induced diabetes in ICR mice. Lab animal. 2009;38:55–60. doi: 10.1038/laban0209-55. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi K, Kojima R, Ito M. Strain differences in the diabetogenic activity of streptozotocin in mice. Biological & pharmaceutical bulletin. 2006;29:1110–9. doi: 10.1248/bpb.29.1110. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 29.Jarcho MR, Massner KJ, Eggert AR, Wichelt EL. Behavioral and physiological response to onset and termination of social instability in female mice. Hormones and behavior. 2016;78:135–40. doi: 10.1016/j.yhbeh.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Scorrano F, Carrasco J, Pastor-Ciurana J, Belda X, Rami-Bastante A, Bacci ML, et al. Validation of the long-term assessment of hypothalamic-pituitary-adrenal activity in rats using hair corticosterone as a biomarker. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:859–67. doi: 10.1096/fj.14-254474. [DOI] [PubMed] [Google Scholar]
- 31.Woolsey ID, Bune NE, Jensen PM, Deplazes P, Kapel CM. Echinococcus multilocularis infection in the field vole (Microtus agrestis): an ecological model for studies on transmission dynamics. Parasitology research. 2015;114:1703–9. doi: 10.1007/s00436-015-4355-9. [DOI] [PubMed] [Google Scholar]
- 32.Woolsey ID, Jensen PM, Deplazes P, Kapel CM. Establishment and development of Echinococcus multilocularis metacestodes in the common vole (Microtus arvalis) after oral inoculation with parasite eggs. Parasitology international. 2015;64:571–5. doi: 10.1016/j.parint.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Yu T, Xu H, Wang W, Li S, Chen Z, Deng H. Determination of endogenous corticosterone in rodent’s blood, brain and hair with LC-APCI-MS/MS. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2015;1002:267–76. doi: 10.1016/j.jchromb.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and comparative endocrinology. 2006;147:255–61. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Meyer J, Novak M, Hamel A, Rosenberg K. Extraction and analysis of cortisol from human and monkey hair. Journal of visualized experiments : JoVE. 2014:e50882. doi: 10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alrefai H, Allababidi H, Levy S, Levy J. The endocrine system in diabetes mellitus. Endocrine. 2002;18:105–19. doi: 10.1385/ENDO:18:2:105. [DOI] [PubMed] [Google Scholar]
- 37.Castillo-Quan JI, Perez-Osorio JM. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications: response to Chiodini et al. Diabetes care. 2007;30:e49. doi: 10.2337/dc07-0104. author reply e50. [DOI] [PubMed] [Google Scholar]
- 38.Elahi-Moghaddam Z, Behnam-Rassouli M, Mahdavi-Shahri N, Hajinejad-Boshroue R, Khajouee E. Comparative study on the effects of type 1 and type 2 diabetes on structural changes and hormonal output of the adrenal cortex in male Wistar rats. Journal of Diabetes & Metabolic Disorders. 2013;12:1–6. doi: 10.1186/2251-6581-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan O, Chan S, Inouye K, Vranic M, Matthews SG. Molecular regulation of the hypothalamo-pituitary-adrenal axis in streptozotocin-induced diabetes: effects of insulin treatment. Endocrinology. 2001:142. doi: 10.1210/endo.142.11.8474. [DOI] [PubMed] [Google Scholar]
- 40.Ho N, Balu DT, Hilario MR, Blendy JA, Lucki I. Depressive phenotypes evoked by experimental diabetes are reversed by insulin. Physiology & behavior. 2012;105:702–8. doi: 10.1016/j.physbeh.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho N, Brookshire BR, Clark JE, Lucki I. Indomethacin reverses decreased hippocampal cell proliferation in streptozotocin-induced diabetic mice. Metabolic brain disease. 2015;30:555–62. doi: 10.1007/s11011-014-9611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosbah AA, Abd-Ellatif NA, Sorour EI. Influence of serum cortisol levels on glycemic control in children with type 1 diabetes. Journal of the Egyptian Society of Parasitology. 2011;41:777–84. [PubMed] [Google Scholar]
- 43.Chan O, Inouye K, Vranic M, Matthews SG. Hyperactivation of the hypothalamo-pituitary-adrenocortical axis in streptozotocin-diabetes is associated with reduced stress responsiveness and decreased pituitary and adrenal sensitivity. Endocrinology. 2002;143:1761–8. doi: 10.1210/endo.143.5.8809. [DOI] [PubMed] [Google Scholar]
- 44.Gaete X, Iniguez G, Linares J, Avila A, Mericq V. Cortisol hyporesponsiveness to the low dose ACTH test is a frequent finding in a pediatric population with type 1 diabetes mellitus. Pediatric diabetes. 2013;14:429–34. doi: 10.1111/pedi.12021. [DOI] [PubMed] [Google Scholar]
- 45.Sharma AN, Wigham J, Veldhuis JD. Corticotropic axis drive of overnight cortisol secretion is suppressed in adolescents and young adults with type 1 diabetes mellitus. Pediatric diabetes. 2014;15:444–52. doi: 10.1111/pedi.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steptoe A, Hackett RA, Lazzarino AI, Bostock S, La Marca R, Carvalho LA, et al. Disruption of multisystem responses to stress in type 2 diabetes: investigating the dynamics of allostatic load. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:15693–8. doi: 10.1073/pnas.1410401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Straile WE, Chase HB, Arsenault C. Growth and differentiation of hair follicles between periods of activity and quiescence. Journal of Experimental Zoology. 1961;148:205–21. doi: 10.1002/jez.1401480304. [DOI] [PubMed] [Google Scholar]
- 48.Arnon L, Hazut N, Tabachnik T, Weller A, Koren L. Maternal testosterone and reproductive outcome in a rat model of obesity. Theriogenology. 2016 doi: 10.1016/j.theriogenology.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 49.Porter RM. Mouse models for human hair loss disorders. Journal of Anatomy. 2003;202:125–31. doi: 10.1046/j.1469-7580.2003.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]