Abstract
Asymmetry in cortical activation was tested for short- and long-term stability during the first year of life. Infants (N = 129) completed a total of four laboratory visits: 2 visits occurred about 1 week apart when infants were 6 months old and 2 visits occurred about 1 week apart when infants were 12 months of age. At each laboratory visit, electroencephalogram readings were taken during 5 one-minute, neutral baselines as well as during a negative and a positive emotion-eliciting task. The stability of hemispheric asymmetry was assessed at two midfrontal (F3/4, F7/8) and one parietal (set of electrodes P3/4). Asymmetry in baseline and fear-eliciting episodes showed moderate short-term stability. Long-term stability was apparent when assessments were composited at 6 months and 12 month. Frontal asymmetry was greater than parietal asymmetry for baseline recordings. There was minimal evidence for stability in asymmetry during positive emotion tasks. Results are discussed with regard to the collection and interpretation of alpha asymmetry measures during infancy.
Keywords: EEG asymmetry, Emotion, Infancy, Development
Asymmetric activity in the brain’s frontal hemispheres within the alpha frequency range is generally considered a reliable means of assessing trait-level individual differences in approach-withdrawal tendencies and emotion behaviors from infancy through adulthood (Davidson, 1992, 1994; Davidson & Fox, 1989). In general, greater activity in the right, relative to left, frontal hemisphere (i.e., right frontal asymmetry) during baseline assessments is linked with greater propensities for withdrawal and negativity (Davidson & Irwin, 1999). Conversely, left frontal asymmetry is associated with enhanced propensities for approach and certain types of positive affect (Davidson & Irwin, 1999; Harmon-Jones & Allen, 1998).
Lateralized activity at baseline is apparent in infants within the first 5 days of life (Field, Diego, Hernandez-Reif, Schanberg, & Kuhn, 2002) and appears to be linked to similar approach-withdrawal tendencies in both infants and adults. For example, greater baseline right frontal asymmetry is observed between 3 and 13 months of age in infants of depressed relative to nondepressed mothers; these infants of depressed mothers tend to exhibit high propensities for withdrawal and negative affect (Dawson et al., 1999; Field, Fox, Pickens, & Nawrocki, 1995). Additionally, infants between 10 and 24 months of age who cried during maternal separation showed greater right frontal asymmetry at baseline than did noncriers (Davidson & Fox, 1989; Fox, Bell, & Jones, 1992).
In adults, measures of asymmetry from baseline recordings show high test-retest reliability and internal consistency (Tomarken, Davidson, Wheeler, & Kinney, 1992). High levels of stability in measures of baseline asymmetry are consistent with the interpretation of asymmetry as a marker of trait-level propensities that manifest in consistent ways over time. Despite apparent similarities in the implications for baseline asymmetry over time, the stability of asymmetry measures in infants is not well understood. Lower levels of stability may reasonably be expected in infants given the vast changes in both neural structure (Barkovich, Kjos, Jackson, & Norman, 1988) and displays of affective behaviors visible during this period (Goldsmith et al., 1987; Shiner et al., 2012).
To our knowledge, only three studies have investigated the stability in measures of frontal asymmetry over time in young children. High levels of stability in baseline asymmetry were apparent in a special population, children of depressed mothers, from 3 to 6 months of age (Jones, Field, Davalos, & Pickens, 1997). However, as suggested by the authors, the long-term effects of maternal depression on infant development may lead to higher levels of stability in frontal asymmetry for infants of depressed mothers relative to typically developing infants. Separate work by Fox and colleagues (1992) partially addresses this issue. They reported moderate to high levels of stability in asymmetry scores across short (1 month) periods of time for typically developing infants between 7 and 12 months of age. Stability in asymmetry across longer periods (2–3 months) was generally apparent only after 9 months of age. Similarly, Howarth and colleagues (2016) reported moderate stability in asymmetry from 10 to 24 months of age and limited stability between 24 and 36 months. Such findings illustrate the need to systematically compare levels of short- and long-term stability in frontal asymmetry measures across the first year of life. Differences in levels of short- and long-term term stability may have implications for planning, executing, and interpreting research that includes assessments of asymmetry in infants. Therefore, examining short- and long-term stability in infant frontal asymmetry during the first year of life was one goal of our study.
No studies have systematically examined stability in measures of frontal asymmetry during nonbaseline episodes. The capability model of frontal EEG asymmetry posits that measures of asymmetry derived from emotion contexts offer unique information about an individual’s capacity to employ or inhibit approach or withdrawal behaviors in response to contextual demands (Coan, Allen, & McKnight, 2006). A limited amount of work has attempted to associate infants’ affective and approach-withdrawal behaviors with asymmetry in nonbaseline, emotion-eliciting episodes. Increased left frontal asymmetry has been observed in 10–12 month-old infants in response to a positive film clip (Davidson & Fox, 1982) and in 10-month old infants who reached for their mothers during an approach task (Fox & Davidson, 1987). Similarly, increased right frontal asymmetry in 10-month old infants has been linked to increased fear behavior in multiple fear during both a social (i.e., interaction with a stranger) and nonsocial (i.e., presentation of a toy spider) fear-eliciting episodes (Diaz & Bell, 2012). However, none of this work provided longitudinal estimates of stability in asymmetry during infancy. The capability model suggests that measures of asymmetry during emotional challenges are highly robust to error and thus more stable than baseline asymmetry (Coan et al., 2006). If this is the case, such measures may be preferable during the infant period, when psychophysiological methods often result in substantial artifact and/or missing data. Alternatively, such measures may be less stable during infancy, as emotions and emotion behaviors undergo vast developmental change (Camras & Fatani, 2008; Sroufe, 1996). Therefore, examining stability in frontal asymmetry during periods of positive and negative emotion elicitation was a second goal of our study.
Like emotion-based assessments of frontal asymmetry, measures of posterior asymmetry provide unique information about individual differences in behavioral propensities. While frontal asymmetry is linked to individual differences in approach/withdrawal and emotional valence, asymmetry in posterior regions has been associated with individual differences in arousal (Heller & Nitscke, 1997). Specifically, right parietal activity is linked with high levels of arousal while left parietal activity is linked to low arousal. Although anterior and posterior activity may be related, they are believed to reflect separate dimensions of processing and predict distinct outcomes. As such, frontal and parietal asymmetry may evidence different levels of stability over time. If parietal asymmetry gauges a more general process relative to frontal asymmetry, it may show less stability over time than frontal measures (Coan et al., 2006). Indeed, results from past research including both frontal and parietal measures suggest less stability in parietal, relative to frontal, asymmetry in both children (Fox et al., 1992; Jones et al., 1997) and adults (Tomarken et al., 1992). However, assessments of stability in parietal measures have focused only on baseline assessments. Thus, our final goal was to examine stability of parietal asymmetry across both baseline and emotion-eliciting contexts.
In sum, we aimed to expand understanding of the stability of frontal and parietal asymmetry measures (1) over short and long periods during early infancy and (2) in both neutral and emotion-eliciting contexts.
Method
Procedure
Participants were drawn from a longitudinal twin study of emotional development across infancy (Schmidt et al., 2013). To be included in the analyses, infants were required to be free of known neurological impairments and have a history of right-handedness in their immediate family. Infants (N = 129) visited the laboratory on four occasions between 6 and 12 months of age. When infants were approximately 6-months old, they participated in a series of behavioral episodes while electroencephalograph (EEG) data were collected. They repeated the procedure 1–2 weeks later. This procedure was repeated when infants reached 12 months of age, with one visit closely following the child reaching 12 months of age and an additional visit occurring 1–2 weeks later. Children’s mean ages at each visit were 30.05 (SD = 4.42), 30.74 (SD = 2.45), 54.70 (SD = 3.99), and 56.16 (SD = 3.12) weeks.
Measures
At each laboratory visit, the child was seated in a highchair and fitted with a Lycra electrode cap (Electro-Cap International; Eaton, OH) according to anatomical landmarks. Electroconductive gel (OMNIPREP) and Ag/AgCl electrodes were applied to the following sites per the standard 10/20 system: Fp1, Fp2, F3, F4, F7, F8, T3, T4, T5, T6, C3, C4, P3, P4, O1, O2, Pz, Fz, and Cz. The site for electrode Cz, which served as the reference during recording, was lightly abraded prior to electrode application. All impedances were reduced to less than 20kΩ prior to recording and electrodes at homologous sites were kept within 5kΩ of one another. EEG data were sampled at a rate of 500Hz and amplified with a gain of 20,000. High-pass and low-pass filters were applied during recording at cutoffs of 1Hz and 200Hz, respectively.
Participants then completed a resting baseline assessment followed by three emotion-eliciting episodes. As is typical for studies of individual differences in emotion, all participants completed the episodes in the same order. The first two emotion episodes were designed to elicit fear (first episode) and positive affect (second episode) while physiological data were recorded. A third episode, designed to elicit frustration, did not produce enough artifact-free data to be included in the analyses, as this task elicited high levels of movement in infants. Therefore, the frustration episode is not discussed further.
Baseline
Infants first completed a series of five one-minute recording periods. For this, infants and their parent received instructions from the experimenter, who then left them alone together in the experimental room. An auditory signal indicated the beginning of each recording period, during which parents were instructed to refrain from talking, but to hold up a series of interesting toys to keep the infant’s attention captured. A second auditory signal indicated the end of each recording period.
Stranger Approach
Following the conclusion of the baseline period, a Stranger Approach procedure that elicits fear and wariness in young children was employed (Goldsmith & Rothbart, 1999). For this, a male stranger entered the experimental room. The stranger turned to look at the child and then paused for 10 seconds. After this, the stranger moved approximately half of the distance toward the child, where he paused for 5 seconds and then said “Hello (child name), I’m going to come a little closer to you.” The stranger then approached to within 1 foot of the child and stood next to him/her for two minutes. Following this, the stranger turned away from the child and left the room. Parents remained in the experimental room but were asked to refrain from interacting with the child. Recording was discontinued if the child displayed 30 seconds of intense crying.
Peek-a-boo
A Peek-a-Boo task was used to elicit positive affect in infants (Goldsmith & Rothbart, 1999). At the beginning of this task, the primary experimenter re-entered the room and instructed the parent and child in a game of peek-a-boo. The task involved the parent moving behind a wooden screen while the experimenter asked, “Where’s Mommy?” After a 3 second delay, on the experimenter’s cue, the parent emerged from behind the screen, smiling and saying “Peek-a-boo.” After three seconds, the next trial began. Parent-infant dyads each completed a series of 3 trials.
EEG Data Scoring
Data were re-referenced offline to a whole-head average reference, with a minimum of 12 sites evenly distributed across the head (Bertrand, Perrin, & Pernier, 1985). All data were visually scored and edited to remove artifacts resulting from eye movements, muscle activity, and/or gross motor movements. A Fast Hartley Transform (Bracewell, 1984) was applied to artifact-free chunks that were a minimum of 1.024 seconds in duration. Alpha power was defined as power density in the 5–9 Hz frequency band. This band of frequencies was selected because includes those frequencies which approximate the adult alpha frequency band (Marshall, Bar-Haim, & Fox, 2002) and in order to maintain consistency with previous published work from this sample (Buss et al., 2003). Alpha power was not calculated for infants who had less than 30 seconds of usable EEG data. Although the 30 second requirement may be longer than needed (Möcks & Gasser, 1984), it is roughly half of that typically used for adults. This cutoff was applied given the short duration of the emotion episodes. To account for possible differences in episode length and available data, alpha power was weighted by the proportion of the total number of seconds of artifact free data available. This procedure is similar to that employed by Tomarken and colleagues (1992). The number of seconds of clean data was unrelated to asymmetry scores at all visits (|r|s < 0.28, ps > 0.05). Asymmetry scores were computed at homologous electrodes by subtracting log left alpha power from log right alpha power.
Missing Data
All children in the data set provided usable EEG during a minimum of one of the three previously described episodes. Little’s MCAR χ2[1131] = 972.45, p > 0.10 suggested that data were missing completely at random. Therefore, we used a Full Information Maximum Likelihood (FIML) procedure to account for all missing data. FIML reconstructs the variance-covariance matrix of the requested parameters based on the likelihood of obtaining the observed dataset from the reconstructed matrix. The procedure uses all available data in the estimation algorithm. Maximum likelihood methods are regarded as state-of-the-art procedures for producing unbiased parameter estimates when data are missing at random (Enders, 2010; Schafer & Graham, 2002).
Plan for Analysis
All analyses used MPlus Version 7, using the TYPE=COMPLEX command to account for twin relatedness, and were conducted in three steps. For each episode, we first examined short-term stability in asymmetry. To do this, we calculated the Pearson correlation between asymmetry scores at the two visits at 6 months of age (short term stability at age 6 months) and 12 months of age (short term stability at 12 months of age). Fisher’s r to z transform was used to test for significant differences in stability across electrode sites at contemporaneous assessments (Cohen & Cohen, 1983).
Second, we calculated 6-month and 12-month asymmetry composites using the 2 visits at age 6 months (6 month composite asymmetry) and the 2 visits at age 12 months (12 month composite asymmetry). These composites were used to test long-term stability by calculating the Pearson correlation between composite asymmetry scores at 6 and 12 months of age. Again, Fisher’s r to z transform was used to test for significant differences in stability across electrode sites.
Finally, we recognize that composites that aggregate two assessments may enhance the reliability of measures relative to single assessments. Thus, we also examined long-term stability in asymmetry by calculating the Pearson correlation between asymmetry scores at visit 1 at age 6 months and visit 1 and age 12 months and the correlation between visit 2 at age 6 months and visit 2 at age 12 months. We report mean correlations to allow for the comparison of measures of long-term stability based on single versus dual assessments.
Results
Stability of Baseline Asymmetry Between 6 and 12 Months of Age
As shown in Table 1, significant short-term stability in baseline asymmetry assessed over a period of one week was seen at F7/8 and P3/4 but not at F3/4. INSERT TABLE 1 ABOUT HERE Fisher’s r to z transform suggested that, at 6 months of age, short-term stability in asymmetry at F7/8 was greater than stability in asymmetry at F3/4 (z = −3.79, p < 0.01) and stability in asymmetry at P3/4 (z = 2.31, p = 0.01). Short-term stability at P3/4 was marginally greater than stability at F3/4 (z = −1.48, p < 0.07). Thus, the greatest short-term stability in baseline asymmetry at 6 months of age was observed at F7/8 electrodes and the least short-term stability was observed at F3/4 electrodes.
Table 1.
Short and long term stability in frontal asymmetry during baseline.
| F3/4 | F7/8 | P3/4 | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Short-term stability (1–2 weeks) | ||||||
| 6 months | 0.253 | 0.10 | 0.686** | <0.01 | 0.451** | <0.01 |
| 12 months | 0.377* | <0.04 | 0.426* | <0.01 | 0.162 | 0.12 |
| Long-term stability (6 months) | ||||||
| 6 months to 12 months | 0.319* | 0.01 | 0.604** | <0.01 | 0.174* | 0.03 |
| Average long-term stability (6 months) when only one occasion at each age is used | ||||||
| 6 months to 12 months | 0.214 | 0.506 | 0.285 | |||
Note:
p < 0.05,
p < 0.01
At 12 months of age (Table 1), short-term stability in baseline asymmetry over a one-week period was significant only at frontal electrodes. Fisher’s r to z transform suggested that stability in baseline asymmetry at F7/8 was not different from stability in asymmetry at F3/4 (z = −0.37, p = 0.36) but was greater than stability in asymmetry at P3/4 (z = 1.86, p = 0.03). Short-term stability in asymmetry at F3/4 was marginally greater than stability at P3/4 (z = 1.48, p = 0.07). Therefore, the greatest short-term stability in baseline asymmetry at age 12 months was observed at F3/4 and F7/8.
We then used the composites from the 6-month and 12-month visits to examine long-term stability in asymmetry over a period of six months (Table 1). The composites suggested significant stability at all electrodes. Fisher’s r to z test suggested that long-term stability in asymmetry at F7/8 was greater than stability in asymmetry at F3/4 (z = −2.93, p < 0.01) and long-term stability at P3/4 (z = −4.16, p < 0.01). Long-term stability at F3/4 was marginally greater than long-term stability at P3/4 (z = 1.23, p = 0.10). Thus, the greatest long-term stability, as reflected by the composites, was seen at frontal electrodes with maximal long-term stability observed at F7/8 (r = .60).
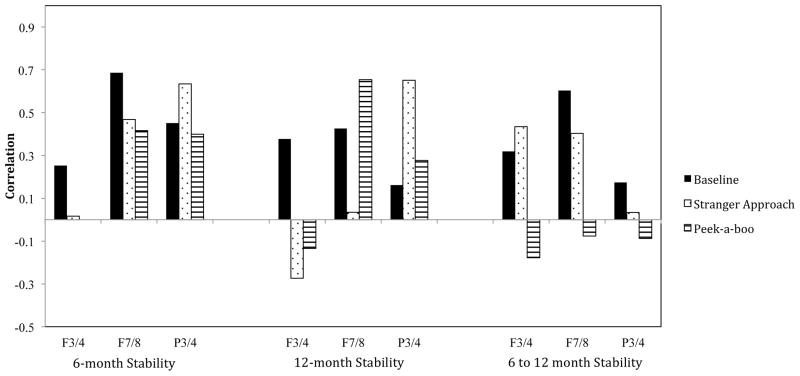
For comparison, we also derived estimates of 6–12 month stability using only one measure per age. These single occasion stability estimates were not significantly lower than those derived using the composite measure (all zs < 1.11, ps > 0.10). Comparisons of stability estimates are illustrated in Figure 1.
Figure 1.
Alpha asymmetry stability across contexts
Stability of Asymmetry During Fear Episode Between 6 and 12 Months of Age
As shown in Table 2, significant short-term stability in asymmetry during a fear episode at 6 months of age was seen at F7/8 and P3/4 but not at F3/4. INSERT TABLE 2 ABOUT HERE Short-term stability in asymmetry during a fear episode at F7/8 was greater than stability at F3/4 (z = 2.84, p < 0.01), and marginally less than stability at P3/4 (z = −1.40, p = 0.08). Short-term stability at P3/4 was greater than stability at F3/4 (z = 4.25, p < 0.01). Thus, during a fear episode at 6 months of age, the greatest short-term stability in asymmetry was apparent at P3/4 and the least stability was at F3/4.
Table 2.
Short and long term stability in frontal asymmetry during stranger approach.
| F3/4 | F7/8 | P3/4 | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Short-term stability (1–2 weeks) | ||||||
| 6 months | 0.016 | 0.86 | 0.468** | <0.01 | 0.635** | <0.01 |
| 12 months | −0.273 | 0.19 | 0.035 | 0.58 | 0.651** | <0.01 |
| Long-term stability using all data (6 months) | ||||||
| 6 months to 12 months | 0.434* | <0.01 | 0.403** | <0.01 | 0.034 | 0.83 |
| Average long-term stability (6 months) when only one occasion at each age is used | ||||||
| 6 months to 12 months | 0.168 | 0.266 | 0.119 | |||
Note:
p < 0.05,
p < 0.01
At 12 months of age, correlations suggested significant short-term stability only at P3/4. Short-term stability in asymmetry in a fear episode was greater at F7/8 than at F3/4 (z = −1.80, p = 0.04). Again, stability in asymmetry at F7/8 was less than stability at P3/4 (z = 4.23, p < 0.01). Stability in asymmetry at F3/4 was significantly less than stability in asymmetry at P3/4 (z = −3.19, p < 0.01). Therefore, at 12 months of age, the greatest short-term stability was observed at P3/4.
We then examined long-term stability in asymmetry during a fear episode assessed over a period of six months using the composites formed at each age (Table 2). Composites suggested greater stability at frontal but not parietal electrodes. Consistent with this, Fisher’s r to z showed that stability was greater at both F3/4 (z = 3.19, p < 0.01) and F7/8 (z = 2.92, p < 0.01) relative to P3/4. Stability at F3/4 did not differ from stability at F7/8 (z = 0.28, p = 0.39).
For comparison, we also derived estimates of 6–12 month stability using only one measure per age. Stability estimates using the composite measures were greater than estimates derived from single assessments at F3/4 (z = −2.13, p = 0.02) but were similar at other electrode sites (zs < 1.12 ps > 0.13).
Stability of Asymmetry During Pleasure Episode Between 6 and 12 Months of Age
As suggested by Table 3, the magnitudes of correlations suggested somewhat limited short-term stability in asymmetry during the positive episode across all electrode pairs. At 6 months of age, Short-term stability was greater at F7/8 (z = −2.75, p < 0.01) and P3/4 (z = −2.62, p < 0.01) relative to F3/4. Short term stability in asymmetry did not differ between F7/8 and P3/4 (z = 0.13, p = 0.45). INSERT TABLE 3 ABOUT HERE
Table 3.
Short and long term stability in frontal asymmetry during peek-a-boo.
| F3/4 | F7/8 | P3/4 | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Short-term stability (1–2 weeks) | ||||||
| 6 months | 0.000 | 1.00 | 0.416** | <0.01 | 0.399 | 0.21 |
| 12 months | −0.134 | 0.35 | 0.654** | <0.01 | 0.277** | 0.01 |
| Long-term stability (6 months) | ||||||
| 6 months to 12 months | −0.178 | 0.28 | −0.077 | 0.26 | −0.087 | 0.61 |
| Average long-term stability (6 months) when only one occasion at each age is used | ||||||
| 6 to 12 months | −0.230 | −0.251 | −0.075 | |||
Note:
p < 0.05
At 12 months of age, patterns of correlations suggested significant short-term stability in asymmetry during a positive episode at F7/8 and P3/4. Fisher’s r to z showed that short-term stability was significantly lower at F3/4 than at F7/8 (z = 5.54, p < 0.01) and P3/4 (z = −2.53, p = 0.01). Short-term stability in asymmetry at F7/8 was significantly greater than stability in asymmetry at P3/4 (z = 3.01, p < 0.01). Thus, at 12 months of age, short-term stability in asymmetry during a positive episode was greatest at F7/8.
We then examined long-term stability in asymmetry during a positive episode assessed over a period of six months using the composites formed at each age (Table 3). None of the estimates of 6-month stability were significant (ps > 0.28) and none of the estimates differed from one another (zs < 0.80, ps > 0.21).
Finally, for comparison, we also derived estimates of 6–12 month stability using only one measure per age. Stability estimates using the composite measures were marginally greater than estimates derived from single assessments at F7/8 (z = 1.33, p = 0.09) but were similar at other electrode sites (zs < 0.40 ps > 0.34).
Discussion
Our results suggested unique patterns of stability in alpha asymmetry for neutral (i.e., baseline) and emotion-eliciting contexts. The stability of resting infant asymmetry at midfrontal sites was comparable to stability estimates previously observed in adults across a 3-week test-retest period (Tomarken et al., 1992) and between infancy and toddlerhood in a sample enriched for chronic maternal depression (Jones et al., 1997). Stability was particularly evident across shorter periods, which spanned a test-retest period of 1 week in this work. However, relatively high levels of stability in asymmetry were also observed at frontal and midfrontal sites across a period of 6 months when asymmetry scores were based on composite estimates. Specifically, when scores of frontal asymmetry from each 6-month visit were composited and correlated with frontal asymmetry composites created from each 12-month visit, we observed moderate-to-high stability in frontal asymmetry across the second half of the first year of life. This moderate-to-high stability likely reflects increased internal consistency of asymmetry estimates as the number of baseline segments are increased, as has been reported in adults (Tomarken et al., 1992). Such an effect had not previously been demonstrated during infancy. This effect was less robust when single assessments were used. Therefore, for researchers who are interested in estimates of infant frontal asymmetry that reflect trait-level stability, rather than state-level measures, including at least two recordings as the time between assessments lengthens may be necessary to observe the trait-level effects.
Stability in asymmetry during baseline recordings was greatest and most consistently observed at midfrontal recording locations. Measures of frontal and parietal asymmetry appear to reflect different individual propensities, particularly with regard to tendencies for emotional experience. As previously noted, frontal asymmetry may be a unique trait-level marker of affective and approach-withdrawal tendencies in infants (Buss et al., 2003; Davidson & Fox, 1989; Hane, Fox, Henderson, & Marshall, 2008). In contrast, parietal asymmetry may reflect more cognitive processing and general emotional arousal, regardless of valence (Heller, Nitschke, & Miller, 1998; Solomon, O’Toole, Hong, & Dennis, 2014). Therefore, to the degree that posterior asymmetry may reflect cognitive or emotional arousal to a task-at-hand rather than trait-like pattern of individual differences in behavioral propensities, less consistency over time – particularly during baseline tasks—may be expected.
In contrast to measures of baseline asymmetry, we observed less stability in frontal asymmetry measured during emotion-eliciting episodes. Namely, we observed a moderate degree of short-term stability in asymmetry at midfrontal and parietal sites during fear and positive affect-eliciting episodes. One reason for limited short-term stability may be the vast changes in emotional development that occur from 6 to 12 months of age. For example, differential emotions theory suggests that across the first year of life, expressions of pleasure move from a precursory form that is dependent primarily on stimulus recognition to a true basic emotional response that include more complex cognitive processing (Sroufe, 1996). Similarly, both mean levels and individual differences in fearfulness undergo vast developmental change between 3 and 36 months of age (Brooker et al., 2013; Sroufe, 1977). Other theories on the emergence and development of emotions also suggest inconsistent links between observable facial expressions and other components of emotion, such as physiological systems (Camras & Fatani, 2008). Thus, it is sensible that stability in asymmetry as elicited in emotional contexts may show low levels of stability during this period of developmental change in both positive and fear-based emotion systems.
Short-term stability was observed at parietal sites, suggesting that neural patterns of arousal are consistent across closely spaced assessments. These neural patterns of arousal may develop earlier than physiological responses associated with differentiated emotions. Unfortunately, little research has investigated the timing by which emotion systems become stably aligned with physiological profiles such as measures of frontal and parietal asymmetry. This will be an important avenue for future work.
Our results also suggest that long-term stability was evident during a fear-eliciting but not a positive-affect eliciting episode. This was true at frontal and midfrontal electrode sites and was evident when composite scores of asymmetry were used to compare 6 and 12 month assessments. One reason for this difference may lay in differences in the timing of the development of positive affect and fear. Fear—at least of the type elicited by a stranger approach—is thought to emerge around 6 months of age (Waters, Matas, & Sroufe, 1975) and increase through age 36 months (Brooker et al., 2013; Sroufe, 1977). Unfortunately, the timing of developing positive affect in early infancy has received scant research attention, and the direction of developing associations between EEG asymmetry and affect are only beginning to be investigated (Howarth, Fettig, Curby, & Bell, 2016). Moreover, the possibility of vast individual differences in the differentiation of emotions (Feldman Barrett, Gross, Conner Christensen, & Benvenuto, 2001) may also lead to inconsistencies in patterns of physiological arousal during different emotion-eliciting episodes. A second possibility is that less stability in asymmetry associated with positive emotions is related to the limited heritability of positive affect during infancy. In infancy, greater genetic influences are observed for aspects of distress and negative emotion relative to positive emotion (Goldsmith, Buss, & Lemery, 1997; Goldsmith, Lemery, Buss, & Campos, 1999). Positive affect, in contrast, appears to strongly influenced by the early environment. Importantly, stable influences in infant affective behavior tend to be heritable influences. Thus, in early life, biological signatures may be more readily apparent for fear propensities than for tendencies for positive affect.
Although a replication of our findings is needed, results offer suggestions for the design of future investigations that incorporate longitudinal assessments of asymmetry. First, our results suggest that multiple baseline assessments or longer baseline recordings may lead to more stable estimates of baseline asymmetry in the first year of life. Although this guideline feels intuitive, it has been offered previously in the absence of empirical support, potentially making it easier to dismiss as subjective opinion. Additionally, longer or greater numbers of baselines are difficult to incorporate at very young ages. We note that several helpful suggestions regarding strategies for keeping infants engaged and reducing artifacts during EEG recording exist in the literature (DeBoer, Scott, & Nelson, 2007; Marshall & Fox, 2008; Trainor, 2008). Many of these are particularly beneficial for baseline recordings.
In addition, our work suggests that measures of EEG asymmetry during the first year of life may not be similarly associated with emotional or physiological responses in the same individual over time. In other words, biology-behavior associations are dynamic rather than fixed. Similarly, temperament theorists argue that a certain degree of instability may be expected as systems develop (Goldsmith et al., 1987; Shiner et al., 2012). Thus, future research should exercise caution in assuming that early findings are consistent with what is known about the development of emotional and physiological systems during the period of development under study.
Finally, the results underscore previous assertions that EEG measures in emotional relative to nonemotional episodes or at frontal relative to parietal electrode sites may reflect different psychological phenomena (Brooker, Phelps, Davidson, & Goldsmith, 2016; Coan, Allen, & McKnight, 2006; Heller et al., 1998). Moreover, neural correlates of emotion may follow distinct trajectories of development. Again, the appropriate cautions should be taken in the interpretation and generalization of findings.
Although our study provides needed empirical evidence for differing stability of asymmetry measures in early life, it is not without limitations. First, although our sample is larger than (Jones et al., 1997; Tomarken et al., 1992) or comparable to (Howarth et al., 2016) samples used in previous investigations of stability in asymmetry, it is still only moderate in size for the questions being addressed. Thus, replication studies will likely lead to more precise estimates of effect sizes for the types of analyses used here. Second, this work was conducted in a sample of typically developing twins. Although twins are generally representative of nontwin populations recruited in a similar way (Andrew et al., 2001), the degree to which our results may translate to atypical populations is unknown.
In sum, our results show a moderate level of short and long-term stability in frontal asymmetry from 6 to 12 months of age when EEG was measured during baseline and fear-eliciting conditions. Stability for long-term measures was greatest when multiple baseline assessments were used. In contrast, stability over the 6-month period was not apparent under positive affect eliciting conditions. This work has clear methodological implications for future longitudinal research including measures of EEG asymmetry in the first year of life.
Acknowledgments
Data collection for this project was supported by R01 MH50560 from the National Institute of Mental Health (PI: Goldsmith). The preparation of this manuscript was supported by K01 MH100240 (PI: Brooker) from the National Institute of Mental Health and P20 GM104417 (PI: Adams) from the National Institute of General Medical Sciences. Infrastructure support was provided by P50 MH094051 and P30 HD03352. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
We thank the families who participated in this study and the staff members who helped with the recruitment of study participants and data collection. Data collection for this project was supported by R01 MH50560 from the National Institute of Mental Health (PI: Goldsmith). Infrastructure support was also provided by P30 HD03352 and P50 MH084051. The writing of this manuscript was supported by K01 MH100240 from the National Institute of Mental Health (PI: Brooker). The authors have no conflicts of interest to report.
Contributor Information
Rebecca J. Brooker, Montana State University
Mara J. Canen, Montana State University
Richard J. Davidson, University of Wisconsin –Madison
H. Hill Goldsmith, University of Wisconsin –Madison.
References
- Andrew T, Hart DJ, Snieder H, de Lange M, Spector TD, MacGregor AJ. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Research and Human Genetics. 2001;4(06):464–477. doi: 10.1375/twin.4.6.464. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Kjos BO, Jackson DE, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166(1):173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Gross J, Conner Christensen T, Benvenuto M. Knowing what you’re feeling and knowing what to do about it: Mapping the relation between emotion differentiation and emotion regulation. Cognition & Emotion. 2001;15(6):713–724. doi: 10.1080/02699930143000239. [DOI] [Google Scholar]
- Bertrand O, Perrin F, Pernier J. A theoretical justification of the average reference in topographic evoked potential studies. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1985;62(6):462–464. doi: 10.1016/0168-5597(85)90058-9. [DOI] [PubMed] [Google Scholar]
- Bracewell RN. The fast Hartley transform. Proceedings of the IEEE. 1984;72(8):1010. doi: 10.1109/PROC.1984.12968. [DOI] [Google Scholar]
- Brooker RJ, Buss KA, Lemery-Chalfant K, Aksan N, Davidson RJ, Goldsmith HH. The development of stranger fear in infancy and toddlerhood: Normative development, individual differences, antecedents, and outcomes. Developmental Science. 2013;16(6):864–878. doi: 10.1111/desc.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Phelps RA, Davidson RJ, Goldsmith HH. Context differences in delta beta coupling are associated with neuroendocrine reactivity in infants. Developmental Psychobiology. 2016 doi: 10.1002/dev.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Schumacher JRM, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003;117(1):11–20. doi: 10.1037/0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Camras LA, Fatani SS. The development of facial expressions: Current perspectives on infant emotions. In: Lewis M, Haviland-Jones JM, Feldman Barrett L, editors. Handbook of Emotions. 3. New York, New York: Guilford Press; 2008. pp. 291–303. [Google Scholar]
- Coan JA, Allen JJB, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72(2):198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analyses for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1983. [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20(1):125–151. doi: 10.1016/0278-2626(92)90065-T. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology. 1994;6(04):741–758. doi: 10.1017/S0954579400004764. [DOI] [Google Scholar]
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218(4578):1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of Abnormal Psychology. 1989;98(2):127–131. doi: 10.1037/0021-843X.98.2.127. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/S1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Yamada E, Hessl D, Osterling J. Infants of depressed mothers exhibit atypical frontal electrical brain activity during interactions with mother and with a familiar, nondepressed adult. Child Development. 1999;70(5):1058–1066. doi: 10.1111/1467-8624.00078. [DOI] [PubMed] [Google Scholar]
- DeBoer T, Scott LA, Nelson CA. Methods for acquiring and analyzing infant event-related potentials. In: DeHaan M, editor. Infant EEG and Event-Related Potentials. New York: Psychology Press; 2007. pp. 5–38. [Google Scholar]
- Diaz A, Bell MA. Frontal EEG asymmetry and fear reactivity in different contexts at 10 months. Developmental Psychobiology. 2012;54(5):536–545. doi: 10.1002/dev.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders C. Applied missing data analysis. New York: The Guilford Press; 2010. [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Schanberg S, Kuhn C. Relative right versus left frontal EEG in neonates. Developmental Psychobiology. 2002;41(2):147–155. doi: 10.1002/dev.10061. [DOI] [PubMed] [Google Scholar]
- Field T, Fox NA, Pickens J, Nawrocki T. Relative right frontal EEG activation in 3- to 6-month-old infants of “depressed” mothers. Developmental Psychology. 1995;31(3) doi: 10.1037/0012-1649.31.3.358. [DOI] [Google Scholar]
- Fox NA, Bell MA, Jones NA. Individual differences in response to stress and cerebral asymmetry. Developmental Neuropsychology. 1992;8(2–3):161–184. doi: 10.1080/87565649209540523. [DOI] [Google Scholar]
- Fox NA, Davidson RJ. Electroencephalogram asymmetry in response to the approach of a stranger and maternal separation in 10-month-old infants. Developmental Psychology. 1987;23(2):233–240. doi: 10.1037/0012-1649.23.2.233. [DOI] [Google Scholar]
- Goldsmith HH, Buss AH, Plomin R, Rothbart MK, Thomas A, Chess S, … McCall RB. Roundtable: What is temperament? Four approaches. Child Development. 1987;58(2):505–529. doi: 10.2307/1130527. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Buss KA, Lemery KS. Toddler and childhood temperament: Expanded content, stronger genetic evidence, new evidence for the importance of environment. Developmental Psychology. 1997;33(6):891–905. doi: 10.1037/0012-1649.33.6.891. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS, Buss KA, Campos JJ. Genetic analyses of focal aspects of infant temperament. Developmental Psychology. 1999;35(4):972–985. doi: 10.1037/0012-1649.35.4.972. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. The Laboratory Temperament Assessment Battery (Lab-TAB): Pre-locomotor Version 3.1. Department of Psychology, University of Oregon; 1999. [Google Scholar]
- Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology. 2008;44(5):1491–1496. doi: 10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74(5):1310–1316. doi: 10.1037/0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Miller GA. Lateralization in emotion and emotional disorders. Current Directions in Psychological Science. 1998;7(1):26–32. doi: 10.1111/1467-8721.ep11521823. [DOI] [Google Scholar]
- Heller W, Nitscke JB. Regional brain activity in emotion: A framework for understanding cognition in depression. Cognition and Emotion. 1997;11(5–6):637–661. doi: 10.1080/026999397379845a. [DOI] [Google Scholar]
- Howarth GZ, Fettig NB, Curby TW, Bell MA. Frontal Electroencephalogram Asymmetry and Temperament Across Infancy and Early Childhood: An Exploration of Stability and Bidirectional Relations. Child Development. 2016;87(2):465–476. doi: 10.1111/cdev.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NA, Field T, Davalos M, Pickens J. EEG stability in infants/children of depressed mothers. Child Psychiatry & Human Development. 1997;28(2):59–70. doi: 10.1023/A:1025197101496. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113(8):1199–1208. doi: 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA. Electrophysiological measures in research on social and emotional development. In: Schmidt LA, Segalowitz SJ, editors. Developmental psychophysiology: Theory, systems, and methods. New York: Cambridge University Press; 2008. pp. 127–149. [Google Scholar]
- Möcks J, Gasser T. How to select epochs of the EEG at rest for quantitative analysis. Electroencephalography and Clinical Neurophysiology. 1984;58(1):89–92. doi: 10.1016/0013-4694(84)90205-0. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7(2):147–177. doi: 10.1037/1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- Schmidt NL, Van Hulle CA, Brooker RJ, Meyer LR, Lemery-Chalfant K, Goldsmith HH. Wisconsin twin research: Early development, childhood psychopathology, autism, and sensory over-responsivity. Twin Research and Human Genetics. 2013;16(Special Issue 01):376–384. doi: 10.1017/thg.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiner RL, Buss KA, McClowry SG, Putnam SP, Saudino KJ, Zentner M. What is temperament now? Assessing progress in temperament research on the twenty-fifth anniversary of Goldsmith et al. (1987) Child Development Perspectives. 2012;6(4):436–444. doi: 10.1111/j.1750-8606.2012.00254.x. [DOI] [Google Scholar]
- Solomon B, O’Toole L, Hong M, Dennis TA. Negative affectivity and EEG asymmetry interact to predict emotional interference on attention in early school-aged children. Brain and Cognition. 2014;87(0):173–180. doi: 10.1016/j.bandc.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA. Wariness of strangers and the study of infant development. Child Development. 1977;48(3):731–746. doi: 10.2307/1128323. [DOI] [Google Scholar]
- Sroufe LA. Emotional development. New York, New York: Cambridge University Press; 1996. [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology. 1992;29(5):576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Trainor LJ. Event-related potential (ERP) measures in auditory development research. In: Schmidt LA, Segalowitz SJ, editors. Developmental psychophysiology: Theory, systems, and methods. New York, New York: Cambridge University Press; 2008. p. 461. [Google Scholar]
- Waters E, Matas L, Sroufe LA. Infants’ Reactions to an Approaching Stranger: Description, Validation, and Functional Significance of Wariness. Child Development. 1975;46(2):348–356. doi: 10.2307/1128127. [DOI] [PubMed] [Google Scholar]