Abstract
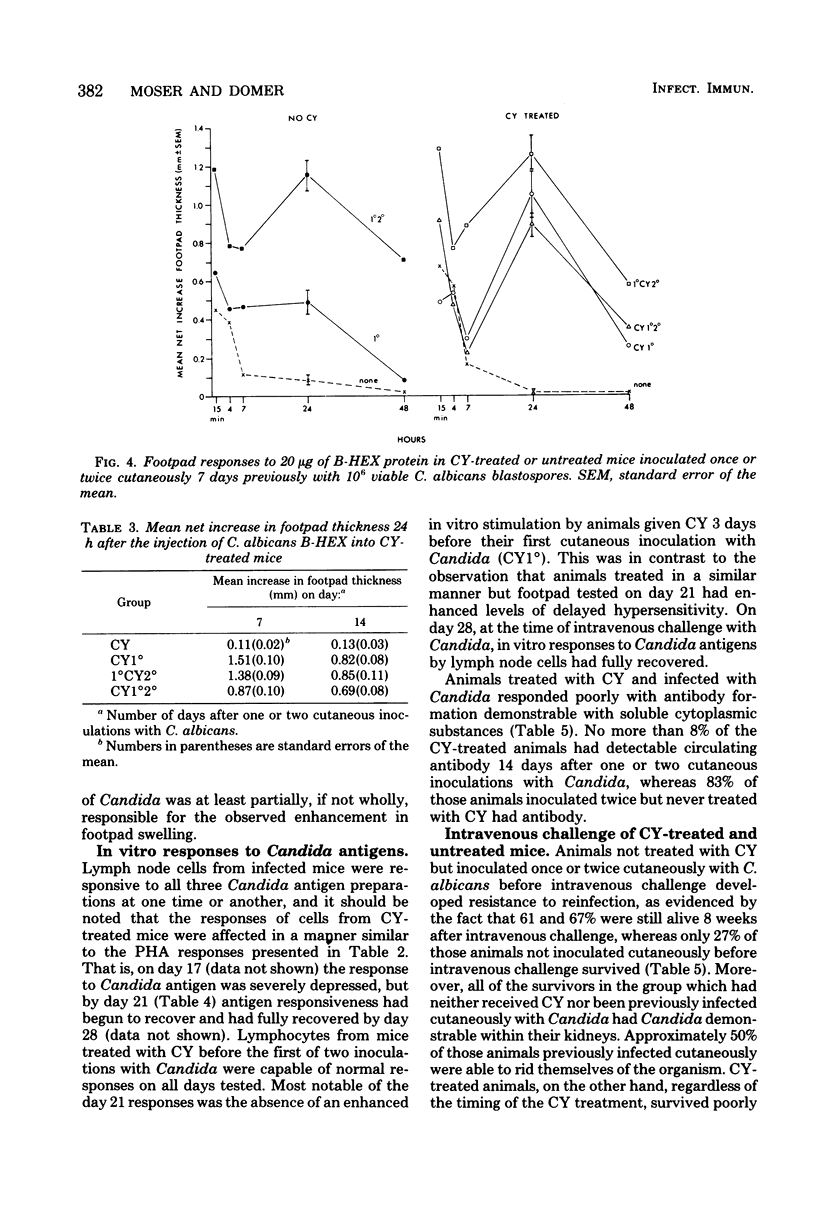
Male CBA/J mice were given a single dose of 200 mg of cyclophosphamide (CY) per kg 3 days before a first or second cutaneous inoculation with viable Candida albicans in an attempt to suppress antibody formation and determine the effects of such suppression on the development of acquired immunity. After cutaneous inoculation, mice not treated with CY developed acquired immunity to intravenous challenge, which was accompanied by the development of circulating antibodies, delayed hypersensitivity, and in vitro responsiveness of lymph node cells to Candida antigens. CY treatment resulted in an immediate depression of peripheral blood leukocytes, with polymorphonuclear leukocytes and monocytes rebounding quickly to normal or above normal levels while lymphocyte remained depressed throughout the 4-week observation period. In vitro stimulation of lymph node cells from CY-treated mice was depressed shortly after treatment; however, responses to phytohemagglutinin and three Candida antigens (a cell wall preparation, a membrane preparation, and soluble cytoplasmic substances) recovered, whereas the responses to lipopolysaccharide did not. CY effects on the cutaneous lesion were twofold; first, the number of viable Candida cells in the lesions was much higher in animals receiving CY 3 days before Candida inoculation, and second, the size of the dermal lesion was either greatly enhanced or reduced depending upon the time of CY treatment relative to the number of cutaneous Candida inoculations. CY-treated animals developed higher levels of delayed hypersensitivity to the membrane preparation when infected once cutaneously than did corresponding untreated animals. The number of mice responding with circulating antibodies to soluble cytoplasmic substances after cutaneous inoculation was greatly reduced in CY-treated groups, and this impaired ability to produce antibodies correlated with the poor survival of these mice after intravenous challenge. Our results suggest that the ability to produce antibody at the time of challenge is crucial to successful defense against systemic candidiasis in this murine model.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brummer E., Vris T. W., Lawrence H. S. A microculture system for the measurement of antigen-induced murine lymphocyte proliferation: advantages of 5% horse serum and 5 X 10(-5) M mercaptoethanol. J Immunol Methods. 1977;17(3-4):319–327. doi: 10.1016/0022-1759(77)90114-4. [DOI] [PubMed] [Google Scholar]
- Buhles W. C., Jr, Shifrine M. Effects of cyclophosphamide on macrophage numbers, functions and progenitor cells. J Reticuloendothel Soc. 1977 May;21(5):285–297. [PubMed] [Google Scholar]
- COHEN J. A., WARRINGA M. G. P. J. Purification of cholinesterase from ox red cells. Biochim Biophys Acta. 1953 Jan;10(1):195–196. doi: 10.1016/0006-3002(53)90234-0. [DOI] [PubMed] [Google Scholar]
- CUTLER S. J., EDERER F. Maximum utilization of the life table method in analyzing survival. J Chronic Dis. 1958 Dec;8(6):699–712. doi: 10.1016/0021-9681(58)90126-7. [DOI] [PubMed] [Google Scholar]
- Cooper M. G. Delayed-type hypersensitivity in the mouse. I. Induction and elicitation by Salmonella adelaide flagellin and its derivatives. Scand J Immunol. 1972;1(2):167–178. doi: 10.1111/j.1365-3083.1972.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Crowle A. J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Domer J. E., Hamilton J. G., Harkin J. C. Comparative study of the cell walls of the yeastlike and mycelial phases of Histoplasma capsulatum. J Bacteriol. 1967 Aug;94(2):466–474. doi: 10.1128/jb.94.2.466-474.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E., Moser S. A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978 Apr;20(1):88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont F. Destruction and regeneration of lymphocyte populations in the mouse spleen after cyclophosphamide treatment. Int Arch Allergy Appl Immunol. 1974;47(1):110–123. doi: 10.1159/000231206. [DOI] [PubMed] [Google Scholar]
- Easmon C. S., Glynn A. A. Effect of cyclophosphamide on delayed hypersensitivity to Staphylococcus aureus in mice. Immunology. 1977 Nov;33(5):767–776. [PMC free article] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses to cutaneous inoculation with Candida albicans. Infect Immun. 1978 Feb;19(2):499–509. doi: 10.1128/iai.19.2.499-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., Moser S. A., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses in T-lymphocyte-depleted mice. Infect Immun. 1978 Sep;21(3):729–737. doi: 10.1128/iai.21.3.729-737.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill H. K., Liew F. Y. Regulation of delayed-type hypersensitivity. III. Effect of cyclophosphamide on the suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1978 Mar;8(3):172–176. doi: 10.1002/eji.1830080306. [DOI] [PubMed] [Google Scholar]
- Gordon M. A., Almy R. E., Greene C. H., Fenton J. W., 2nd Diagnostic mycoserology by immunoelectroosmophoresis: a general, rapid, and sensitive microtechnic. Am J Clin Pathol. 1971 Oct;56(4):471–474. doi: 10.1093/ajcp/56.4.471. [DOI] [PubMed] [Google Scholar]
- Hurtrel B., Lagrange P. H. Réactions d'hypersensibilité de type retardé induites par Candida albicans chez la souris. Ann Immunol (Paris) 1978 Jul-Sep;129 100(5):653–668. [PubMed] [Google Scholar]
- Joyce R. A., Chervenick P. A. Corticosteroid effect on granulopoiesis in mice after cyclophosphamide. J Clin Invest. 1977 Aug;60(2):277–283. doi: 10.1172/JCI108775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Smith T. K. Chronic mucocutaneous candidiasis: immunologic and antibiotic therapy. Ann Intern Med. 1974 Mar;80(3):310–320. doi: 10.7326/0003-4819-80-3-310. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y. Regulation of delayed-type hypersensitivity. I. T suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1977 Oct;7(10):714–718. doi: 10.1002/eji.1830071013. [DOI] [PubMed] [Google Scholar]
- Marbrook J., Baguley B. C. The recovery of immune responsiveness after treatment with cyclophosphamide. Int Arch Allergy Appl Immunol. 1971;41(6):802–812. doi: 10.1159/000230572. [DOI] [PubMed] [Google Scholar]
- Milton J. D., Carpenter C. B., Addison I. E. Depressed T-cell reactivity and suppressor activity of lymoid cells from cyclophosphamide-treated mice. Cell Immunol. 1976 Jun 15;24(2):308–317. doi: 10.1016/0008-8749(76)90214-8. [DOI] [PubMed] [Google Scholar]
- Mitsuoka A., Baba M., Morikawa S. Enhancement of delayed hypersensitivity by depletion of suppressor T cells with cyclophosphamide in mice. Nature. 1976 Jul 1;262(5563):77–78. doi: 10.1038/262077a0. [DOI] [PubMed] [Google Scholar]
- Mourad S., Friedman L. Passive immunization of mice against Candida albicans. Sabouraudia. 1968 Feb;6(2):103–105. [PubMed] [Google Scholar]
- Mukherji A. K., Mallick K. C. Disseminated candidosis in cyclophosphamide induced leucopenic state: an experimental study. Indian J Med Res. 1972 Nov;60(11):1584–1591. [PubMed] [Google Scholar]
- Pearsall N. N., Adams B. L., Bunni R. Immunologic responses to Candida albicans. III. Effects of passive transfer of lymphoid cells or serum on murine candidiasis. J Immunol. 1978 Apr;120(4):1176–1180. [PubMed] [Google Scholar]
- Remington J. S., Gaines J. D., Gilmer M. A. Demonstration of Candida precipitins in human sera by counterimmunoelectrophoresis. Lancet. 1972 Feb 19;1(7747):413–413. doi: 10.1016/s0140-6736(72)90860-4. [DOI] [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Snippe H., Davidse R. P., Belder M., Willers J. M. Effects of cyclophosphamide treatment on the in vitro activity of mouse lymphoid cells after nonspecific and specific stimulation. Int Arch Allergy Appl Immunol. 1976;50(5):536–547. doi: 10.1159/000231558. [DOI] [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]
- Valdimarsson H., Higgs J. M., Wells R. S., Yamamura M., Hobbs J. R., Holt P. J. Immune abnormalities associated with chronic mucocutaneous candidiasis. Cell Immunol. 1973 Mar;6(3):348–361. doi: 10.1016/0008-8749(73)90035-x. [DOI] [PubMed] [Google Scholar]
- Willers J. M., Sluis E. The influence of cyclophosphamide on antibody formation in the mouse. Ann Immunol (Paris) 1975 Apr;126(3):267–279. [PubMed] [Google Scholar]



