Abstract
AIMS
To determine the spinal segmental afferent contributions to tibial and pudendal inhibition of bladder overactivity.
METHODS
Intravesical infusion of 0.5% acetic acid was used to irritate the bladder and induce bladder overactivity in anesthetized cats. Tibial or pudendal nerve stimulation was used to suppress the bladder overactivity and increase bladder capacity during cystometry. L5-S3 dorsal roots ipsilateral to the stimulation were exposed by a laminectomy and transected sequentially during the experiments to determine the role of individual dorsal roots in tibial or pudendal neuromodulation.
RESULTS
Transection of L5 dorsal root had no effect. Transection of L6 dorsal root in four cats produced an average 18% reduction in tibial inhibition, which is not a significant change when averaged in the group of 10 cats. Transection of L7 dorsal root completely removed the tibial inhibition without changing reflex bladder activity or pudendal inhibition. Transection of S1 dorsal root reduced the pudendal inhibition, after which transection of S2 dorsal root completely removed the pudendal inhibition. Transection of S3 dorsal root had no effect. The control bladder capacity was increased only by transection of S2 dorsal root.
CONCLUSIONS
This study in cats revealed that tibial and pudendal neuromodulation of reflex bladder overactivity depends on activation of primary afferent pathways that project into different spinal segments. This difference may be related to the recent observation in cats that the two types of neuromodulation have different mechanisms of action.
Keywords: bladder, cat, neuromodulation, pudendal, tibial
1 | INTRODUCTION
Overactive bladder (OAB) is described as a constellation of symptoms that include urinary urgency with or without urge incontinence, usually with frequency and nocturia.1 Approximately 33 million adults suffer from OAB in the United States.2 As the etiology of the condition has largely remained unknown, treating OAB has been a major challenge. Sacral neuromodulation, an FDA approved treatment for OAB, involves electrical stimulation of the S3 sacral spinal root by an implanted stimulator.3,4 In addition, stimulation of tibial or pudendal nerves has also been shown to be clinically effective in OAB treatment.5–8 Despite the clinical success of sacral, tibial, and pudendal neuromodulation in treating OAB, the possible mechanisms of action are currently still unknown.
Sacral neuromodulation is believed to stimulate the afferent nerves in the sacral root to modulate bladder activity.9 As both tibial and pudendal afferent nerves travel in multiple spinal roots including the S3 root, it is possible that sacral neuromodulation produces therapeutic effects by stimulating the central projections of both tibial and pudendal afferent nerves. However, whether the axonal projections of these nerves in the S3 root play a role in sacral neuromodulation or how much they contribute to sacral neuromodulation has never been determined.
The purpose of this study was to identify the spinal roots containing primary afferents that contribute functionally to tibial and pudendal neuromodulation of bladder overactivity in α-chloralose anesthetized cats. Bladder overactivity was induced by intravesical infusion of dilute (0.5%) acetic acid to irritate the bladder. Tibial and pudendal nerves on the right side were electrically stimulated to inhibit the irritation-induced bladder overactivity. Lumbosacral dorsal roots on the same side of the stimulated nerves were transected sequentially from L5 to S3 to determine the contribution of each dorsal root to the inhibition of bladder overactivity induced by tibial or pudendal nerve stimulation. The results are important for a functional comparison between sacral, tibial, and pudendal neuromodulation, which will be helpful to understand the possible mechanisms underlying different neuromodulation therapies.
2 | MATERIALS AND METHODS
The protocol involving the experimental use of animals in this study was approved by the Animal Care and Use Committee at the University of Pittsburgh.
2.1 | Surgical procedures
This study used a total of 10 cats (five males and five females, 2.9–4.4 kg; Liberty Research, Waverly, NY) that were anesthetized with isoflurane (2–5% in O2) during surgery followed by α-chloralose anesthesia (65 mg/kg intravenously, and supplemented as needed) during data collection. Heart rate and oxygen saturation were monitored by a pulse oximeter (9847V, NONIN Medical, Plymouth, MN) placed on the tongue/ear. Arterial blood pressure was monitored via a catheter inserted into the right carotid artery. Airway access was secured via a tracheostomy and an endotracheal tube. Drugs and fluid were given intravenously through a catheter placed in the left cephalic vein. The ureters were isolated, cut, and drained externally via an open laparotomy approach. The bladder was then cannulated by a double lumen catheter via a small cut at the proximal urethra and secured in place with a ligature to prevent leakage. One lumen of the catheter was connected to a pump that infused (1 or 2 mL/min) saline or 0.5% acetic acid (AA) while the other lumen was connected to a pressure transducer to monitor the pressure within the bladder. The tibial nerve was then isolated on the medial side of the right hindlimb and a tripolar cuff electrode (NC223pt, MicroProbe, Gaithersburg, MD) was implanted on the nerve for stimulation. Another tripolar cuff electrode was implanted on the right pudendal nerve that was dissected in the region of the sciatic notch. All incisions were closed with suture.
The spinal cord and cauda equina were exposed between the L4 and Cx1 vertebrae via a dorsal laminectomy. The spinal dura was cut and L5-S3 dorsal roots on the right side were separated and looped with sutures for transection during the experiments. The animal was mounted in a modified Narishige “Eccles” spinal cord frame in which the hip was supported by metal pins, and the spinous process at the rostral end of the laminectomy was secured with a clamp. The skin, cut mid-sagittally from L4 to Cx1, was tied along each margin to form a pool that was filled with warmed (35–37°C) mineral oil. The temperature of the animal was maintained at 36–38°C using a heating pad during the experiments.
2.2 | Experimental protocol
Based on our previous studies,10,11 uniphasic rectangular electrical pulses of 0.2 ms pulse width and 5 Hz frequency were used for tibial nerve stimulation (TNS) and pudendal nerve stimulation (PNS). The stimulation threshold (T), which is defined as the minimal intensity to induce a toe twitch for TNS or an anal twitch for PNS, was determined at the beginning of the experiment. Then the stimulation intensity of 4T was used for both TNS and PNS during the experiment to inhibit bladder activity and increase bladder capacity.
At the beginning of each experiment, multiple cystometrograms (CMGs) were performed by slowly infusing the bladder with saline to determine the bladder capacity that was defined as the bladder volume threshold to induce a bladder contraction of large amplitude (>30 cm H2O) and long duration (>20 s). Then, 0.5% AA was infused into the bladder to irritate the bladder and induce bladder overactivity. Once the control bladder capacity stabilized during repeated AA CMGs, the inhibitory effect of TNS or PNS was determined by four AA CMGs: (1) Control CMG without stimulation; (2) CMG during TNS; (3) CMG during PNS; and (4) Control CMG again to examine any post-stimulation effect. Then, the spinal dorsal roots were transected sequentially from L5 to S3. Ten minutes after each root transection, the four CMGs (control, TNS, PNS, and control) were performed again to determine the effect of root transection.
2.3 | Data analysis
The bladder capacities measured during each CMG were compared before and after each root transection. For each comparison, the bladder capacities were normalized to the capacity measured during the first control CMG before a specific root transection. Repeated measurements in the same animal under the same conditions were averaged. The normalized data from different animals are presented as mean ± standard error. Statistical significance (P < 0.05) was determined by repeated-measures ANOVA followed by Bonferroni (two-way) multiple comparison to determine the effects of each root transection on reflex bladder activity and TNS/PNS inhibition or by Dunnett (one-way) multiple comparison to determine the effects of TNS and PNS on reflex bladder activity. Student t-test was used to detect the significant (P < 0.05) change on reflex bladder activity induced by AA irritation.
3 | RESULTS
3.1 | Inhibition of bladder overactivity by TNS and PNS
Intravesical infusion of dilute (0.5%) AA irritated the bladder, induced bladder overactivity, and significantly (P < 0.01) reduced bladder capacity to 13.7 ± 3.3 mL from 21.7 ± 5.2 mL measured during saline CMGs (N = 10 cats). TNS or PNS inhibited AA-induced bladder overactivity and significantly (P < 0.01) increased bladder capacity by 4.1 ± 0.5 mL or 10.0 ± 3.0 mL, respectively. Typical CMG traces before any root transection are shown on the first row in Fig. 1A. After TNS and PNS, the following AA control CMG showed that the bladder capacity returned to the pre-stimulation level, that is no post-stimulation effect. A post-stimulation effect was also not observed after each root transection as presented in the following section.
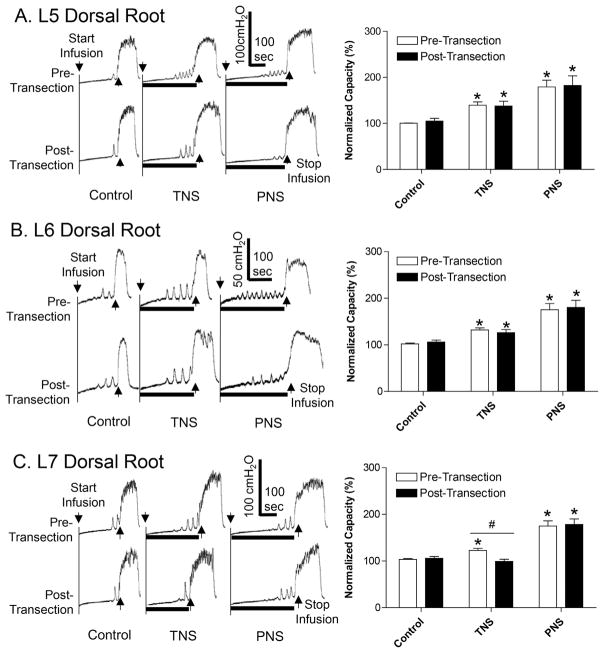
FIGURE 1.
Effect of sequential L5–L7 dorsal root transection on inhibition of bladder overactivity induced by tibial nerve stimulation (TNS) or pudendal nerve stimulation (PNS). A. Transection of L5 dorsal root. B. Transection of L6 dorsal root. C. Transection of L7 dorsal root. Left column: repeated CMG traces with or without TNS/PNS. The black bar under the bladder pressure trace indicates the stimulation duration. Right column: normalized bladder capacity (N = 10 cats). The bladder capacities were always normalized to the pre-transection control for each root transection. * Indicates significantly (P < 0.05) different from the respective control (one-way ANOVA). # Indicates a significant (P < 0.05) difference before and after root transection (two-way ANOVA). TNS: 4T = 3.9 ± 0.65 V, 5 Hz, 0.2 ms. PNS: 4T = 2.1 ± 0.42 V, 5 Hz, 0.2 ms. T is the threshold intensity for inducing anal or toe twitching
3.2 | Effects of L5-S3 dorsal root transection on TNS and PNS inhibition
Transection of L5 dorsal root did not change the control bladder capacity and the increased capacities measured during either TNS or PNS (Fig. 1A). Transection of L6 dorsal root in four cats produced an average 18% reduction in tibial inhibition, but this reduction is not a significant change when averaged in the group of 10 cats (Fig. 1B). However, subsequent transection of L7 dorsal root removed the TNS inhibition by significantly (P < 0.01) reducing the bladder capacity measured during TNS from 122.6 ± 4.4% to 99.2 ± 4.7% of control capacity, while the control bladder capacity and the capacity measured during PNS were not changed (Fig. 1C).
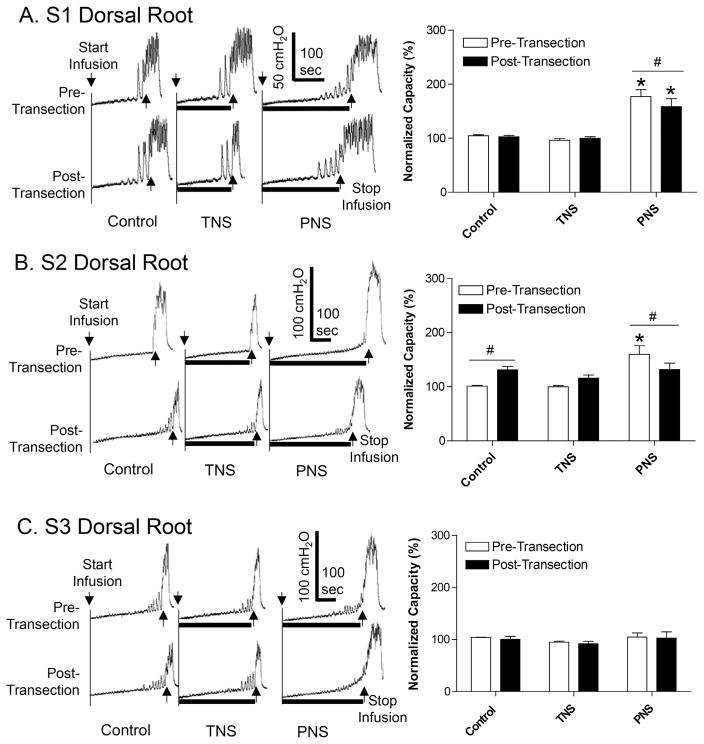
After transection of L5–L7 dorsal roots, subsequent transection of the S1 dorsal root significantly (P < 0.01) reduced the bladder capacity measured during PNS from 177.1 ± 12.8% to 158.5 ± 15.0% of control capacity (Fig. 2A). After transection of S2 dorsal root which significantly (P = 0.02) increased the control bladder capacity to 131.1 ± 6.3% (Fig. 2B), PNS failed to increase bladder capacity, that is PNS inhibition was eliminated (Fig. 2B). The final transection of the S3 dorsal root had no effect on either the control capacity or the bladder capacities measured during TNS or PNS (Fig. 2C).
FIGURE 2.
Effect of sequential S1–S3 dorsal root transection on inhibition of bladder overactivity induced by tibial nerve stimulation (TNS) or pudendal nerve stimulation (PNS). A. Transection of S1 dorsal root. B. Transection of S2 dorsal root. C. Transection of S3 dorsal root. Left column: repeated CMG traces with or without TNS/PNS. The black bar under the bladder pressure trace indicates the stimulation duration. Right column: normalized bladder capacity (N = 10 cats). The bladder capacities were always normalized to the pre-transection control for each root transection. * Indicates significantly (P < 0.05) different from the respective control (one-way ANOVA). # Indicates a significant (P < 0.05) difference before and after root transection (two-way ANOVA). TNS: 4T = 3.9 ± 0.65 V, 5 Hz, 0.2 ms. PNS: 4T = 2.1 ± 0.42 V, 5 Hz, 0.2 ms. T is the threshold intensity for inducing anal or toe twitching
4 | DISCUSSION
This study in cats shows, that TNS inhibition of bladder overactivity is solely dependent on afferent axons projecting to the spinal cord through the L7 dorsal root, while the afferent axons in L5 and L6 dorsal roots are not necessary (Fig. 1). Meanwhile, PNS inhibition of bladder overactivity is dependent on afferent axons in both the S1 and S2 dorsal roots (Fig. 2). These results indicate clearly that TNS or PNS activates completely different spinal segmental afferent inputs to inhibit bladder overactivity in cats.
It is surprising that only the tibial afferent nerves in L7 dorsal root are responsible for TNS inhibition (Fig. 1), because it is known that tibial afferent nerves project to L5-S1 spinal segments.12 The stimulation intensity used in this study is four times threshold intensity for inducing a toe twitching response, which should be strong enough to activate afferent inputs to multiple spinal segments. Therefore, one possible explanation could be that only the tibial afferent nerves in L7 dorsal root have a synaptic interaction with the micturition reflex pathway due to the proximity of the L7 spinal segment to the sacral cord that controls the bladder. The number of tibial afferent nerves projecting to the S1 spinal segment is also much less than the number projecting to the L7 spinal segment,12 which may explain the major role of the L7 dorsal root in tibial inhibition of bladder overactivity. Another possibility is that tibial inhibition only requires partial activation of the total number of afferent nerves in the tibial nerve. As L6 and L7 spinal segments are the major afferent projections from the tibial nerve,12 TNS may be able to achieve full inhibition through either one of the dorsal roots. However, testing this possibility will require additional experiments to transect the L7 dorsal root before L6 dorsal root. A possible contribution of afferents in the L6 dorsal root to tibial inhibition was also suggested by the observation that four of the 10 tested cats showed an average 18% reduction in the effect of TNS after transection of the L6 dorsal root. Nevertheless, it is clear that tibial inhibition is dependent on afferent projections to the lumbar cord but not on projections to the sacral cord. This result indicates that tibial neuromodulation does not share the same afferent pathway with sacral neuromodulation that only requires activation of afferent nerves in the sacral spinal roots.
In contrast to tibial inhibition, pudendal inhibition requires afferent projections to both S1 and S2 spinal segments but not the S3 segment (Fig. 2). This result agrees very well with a previous study in cats showing that a majority of pudendal afferent nerves projects to the spinal cord via S1 and S2 dorsal roots.13 It is also similar to sacral neuromodulation that requires activation of afferent nerves in S1 or S2 dorsal root but not S3 dorsal root in cats.14 Thus, it is possible that sacral neuromodulation may activate some of same afferent axons as those activated during pudendal neuromodulation in cats. In humans, sacral neuromodulation is applied to sacral S3 root3,4 that is equivalent to S1/S2 roots in cats due to spinal segmental differences between cats and humans. However, because sacral neuromodulation normally involves stimulation of only one root, it probably can only activate a portion of the pudendal afferent axons due to the multi-segmental projections of these axons to the sacral spinal cord.
Furthermore, as transection of S1 and S2 dorsal roots removed both pudendal and bladder afferent inputs to the spinal cord, it is difficult to know if the removal of pudendal inhibition (Fig. 2A and B) is due to elimination of pudendal afferents or bladder afferents. It is possible that removal of bladder afferents alone can also eliminate the pudendal inhibition if the pudendal afferent input only blocks the ipsilateral bladder afferent input and/or the ipsilateral spinal circuitry controlling the bladder. This idea can be tested in future experiments by transection of bladder afferent and efferent pathways in the pelvic nerve ipsilateral to the stimulated pudendal nerve to determine if pudendal inhibition persists after the transection.
It is worth noting that pudendal inhibition does not require lumbar L5–L7 dorsal roots (Fig. 1), indicating completely different spinal segmental afferent pathways than those involved in tibial neuromodulation. This difference may explain the different mechanisms of action in pudendal and tibial neuromodulation that were identified in our previous studies in cats. For example, opioid receptors are involved in tibial but not pudendal neuromodulation,15 while pudendal but not tibial neuromodulation can inhibit spinal reflex bladder activity16 and the bladder contractions induced by descending efferent input from the pontine micturition center.17 Although our current study did not determine the CNS site of action for tibial/pudendal neuromodulation, it revealed which spinal dorsal roots contain the afferent nerve fibers important for tibial/pudendal neuromodulation.
The control bladder capacity was changed only by transection of S2 but not S1/S3 dorsal roots (Fig. 2). However, it is known that bladder afferent nerves project into the spinal cord via S1–S3 dorsal roots in cats.18 It is possible that the bladder afferent projections to multiple spinal segments may be redundant for inducing micturition, so that it requires transection both S1 and S2 dorsal roots to change the bladder capacity. Similar to the tibial inhibitory effect as discussed above, additional experiments will be needed to determine whether a different effect on control bladder capacity will be observed when the S2 dorsal root is transected before the S1 dorsal root. It is also worth noting that the bladder reflex is mediated by bilateral afferent inputs to the spinal cord, which is different from TNS or PNS that activates ipsilateral afferent inputs to the spinal cord.
It is worth noting that the sacral dorsal roots are transected sequentially from L5 to S3 in this study. The roots transected early might have influenced the effects of the root transection that is performed later. However, this seems less likely because: (1) 10 min are allowed after each root transection to avoid the acute effect of nerve transection; (2) the control bladder capacity is not changed by the root transections except the S2 dorsal root that is known to be the major dorsal root for triggering micturition reflex activity. Despite this possible influence, it is clear that the tibial neuromodulation is dependent on afferent inputs to the lumbar spinal segments, while the pudendal neuromodulation is dependent on the afferent inputs to the sacral spinal segments. Furthermore, any order of nerve transection (randomly or reverse order) will not eliminate the possible effect of previous transections unless we design each root transection as a separate group of experiments, which will require a very large number of animals for the entire study.
The effects of dorsal root transections on TNS/PNS were investigated during repeated AA CMGs but not during saline CMGs in this study. This is because our previous study (11) has shown that repeated TNS can produce a significant amount of post-stimulation inhibition during saline CMGs but not during AA CMGs. Post-stimulation inhibition changes control CMGs by increasing bladder capacity during TNS and therefore precludes testing TNS after sequential dorsal root transection. This study using AA CMGs eliminates the post-TNS effect on control bladder capacity. Furthermore, the cat model of bladder overactivity induced by AA irritation has been used in many previous studies in which drugs and neuromodulation were tested,10,11,19 showing that the control bladder capacity is stable during repeated AA CMGs lasting for 5–6 h.
This study in the anesthetized cat revealed that completely different spinal segmental primary afferents contribute to tibial or pudendal neuromodulation of bladder overactivity, indicating that different neural pathways and mechanisms may be involved in different types of neuromodulation. More importantly, this study allows a comparison to be made between the afferent pathways of three types of neuromodulation (tibial, pudendal, and sacral) and their respective mechanisms of action.
Acknowledgments
Funding information
National Institutes of Diabetes and Digestive and Kidney Diseases, Grant numbers: DK-094905, DK-102427, DK-091253
This study is supported by the National Institutes of Diabetes and Digestive and Kidney Diseases under Grants DK-094905, DK-102427, and DK-091253.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
Nothing to disclose.
References
- 1.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt RA, Jonas U, Oleson KA, et al. Sacral nerve stimulation for treatment of refractory urinary urge incontinence. J Urol. 1999;162:352–357. [PubMed] [Google Scholar]
- 4.van Kerrebroeck PEV, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029–2034. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol. 2010;183:1438–1443. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol. 2009;182:1055–1061. doi: 10.1016/j.juro.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 7.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn. 2005;24:643–647. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 8.Peters KM, Killinger KA, Boguslawski BM, Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn. 2010;29:1267–1271. doi: 10.1002/nau.20823. [DOI] [PubMed] [Google Scholar]
- 9.Kessler TM, Fowler CJ. Sacral neuromodulation for urinary retention. Nat Clin Pract Urol. 2008;5:657–666. doi: 10.1038/ncpuro1251. [DOI] [PubMed] [Google Scholar]
- 10.Larson JA, Ogagan PD, Chen G, et al. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol. 2011;589:5833–5843. doi: 10.1113/jphysiol.2011.215657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tai C, Larson JA, Ogagan PD, et al. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and non-nociceptive bladder reflexes in cats. Am J Physiol Renal Physiol. 2012;302:F1090–F1097. doi: 10.1152/ajprenal.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafson KJ, Moffitt MA, Wang X, Sun J, Snyder S, Grill WM. Topography of spinal neurons active during hindlimb withdrawal reflexes in the decerebrate cat. Neurosci. 2006;141:1983–1994. doi: 10.1016/j.neuroscience.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Thor KB, Morgan C, Nadelhaft I, Houston M, de Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989;288:263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F, Zhao S, Shen B, et al. Neural pathways involved in sacral neuromodulation of reflex bladder activity in cats. Am J Physiol Renal Physiol. 2013;304:F710. doi: 10.1152/ajprenal.00334.2012. [DOI] [PubMed] [Google Scholar]
- 15.Mally AD, Matsuta Y, Zhang F, et al. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol. 2013;189:1574–1579. doi: 10.1016/j.juro.2012.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao Z, Rogers MJ, Shen B, et al. Somatic modulation of spinal reflex bladder activity mediated by nociceptive bladder afferent nerve fibers in cats. Am J Physiol Renal Physiol. 2014;307:F673–F679. doi: 10.1152/ajprenal.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyon TD, Ferroni MC, Kadow BT, et al. Pudendal but not tibial nerve stimulation inhibits bladder contractions induced by stimulation of pontine micturition center in cats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R366–R374. doi: 10.1152/ajpregu.00490.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 19.Kullmann FA, Wells GI, Langdale CL, Zheng J, Thor KB. Stability of the acetic acid-induced bladder irritation model in alpha chloralose-anesthetized female cats. PLoS ONE. 2013;8:e73771. doi: 10.1371/journal.pone.0073771. [DOI] [PMC free article] [PubMed] [Google Scholar]