Abstract
Objective
This study aimed to compare the incidence of radiologically unrecognized (occult) hepatocellular carcinoma (HCC) lesions in explant hepatectomy specimens from orthotopic liver transplants (OLTs) performed for HCC with rates of HCC intrahepatic recurrence after resection.
Summary of Background Data
Resection of HCC is associated with high rates of intrahepatic HCC recurrence. However, it is unclear whether these recurrences represent incomplete resection of unrecognized metastatic lesions from the primary tumor or subsequent de novo tumor formation due to inherent biological proclivity for HCC formation.
Methods
We collected patient, tumor, and pathology data on HCC patients treated surgically from 3696 OLTs in the Organ Procurement and Transplantation (OPTN) national database, 299 OLTs at a single transplant center, and 232 partial hepatectomies from a hepatobiliary cancer center.
Results
In the OPTN and high-volume transplant center cohorts, 37% and 42% of patients had occult HCC lesions on explant pathology, respectively. Among cancer center patients, the 2-year recurrence rate was 46%, and 74% of patients who recurred presented with liver only recurrence.
Conclusion
Although the transplant and resection populations differ, occult multifocality is common in transplant explants and similar to the 46% early recurrence rate following partial hepatectomy. These data suggest that non-curative resection often results from occult intrahepatic multifocality present at the time of resection rather than a malignant predisposition of the remnant liver with de novo tumorigenesis.
Keywords: hepatocellular carcinoma, liver transplant, outcomes, resection
Resection of hepatocellular carcinoma (HCC) in cirrhotic patients is commonly performed despite a higher risk of recurrence and decreased survival than orthotopic liver transplantation (OLT), largely because of organ supply limitations and limited access to organs for patients with tumors exceeding parameters for Model of End-Stage Liver Disease (MELD) exception points.1–13 The high risk of recurrence is due to incomplete resection of a primary lesion (positive margin), failure to resect all present tumors in the remaining liver at the time of resection (occult multifocality), and development of de novo tumor after resection attributable to malignant predisposition of the remnant liver, commonly referred to as a “field defect.”14,15
These distinct patterns of recurrence complicate individualized pre-resection assessment of risk of recurrence. Retrospective genetic analyses of HCC lesions can identify recurrences as either clonally related to the primary tumor or clonally distinct and thus de novo and suggest that recurrences due to occult multifocality tend to occur early after resection, whereas de novo tumor formation may occur later.16 Other retrospective studies have identified gene expression patterns in nonmalignant liver parenchyma surrounding HCC lesions that predict risk of late but not early recurrence.15 However, neither the kinetics of recurrence nor modern genetic approaches offer prospective guidance in assessing the risk of HCC recurrence following partial hepatectomy.
The application of transplantation to HCC patients, by contrast, is guided by the Milan17 and UCSF18 criteria, which prospectively define eligibility for OLT on the basis of HCC lesion numbers and sizes on imaging in an effort to optimize post-transplant outcomes. Although divergence between pathological and radiographic assessment of HCC burden is well-recognized in the transplant patient population, established correlation between the radiographic standards of the Milan and UCSF criteria and post-transplant outcomes as well as the complete resection of the liver decreases the clinical relevance of occult multifocality.19–21 In partial hepatectomy, however, inherent tension between achieving complete oncologic clearance of HCC and preserving maximal liver volume and function increases the clinical relevance of occult HCC lesions, especially in the presence of cirrhosis. Cross-sectional imaging often guides decisions between nonanatomic, segmental, or lobar liver resections.
We wished to assess whether the transplant experience in HCC could help inform expected outcomes and possibly informed consent in surgical resection. Specifically, we aimed to answer 3 key questions. First, what it the incidence of occult multifocality in OLT hepatectomy explant specimens? Second, is there evidence of similar occult multifocality in resection patients? Third, do any transplant recipient factors predict an increased risk of occult multifocality?
METHODS
Data Sources
US national data from all OLTs performed for HCC with exception points from January 1, 2012, through December 31, 2014, were obtained through a Standard Transplant Analysis and Research (STAR) file from the United Network for Organ Sharing (UNOS)/ Organ Procurement and Transplantation Network (OPTN). The start date of January 1, 2012, marks the addition of explant pathology data from all HCC patients listed with exception points to the OPTN database. Tumor number was assessed on the basis of pathologist-reported number of distinct lesions. Microsatellitosis reported in the national database was treated as a single lesion.
Single transplant center data were collected from all OLTs performed for preoperatively diagnosed HCC at the University of Pennsylvania between March 1, 2002, and December 31, 2011 (all within the “MELD era”). Listed patients with known HCC were followed with abdominal magnetic resonance imaging (MRI) or computed tomography (CT) if MRI was contraindicated every 3 months; cirrhotic patients listed without known HCC were followed with ultrasound (US), CT, or MR every 6 months. Diffusion-weighted imaging was added to the routine abdominal MRI examination in 2005. Gadoxetate disodium use was introduced in 2008 in selective cases due to concerns about suboptimal arterial-phase imaging and the possibility of increased false-positive findings. Images underwent standard review by 2 radiologists at a tumor selection conference to reduce operator bias. Decisions about the use of locoregional therapy to control progression or downstage a patient were made by a multi-disciplinary tumor board consisting of interventional and diagnostic radiologists, hepatologists, and surgeons. The parenchyma of each explanted liver was serially sectioned at 3- to 5-mm intervals and examined for number and size of macroscopic HCC lesions; macroscopically identified lesions were examined microscopically with at least 1 cm of surrounding liver by a single hepatopathologist.
For all transplant patients, demographic information, biochemical data, tumor imaging characteristics, hepatectomy explant pathology characteristics, and patient outcomes from each database were collected and analyzed. Patients were excluded if they were less than 18 at time of HCC diagnosis, preoperative radiographic tumor data were missing, the explant pathology data were missing, or HCC was discovered incidentally on explant pathology and not diagnosed preoperatively.
Data were collected from all HCC patients treated with partial hepatectomy at Memorial Sloan Kettering Cancer Center from January 1, 1992, through January 1, 2012. Demographic information, biochemical data, tumor imaging characteristics, resection pathology characteristics, and patient outcomes were collected and analyzed. Exclusion criteria were death within 30 days from resection, loss to follow-up within 30 days from resection, preoperative radiographic tumor size >10 cm, preoperative radiographic tumor size missing, and grossly or microscopically positive margins of the resection (R1/ R2). All patients underwent preoperative CT or MRI of the abdomen and pelvis.
Statistical Analysis
Descriptive and comparative statistics were performed using Stata version 14.0 software (College Station, TX). Continuous variables were compared using the Student t test or Mann-Whitney test, as appropriate by the type of distribution. Categorical variables were compared using χ2 or the Fisher exact test depending on the number of observations. A P value <0.05 was considered significant. Significant variables identified in univariate analysis were entered into a logistic regression model to study associations between clinical characteristics and the risk of HCC. Survival distributions were estimated using the Kaplan-Meier method. Associations with death and recurrence were analyzed by Cox proportional hazards model. Time to event was calculated from date of transplant or resection to date of event. An event for recurrence-free survival (RFS) was defined as tumor recurrence or death. Patients without the event of interest at last follow-up were censored at the time of last follow-up.
RESULTS
Transplant Populations
We identified 4001 patients in the OPTN database who were transplanted with HCC MELD exception points from January 1, 2012, through December 31, 2014, of whom 3696 met inclusion criteria. Three hundred eight HCC patients were transplanted in a single transplant center between 2002 and 2011 of whom 299 met inclusion criteria. Descriptive statistics of each group are detailed in Table 1.
TABLE 1.
Descriptive Statistics of Surgically Treated HCC Groups
| Characteristic | National OLT Data (2012–2014) | OLT Center (2002–2011) | Resection Center (1992–2012) |
|---|---|---|---|
| n = 3696 | n = 299 | n = 232 | |
| Age, y, median (range) | 60 (19–81) | 56 (26–74) | 68 (26–89) |
| Male, % (n) | 77% (2856) | 81% (242) | 71% (164) |
| Biochemical MELD, median (range) | 11 (6–40) | 12 (6–40) | 8 (0–29) |
| MELD >10, % (n) | 57% (2096) | 71% (212) | 20% (45) |
| Single lesion on preoperative imaging | 78% (2872) | 71% (213) | 91% (210) |
| Tumor >5 cm, % (n) | 2% (89) | 9% (28) | 59% (126) |
| AFP >20 ng/mL, % (n) | 33% (1213) | 59% (176) | 45% (94) |
| Waitlist time, mo, median (range) | 6.7 (0.0–188) | 4.1 (0–132) | NA |
| Within Milan Criteria by Imaging, % (n) | 95% (3507) | 86% (258) | 41% (96) |
| Within UCSF criteria by imaging, % (n) | 97% (3592) | 96% (286) | |
| Etiology of liver disease, % (n) | |||
| HCV | 59% (2188) | 69% (205) | 22% (52) |
| HBV | 5% (190) | 9% (26) | 23% (54) |
| Alcohol | 8% (302) | 6% (19) | 12% (29) |
| PSC/PBC/AIH | 2% (89) | 2% (5) | 0% (0) |
| NASH/Cryptogenic | 11% (398) | 15% (44) | 1% (1) |
| Other/None | 14% (529) | 0% (0) | 42% (96) |
| Last pre-OLT imaging modality, % (n)* | |||
| MR | 61% (2260) | 88% (194) | 76% (177) |
| CT | 38% (1410) | 11% (24) | 20% (46) |
| US | 0% (0) | 1% (2) | 4 (9) |
| Received regional treatment of HCC, % (n) | 51% (1880) | 71% (212) | 7% (18) |
| TACE | 40% (1491) | 52% (155) | 7% (16) |
| RFA | 11% (392) | 12% (36) | 1% (2) |
| Liver resection | 1% (27) | 7% (21) | NA |
AFP indicates alpha fetoprotein; AIH, autoimmune hepatitis; CT, computed tomography; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; MR, magnetic resonance; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; OLT, orthotopic liver transplant; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; UCSF, University of California in San Francisco; US, ultrasound.
Not recorded in 79 single OLT center patients.
Incidence of Occult Multifocal HCC
In the OPTN cohort, the incidence of occult multifocality on explant pathology was 37%. The single-center data on occult multi-focality had a similar rate of 42%. The median (range) number of additional “unidentified” lesions observed in patients with occult multifocality was 2 (1 to 6) in the OPTN cohort and 3 (1 to 20) in the single transplant center cohort. In the OPTN cohort, 95% of patients were within Milan criteria by imaging on their last pre-operative study and 69% were within an extrapolated version of Milan criteria as applied to the pathologic analysis. In the OLT center cohort, 86% of patients were within Milan criteria by imaging and 71% were within Milan criteria by pathology. The incidence of occult multifocality did not change in the years encompassing the addition of diffuse-weighted imaging or selective gadoxetate administration to MR protocols in 2005 and 2008, respectively (P = 0.76). Detailed tumor characteristics including disease burden on preoperative imaging are described in Table 2.
TABLE 2.
Incidence of Occult Multifocality in OLT Patients
| Characteristic | All Patients
|
MELD ≤10 and Single Tumor
|
||
|---|---|---|---|---|
| National Data (2012–2014) | OLT Center (2002–2011) | National Data (2012–2014) | OLT Center (2002–2011) | |
| n = 3696 | n = 299 | n = 1287 | n = 61 | |
| Occult Multifocality, % (n) | 37% (1342) | 42% (127) | 35% (452) | 43% (26) |
| Preoperative tumor Number, median (range) | 1 (1–5) | 1 (0–5) | 1 | 1 |
| Preoperative largest lesion (cm), median (range) | 2.5 (0.4–14.6) | 2.5 (0.7–7) | 2.4 (0.4–14.6) | 2.9 (0.9–6) |
| Number of additional lesions, median (range) | 2 (1–6) | 3 (1–20) | 1 (1–5) | 2 (2–10) |
MELD indicates Model for End-Stage Liver Disease; OLT, orthotopic liver transplant.
To assess the incidence of occult multifocality in a subset of better-compensated transplant recipients who would have been more likely to qualify for HCC resection, we analyzed the subgroup of OLT recipients with a MELD ≤10 and a single tumor on pre-operative imaging. The incidence of occult multifocal HCC among this OPTN subgroup (35%) was not different from patients not included in the subgroup (P = 0.27). Similarly, in OLT center cohort, the proportion of occult multifocality in this subgroup was of 43% and not different from patients who did not meet the more selective criteria (P = 0.98).
Risk Factors for Occult Multifocal HCC
Factors associated with risk of occult multifocality on explant pathology from the OPTN database are summarized in Table 3. Male sex [RR: 1.36; 95% confidence interval (95% CI) 1.21–1.53], single lesion on preoperative imaging (RR: 1.15; 95% CI 1.03–1.28), biochemical MELD >10 (RR: 1.14; 95% CI 1.04–1.24), and previous locoregional treatment (RR: 1.22; 95% CI 1.12–1.33) were significantly associated with an increased risk of occult multifocality. Of note, neither large tumor size (≥5 cm) nor tumor burden outside the Milan or UCSF criteria significantly increased risk of multifocality using OPTN data. There was no association between imaging modality and the risk of occult multifocality.
TABLE 3.
OLT Recipient Factor Associations with Occult Multifocal HCC on Univariate Analysis.
| National Data (2012–2014)
|
OLT Center (2002–2011)
|
|||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% C.I. | P | |
| Male | 1.36 | 1.21–1.53 | <0.001 | 0.96 | 0.75–1.45 | 0.81 |
| Age ≥60 y | 1.04 | 0.96–1.13 | 0.36 | 0.96 | 0.76–1.22 | 0.77 |
| History of prior malignancy | 1.07 | 0.56–2.04 | 0.84 | |||
| Single lesion on preoperative imaging | 1.15 | 1.03–1.28 | 0.01 | 1.27 | 0.92–1.74 | 0.13 |
| Tumor >5 cm | 1.13 | 0.87–1.45 | 0.37 | 1.44 | 1.09–1.90 | 0.05 |
| MELD >10 | 1.14 | 1.04–1.24 | 0.003 | 1.18 | 0.87–1.60 | 0.28 |
| AFP >20 ng/mL | 1.05 | 0.96–1.14 | 0.32 | 1.1 | 0.89–1.38 | 0.36 |
| Waitlist time >12 mo | 1.03 | 0.94–1.13 | 0.56 | 1.02 | 0.80–1.30 | 0.9 |
| Outside Milan Criteria | 1.18 | 0.98–1.42 | 0.10 | 1.34 | 1.06–1.69 | 0.03 |
| Outside UCSF criteria | 1.25 | 0.98–1.61 | 0.10 | 1.31 | 0.90–1.91 | 0.24 |
| Etiology of liver disease | ||||||
| HCV | 1.06 | 0.97–1.15 | 0.22 | 0.97 | 0.77–1.22 | 0.79 |
| HBV | 0.94 | 0.76–1.15 | 0.52 | 1.01 | 0.69–1.46 | 0.97 |
| Alcohol | 0.96 | 0.81–1.12 | 0.58 | 0.87 | 0.54–1.42 | 0.58 |
| PSC/PBC/AIH | 0.82 | 0.59–1.13 | 0.17 | 1.12 | 0.54–2.32 | 0.77 |
| NASH/Cryptogenic | 1.05 | 0.92–1.20 | 0.45 | 1.11 | 0.82–1.51 | 0.51 |
| Other | 0.94 | 0.83–1.06 | 0.32 | NA | NA | NA |
| Last pre-OLT imaging modality | ||||||
| MR | 0.93 | 0.85–1.01 | 0.10 | 0.98 | 0.78–1.22 | 0.83 |
| CT | 1.07 | 0.99–1.17 | 0.1 | 1.01 | 0.69–1.46 | 0.97 |
| US | NA | NA | NA | 0.93 | 0.23–3.75 | 0.92 |
| Received regional treatment of HCC | 1.22 | 1.12–1.33 | <0.001 | 0.97 | 0.77–1.222 | 0.79 |
AFP indicates alpha fetoprotein; AIH, autoimmune hepatitis; CT, computed tomography; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; NASH, nonalcoholic steatohepatitis; MR, magnetic resonance; OLT, orthotopic liver transplant; PBS, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; UCSF, University of California in San Francisco; US, ultrasound.
Covariates that were significantly associated with increased or decreased risk of multifocality were examined in a logistic regression model to assess risk of multifocality on the explant. All significant variables identified on univariate analysis remained significant in multivariate logistic analysis. The overall predictive value of this model was limited, with a c statistic on 0.58.
Assessment of variables associated with an increased risk of multifocality was also performed in the single transplant center cohort (Table 4). In this database, primary tumor size >5 cm (RR 1.44; 95% CI 1.09–1.90) and tumor beyond Milan criteria (RR 1.34; 95% CI 1.06 – 1.69) were significantly associated with an increased risk of occult multifocality (Table 3).
TABLE 4.
Multivariate Model of OLT Recipient Factors Significantly Associated With Occult Multifocal HCC in the OPTN National Database
| Characteristic | OR | 95% CI | P |
|---|---|---|---|
| Male | 1.61 | 1.37–1.92 | <0.001 |
| Single lesion on preoperative imaging | 1.29 | 1.09–1.52 | <0.001 |
| MELD >10 | 1.29 | 1.12–1.48 | 0.003 |
| Received regional treatment of HCC | 1.41 | 1.23–1.62 | <0.001 |
AFP indicates alpha fetoprotein; MELD, Model for End-Stage Liver Disease; OLT, Orthotopic Liver Transplant; RFA, radiofrequency ablation.
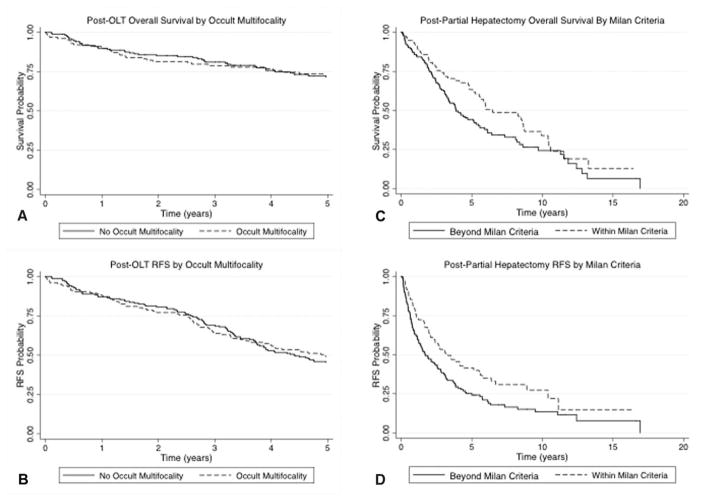
Overall and RFS in the single transplant center cohort at 2 years were 83% (95% CI 79–87) and 80% (95% CI 75–84), respectively, with a median follow-up of 4.6 (2.5–7.4) years and were not significantly impacted by the presence of occult multifocality (Figs. 1A, B). Patients outside of Milan criteria by either imaging [hazard ratio (HR) 2.17; 95% CI 1.31–3.59; P = 0.003] or pathology (HR 2.11; 95% CI 1.37–3.26; P = 0.001) had significantly reduced RFS.
FIGURE 1.
Survival and recurrence after OLT and partial hepatectomy. After OLT, overall survival (A) and RFS (B), and recurrence are not impacted by presence of occult multifocality in OLT patients (P = 0.45 and 0.76, respectively). After partial hepatectomy, patients beyond MC had worse overall survival (C) and RFS (D) than patients within MC (P = 0.03 and P = 0.006, respectively). Patients with HCC within the Milan criteria had significant lower rates of recurrence after partial hepatectomy than those beyond Milan criteria (P = 0.006).
HCC Resection
Two hundred thirty-two patients underwent partial hepatectomy with complete resection of HCC lesions (R0) at the single resection center between 1992 and 2011 and met inclusion criteria. Median follow-up among the patients included in the analysis was 45 months.
The patients in the resection cohort differed in several substantial ways from patients in the OLT cohorts (Table 1). Resection patients were older, more likely to have a single HCC lesion on pre-operative imaging, had lower rates of HCV positivity, higher rates of HCC without identified liver disease, lower rates of cirrhosis, and lower rates of MELD ≥10.
The majority of resection patients (59%; n = 136) were beyond Milan criteria by preoperative imaging and would have therefore been unlikely to meet selection criteria for transplantation, highlighting intrinsic differences between the cohorts. Resection patients were, therefore, stratified by Milan criteria and compared (Table 5 and Supplemental Table 1, http://links.lww.com/SLA/B170). Patients within Milan criteria had significantly increased proportions of viral etiologies of their primary liver disease (58% vs 37%; P = 0.02), increased cirrhosis on preoperative imaging and pathology (40% vs 27%; P = 0.05 and 40% vs 20%; P < 0.001, respectively), decreased median tumor size (3.5 vs 7.0; P < 0.001), and decreased rates of single tumor on preoperative imaging (85% vs 97%; P = 0.006).
TABLE 5.
HCC Resection Characteristics Stratified by Preoperative Radiographic Milan Criteria
| Characteristic | All Patients | Patients Stratified by MC
|
P | Patients Stratified by Cirrhosis
|
P | ||
|---|---|---|---|---|---|---|---|
| Beyond MC | Within MC | No Cirrhosis | Cirrhosis | ||||
|
|
|
||||||
| n = 232 | n = 136 | n = 96 | n = 140 | n = 66 | |||
| Resection period, % (n) | |||||||
| 1992–1998 | 22% (51) | 21% (29) | 23% (22) | 22% (31) | 30% (20) | ||
| 1999–2005 | 32% (75) | 36% (49) | 27% (26) | 0.3 | 33% (46) | 41% (27) | 0.08 |
| 2006–2012 | 46% (106) | 43% (58) | 50% (48) | 45% (63) | 29% (19) | ||
| Female, % (n) | 29% (68) | 40% (42) | 27% (26) | 0.5 | 32% (45) | 26% (17) | 0.4 |
| Age, y, median (range) | 68 (26–89) | 68 (26–89) | 67 (32–85) | 0.4 | 68 (26–89) | 65 (37–79) | 0.07 |
| History of prior malignancy, % (n) | 25% (58) | 23% (31) | 28% (27) | 0.4 | 24% (33) | 27% (18) | 0.6 |
| Previous liver resection, % (n) | 5% (10) | 2% (2) | 8% (8) | 0.06 | 5% (7) | 5% (3) | 0.9 |
| MELD ≥10 | 20% (45) | 24% (31) | 15% (14) | 0.1 | 19% (26) | 20% (12) | 0.9 |
| AFP, ng/mL, median (range) | 14 (0–209,000) | 14 (0–209,000) | 14 (2–27,703) | 0.5 | 9 (0–209,000) | 29 (2–143,416) | 0.4 |
| AFP >20 ng/mL | 45% (94) | 45% (57) | 44% (37) | 0.9 | 39% (49) | 59% (34) | 0.01 |
| Etiology of primary liver disease, % (n) | |||||||
| HCV | 22% (52) | 17% (23) | 30% (29) | 14% (19) | 32% (21) | ||
| HBV | 23% (54) | 20% (27) | 28% (27) | 20% (28) | 35% (23) | ||
| Alcohol | 12% (29) | 12% (17) | 13% (12) | 0.02 | 13% (18) | 15% (10) | <0.001 |
| PSC/PBC/AIH | 0 | 0 | 0 | 0 | 0 | ||
| NASH/Cryptogenic | 1% (1) | 1% (1) | 0 | 0 | 0 | ||
| Other/None | 42% (96) | 50% (68) | 29% (28) | 54% (75) | 18% (12) | ||
| Child-Pugh Score*, median (range) | 5 (5–8) | 5 (5–8) | 5 (5–8) | 0.4 | 5 (5–8) | 5 (5–6) | 0.5 |
| Child-Pugh Score*, % (n) | |||||||
| A | 96% (212) | 95% (122) | 98% (90) | 0.3 | 96% (129) | 100% (61) | 0.3 |
| B | 4% (9) | 5% (7) | 2% (2) | 4% (5) | 0 | ||
| Preoperative cirrhosis†, % (n) | 32% (66) | 27% (34) | 40% (32) | 0.05 | NA | NA | NA |
| Preoperative tumor size, cm, median (range) | 5.4 (1–10) | 7 (3–10) | 3.5 (1–5) | <0.001 | 6.2 (1–10) | 4.8 (1.1–10) | 0.002 |
| Preoperative single tumor, % (n) | 91% (210) | 85% (117) | 97% (93) | 0.006 | 95% (133) | 82% (54) | 0.002 |
| Major hepatectomy, % (n)‡ | 37% (86) | 51% (69) | 18% (17) | <0.001 | 48% (67) | 21% (14) | <0.001 |
| Pathology | |||||||
| Tumor size, cm, mean (range) | 5.5 (0.9–16) | 7 (1–16) | 3.5 (1–13) | <0.001 | 6.5 (1.5–16) | 5 (1–12) | <0.001 |
| Single tumor, % (n) | 87% (201) | 82% (112) | 93% (89) | 0.02 | 86% (120) | 91% (60) | 0.3 |
| Cirrhosis present | 28% (65) | 20% (27) | 40% (38) | <0.001 | 11% (16) | 59% (39) | <0.001 |
| Within Milan criteria | 44% (102) | 17% (23) | 82% (79) | <0.001 | 34% (48) | 56% (37) | 0.003 |
| Outcome | |||||||
| Recurrence status, % (n) | |||||||
| Yes | 55% (127) | 60% (81) | 48% (46) | 52% (73) | 70% (46) | ||
| No | 37% (87) | 30% (41) | 48% (46) | 0.01 | 40% (56) | 20% (13) | 0.02 |
| Unknown | 8% (18) | 10% (14) | 4% (4) | 8% (11) | 11% (7) | ||
| Survival | |||||||
| Follow-up, mo, median (range) | 45 (1–203) | 41 (1–203) | 56 (1–197) | NA | 55 (1–200) | 44 (1–203) | NA |
| Follow-up for survivors, mo, median (range) | 58 (2–200) | 60 (2–200) | 58 (5–197) | NA | 69 (2–200) | 73 (43–135) | NA |
| Overall survival, mo, median (95% CI) | 62 (48–76) | 47 (34–59) | 78 (46–110) | 0.03 | 78 (51–104) | 45 (34–56) | 0.006 |
| RFS, mo, median (95% CI) | 29 (22–36) | 20 (12–29) | 39 (23–56) | 0.006 | 34 (21–47) | 20 (12–28) | 0.002 |
| 2-year RFS, % (95% CI) | 54 (47–61) | 47 (39–56) | 64 (54–74) | 0.006 | 59 (51–67) | 42 (30–54) | 0.002 |
AFP indicates alpha fetoprotein; AIH, autoimmune hepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; NASH, nonalcoholic steatohepatitis; PBS, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; RFS, recurrence-free survival.
Not recorded in 11 patients.
Not recorded in 26 patients.
Major hepatectomy consisted of resection of ≥3 liver segments.
Because many resection patients did not have pre-operative diagnoses of cirrhosis, subgroup analysis was also performed in partial hepatectomy patients with cirrhosis (Table 5 and Supplemental Table 1, http://links.lww.com/SLA/B170). Compared with noncirrhotic resection patients, patients with cirrhosis had higher incidence of elevated AFP (59% vs 39%, P = 0.01), higher incidence of viral liver disease (P < 0.001), smaller median tumor size (4.8 vs 6.2 cm, P = 0.002), and decreased incidence of solitary liver lesions (82% vs 95%, P < 0.001). They were less likely to undergo a major hepatectomy (21% vs 48%, P = 0.003).
In all resection patients, the 2-year RFS was 54% (95% CI 47–61) and the 3-year RFS was 45% (95% CI 38–51). Within the subgroup of patients with MELD ≤10 and a single tumor on pre-operative imaging, 2-year RFS was 61% (95% CI 53–69). Seventy-four percent (n = 67) of recurrences occurred in the liver only. Overall and RFS was similar in patients who underwent major hepatectomies (resection of ≥3 liver segments) and those who had less extensive resection (median of 71 vs 61 months, P = 0.90; and 31 vs 27, P = 0.80, respectively). Median overall and RFS was superior in patients within Milan criteria (78 vs 47 months, P = 0.03; and 39 vs 20 months, P = 0.06; respectively; Figs. 1C, D) and in patients without cirrhosis (78 vs 45 months, P = 0.006; and 34 vs 20 months, P = 0.002). Lymphadenectomy was performed in 40 cases; 1 patient had metastatic HCC in the resected lymph nodes.
DISCUSSION
In this study, the incidence of occult multifocality HCC lesions identified on explant hepatectomy specimens from 2 cohorts of liver transplant patients was 37% to 42% and closely corresponded to the 46% incidence of 2-year HCC recurrence observed in partial hepatectomy patients at a high volume resection center. This similarity was essentially equivalent in the more comparable subgroup of patients with MELD ≤10 and a single tumor on pre-operative imaging from each cohort; within this subgroup, transplant patients had a 35% to 43% incidence of occult multifocality and partial hepatectomy patients had a 39% rate of 2-year HCC recurrence. HCC recurrence can result from multiple mechanisms, but the similar incidence of occult lesions in OLT patients and early trajectory of recurrence in resection patients suggests that residual tumor burden after resection is the important contributor.
This observation suggests that occult multifocal HCC is of critical significance in resection candidates. Although transplantation generally results in clearance of all intrahepatic HCC, recognized or not, the anatomic parameters of resections are often dictated by the pre-operative imaging. Undetected HCC lesions may be left behind, particularly in cirrhotic patients for whom minimization of resection volume is required. Although the patients treated by resection and transplant in this study differed in important ways, our subgroup analysis of transplant patients with a MELD ≤10 and a solitary HCC lesion on pre-operative imaging revealed no reduction in the incidence of occult multifocality, indicating that occult multifocality is not a phenomenon restricted to high MELD or multifocal tumor patients. In discussing risks of recurrence with patient pre-operatively, the difference between a malignant predisposition in the remaining liver leading to an increased risk of new tumor at a later time and a 35% to 45% chance of leaving existing tumor behind at the time of resection is significant.
Despite the high incidence of occult multifocal HCC, prognostic tools to predict its presence remain inadequate. Our analysis identified several variables significantly associated with an increased risk of multifocality in the national data (male sex, single lesion on pre-operative imaging, MELD >10, and previous locoregional treatment of HCC). Most of these factors also displayed a tendency toward increased risk of occult multifocality in the single center cohort, although they fail to meet the threshold for significance in this smaller group of patients. However, even the combined logistic regression model using these variables had poor predictive power (c statistic of 0.58), belying the weakness of the individual associations and the limited utility to aid the clinical decisions about which modality to offer early-stage HCC patients.
This study has several noteworthy limitations. First and foremost, the 3-database design of this research used independent data sets that are not directly comparable and which have selection criteria that are not totally analogous. However, analysis of the data from each set independently points to the same conclusion that unrecognized HCC is common in patients regardless of which treatment modality (resection or transplantation) is applied. The nature of the surgical procedures intrinsically prevents the direct comparison of equivalent groups and tumor explant specimens, as no randomized trial of resection versus transplant is feasible and resection by definition does not allow for total examination of the remaining liver. Second, a cohort of resection patients did not have imaging or pathologic evidence of cirrhosis. The transplant population did not yield explants from noncirrhotic patients and therefore the incidence of occult multifocality maybe different in the noncirrhotic population. Third, the epidemiology and etiology of HCC may change substantially with new approaches to HCV treatment. These shifts may affect recurrence patterns and multifocality. Previous work has described increased post-OLT recurrence rates in HCV and HCC patients compared with HCV-HCC patients as well as higher rates of recurrence following hepatectomy.22,23 The progression from HCV infection to HCC development occurs over years, and the effects of new antiviral medication on HCC will likely only become clear after years of widespread use.
The data presented in this paper reinforce the case for transplantation to be considered as the primary option for early-stage HCC patients with cirrhosis. The superior oncologic outcomes of liver transplantation compared with resection for HCC in cirrhotic patients are well documented, and the recognition that a partial hepatectomy may fail to achieve gross oncologic clearance in nearly 35% to 45% of patients despite complete resection of identified HCCs lesions strengthens the understanding of this benefit. With limited organ supply, resection will remain an essential therapy for many HCC patients. More extensive use of living donor liver transplantation has the potential to expand access to OLT for HCC patients, although some prior studies have raised concerns of worse oncologic outcomes after living donation compared with deceased donation.24–26 These tumor-specific outcomes differences are relatively small compared with the outcomes difference between transplantation and resection and this study may encourage a more focused pursuit of live donor liver transplantation to help achieve more complete surgical HCC clearance. We believe that this study may alter how the risk of postresection recurrence is described to patients to facilitate fully informed consent and selection of the optimal procedure for each patient.
Acknowledgments
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers K08-DK092282 (PI: MHL) and R01-DK106243 (PI: MHL) and Health Resources and Services Administration contract 234-2005-37011C.
Footnotes
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
There are no conflicts of interest to report.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
References
- 1.Esquivel CO. Is liver transplantation justified for the treatment of HCC in Child’s A patients? Not always. Liver Transpl. 2003;9:521–522. doi: 10.1053/jlts.2003.50112. [DOI] [PubMed] [Google Scholar]
- 2.Pawlik TM. Debate: resection for early hepatocellular carcinoma. J Gastrointest Surg. 2009;13:1026–1028. doi: 10.1007/s11605-008-0779-1. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 4.Hanazaki K, Kajikawa S, Shimozawa N, et al. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381–388. doi: 10.1016/s1072-7515(00)00700-6. [DOI] [PubMed] [Google Scholar]
- 5.Ramacciato G, Mercantini P, Corigliano N, et al. Hepatic resections for hepatocellular carcinoma (HCC): short and long-term results on 106 cirrhotic patients. J Exp Clin Cancer Res. 2003;22(4 suppl):233–241. [PubMed] [Google Scholar]
- 6.Shimozawa N, Hanazaki K. Longterm prognosis after hepatic resection for small hepatocellular carcinoma. J Am Coll Surg. 2004;198:356–365. doi: 10.1016/j.jamcollsurg.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Yeh CN, Chen MF, Lee WC, et al. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol. 2002;81:195–202. doi: 10.1002/jso.10178. [DOI] [PubMed] [Google Scholar]
- 8.Belghiti J, Panis Y, Farges O, et al. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114–117. doi: 10.1097/00000658-199108000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–543. doi: 10.1097/01.SLA.0000059988.22416.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grazi GL, Ercolani G, Pierangeli F, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71–78. doi: 10.1097/00000658-200107000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Chen Y, Wurmbach E, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758–1767. doi: 10.1053/j.gastro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 13.Cha CH, Ruo L, Fong Y, et al. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg. 2003;238:315–321. doi: 10.1097/01.sla.0000086548.84705.ef. discussion 321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feo F, Pascale RM. Multifocal hepatocellular carcinoma: intrahepatic metastasis or multicentric carcinogenesis? Ann Transl Med. 2015;3:4. doi: 10.3978/j.issn.2305-5839.2014.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelstein SD, Marsh W, Demetris AJ, et al. Microdissection-based allelo-typing discriminates de novo tumor from intrahepatic spread in hepatocellular carcinoma. Hepatology. 2003;37:871–879. doi: 10.1053/jhep.2003.50134. [DOI] [PubMed] [Google Scholar]
- 17.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 18.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharjya S, Bhattacharjya T, Quaglia A, et al. Liver transplantation in cirrhotic patients with small hepatocellular carcinoma: an analysis of pre-operative imaging, explant histology and prognostic histologic indicators. Dig Surg. 2004;21:152–159. doi: 10.1159/000078741. discussion 159–160. [DOI] [PubMed] [Google Scholar]
- 20.Burrel M, Llovet JM, Ayuso C, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034–1042. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 21.Freeman RB, Mithoefer A, Ruthazer R, et al. Optimizing staging for hepatocellular carcinoma before liver transplantation: a retrospective analysis of the UNOS/OPTN database. Liver Transpl. 2006;12:1504–1511. doi: 10.1002/lt.20847. [DOI] [PubMed] [Google Scholar]
- 22.Bozorgzadeh A, Orloff M, Abt P, et al. Survival outcomes in liver transplantation for hepatocellular carcinoma, comparing impact of hepatitis C versus other etiology of cirrhosis. Liver Transpl. 2007;13:807–813. doi: 10.1002/lt.21054. [DOI] [PubMed] [Google Scholar]
- 23.Hanazaki K, Wakabayashi M, Sodeyama H, et al. Surgical outcome in cirrhotic patients with hepatitis C-related hepatocellular carcinoma. Hepato-gastroenterology. 2000;47:204–210. [PubMed] [Google Scholar]
- 24.Lo CM, Fan ST, Liu CL, et al. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007;94:78–86. doi: 10.1002/bjs.5528. [DOI] [PubMed] [Google Scholar]
- 25.Fisher RA, Kulik LM, Freise CE, et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7:1601–1608. doi: 10.1111/j.1600-6143.2007.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park MS, Lee KW, Suh SW, et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation. 2014;97:71–77. doi: 10.1097/TP.0b013e3182a68953. [DOI] [PubMed] [Google Scholar]